Abstract
Background
Insulin resistance is a causal factor in pre-diabetes and type 2 diabetes (T2D), and also increases the risk of developing Alzheimer’s disease (AD). Reductions in cerebral glucose metabolic rate (CMRglu) as measured by fluorodeoxyglucose positron emission tomography (FDG PET) in parietotemporal, frontal, and cingulate cortex are also associated with increased AD risk, and can be observed years before dementia onset.
Objectives
We examined whether greater insulin resistance as indexed by the homeostasis model assessment (HOMA-IR) would be associated with reduced resting CMRglu in areas known to be vulnerable in AD in a sample of cognitively normal adults with newly diagnosed pre-diabetes or T2D (P-D/T2D). We also determined whether P-D/T2D adults have abnormal patterns of CMRglu during a memory encoding task.
Design
Randomized crossover design of resting and activation [F-18] FDG-PET.
Setting
University Imaging Center and VA Clinical Research Unit.
Participants
Participants included 23 older adults (mean age±SEM=74.4±1.4) with no prior diagnosis of or treatment for diabetes, but who met American Diabetes Association glycemic criteria for pre-diabetes (n=11) or diabetes (n=12) based on fasting or 2-h oral glucose tolerance test (OGTT) glucose values, and 6 adults (mean age±SEM=74.3±2.8) with normal fasting glucose and glucose tolerance. No participant met Petersen criteria for mild cognitive impairment (MCI).
Intervention
Fasting participants rested with eyes open in a dimly lit room and underwent resting and cognitive activation [F-18]FDG PET imaging on separate days, in randomized order, at 9 am. Following a 30-min transmission scan, subjects received an intravenous injection of 5 mCi [F-18]FDG, and the emission scan commenced 40 min post-injection. In the activation condition, a 35-min memory encoding task was initiated at the time of tracer injection. Subjects were instructed to remember a repeating list of 20 words that were randomly presented in series through earphones. Delayed free recall for items on the word list was assessed once the emission scan was complete.
Main Outcome Measures
HOMA-IR was calculated for each participant using fasting glucose and insulin values obtained during OGTT screening, and then correlated with CMRglu values obtained during the resting scan. Resting CMRglu values were also subtracted from CMRglu values obtained during the memory encoding/activation scan to examine task-related patterns of CMRglu.
Results
Greater insulin resistance as indexed by HOMA-IR was associated with an AD-like pattern of reduced CMRglu in frontal, temporal-parietal, and cingulate regions in adults with P-D/T2D. The relationship between CMRglu and HOMA-IR was independent of age, 2-h OGTT glucose concentration, or apolipoprotein E-ε4 allele carriage. During the memory encoding task, normal adults showed activation in right anterior and inferior prefrontal cortex, right inferior temporal cortex, and medial and posterior cingulate regions. Compared to the normal group, adults with P-D/T2D showed a different pattern during the memory encoding task, characterized by more diffuse and extensive activation, and recalled fewer items on the delayed memory test.
Conclusions
Our results suggest that insulin resistance may be a marker of AD risk that is associated with reduced CMRglu and subtle cognitive impairments at the earliest stage of disease, even before the onset of MCI.
Keywords: Alzheimer’s disease, FDG PET, insulin, insulin resistance, diabetes, pre-diabetes, memory
BACKGROUND
Insulin modulates numerous physiologic actions in the periphery and central nervous system (CNS) that are related to late-life neurodegenerative disease, including glucose metabolism, vascular function, synaptic maintenance, β-amyloid regulation, and tau phosphorylation.1 Insulin resistance, a condition characterized by an insufficient response to insulin in target tissues, is a causal factor in pre-diabetes (P-D) and type 2 diabetes (T2D), and also increases the risk of developing Alzheimer’s disease (AD).1 Reductions in regional cerebral glucose metabolic rate (CMRglu) as measured by fluorodeoxyglucose positron emission tomography (FDG PET) are also associated with increased AD risk, and can be observed years before dementia onset.2,3 A recent large study comparing FDG PET patterns in normal adults and adults with prodromal AD (amnestic mild cognitive impairment, aMCI) demonstrated lower CMRglu in posterior cingulate, precuneous, parietotemporal and frontal cortices.4 Similar patterns have been reported in cognitively normal carriers of the apolipoprotein E- ε4 allele AD risk factor (APOE-ε4).5
Insulin regulates aspects of cerebral glucose metabolism, including basal CMRglu as well as glucose uptake within the hippocampus and cortex.6,7 These regions play an important role in memory, and thus it is not surprising that inducing insulin resistance in animal models and thereby disrupting cerebral insulin function causes memory impairment, a hallmark symptom of AD and a frequently reported characteristic of T2D in older adults.8 In the present study, we examined the hypothesis that, in a sample of cognitively normal adults with newly diagnosed pre-diabetes or T2D (hereafter referred to as P-D/T2D), greater insulin resistance as indexed by the homeostasis model assessment (HOMA-IR) would be associated with reduced resting CMRglu in areas known to predict AD vulnerability. We also determined whether adults with P-D/T2D have abnormal patterns of CMRglu during a memory encoding task.
METHODS
Participants
All study procedures were approved by the Institutional Review Boards of the University of Washington and the Veterans Affairs Puget Sound Health Care System (VAPSHCS) and written informed consent was obtained from all participants prior to study enrollment. Participants were solicited through community advertising and underwent oral glucose tolerance testing (OGTT), physical examination and neuropsychological assessment. Participants were excluded if they had previously received a diagnosis of diabetes or had ever been treated with a medication for diabetes, or if they met criteria for mild cognitive impairment (MCI) as described by Petersen.9 Subjects with neurological conditions, uncontrolled hypertension, cardiac disease or dyslipidemia, renal dysfunction, liver dysfunction, or any other significant health condition were excluded. Twenty-three adults met American Diabetes Association glycemic criteria for pre-diabetes (n=11; 1 with isolated impaired fasting glucose and 10 with impaired glucose tolerance) or diabetes (n=12; all with impaired glucose tolerance) based on fasting or 2-hr OGTT glucose values.10 Six adults with normal fasting glucose and normal glucose tolerance were included in the cohort for comparison. A well-validated index of insulin resistance (homeostasis model assessment of insulin resistance, HOMA-IR) was calculated using fasting glucose and insulin values obtained prior to administration of the beverage for the OGTT.11 Demographic and cognitive characteristics are presented in Table 1. Normal and P-D/T2D groups were comparable for age, BMI, pre-PET fasting glucose level, and all cognitive measures (all ps>0.27).
Table 1.
Sample demographic, metabolic and cognitive characteristics
| Normal | Pre-Diabetes/T2D | |
|---|---|---|
| Age, yrs | 74.33 (6.3) | 74.39 (7.1) |
| BMI, kg/m2 | 28.5 (3.4) | 27 (2.9) |
| Pre-PET fasting blood glucose, mg/dL | 100.95 (6.1) | 107.14 (12.3) |
| 2-h OGTT blood glucose, mg/dL* | 110.66 (14.6) | 187.78 (48.3) |
| Fasting insulin, µU/mL | 9.66 (2.5) | 17.06 (10.3) |
| 2 hour OGTT insulin, µU/mL* | 99 (87.8) | 197.46 (132.2) |
| Dementia Rating Scale score | 139 (2.1) | 137.5 (1.1) |
| Immediate Story Recall | 20.05 (3.3) | 19.39 (1.5) |
| Delayed Story Recall | 19.05 (2.5) | 15.91 (1.2) |
| Immediate Hopkins List Recall | 21.80 (2.3) | 23.91 (1.1) |
| Delayed Hopkins List Recall | 7.2 (1.2) | 8.26 (0.5) |
| Forward Digit Span | 6.0 (0.6) | 6.39 (0.3) |
| Reverse Digit Span | 5.0 (0.5) | 5.13 (0.2) |
| Letter Fluency | 22.8 (2.5) | 21.43 (1.2) |
| Category Fluency | 27.8 (2.9) | 30.22 (1.4) |
| Digit Symbol Total Correct | 57.6 (5.3) | 59.39 (2.5) |
P-D/T2D>normal group, p<0.05.
Procedures
Participants underwent resting and cognitive activation PET imaging using [F-18]FDG on separate days approximately two weeks apart, in counterbalanced order, at 9 am after an overnight fast. Participants were placed in the PET scanner in a supine position, with a venous line maintained for radiotracer injection, and plasma glucose was sampled. Throughout the imaging procedure for both the rest and activation conditions, the head was restrained using a neck-conforming support, and subjects rested silently with eyes open in a dimly lit and quiet room. Following a 30-min transmission scan for attenuation correction, subjects received an intravenous injection of 5 mCi [F-18]FDG. Forty minutes later, the emission scan commenced. In the cognitive activation session, a 35-min cognitive task was administered immediately after the tracer injection. This allowed brain FDG uptake to represent neuronal activity during cognitive task performance.12
The task used in the activation condition was based on previous work by Alkire and colleagues.13 Subjects were instructed to remember a repeating list of 20 words that were randomly presented in series through earphones, at a rate of one word every 3 seconds. The words were 4 to 8 letters in length, of average linguistic frequency, and neutral in emotional valence. Delayed free recall was assessed when the emission scan was complete and the intravenous lines were removed.
PET image sets were co-registered and anatomically standardized to Talairach and Tournoux stereotactic coordinates using NEUROSTAT (University of Washington). Pixel intensity was normalized to global activity and smoothed using a 3-dimensional gaussian kernel (2.25-mm SD) to reduce residual anatomic variances.
Correlations between normalized CMRglu at rest and HOMA-IR values were calculated on a voxelwise basis, and the correlation coefficients were transformed to Z scores (Fisher transformation). Coordinates for which Z values exceeded 3.5 were considered to be significant, controlling type I error rate at approximately P=.05 for multiple comparisons.15 Associations between regional CMRglu values and HOMA-IR, age, APOE-ε4 carriage, fasting plasma glucose levels measured prior to radiotracer injection, and degree of hyperglycemia indexed by 2-h OGTT glucose concentration were examined with correlational and multiple regression analyses.
Group-wise paired subtraction analysis allows the statistical comparison of activation versus a resting baseline in the same subjects. In this study, resting scans were subtracted from activation scans across subjects. One-sample t-statistic values were calculated across subjects for each subtracted pixel value. The calculated t statistic values were then converted to Z-statistic maps using a probability integral transformation.14 The resultant Z-statistic maps represent the extent and significance of task-related brain activity averaged across all subjects in the group.
To determine whether recall performance was associated with CMRglu, Pearson correlations were conducted between delayed recall scores and mean global-normalized CMRglu values of stereotactically defined volumes of interest (VOI) for frontal, temporal, parietal, and posterior cingulate cortices.
RESULTS
Resting CMRglu
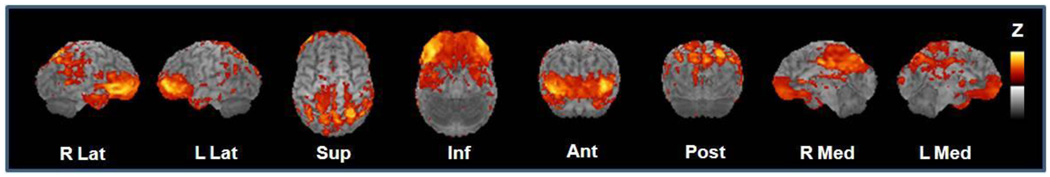
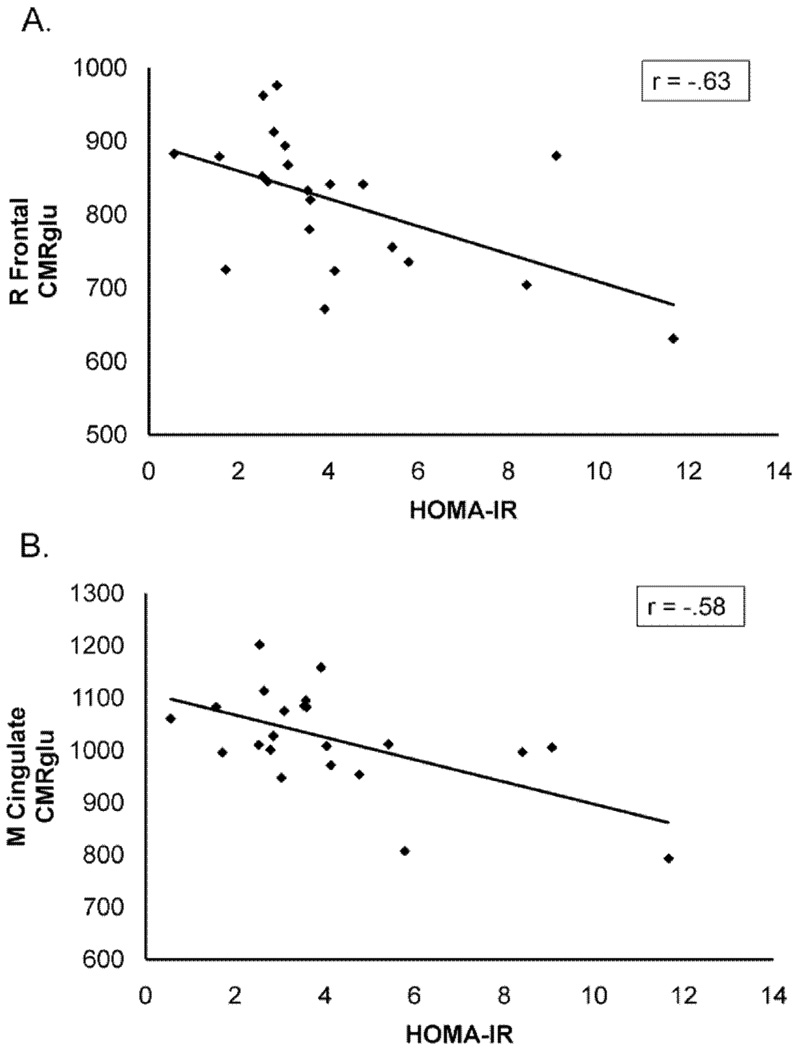
For adults with P-D/T2D, greater insulin resistance, as indexed by HOMA-IR values, was associated with reduced CMRglu in regions known to be affected early in AD, including posterior cingulate cortex, the precuneus region, parietal cortices (Brodmann areas 7 and 40), the temporal/angular gyri (Brodmann area 39), and the anterior and inferior prefrontal cortices (Brodmann areas 10, 45, 47; Figure 1). Sterotactic coordinates for brain regions exhibiting signficant correlations between resting CMRglu and HOMA-IR and the associated significance values are provided in Table 2. Multiple regression analyses indicated that the significant relationship between HOMA-IR and CMRglu for adults with P-D/T2D was unaffected by age, pre-PET fasting glucose values, hyperglycemia (2-h OGTT glucose values) or APOE-ε4 allele carriage. Scatterplots showing the negative relationship between insulin resistance and CMRglu for the P-D/T2D group in two representative areas (frontal cortex and cingulate) are shown in Figure 2. Correlations between CMRglu and HOMA-IR was not significant for normal adults, likely due to the restricted range of HOMA-IR values and small sample size.
Figure 1.
Brain regions in which lower CMRglu was associated with greater insulin resistance as indexed by HOMA-IR. Regions in which the strongest negative associations were observed are represented in yellow.
Table 2.
Talairach and Tournoux stereotactic atlas coordinates and associated p values for brain regions in which lower CMRglu was associated with greater insulin resistance
| Brain Region | Atlas coordinates (mm)3 |
p-value | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Posterior Cingulate/Precuneus | −6 | −76 | 40 | .000365 |
| Medial Cingulate | −3 | −8 | 38 | .000131 |
| R Parietal | −26 | −67 | 50 | .000036 |
| L Temporal | 37 | −55 | 25 | .000142 |
| R Frontal | ||||
| BA10 | −39 | 44 | −2 | .000048 |
| BA45 | −55 | 32 | 0 | .000025 |
| BA47 | −39 | 32 | −4 | .000032 |
| L Frontal | ||||
| BA10 | 35 | 48 | 2 | .000024 |
| BA47 | 42 | 35 | −4 | .000630 |
Figure 2.
Scatterplots for CMRglu and HOMA-IR values in (A) frontal and (B) cingulate cortex for adults with P-D/T2D.
Cognitive Task-Activated CMRglu
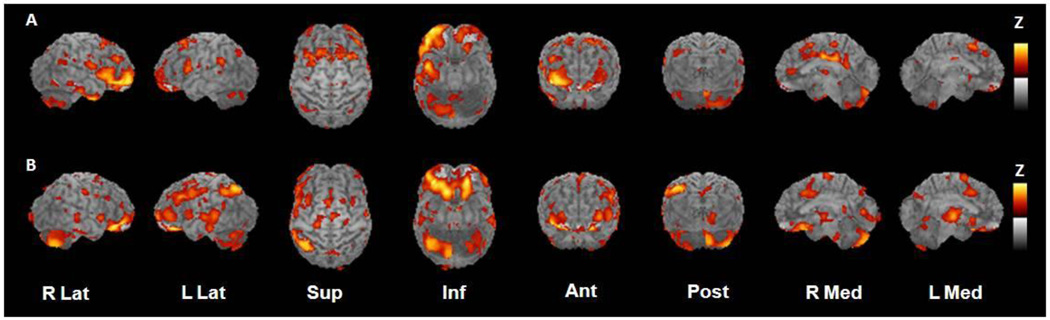
Normal adults showed task-related change in CMRglu activity in right anterior and inferior prefrontal cortex (Brodmann areas 10, 45, 47), right inferior temporal cortex (Brodmann area 20), and medial and posterior cingulate regions (Brodmann areas 23, 24, and 31; Table 3 and Figure 3A). This pattern is consistent with previous imaging results using similar encoding paradigms in normal adults.16 In contrast, the P-D/T2D group had a more widespread pattern of activation including bilateral orbital-medial and inferior prefrontal regions (Brodmann areas 11, 25, 47) that were adjacent to regions activated for normal adults. The P-D/T2D group also showed task-specific activation of subcortical regions (right putamen, left thalamus) and right cerebellar vermis (Figure 3B). Delayed recall for words presented during the activation was recorded after the scan was completed. The normal group recalled more words than did the P-D/T2D group (normal group mean recall±SEM = 19.60±2.45, P-D/T2D group recall = 13.96±1.17, p=0.047). To determine whether recall performance was related to task-related CMRglu for the P-D/T2D, delayed recall scores were correlated with mean global-normalized CMRglu for frontal, temporal, parietal, and posterior cingulate VOIs. Better recall was associated with greater CMRglu for right frontal and posterior cingulate cortices (rs=0.52 and 0.46, ps<0.04) and for left frontal, temporal, and parietal cortices (rs=0.63, 0.47, and 0.51, ps<0.03).
Table 3.
Talairach and Tournoux stereotactic atlas coordinates and associated p values for brain regions activated during the memory encoding task compared with resting baseline.
| Brain Region | Atlas coordinates (mm)3 |
p-value | ||
|---|---|---|---|---|
| X | Y | Z | ||
| NORMAL | ||||
| Medial Cingulate | −8 | −10 | 34 | .000054 |
| R Frontal | ||||
| BA10 | −35 | 57 | −4 | .000003 |
| BA45 | −53 | 26 | 0 | .000093 |
| BA47 | −44 | 44 | −9 | .000002 |
| R Temporal | −44 | 1 | −36 | .000028 |
| P-D/T2D | ||||
| L Frontal | ||||
| BA11 | 21 | 41 | −18 | .000002 |
| BA25 | 19 | 23 | −16 | .000002 |
| R Frontal | ||||
| BA11 | −35 | 41 | −14 | .000007 |
| BA47 | −44 | 50 | −9 | .000017 |
| R Putamen | −24 | 1 | 0 | .000084 |
| R Cerebellum | −37 | −60 | −38 | .000104 |
| L Thalamus | 8 | −13 | 0 | .000172 |
Figure 3.
Statistical parametric maps of task-specific activation for (A) normal adults and (B) adults with P-D/T2D. Maps were constructed by subtracting resting scans from scans obtained while participants performed a memory encoding task. Yellow represents areas of greatest activation.
DISCUSSION
Insulin resistance was associated with a pattern of reduced CMRglu in frontal, temporal-parietal, and cingulate regions in cognitively intact adults with P-D/T2D. This pattern of hypometabolism has also been observed in patients with MCI and AD, in middle-aged non-demented carriers of the APOE-ε4 genetic risk factor, and in pre-symptomatic adults with the AD-causative presenilin-1 gene.5,17 In our sample, the relationship between CMRglu and insulin resistance indexed by HOMA-IR was independent of age, 2-h OGTT glucose concentration, or APOE-ε4 allele carriage. Adults with P-D/T2D also showed a different activation pattern during a memory encoding task compared with normal adults, characterized by more widespread activation. Our participants received careful neuropsychological assessment and were not cognitively impaired according to current criteria for MCI.9 However, their ability to recall words encoded during scanning was reduced relative to adults who were not insulin resistant, despite being of similar age and education levels. Taken together, these results suggest that increased insulin resistance may be a marker of AD risk that is associated with reduced CMRglu and subtle cognitive impairments at the earliest stage of disease, even before the onset of MCI.
Although reduced CMRglu has been reported in several rodent models of diabetes,18,19 few human studies have examined this possibility in T2D, and none in P-D. In an early FDG-PET study in humans, a small sample of Pima Indians with T2D (n=4) showed no overall differences compared with caucasian participants without T2D.20 Regional differences were not examined, however, and the diabetic group was heterogenous with respect to treatment. Nagamachi et al. examined cerebral blood flow (CBF) using SPECT in adults with T2D with no evidence of cerebral infarction on CT.21 They reported reduced CBF in all cortical areas, with prominent reduction in frontal areas for more severely affected patients who were treated with insulin. Generalized reductions in CBF and CMRglu were related to an atrophy index for a group of subjects with microvascular disease and treated diabetes.22 In contrast, participants in our study had milder, newly-diagnosed P-D or T2D, and had never received treatment for diabetes. Thus, the AD-like pattern of hypometabolism we observed is likely related to the underlying pathophysiology of insulin resistance and diabetes, as opposed to secondary effects of diabetic treatment.
The identification of specific metabolic factors that are associated with abnormal CMRglu patterns may elucidate important pathogenetic pathways. Reiman et al. investigated the relationship between cholesterol and CMRglu in late middle-aged adults of varying APOE genotypes.23 They reported an AD-like pattern of temporo-parietal and frontal hypometabolism that was more prominent in APOE-ε4 carriers than in non-carriers. In our study, the relationship between insulin resistance and CMRglu was not mediated by APOE-ε4 carriage status, suggesting that insulin resistance and APOE-ε4 carriage may be independent factors associated with AD-related CMRglu abnormalities. This possibility has been suggested in other studies in which factors related to insulin resistance and APOE genotype have been shown to be independent risk factors for AD. Support for insulin resistance-related CMRglu reductions was also provided by a recent study in which a cognitively mixed group of adults showed reduced frontal CMRglu that was associated with increased cardiovascular risk, as assessed by the Framingham Cardiovascular Risk Profile.24 This index includes components such as diabetes, hypertension, cholesterol, and age, and is strongly related to measures of insulin resistance.25
There are several possible mechanisms through which insulin resistance may affect CMRglu.26 Insulin resistance is associated with reduced insulin levels and/or activity in the CNS, due to reduced transport of insulin across the BBB in the context of chronically elevated peripheral insulin levels, or to reduced CNS insulin signalling. Insulin modulation affects brain glucose utilization in animal models,7,27 and thus reduced insulin levels or activity may interere with this process. Insulin resistance is also associated with impaired cerebrovascular function which may affect glucose delivery to the CNS, even in the absence of frank infarcts.1 Other indirect effects on neurotransmitter modulation may negatively impact glucose utilization.1 A final potential mechanism with direct relevance to AD concerns the relationship between insulin and Aβ. Increased Aβ burden has been linked to reduced CMRglu, and insulin modulates levels of Aβ, in part through its effects on Aβ clearance.1,28
Interesting differences in activation patterns were observed during the memory encoding task for normal and P-D/T2D groups. For normal adults, activation was observed in Brodmann areas 10, 45, and 47 in right frontal cortex, in right inferior temporal cortex, and in medial and posterior cingulate regions. This right-sided lateralization may initially appear surprising given that the encoding paradigm used auditorally-presented verbal stimuli. Several reviews have noted however, that lateralization patterns during encoding differ for older adults and include regions observed in our study.16 Furthermore, selective right prefrontal activation, particularly involving Brodmann area 10, has been noted in a variety of verbal memory tasks.29 These patterns may reflect different strategic approaches to encoding, or recruitment of different regions as a compensatory mechanism for age-related metabolic dysfunction. Additionally, because it was necessary to obtain a resting scan to explore relationships of basal CMRglu with insulin resistance, task activation was compared to a resting state, rather than to a control task that accounted for non-specific attentional and working memory demands; consequently, activation includes cognitive processes in addition to memory encoding, processes that may preferentially activate right hemisphere neurocognitive networks. Use of a matched control task in future studies may more clearly delineate activation patterns due specifically to encoding processes. It is also worth noting that our normal group may be healthier than control groups included in many neuroimaging studies; it is likely that many studies of “normal” older adults include adults with undiagnosed P-D/T2D in their sample, as it is estimated that as more than 50% of adults over the age of 60 with these conditions are unaware of their abnormal glycemic status,30 and screening OGTTs are not routinely administered. The inclusion of such subjects undoubtedly contributes to heterogenous results in neuroimaging studies of older adults.
In contrast to the pattern observed for the normal group, the P-D/T2D group showed more diffuse activation that included bilateral medial and inferior frontal regions that were adjacent to regions activated for normal adults. The P-D/T2D group also showed activation of subcortical regions (right putamen, left thalamus) and right cerebellar vermis. Diffuse activation or hyperactivation of areas not typically engaged in a cognitive task have been reported in adults with prodromal or early AD as well as in non-symptomatic APOE-ε4 carriers, and may be a compensatory mechanism that is invoked following dysfunction of the neuroarchitectural network that typically would support a cognitive task.31,32 A recent meta-analysis reported that patients with AD showed extensive prefrontal activation during memory encoding tasks, including activation in Brodmann area 11, as well as thalamic and cerebellar activation, a pattern very similar to that observed in our P-D/T2D group.33 Postive correlations were also observed between recall and CMRglu for several stereotactically defined globally-normalized VOIs that included frontal, parietal, temporal, and posterior cingulate cortices.
Our results suggest that insulin resistance may be a risk factor for AD in part due to detrimental effects on CMRglu. Screening for insulin resistance may thus provide a relatively low-cost, non-invasive means for identifying adults at risk, as well as providing a rationale for examining the potential benefits of interventions directed at improving insulin resistance. Many such interventions, such as exercise, are low-risk, with numerous documented health benefits, and improve cognitive function in adults with MCI and AD.34 Our results also provide a strong rationale for further study of the mechanisms underlying the association between insulin resistance and reduced CMRglu.
ACKNOWLEDGMENTS
This study was supported by NIDDK RO1 DK061606 (SC) and by the Department of Veterans Affairs. Neither funding source provided scientific input to the study. The first author, Dr. Baker, had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis which was conducted without input from the funding agencies.
REFERENCES
- 1.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009 Mar;66(3):300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mistur R, Mosconi L, Santi SD, et al. Current Challenges for the Early Detection of Alzheimer's Disease: Brain Imaging and CSF Studies. J Clin Neurol. 2009 Dec;5(4):153–166. doi: 10.3988/jcn.2009.5.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997 Jul;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 4.Langbaum JB, Chen K, Lee W, et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009 May 1;45(4):1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996 Mar 21;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 6.Bingham EM, Hopkins D, Smith D, et al. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002 Dec;51(12):3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 7.Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009 Nov 3;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007 Dec 14;282(50):36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004 Sep;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 10.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004 Jan;27 Suppl 1:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg MD, Yoshii F, Vibulsresth S, et al. Human task-specific somatosensory activation. Neurology. 1987 Aug;37(8):1301–1308. doi: 10.1212/wnl.37.8.1301. [DOI] [PubMed] [Google Scholar]
- 13.Alkire MT, Haier RJ, Fallon JH, Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14506–14510. doi: 10.1073/pnas.95.24.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friston KJ, Passingham RE, Nutt JG, Heather JD, Sawle GV, Frackowiak RS. Localisation in PET images: direct fitting of the intercommissural (AC-PC) line. J Cereb Blood Flow Metab. 1989 Oct;9(5):690–695. doi: 10.1038/jcbfm.1989.97. [DOI] [PubMed] [Google Scholar]
- 15.Worsley K, Marrett S, Neelin P, Evans A. Quantification of Brain Function Using PET. San Diego: Academic Press; 1996. A unified statistical approach for determining significant signals in location and scale space images of cerebral activation; pp. 327–333. [Google Scholar]
- 16.Beason-Held LL, Golski S, Kraut MA, Esposito G, Resnick SM. Brain activation during encoding and recognition of verbal and figural information in older adults. Neurobiol Aging. 2005 Feb;26(2):237–250. doi: 10.1016/j.neurobiolaging.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Mosconi L, Sorbi S, de Leon MJ, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J Nucl Med. 2006 Nov;47(11):1778–1786. [PubMed] [Google Scholar]
- 18.Garris DR, Williams SK, Coleman DL, Morgan CR. Glucose utilization by the mouse brain: influence of age and diabetes. Brain Res. 1984 Aug;317(2):141–146. doi: 10.1016/0165-3806(84)90091-9. [DOI] [PubMed] [Google Scholar]
- 19.Vannucci SJ, Gibbs EM, Simpson IA. Glucose utilization and glucose transporter proteins GLUT-1 and GLUT-3 in brains of diabetic (db/db) mice. Am J Physiol. 1997 Feb;272(2 Pt 1):E267–E274. doi: 10.1152/ajpendo.1997.272.2.E267. [DOI] [PubMed] [Google Scholar]
- 20.Eastman RC, Carson RE, Gordon MR, et al. Brain glucose metabolism in noninsulin-dependent diabetes mellitus: a study in Pima Indians using positron emission tomography during hyperinsulinemia with euglycemic glucose clamp. J Clin Endocrinol Metab. 1990 Dec;71(6):1602–1610. doi: 10.1210/jcem-71-6-1602. [DOI] [PubMed] [Google Scholar]
- 21.Nagamachi S, Nishikawa T, Ono S, et al. Regional cerebral blood flow in diabetic patients: evaluation by N-isopropyl-123I-IMP with SPECT. Nucl Med Commun. 1994 Jun;15(6):455–460. doi: 10.1097/00006231-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Sabri O, Hellwig D, Schreckenberger M, et al. Influence of diabetes mellitus on regional cerebral glucose metabolism and regional cerebral blood flow. Nucl Med Commun. 2000 Jan;21(1):19–29. doi: 10.1097/00006231-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Reiman EM, Chen K, Langbaum JB, et al. Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer's disease and normal aging. Neuroimage. 2010 Jan 1;49(1):169–176. doi: 10.1016/j.neuroimage.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczynski B, Jagust W, Chui HC, Reed B. An inverse association of cardiovascular risk and frontal lobe glucose metabolism. Neurology. 2009 Feb 24;72(8):738–743. doi: 10.1212/01.wnl.0000343005.35498.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson PW, Meigs JB. Cardiometabolic risk: a Framingham perspective. Int J Obes (Lond) 2008 May;32 Suppl 2:S17–S20. doi: 10.1038/ijo.2008.30. [DOI] [PubMed] [Google Scholar]
- 26.Doyle P, Cusin I, Rohner-Jeanrenaud F, Jeanrenaud B. Four-day hyperinsulinemia in euglycemic conditions alters local cerebral glucose utilization in specific brain nuclei of freely moving rats. Brain Res. 1995 Jun 26;684(1):47–55. doi: 10.1016/0006-8993(95)00402-c. [DOI] [PubMed] [Google Scholar]
- 27.Lucignani G, Namba H, Nehlig A, Porrino LJ, Kennedy C, Sokoloff L. Effects of insulin on local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab. 1987 Jun;7(3):309–314. doi: 10.1038/jcbfm.1987.68. [DOI] [PubMed] [Google Scholar]
- 28.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004 Mar;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 29.MacLeod AK, Buckner RL, Miezin FM, Petersen SE, Raichle ME. Right anterior prefrontal cortex activation during semantic monitoring and working memory. Neuroimage. 1998 Jan;7(1):41–48. doi: 10.1006/nimg.1997.0308. [DOI] [PubMed] [Google Scholar]
- 30.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009 Feb;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med. 2010 Mar;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000 Aug 17;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwindt GC, Black SE. Functional imaging studies of episodic memory in Alzheimer's disease: a quantitative meta-analysis. Neuroimage. 2009 Mar 1;45:181–190. doi: 10.1016/j.neuroimage.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010 Jan;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]