Abstract
Obesity is not uniformly associated with the development of metabolic sequelae. Specific patterns of body fat distribution, in particular fatty liver, may preferentially predispose at-risk individuals to disease. Here we characterize the metabolic correlates of fat in the liver in a large community-based sample with and without respect to visceral fat. Fatty liver was measured by multi-detector computed tomography of the abdomen in 2589 individuals from the community-based Framingham Heart Study (FHS). Logistic and linear regression were used to determine the associations of fatty liver with cardio-metabolic risk factors adjusted for covariates with and without adjustment for other fat depots (body mass index [BMI], waist circumference [WC], and visceral adipose tissue [VAT]). The prevalence of fatty liver was 17%. Compared to participants without fatty liver, individuals with fatty liver had a higher adjusted odds ratio (OR) of diabetes (DM; OR 2.98; 95% confidence interval [CI], 2.12–4.21), metabolic syndrome (MetS; OR, 5.22; 95% CI, 4.15–6.57), hypertension (HTN; OR 2.73; 95% CI, 2.16–3.44), impaired fasting glucose (IFG; OR 2.95; 95% CI, 2.32–3.75), insulin resistance (IR; OR, 6.16; 95% CI, 4.90 – 7.76), higher triglycerides (TG) and systolic and diastolic blood pressure (SBP, DBP) and lower high density lipoprotein (HDL) and adiponectin levels (p<0.001 for all). After adjustment for other fat depots, fatty liver remained associated with DM, HTN, IFG, MetS, HDL, TG and adiponectin levels (all p<.001), whereas associations with SBP and DBP were attenuated (p >0.05).
Conclusion
Fatty liver is a prevalent condition and is characterized by dysglycemia and dyslipidemia independent of VAT and other obesity measures. This work begins to dissect the specific links between fat depots and metabolic disease.
Keywords: NAFLD, lipid, glucose, fat depot
Background
Obesity is a global epidemic. In the United States more than 66% of individuals are overweight or obese and more than 33% are obese (1). Obesity affects more than a billion people worldwide and is expected to increase to 1.5 billion by 2115(2). Obesity is associated with metabolic complications, although not all obese individuals develop medical sequelae (3). Why some people develop obesity-related illnesses and others do not has not been well-characterized.
One hypothesis for why some individuals develop medical problems from obesity is that specific fat depots may predispose some individuals to getting particular ailments. Abdominal obesity, as estimated by waist circumference, has been associated with metabolic syndrome, insulin resistance, and cardiovascular complications (4). Subcutaneous and visceral adipose tissue as well as fat in liver are all correlated with waist circumference (5, 6), but how these depots selectively contribute to the development of metabolic complications is not clear. One possibility is that these depots produce adipocytokines including adiponectin and resistin that can affect steatosis as well as metabolic traits. Adiponectin in rodents for example can alleviate steatosis and improve insulin sensitivity (7, 8) while resistin may promote steatosis and insulin resistance (9, 10). Further, fatty liver has been considered by some to be a by-product of fat deposition in the viscera, blood from which drains to the liver where it is deposited (11). Alternatively, fat in the liver may confer independent metabolic consequences above and beyond the effects of visceral fat.
Therefore, the goal of this study is to examine the correlation of fatty liver with metabolic risk factors for cardiovascular disease, and in particular to assess the association of metabolic risk factors for cardiovascular disease with fatty liver above and beyond standard anthropometric measures and visceral abdominal fat. Here we report the measurement, prevalence, metabolic and anthropometric correlates of fatty liver in the Framingham Heart Study.
Participants and Methods
Participants
Participants were drawn from the Framingham Heart study, a prospective cohort study initiated to evaluate risk factors for the development of cardiovascular disease. The selection criteria for this cohort has been previously published (12). The study was initiated in 1948 when 5209 residents of Framingham Massachusetts were enrolled. These individuals have been followed since then with multiple serial examinations and collection of risk factor data. In 1971, 5124 offspring and their spouses were recruited into the Offspring Study and have been followed every four to eight years since then (13). In 2002, 4095 Third Generation members and their spouses were enrolled (14). Between 2002 and 2005, multidetector computed tomography of the chest and abdomen were performed in 3529 individuals drawn from families including both Offspring and Third Generation participants.
Multidetector computed tomography scan cohort
Overall 3529 individuals underwent multidetector computed tomography scanning, 1418 from the Offspring and 2111 from the Third Generation. Inclusion criteria for the study favored individuals who still resided in the greater New England area and included 755 families. Minimum age cutoffs were 35 years in men and 40 years in women. All women of child-bearing age completed a pregnancy screening, and pregnant women (for risk to the fetus) and individuals >160 kg were excluded from scanning. Individuals undergoing scans were excluded from this analysis if their Multidetector computed tomography scans were not interpretable for fatty liver (n=323), did not attend Offspring Examination 7 (n=23) or if individuals reported greater than 7 drinks for men or 14 drinks for women per week (n=487). Of these, 107 were missing a complete covariate profile and were further excluded, resulting in a total sample size of 2589.
Multidetector computed tomography scan protocol and measurement of fatty liver, visceral and subcutaneous adipose tissue
Multidetector computed tomography scanning was conducted as previously reported (5, 15, 16). A calibration phantom (Image Analysis, Lexington, KY, USA) with a water equivalent compound (CT-Water, Light Speed Ultra, General Electric, Milwaukee, WI, USA) and calcium hydroxyapatite at 0, 75, and 150 mg/cm3 was placed under each participant (16).
Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were measured(5, 15); briefly, 25 contiguous 5mm thick sections (120kVP, 400mA, gantry rotation time 500ms, table feed 3:1 were acquired covering 125mm above S1). Fat was identified using an image display window of −195 to −45 HU and a window center of −120 HU. After manually tracing the abdominal muscular wall separating the visceral from the subcutaneous compartment high resolution volumetric measures SAT and VAT were defined as the volumetric fat content outside and inside of this dividing line. The intra-class correlation coeficient was 0.992 for VAT and 0.997 for SAT.
Fatty liver was measured on Multidetector computed tomography scans of the abdomen and has been described elsewhere(16). Briefly, three areas from the liver, two from the spleen and one from the external phantom were measured. The average of the liver and spleen measures were then calculated and used to create liver/spleen ratios and liver/phantom ratios. The intra-class correlation coefficient was 0.99 (16). Given that the phantom but not the spleen was visualized on all scans, primary analyses were conducted with a liver phantom ratio as the indexed standard; secondary analyses were conducted on the liver-spleen ratio. Only participants with abdominal scans were used in the current analysis, since data from the abdominal scans had better reproducibility than chest scans (16).
Distribution of the fatty liver phenotype
The distributions of liver phantom ratio and liver spleen ratio were left skewed with a median [Lower–Upper Quartile] of 0.37[0.34–0.39] and 1.21[1.13–1.28] respectively (Supplemental Figure 1A). The 95th percentiles were 0.41 and 1.37 for the liver phantom ratio and liver spleen ratio, respectively. In the literature, an liver spleen ratio of 1.1 corresponds to the presence of 30% fatty liver (17). We found that a liver phantom ratio cutoff of 0.33 had a 98% sensitivity and 70% specificity using liver spleen ratio cutpoint of 1.1 as the gold standard (Supplemental Figure 1B).
Measurement of covariates
Risk factors used in analyses in this paper were measured at the seventh examination cycle of the Offspring cohort (1998–2001) or the first examination of the Third Generation cohort (2002–2005). Body mass index (BMI) is defined by weight (kilograms)/height (meter)2; waist circumference (WC) is measured at the level of the umbilicus; diabetes (DM) is defined as a fasting plasma glucose (FPG) of at least 126 mg/dL at examination or treatment with either insulin or a hypoglycemic agent; impaired fasting glucose (IFG) is defined as FPG of 100–125 mg/dL among those not treated for diabetes; hypertension (HTN) is defined as a systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg or on antihypertensive treatment. Triglycerides (TG) and high density lipoprotein (HDL) levels are measured on fasting morning samples. Participants are considered current smokers if they had smoked at least 1 cigarette per day in the year preceding the Framingham Heart Study examination. Alcohol use was assessed through a series of physician administered questions. Physical activity, assessed with a questionnaire, is a score based on the average daily number of hours of sleep and sedentary, slight, moderate, and heavy activity of the participant. Women are considered menopausal if their periods had stopped for at least 1 year. Metabolic syndrome (MetS) is defined from modified Adult Treatment Panel criteria. Obesity is defined as BMI ≥ 30 kg/m2. Insulin resistance was determined as the top quartile of the homeostasis model (HOMA-IR: [fasting glucose × fasting insulin]/22.5] distribution among individuals without diabetes (18, 19). Circulating adiponectin and resistin were measured by enzyme linked immunoassay after an 8 hour fast as previously described (20).
Determination of fatty liver phantom ratio dichotomous cutoff and continuous distribution
The distributions of liver phantom ratios and liver spleen ratios were characterized. Because the liver phantom ratio and liver spleen ratio are likely measures of more than just fat in the liver (i.e water content, iron, etc) the top 5% of points in liver phantom ratio were winsorized for analyses with fatty liver as a continuous variable.
Prior to winsorization, to determine the liver phantom ratio cut-off that mirrored a liver-spleen ratio of 1.1, the cutoff that best discriminates the presence of 30% fat in the liver (17), we minimized misclassification of subjects at various cutoffs for liver phantom ratio. From a Receiver Operating Curve analysis of liver phantom ratio compared to a gold standard of liver spleen ratio of 1.1 we determined the sensitivity and specificity of various cutoffs of liver phantom ratio and established a liver phantom ratio cutoff of 0.33 or lower as our working definition of fatty liver.
Statistical analyses
Differences in participant characteristics between those with (liver phantom ratio ≤ 0.33) and without fatty liver (liver phantom ratio > 0.33) were determined using a t-test for normally distributed traits, Wilcoxon rank sum test for non-normally distributed continuous variables or ordinal variables, and a chi squared test for dichotomous variables. As the liver phantom ratio was not normally distributed, Spearman correlation coefficients were used to determine age- and sex-adjusted correlations of continuous metabolic traits with liver phantom ratio.
Primary multivariable analyses focused on fatty liver (yes/no) as the exposure and individual metabolic risk factors and fat depot measures as the dependent (outcome) variables. For dichotomous outcomes, odds ratios (calculated fatty liver yes vs. fatty liver no) are reported; for continuous outcomes, the regression coefficients for the presence of fatty liver are reported. We also modeled continuous fatty liver as the exposure and odds ratios and regression coefficients for a 1 SD decrease in liver phantom ratio are reported for dichotomous and continuous outcomes, respectively. The following modeling structures were used. In Model 1, age, sex, alcohol consumption (after exclusions mentioned above), menopausal status, hormone replacement therapy, smoking (3-level variable: current/former/never smoker) were included as covariates. In addition, lipid treatment, hypertension treatment, and diabetes treatment were included as covariates in models for HDL cholesterol, log triglycerides, systolic and diastolic blood pressure, and fasting plasma glucose, respectively. In Model 2, we additionally adjusted for body mass index, waist circumference, and VAT. SAT was not included in these multivariate models because it was highly collinear with BMI.
In secondary analyses, we additionally added physical activity and education to the models. Assessment of the significance of sex and age interactions with fatty liver on metabolic risk factors was also assessed. Analyses were performed using SAS version 9.1; a two-sided 0.05 alpha was used to declare statistical significance.
Results
Study Sample Characteristics
The characteristics of the participants are shown in Table 1. Fifty one percent of the sample were women, with an average age of 51 years and a BMI of 27.6 kg/m2. Using liver phantom ratio ≤ 0.33 to define fatty liver, we determined the characteristics of the participants with and without fatty liver (Table 1). Individuals with fatty liver had a substantially more adverse cardiovascular disease risk factor profile (Table 1).
Table 1.
Characteristics of the participants
| Category | All (2589)* | No Fatty Liver (2150)* | Fatty Liver (439)* | p-value# |
|---|---|---|---|---|
| Covariates | ||||
| Female (%) | 51 | 44 | 53 | 0.0002 |
| Age (years) | 51.0(10.6) | 50.8(10.6) | 52.3(10.7) | 0.0015 |
| Drinks per week | 3.0(3.5) | 3.0(3.4) | 3.1(3.9) | 0.7619 |
| Physical Activity | 37.4(6.8) | 37.5(6.9) | 36.9(6.4) | 0.0256 |
| Smoking Category | 0.1376 | |||
| Smoke Never (%) | 49.7 (1287) | 50.6 (1088) | 45.3 (199) | |
| Smoke Former (%) | 38.7 (1002) | 37.9 (815) | 42.6 (137) | |
| Smoke Current (%) | 11.6 (300) | 11.5 (247) | 12.1 (53) | |
| Education Category | 0.0028 | |||
| Education some High School (%) | 1.6 (41) | 1.5 (32) | 2.5 (11) | |
| Education High School Graduate | 21.3 (551) | 20.7 (445) | 24.3 (107) | |
| Education Some College (%) | 29.7 (769) | 29.0 (624) | 32.9 (144) | |
| Education College Graduate (%) | 47.4 (1227) | 48.8 (1049) | 40.3 (177) | |
| Menopause (women only) (%) | 26.1 (676) | 26.1 (561) | 26.2 (115) | 0.0623 |
| HRT (%) | 11.9 (308) | 11.8 (254) | 12.5 (55) | 0.1872 |
| Fat related | ||||
| BMI (kg/m2) | 27.6(5.3) | 26.8(4.8) | 31.4(5.8) | <.0001 |
| Waist Circumference (cm) | 96.5(14.3) | 94.4(13.4) | 106.4(14.1) | <.0001 |
| Subcutaneous Adipose Tissue (cm3) | 2847.7(1399.0) | 2697.5(1327.1) | 3583.4(1506.4) | <.0001 |
| Visceral Adipose Tissue (cm3) | 1749.5(1021.4) | 1568.1(912.9) | 2638.1(1059.6) | <.0001 |
| BMI Category | <.0001 | |||
| Normal weight (BMI<25) | 34.0 (882) | 38.9 (837) | 10.3 (45) | |
| Overweight (25<=BMI<30) | 39.5 (1022) | 40.4(869) | 34.9 (153) | |
| Obese (BMI>=30) | 26.5 (685) | 20.7 (444) | 54.9 (241) | |
| Glucose related | ||||
| Fasting Glucose (mg/dl) | 99.1 (12.9) | 97.1 (19.4) | 108.7 (33.6) | <.0001 |
| Diabetes Mellitus (%) | 6.7 (173) | 5.1 (110) | 14.6 (64) | <.0001 |
| IFG-Non-DM Only(%) | 27.5 (712) | 23.6 (507) | 48.8 (214) | <.0001 |
| HOMA-IR [Median (Q1–Q3)] | 2.63 (2.11 – 3.54) | 2.47 (2.05 – 3.21) | 3.88 (2.95 – 5.39) | <.0001 |
| log HOMA-IR | 1.03 (0.47) | 0.95 (0.43) | 1.40 (0.51) | <.0001 |
| Insulin resistance (%) | 28.9(692) | 21.6(425) | 62.7(267) | <.0001 |
| Adiponectin (μg/ml)** | 9.8(6.0) | 10.5(6.1) | 7.0(4.8) | <.0001 |
| Resistin [Median (Q1–Q3)] | 13.40 (10.60 – 17.10) | 13.25 (10.40 – 17.05) | 14.20 (11.80 – 17.70) | 0.0474 |
| log Resistin (ng/ml)** | 2.62 (0.41) | 2.61 (0.41) | 2.67 (0.39) | 0.1199 |
| Lipid related | ||||
| Triglycerides (mg/dL) | 103[71–155] | 95[67–139] | 157[110–217] | <.0001 |
| HDL Cholesterol (mg/dl) | 52.5(15.8) | 54.0(15.7) | 45.2(14.1) | <.0001 |
| Total Cholesterol (mg/dL) | 195.2(35.4) | 194.6(35.0) | 197.9(37.1) | 0.1065 |
| Blood pressure related | ||||
| SBP (mmHg) | 121.1 (16.2) | 120.0(16.1) | 126.8(15.3) | <.0001 |
| DBP (mmHg) | 75.28(9.3) | 74.7(9.1) | 78.1 (9.5) | <.0001 |
| Hypertension (%) | 27.3 (707) | 23.6 (507) | 45.4 (199) | <.0001 |
| Syndrome related | ||||
| Metabolic Syndrome (%) | 31.4 (813) | 25.0 (538) | 62.9 (276) | <.0001 |
data in parentheses refers to the standard deviation for continuous traits and the number affected for dichotomous traits
represents mean (SD) or median(inter quartile range) or percent (number of individuals)
based on t-test or Wilcoxon Rank Sum Test or Chi squared
based on 857 individuals in offspring only, 157 with fatty liver and 700 without fatty liver
HRT: hormone replacement therapy
BMI: Body mass index
IFG: impaired fasting glucose
HOMA-IR: Homeostasis model assessment of insulin resistance
HDL: high density lipoprotein
SBP: systolic blood pressure
Prevalence
The overall prevalence of fatty liver was 17%. Age- and gender-specific prevalence was higher for men (19%) compared with women (15%), and peaked for men between ages 55–64 years and for women between ages 75–84 years (Table 2). There was little difference in prevalence using liver spleen ratio instead of liver phantom ratio as a measure of fatty liver (data not shown).
Table 2.
Prevalence of Fatty Liver
| Fatty Liver | |||||||
|---|---|---|---|---|---|---|---|
| Age | Total | (%) | Men | (%) | Women | (%) | p value* |
| <35 | 6/45 | 13.3 | 6/40 | 15.0 | 0/5 | 0.0 | >0.9999 |
| 35–44 | 113/779 | 14.6 | 73/436 | 16.7 | 40/343 | 11.7 | 0.0514 |
| 45–54 | 154/926 | 16.6 | 79/420 | 18.8 | 75/506 | 14.8 | 0.1111 |
| 55–64 | 94/486 | 21.1 | 53/207 | 25.6 | 41/279 | 14.7 | 0.0036 |
| 65–74 | 60/290 | 20.7 | 30/134 | 22.4 | 30/156 | 19.2 | 0.5618 |
| 75–84 | 12/63 | 19.1 | 5/27 | 18.5 | 7/36 | 19.4 | >0.9999 |
| all | 439/2589 | 17.0 | 246/1264 | 19.0 | 193/1325 | 15.0 | 0.0010 |
p value for comparison between sexes from Fisher’s Exact test
Correlations Between Fatty Liver and Metabolic/Anthropometric Traits
Fatty liver as measured using liver phantom ratio and liver spleen ratio was associated with all tested metabolic and fat-depot variables. Decreases in liver phantom ratio and liver spleen ratio (i.e. reflecting more fat in the liver) were associated with higher levels of VAT, WC, BMI, TG, weight, SAT, FPG, HOMA-IR, SBP, DBP, and lower adiponectin and HDL (p<0.001 for all; Table 3).
Table 3.
Negative Spearman Correlations of LPR and LSR with continuous Traits
| Trait | LPR(n = 2589) | LSR(n = 1284) | ||
|---|---|---|---|---|
| Corr* | p value | Corr* | p value | |
| BMI (kg/m2) | 0.25 | <.0001 | 0.27 | <.0001 |
| Waist Circumference (cm) | 0.26 | <.0001 | 0.27 | <.0001 |
| SAT (cm3) | 0.20 | <.0001 | 0.21 | <.0001 |
| VAT (cm3) | 0.34 | <.0001 | 0.34 | <.0001 |
| WEIGHT (kg) | 0.23 | <.0001 | 0.26 | <.0001 |
| Glucose (mg/dL) | 0.17 | <.0001 | 0.17 | <.0001 |
| HOMA-IR | 0.32 | <.0001 | 0.32 | <.0001 |
| Adiponectin (μg/ml)** | −0.25 | <.0001 | −0.32 | <.0001 |
| Resistin (ng/ml)** | 0.07 | <.0001 | 0.08 | 0.08 |
| TG (mg/dL) | 0.23 | <.0001 | 0.30 | <.0001 |
| HDL (mg/dL) | −0.19 | <.0001 | −0.23 | <.0001 |
| SBP (mmHg) | 0.14 | <.0001 | 0.11 | <.0001 |
| DBP (mmHg) | 0.11 | <.0001 | 0.10 | 0.0005 |
| HEIGHT (cm) | −0.04 | 0.04 | −0.02 | 0.51 |
negative spearman correlation coeficient
based on 857 individuals in offspring only; 157 with fatty liver and 700 without fatty liver
BMI: body mass index
SAT: subcutaneous adipose tissue
VAT: visceral adipose tissue
HOMA-IR: Homeostasis model assessment of insulin resistance
TG: triglycerides
HDL: high density lipoprotein
SBP: systolic blood pressure
DBP diastolic blood pressure
Multivariable-adjusted Correlations Between Fatty Liver and Metabolic/Anthropometric Traits
Fatty liver (as both continuous and dichotomous measures) was significantly associated with all glucose, lipid and blood pressure traits (p <0.001) except resistin levels in multivariable analyses (Table 4). Compared to participants without fatty liver, individuals with fatty liver had a higher adjusted odds ratio of prevalent DM (odds ratio [OR], 2.98; 95% confidence interval [CI] 2.12–4.21), insulin resistance (IR; OR, 6.16; 95% CI, 4.90 – 7.76), MetS (OR, 5.22; 95% CI 4.15–6.57), HTN (OR, 2.73; 95% CI 2.16–3.44) and IFG (OR, 2.95; 95% CI 2.32–3.75) than individuals without fatty liver (p<0.001 for all).
Table 4.
Multivariate adjusted models for fatty liver
| Dependent Trait | FL +covariates* | FL + covariates’ and obesity traits# | ||||||
|---|---|---|---|---|---|---|---|---|
| N | effect$ | 95% CI | p value | effect$ | 95% CI | p value | ||
| Glucose (mg/dL) | 2588 | 7.7 | [5.73–9.67] | <0.001 | 3.54 | [1.43–5.64] | 0.001 | |
| IFG | 2415 | 2.95 | [2.32 – 3.75] | <0.001 | 1.58 | [1.21 – 2.07] | <0.001 | |
| DM | 2588 | 2.98 | [2.12 – 4.21] | <0.001 | 1.64 | [1.11 – 2.41] | 0.012 | |
| log HOMA-IR | 2395 | 0.44 | [0.40 – 0.49] | <0.001 | 0.21 | [0.17 – 0.25] | <0.001 | |
| Insulin resistance | 2395 | 6.16 | [4.90 – 7.76] | <0.001 | 2.79 | [2.19–3.65] | <0.001 | |
| Adiponectin (mg/ml) | 854 | −3.17 | [−4.09 – −2.24] | <0.001 | −1.59 | −2.57 – −0.62] | 0.001 | |
| log Resistin (ng/ml) | 857 | 0.05 | [−0.02 – 0.12] | 0.147 | 0.00 | [−0.07 – 0.08 | 0.924 | |
| Dichotomous Fatty Liver | log TG (mg/dL) | 2588 | 0.41 | [0.35–0.46] | <0.001 | 0.22 | [0.17 – 0.28] | <0.001 |
| HDL (mg/dL) | 2588 | −7.08 | [−8.44 – −5.73] | <0.001 | −2.48 | [−3.89 – −1.06] | <0.001 | |
| SBP (mmHg) | 2589 | 4.61 | [3.10–6.12] | <0.001 | 1.38 | [−.22 – 2.97] | 0.091 | |
| DBP (mmHg) | 2587 | 2.88 | [1.96– 3.81] | <0.001 | 0.64 | [−0.34 – 1.61] | 0.2 | |
| HTN | 2586 | 2.73 | [2.16–3.44] | <0.001 | 1.52 | [1.17 – 1.97] | 0.002 | |
| MetS | 2587 | 5.22 | [4.15 – 6.57] | <0.001 | 1.95 | [1.48–2.56] | <0.001 | |
| BMI (kg/m2) | 2589 | 4.35 | [3.39–5.30] | <0.001 | ||||
| WC (cm) | 2589 | 4.14 | [3.41–4.86] | <0.001 | ||||
| SAT (cm3) | 2589 | 810 | [636–984 | <0.001 | ||||
| VAT (cm3) | 2589 | 989.2 | 865.5–1113.0] | <0.001 | ||||
| Dependent Trait | FL +covariates* | FL + covariates’ and obesity traits# | ||||||
|---|---|---|---|---|---|---|---|---|
| N | effect& | 95% CI | p value | effect | 95% CI | p value | ||
| Glucose (mg/dL) | 2588 | 2.91 | [2.17–3.65] | <0.001 | 1.36 | [0.56–2.15] | <0.001 | |
| IFG | 2415 | 1.43 | [1.30 – 1.56] | <0.001 | 1.10 | [1 – 1.22] | 0.047 | |
| DM | 2588 | 1.56 | [1.39 – 1.75] | <0.001 | 1.29 | [1.12 – 1.47] | <0.001 | |
| log HOMA-IR | 2395 | 0.17 | [0.16 – 0.19] | <0.001 | 0.08 | [0.07 –0.10] | <0.001 | |
| Insulin resistance | 2395 | 0.44 | [0.39–0.49] | <0.001 | 0.64 | [0.57–0.72] | <0.001 | |
| Adiponectin (mg/ml) | 854 | −1.26 | [−1.61 – −0.90] | <0.001 | −0.62 | [−1.00 – −0.24] | 0.001 | |
| log Resistm (ng/ml) | 857 | 0.03 | [0.00 – 0.06] | 0.032 | 0.01 | [−0.02 – 0.04] | 0.452 | |
| Continuous Fatty Liver | log TG (mg/dL) | 2588 | 0.14 | [0.12–0.16] | <0.001 | 0.07 | [0.05– 0.09] | <0.001 |
| HDL (mg/dL) | 2588 | −2.5 | [−3.01–−1.99] | <0.001 | −0.69 | [−1.23–−0.16] | 0.011 | |
| SBP (mmHg) | 2589 | 1.72 | [1.15 – 2.29] | <0.001 | 0.52 | [−0.08–1.12] | 0.092 | |
| DBP (mmHg) | 2587 | 1.09 | [.75 – 1.44] | <0.001 | 0.25 | [−0.12 – 0.61] | 0.19 | |
| HTN | 2586 | 1.52 | [1.28 – 1.64] | <0.001 | 1.22 | [1.10–1.35] | <0.001 | |
| MetS | 2587 | 1.96 | [1.79 – 2.17] | <0.001 | 1.27 | [1.12 – 1.41] | <0.001 | |
| BMI (kg/m2) | 2589 | 1.6 | [1.41–1.79] | <0.001 | ||||
| WC (cm) | 2589 | 1.62 | [1.42 – 1.81] | <0.001 | ||||
| SAT(cm3) | 2589 | 336.4 | [285.7–397.0] | <0.001 | ||||
| VAT (cm3) | 2589 | 365.5 | [335.1–395.9] | <0.001 | ||||
covariates= age, sex, alcoholic drinks per week, menopausal status, hormone replacement therapy, smoking
obesity traits= BMI, waist circumference, VAT
effect= the change in the continuous dependent trait or in the Odds Ratio of having the dependent trait in individuals with fatty liver compared to those without fatty liver.
effect= the change in the continuous dependent trait or in the Odds Ratio of having the dependent trait in individuals per standard deviation DECREASE in LPR.
FL: Fatty Liver
IFG: impaired fasting glucose
DM: diabetes mellitus
HOMA-IR: Homeostasis model assessment of insulin resistance
TG: triglycerides
HDL: high density lipoprotein
SEP: systolic blood pressure
DBP diastolic blood pressure
HTN: hypertension
MS: metabolic syndrome
BMI: body mass index
WC: waist circumference
SAT: subcutaneous adipose tissue
VAT: visceral adipose tissue
After further adjustment for BMI, WC, and VAT, there remained statistically significant associations of fatty liver with prevalent DM (OR, 1.64; 95% CI 1.11 – 2.41), IFG (OR, 1.58; 95% CI 1.21 – 2.07), insulin resistance (IR; OR, 2.79; 95% CI, 2.14 – 3.65), MetS (OR, 1.95; 95% CI 1.48 – 2.56), log HOMA-IR (beta 0.21 mg/dL 95% CI 0.17 – 0.25 mg/dL), adiponectin (beta −1.59 mg/dL, 95% CI −2.57 – −0.62), log TG (beta 0.22 mg/dL 95% CI 0.17 – 0.28 mg/dL), HDL (beta −2.5 mg/dL 95% CI −3.9 – −1.1 mg/dL) and HTN (OR,1.52; 95% CI 1.17 – 1.97), whereas SBP and DBP were no longer associated with fatty liver (p = 0.09 and 0.19 respectively; Table 4).
Similar associations were observed when we examined decreasing liver phantom ratio as a continuous measure of fatty liver (Table 4). Similar results were also observed after additional adjustment for physical activity and education (data not shown). There was no evidence for an interaction by age or gender in the association of fatty liver with continuous or dichotomous metabolic risk factors except with adiponectin where women had a slightly higher effect than men, and in HOMA-IR where individuals >50 years of age had a higher HOMA-IR values than those <50 years of age but in all cases the effect in these classes was directionally consistent with the overall effect (data not shown).
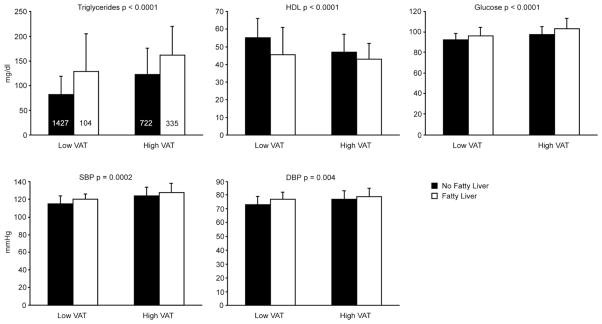
The median glucose, TG, HDL, SBP and DBP values in participants above and below the 90th percentile cut-point for VAT derived from a healthy referent sample(21) (Figure 1) show that lipid and glucose traits were associated with fatty liver ( p<0.0001), while SBP and DBP were associated to a lesser extent (p=0.0002 and 0.004 respectively ) with fatty liver high and low levels of VAT. When fatty liver and VAT were jointly considered in the multivariate models, VAT remained associated with all metabolic correlates (all p<0.0001; data not shown) whereas fatty liver was not associated with SBP and DBP (p=0.06 and 0.16 respectively. However, fatty liver remained associated with all other metabolic traits (p-values<0.004) (data not shown).
Figure 1.
Median and IQR (error bars) for triglycerides, high density lipoproteins (HDL), glucose, systolic blood pressure (SBP) and diastolic blood pressure (DBP) p: age, gender, and VAT corrected p value between fatty liver categories; histogram counts are shown in triglyceride panel apply to all panels. The high VAT category refers to those above the 90th percentile cut-point derived from a healthy referent sample.
Conclusions
Fatty liver is observed in 17% of participants in an unselected community-based sample. Individuals with fatty liver are characterized by a high-risk metabolic profile. After adjustment for other fat depots including VAT, fatty liver remained associated with lipid and glucose traits.
The most compelling and unique finding in our study was the association of fatty liver with lipid and glucose traits independent of VAT. Not all obese individuals develop metabolic disease from their obesity. Understanding how individuals that develop metabolic sequelae from their obesity differ from those that do not may help target at-risk individuals and guide development of novel therapeutics to combat disease. In the present work, we extend previous studies (6, 22–27) and illustrate how fatty liver associates with metabolic syndrome components in the largest study to date of Caucasian individuals that have not been selected for the presence of fatty liver, obesity, or metabolic disease. The association of liver fat with lipid, glucose and blood pressure traits may be indirect and due to generalized adiposity, or to the presence of fat in particular depots including VAT. The size of our cohort and the richness of the covariates and traits measured including VAT gave us the unique opportunity to assess the association of liver fat with these cardiometabolic traits above and beyond VAT. In particular, we found that VAT is the strongest correlate of fatty liver and after adjusting for VAT, fatty liver remains associated with dyslipidemia and dysglycemia. Given the cross sectional, observation nature of our measures, our findings must be considered in light of the fact that association does not prove causality.
The liver is the main source of lipid regulation in the body, plays an important role in glucose metabolism, and overall is known to play little known role in blood pressure regulation. Fat accumulation in the liver is predominantly in the form of triglycerides. Fifteen percent of this fat comes from dietary cholymicrons, 60% from non-esterified fatty acids that come from lipolysis from adipose tissue or from lipoproteins hydrolyzed above a rate that can be taken up by adipose tissue, and 25% from newly synthesized fatty acids (28). Delivery of non esterified fatty acids from visceral adipose tissue has been shown to be as high at 20% of the total delivery of fatty acids to the liver compared to just 5% in lean individuals without visceral fat (29). In our population-based study we show that even though VAT was the strongest correlate of fatty liver, the correlation is at best modest (−0.34), suggesting that VAT is only one component in the pathogenesis of fatty liver. It has been shown that in the absence of peripheral fat stores or in insulin resistant states where peripheral tissues are impaired in their ability to accumulate energy stores, there can be an increase in non-esterified fatty acid delivery to the liver and increased fat accumulation in mice and humans (30–32).. Indeed we find that the second best correlate of fatty liver is insulin resistance. Delivery of excess fatty acids to the liver in energy excess states due to differences in fat storage ability and/or insulin resistance peripherally in the population may result in de novo lipogenesis, fatty acid esterification, and storage of esterified fatty acids as cytoplasmic triglycerides or to formation of VLDL particles (33, 34).. These VLDL particles can be secreted and can lead to the formation of atherogenic small dense lipoprotein particles, cholesterol rich VLDL remnants, and triglyceride rich HDL particles which can be cleared by the kidney leading to lower levels of HDL (33).. In this way, fatty liver may be specifically related to hypertriglyceridemia, low HDL, and impaired glucose utilization above and beyond other fat depots consistent with what we find in our analyses. Further, the lack of peripheral fat storage capacity may be indirectly indicated by low levels of adipokines such as adiponectin, which is inversely corrected with fatty liver. Alternatively, low levels of adiponectin may be related directly to the excess storage of energy in the liver.
The conjoint associations of fatty liver and VAT in association with lipid and glucose traits highlights the independent roles of different metabolic fat depots. Further, our findings that fatty liver was mostly associated with lipid and glucose traits may help explain in part why these abnormalities are often seen together. In addition, understanding why some, but not all individuals, develop fatty liver can shed light into understanding why certain individuals develop metabolic complications of obesity while others do not. Finally, it will be of great interest to determine whether the presence of fat in the liver prospectively is an independent predictor of development of not only metabolic disease in the form of dysglycemia or dyslipidemia but also of cardiovascular disease.
The strength of the current study is the large, well characterized cohort of individuals with a wealth of metabolic traits and covariates measured. Further, our sample is unselected for obesity-related traits, reducing selection bias. Indeed we establish that fatty liver is prevalent at 17%, affects more men than women, and peaks in women at later ages than in men in the largest Caucasian population based study to date. Our study directly measured fatty liver on CT, which allowed us to quantify it more precisely as compared to indirect measures of fatty liver disease such as elevated liver function tests which have a low sensitivity to detect the presence of the condition. We also measured both liver phantom ratio and liver spleen ratio and found that our results were comparable between these two measures, suggesting that these results can be compared with studies that have just the liver spleen ratio. The distribution was skewed with most people having little or no fatty liver. The peak of the distribution may represent a point at which people have no fat in their liver or alternatively low levels of fat that can be considered “normal” for the population. Since high water, glycogen or iron concentrations in the liver increase the attenuation of the liver confounding by these deposits would if anything lead to underestimation of fatty liver; individuals to the left of the peak likely do have high liver fat. Our study was limited by including only individuals of European ancestry and thus cannot be generalized to other ethnicities. Also, these individuals were initially from one geographic area and were part of a health outcomes study which may not be generalizable. Further, covariates were measured at times separate from the CT scans and computed tomography can only indirectly measure fat in the liver; these effects may result in misclassification. However, misclassification would only serve to bias our results towards the null, and would not lead to a positive association, as we have observed. Further, a general limitation of the diagnosis of diabetes in population-based studies is that it is dependent on a one-time assessment of glucose and self-reported medication use, which may include metformin. In particular, metformin might be used for both PCOS and impaired fasting glucose. The exposure and covariate data was measured from 1998–2005, and may not reflect current trends. Lastly, these data are cross sectional and derived from an observational study; therefore we can not draw conclusions
Fatty liver is a prevalent condition and is characterized by dysglycemia and dyslipidemia independent of VAT. These findings highlight the specificity of fat accumulation in particular depots and the presence of metabolic disease.
Supplementary Material
Acknowledgments
Financial Support:
EKS was supported by an NIH T32 DK07191-32 grant to Daniel K. Podolsky in the department of gastroenterology at Massachusetts General Hospital and NIH F32 DK079466-01 and NIH K23DK080145-01 grants to EKS. The Framingham Heart Study is supported by core contract N01-HC25195. Additional research support was provided by an American Diabetes Association Career Development Award (JBM), the General Clinical Research Centers Program (Grant No. M01-RR-01066); and National Institute of Diabetes and Digestive and Kidney Diseases K24 DK080140 (JBM). Dushyant V Sahani has a research agreement with General Electric and is on the Speakers’ Bureau of Bracco Diagnostics. JBM currently has research grants from GlaxoSmithKline and sanofi-aventis, and has consulting agreements with Eli Lilly and Interleukin Genetics.
Abbreviations
- FHS
Framingham Heart Study
- BMI
Body mass index
- WC
Waist circumference
- VAT
Visceral adipose tissue
- OR
Odds ratio
- DM
Diabetes Mellitus
- CI
Confidence Interval
- MetS
Matabolic Syndrome
- p
P value
- HTN
Hypertension
- IFG
Impaired fasting glucose
- TG
Triglycerides
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- HDL
High density lipoprotein
- FPG
Fasting plasma glucose
- Kg
Kilogram
- KY
Kentucky
- USA
United States of America
- Mg
Miligrams
- cm3
Centimerters cubed
- Mm
Milimeters
- WI
Wisconcin
- kVP
Kilovolt potential
- mA
Miliamp
- S1
Sacral spine 1
- HU
Hounsefeld units
- dL
Deciliter
- m2
Milimeter
- SD
Standard Deviation
- TNF
Tumor necrosis factor
- VLDL
Very low density lipoprotein
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. Jama. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Procopiou M, Philippe J. The metabolic syndrome and type 2 diabetes: epidemiological figures and country specificities. Cerebrovasc Dis. 2005;20 (Suppl 1):2–8. doi: 10.1159/000088231. [DOI] [PubMed] [Google Scholar]
- 3.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 4.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 7.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singhal NS, Patel RT, Qi Y, Lee YS, Ahima RS. Loss of resistin ameliorates hyperlipidemia and hepatic steatosis in leptin-deficient mice. Am J Physiol Endocrinol Metab. 2008;295:E331–338. doi: 10.1152/ajpendo.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal NS, Lazar MA, Ahima RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci. 2007;27:12924–12932. doi: 10.1523/JNEUROSCI.2443-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campra JL, Reynolds TB. The Hepatic Circulation. In: Arias IM, Popper H, Schachter D, Shafritz DA, editors. The liver: biology and pathophysiology. New York: Raven Press; 1982. pp. 627–645. [Google Scholar]
- 12.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 14.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Sr, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 15.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 16.Speliotes EK, Massaro JM, Hoffmann U, Foster MC, Sahani DV, Hirschhorn JN, O’Donnell CJ, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–899. doi: 10.1111/j.1440-1746.2008.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Haga H, Maetani Y, Fujii K, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 20.Jain SH, Massaro JM, Hoffmann U, Rosito GA, Vasan RS, Raji A, O’Donnell CJ, et al. Cross-sectional associations between abdominal and thoracic adipose tissue compartments and adiponectin and resistin in the Framingham Heart Study. Diabetes Care. 2009;32:903–908. doi: 10.2337/dc08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pou KM, Massaro JM, Hoffmann U, Lieb K, Vasan RS, O’Donnell CJ, Fox CS. Patterns of Abdominal Fat Distribution: The Framingham Heart Study. Diabetes Care. 2008 doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 23.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 24.Fan JG, Zhu J, Li XJ, Chen L, Lu YS, Li L, Dai F, et al. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol. 2005;20:1825–1832. doi: 10.1111/j.1440-1746.2005.04058.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, Yeh YH, Yueh SK. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol. 2006;40:745–752. doi: 10.1097/00004836-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508–514. doi: 10.1016/j.jhep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 27.Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, Chen MH, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schott M, Scherbaum WA, Bornstein SR. Acquired and inherited lipodystrophies. N Engl J Med. 2004;351:103–104. author reply 103–104. [PubMed] [Google Scholar]
- 31.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 33.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 34.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta. 2000;1483:37–57. doi: 10.1016/s1388-1981(99)00182-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.