Abstract
Black patients receiving dialysis for end-stage renal disease in the United States have lower mortality rates than white patients. Whether racial differences exist in mortality after acute renal failure is not known. We studied acute renal failure in patients hospitalized between 2000 and 2003 using the Nationwide Inpatient Sample and found that black patients had an 18% (95% confidence interval [CI] 16 to 21%) lower odds of death than white patients after adjusting for age, sex, comorbidity, and the need for mechanical ventilation. Similarly, among those with acute renal failure requiring dialysis, black patients had a 16% (95% CI 10 to 22%) lower odds of death than white patients. In stratified analyses of patients with acute renal failure, black patients had significantly lower adjusted odds of death than white patients in settings of coronary artery bypass grafting, cardiac catheterization, acute myocardial infarction, congestive heart failure, pneumonia, sepsis, and gastrointestinal hemorrhage. Black patients were more likely than white patients to be treated in hospitals that care for a larger number of patients with acute renal failure, and black patients had lower in-hospital mortality than white patients in all four quartiles of hospital volume. In conclusion, in-hospital mortality is lower for black patients with acute renal failure than white patients. Future studies should assess the reasons for this difference.
The life expectancy of black Americans is approximately 6 yr shorter than that of white Americans.1 An extensive body of literature in the past two decades has examined the extent to which racial disparities in the quality of health care may contribute to the poorer health of minority groups. Black Americans, for example, are less likely to receive optimal treatment for acute myocardial infarction (AMI),2,3 undergo total hip replacement for severe osteoarthritis,4 undergo mammography for breast cancer screening,5 and receive appropriate hospital care for congestive heart failure (CHF) and pneumonia.6
Racial disparities within nephrology have been investigated primarily in ESRD. For example, compared with white Americans, black Americans receive less intensive maintenance hemodialysis,7 are less likely to be offered kidney transplantation,8,9 and are less likely to receive arteriovenous fistulas compared with grafts for hemodialysis.10
Despite poorer care, however, several studies have shown that black Americans on maintenance hemodialysis have longer survival compared with white Americans, even after adjustment for the effects of age and other conditions (the median age at onset of ESRD is approximately 8 yr younger among black compared with white Americans).11–16 The mechanisms underlying the paradoxical survival advantage for black Americans on hemodialysis are not clear. Potential explanations include racial differences in the response to uremia or its treatment, differences in disease severity and comorbidity, or a “survivor effect,” whereby racial differences in survival before the development of ESRD influence the relative health of the black and white populations after initiation of dialysis.
Because of the notable and paradoxical finding that black Americans have better survival than white Americans with ESRD, we sought to examine whether there are racial differences in the response of patients to episodes of acute renal failure (ARF). We hypothesized that black patients would exhibit lower in-hospital mortality after ARF compared with white patients.
RESULTS
Characteristics of Patients and Hospitals
White patients were on average >8 yr older than black patients with ARF, without ARF, and with ARF that required dialysis (ARF-D; Table 1). Black patients were more likely than white patients to have Medicaid insurance and to reside in a zip code with median income under $25,000. Black patients were most heavily concentrated in the southern United States and were more likely than white patients to be admitted to a teaching hospital. White patients with ARF were evenly distributed across hospitals according to the number of ARF-D discharges per year (from lowest to highest quartile 24.9, 25.0, 26.0, and 24.0%); in contrast, black patients with ARF were more likely to be admitted to hospitals with a higher number of ARF-D discharges (from lowest to highest quartile 16.8, 22.6, 26.8, and 33.7%; P < 0.001).
Table 1.
Demographic and hospital characteristics of patients, NIS 2000 to 2003a
| Characteristic | No ARF | ARF | ARF-D | |||
|---|---|---|---|---|---|---|
| White | Black | White | Black | White | Black | |
| Discharges (n [%]) | 12,998,534 (97.4) | 2,418,016 (96.6) | 323,116 (2.4) | 76,812 (3.1) | 30,254 (0.2) | 9010 (0.4) |
| Mean age (yr) | 59.8 | 50.5 | 71.7 | 63.3 | 66.9 | 58.0 |
| Female (%) | 59.9 | 63.7 | 46.7 | 49.9 | 42.0 | 47.6 |
| Insurance type (%) | ||||||
| Medicare | 50.0 | 35.7 | 73.9 | 58.5 | 66.0 | 51.8 |
| Medicaid | 7.9 | 25.1 | 4.6 | 16.5 | 6.2 | 20.5 |
| private | 35.8 | 28.2 | 18.2 | 17.7 | 23.7 | 20.2 |
| self-pay | 3.3 | 6.7 | 1.7 | 4.4 | 2.1 | 4.5 |
| Median income (%)b | ||||||
| <$25,000 | 2.6 | 18.9 | 3.2 | 21.4 | 2.9 | 21.7 |
| $25,000 to $34,999 | 20.2 | 30.5 | 22.7 | 29.5 | 21.4 | 28.3 |
| $35,000 to $44,999 | 23.6 | 25.3 | 27.1 | 24.8 | 27.1 | 25.7 |
| ≥$45,000 | 38.3 | 25.3 | 47.0 | 24.3 | 48.5 | 24.3 |
| Hospital region (%)c | ||||||
| Northeast | 27.3 | 25.1 | 31.4 | 23.0 | 29.3 | 23.9 |
| Midwest | 17.7 | 12.1 | 15.4 | 13.3 | 15.1 | 12.1 |
| South | 38.5 | 52.7 | 37.1 | 53.9 | 34.5 | 51.5 |
| West | 16.5 | 10.1 | 16.1 | 9.8 | 21.1 | 12.5 |
| Hospital size (%) | ||||||
| small | 12.2 | 9.5 | 10.7 | 8.9 | 7.5 | 7.8 |
| medium | 25.3 | 27.1 | 23.6 | 23.7 | 22.4 | 22.9 |
| large | 62.4 | 63.3 | 65.7 | 67.4 | 70.1 | 69.2 |
| Hospital location (%) | ||||||
| rural | 15.5 | 8.0 | 10.3 | 6.2 | 6.1 | 4.2 |
| urban | 84.5 | 92.0 | 89.7 | 93.8 | 93.9 | 95.8 |
| Teaching hospital (%) | 38.5 | 57.5 | 43.2 | 60.7 | 47.4 | 60.7 |
All results except for number of discharges were calculated with the use of sampling weights.
Median income excludes data from 2003, when reported income quartile cut points were changed.
Northeast states include CT, MA, NH, NJ, NY, PA, and RI; Midwest states include IA, IN, KS, MI, MO, SD, and WI; South states include FL, MD, NC, SC, TN, TX, and VA; West states include AZ, CA, CO, HI, and UT.
A higher proportion of black patients with ARF underwent dialysis than white patients with ARF (11.7 versus 9.4%; P < 0.001). This pattern was observed in every region of the United States, in teaching and nonteaching hospitals, in urban and rural hospitals, and in every quartile of hospital volume of ARF-D (data not shown).
The diagnoses and procedures that accompanied ARF differed according to race (Table 2). For example, coronary artery bypass grafting (CABG) was a listed procedure in 3.6% of white patients with ARF-D but only 1.1% of black patients with ARF-D. White patients with ARF and ARF-D more often had CHF, AMI, and chronic kidney disease (CKD) than their black counterparts. The racial distribution of diagnoses and procedures among patients without ARF generally mirrored the distribution in patients with ARF, with the exception of CKD, which was more common among black than white patients without but not with ARF. The mean Deyo-Charlson Index (D-CI) was similar in both groups, but the distribution varied, with black patients more likely to have D-CI ≥6. White patients were more often discharged to a skilled nursing facility than black patients. Length of stay (LOS) was similar between the two groups.
Table 2.
Clinical characteristics of patients, NIS 2000 to 2003a
| Characteristic | No ARF | ARF | ARF-D | |||
|---|---|---|---|---|---|---|
| White | Black | White | Black | White | Black | |
| Admission type | ||||||
| emergency | 46.5 | 59.3 | 64.1 | 76.8 | 59.7 | 75.3 |
| urgent | 23.6 | 18.6 | 22.4 | 14.0 | 25.6 | 15.7 |
| elective | 29.8 | 21.9 | 13.5 | 9.0 | 14.6 | 8.9 |
| Associated procedures (%)b | ||||||
| mechanical ventilation | 2.4 | 2.8 | 18.7 | 18.6 | 33.0 | 30.4 |
| CABG | 1.3 | 0.5 | 3.4 | 1.4 | 3.6 | 1.1 |
| cardiac catheterization/PCI | 6.1 | 3.6 | 6.2 | 3.9 | 6.4 | 3.4 |
| Associated diagnoses (%)b | ||||||
| CHF | 12.3 | 11.5 | 37.6 | 29.8 | 40.6 | 31.6 |
| AMI | 3.6 | 2.0 | 11.0 | 6.6 | 12.2 | 7.6 |
| Pneumonia | 6.4 | 5.6 | 16.7 | 14.7 | 19 | 17.7 |
| Sepsis | 2.4 | 3.2 | 19.5 | 20.3 | 27 | 28.4 |
| CKD | 0.8 | 1.2 | 7.3 | 5.7 | 10.6 | 9.4 |
| acute pancreatitis | 0.9 | 1.5 | 2.1 | 3.2 | 3.2 | 4.2 |
| acute hepatic failure | 0.1 | 0.1 | 1.8 | 1.4 | 3.9 | 2.7 |
| GI hemorrhage | 1.6 | 1.5 | 5.1 | 4.7 | 6.0 | 5.7 |
| diabetes | 16.8 | 23.5 | 28.2 | 35.7 | 30.1 | 34.1 |
| D-CI (%) | ||||||
| 0 to 2 | 86.8 | 85.7 | 67.9 | 67.2 | 62.3 | 63.2 |
| 3 to 5 | 9.4 | 9.2 | 25.3 | 22.1 | 31.1 | 24.8 |
| ≥6 | 3.9 | 5.1 | 6.8 | 10.7 | 6.6 | 12.0 |
| mean D-CI | 1.2 | 1.2 | 2.2 | 2.3 | 2.3 | 2.4 |
| Discharge to SNF | 14.9 | 11.0 | 28.0 | 23.9 | 24.8 | 21.0 |
| Median LOS (d) | 4.8 | 5.4 | 7.2 | 7.4 | 12.3 | 11.7 |
SNF, skilled nursing facility.
Values are expressed as percentages of white or black patients with the associated procedure or diagnosis. All results were calculated with the use of sampling weights.
In-Hospital Mortality
Age-standardized in-hospital mortality was lower among black patients with ARF and ARF-D than white patients (Table 3). This finding was consistently observed in both genders, across all insurance types, in every geographic region, in rural and urban hospitals, and in teaching and nonteaching hospitals. For nearly every diagnosis and procedure type examined, age-standardized in-hospital mortality was lower among black than white patients with ARF and ARF-D. In every quartile of hospital volume of ARF-D, black patients with ARF had lower age-standardized in-hospital mortality than white patients with ARF (data not shown).
Table 3.
Age-standardized in-hospital mortality of patients with ARF and ARF-D according to selected demographic, hospital, and clinical characteristics
| Parameter | ARF | ARF-D | ||
|---|---|---|---|---|
| White | Black | White | Black | |
| Overall (%) | 21.9 | 19.0 | 29.4 | 26.6 |
| Female (%) | 21.2 | 18.7 | 30.7 | 26.4 |
| Male (%) | 22.5 | 19.4 | 30.3 | 27.0 |
| Insurance type (%) | ||||
| Medicare | 21.0 | 18.4 | 27.7 | 25.1 |
| Medicaid | 25.5 | 21.7 | 36.5 | 32.7 |
| private | 22.6 | 20.1 | 29.3 | 25.5 |
| self-pay | 30.0 | 25.7 | 32.0 | 22.2 |
| Hospital region (%) | ||||
| Northeast | 22.3 | 21.7 | 31.1 | 30.8 |
| Midwest | 20.6 | 16.7 | 27.4 | 25.9 |
| South | 21.7 | 18.4 | 27.2 | 23.5 |
| West | 22.3 | 19.6 | 32.9 | 31.6 |
| Hospital type | ||||
| Rural | 19.7 | 16.3 | 23.5 | 21.2 |
| urban nonteaching | 21.3 | 19.0 | 27.4 | 27.6 |
| urban teaching | 22.9 | 19.4 | 32.1 | 26.2 |
| Associated procedures (%) | ||||
| mechanical ventilation | 55.5 | 56.0 | 55.3 | 58.9 |
| CABG | 23.8 | 20.7 | 37.3 | 28.3 |
| cardiac catheterization/PCI | 19.5 | 14.1 | 28.4 | 21.6 |
| Associated diagnoses (%) | ||||
| CHF | 23.1 | 20.0 | 29.7 | 28.2 |
| AMI | 34.5 | 33.7 | 39.8 | 39.3 |
| pneumonia | 33.6 | 31.4 | 43.5 | 40.0 |
| sepsis | 44.4 | 41.7 | 52.3 | 48.2 |
| CKD | 18.2 | 18.4 | 22.7 | 23.6 |
| acute pancreatitis | 29.0 | 24.1 | 40.3 | 35.7 |
| acute hepatic failure | 61.2 | 62.1 | 64.5 | 64.1 |
| GI hemorrhage | 34.0 | 32.2 | 44.6 | 42.2 |
| diabetes | 13.5 | 12.4 | 20.6 | 21.6 |
All results were calculated with the use of sampling weights.
The results of logistic regression models to examine in-hospital mortality are shown in Table 4. In unadjusted and adjusted models, black patients had significantly lower odds for death than white patients. In the fully adjusted model, black patients with ARF had 18% (95% confidence interval [CI] 16 to 21%) lower odds for death than white patients with ARF; the odds for death for black patients with ARF-D were 16% (95% CI 10 to 22%) lower than those for white patients with ARF-D. Lower odds for death among black patients with ARF were observed consistently across the spectrum of age (data not shown).
Table 4.
Odds ratio for in-hospital mortality among patients with ARF and ARF-D
| Parameter | ARF | ARF-D | ||
|---|---|---|---|---|
| White | Black | White | Black | |
| Unadjusted | 1.0 (ref) | 0.75 (0.73 to 0.77) | 1.0 (ref) | 0.74 (0.70 to 0.80) |
| Age and gender adjusted | 1.0 (ref) | 0.84 (0.82 to 0.87) | 1.0 (ref) | 0.85 (0.79 to 0.91) |
| Age, gender, and mechanical ventilation | 1.0 (ref) | 0.84 (0.82 to 0.87) | 1.0 (ref) | 0.87 (0.81 to 0.93) |
| Age, gender, mechanical ventilation, and D-CI | 1.0 (ref) | 0.82 (0.79 to 0.84) | 1.0 (ref) | 0.84 (0.78 to 0.90) |
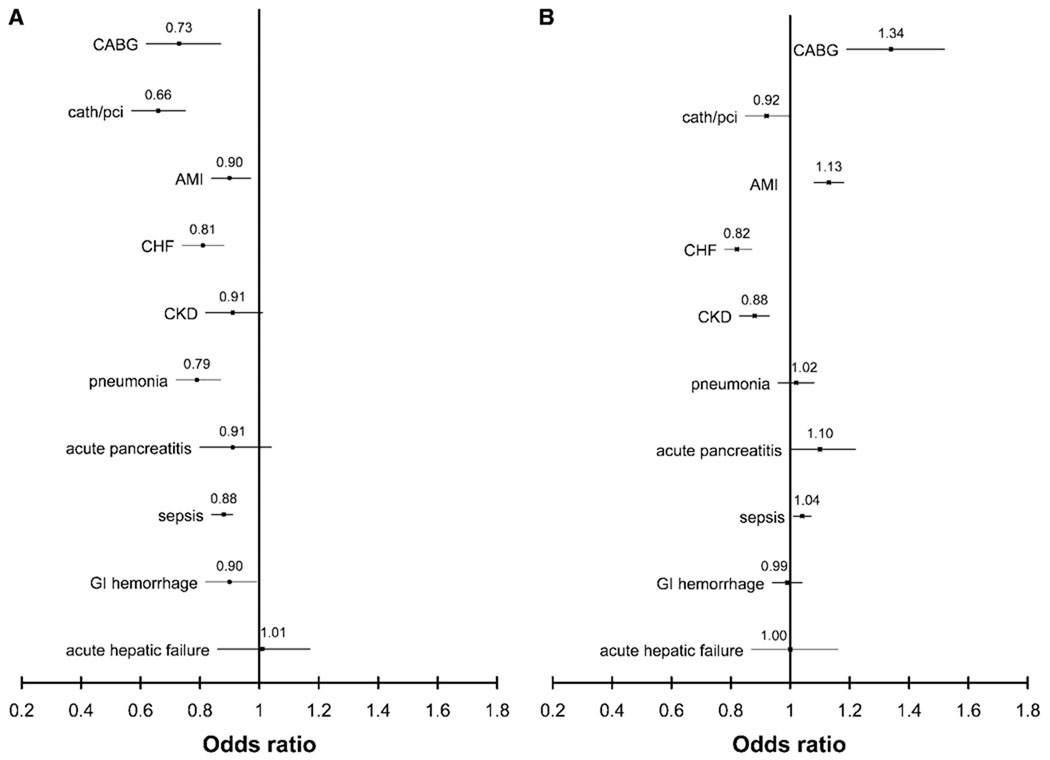
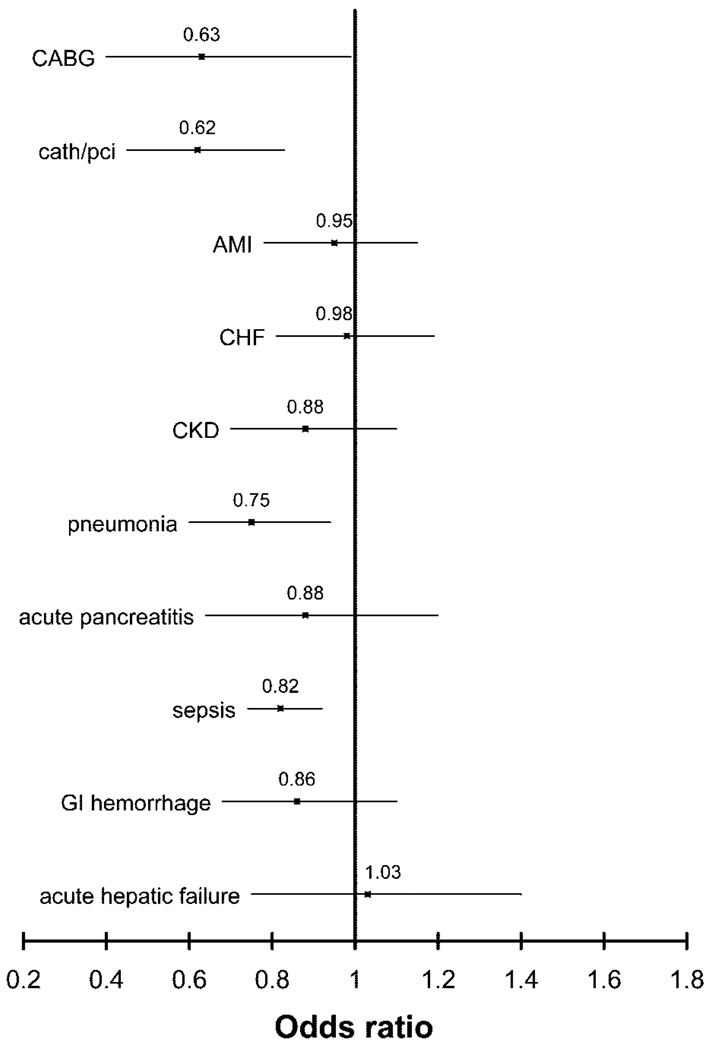
Figure 1 shows the multivariable-adjusted odds for death in black patients with and without ARF compared with white patients with and without ARF across a spectrum of diagnoses and procedures. Black patients who underwent CABG without developing ARF had a 34% (95% CI 19 to 52%) higher odds for death than white patients, but with ARF, black patients had a 27% (95% CI 13 to 38%) lower odds for death than white patients. Similarly for AMI, black patients had a 13% (95% CI 8 to 18%) higher odds for death without ARF but a 10% (95% CI 3 to 16%) lower odds for death with ARF. In every diagnosis or procedure stratum examined except for acute hepatic failure, black patients with ARF had lower odds for death than white patients, although the differences were of borderline statistical significance for patients with CKD or acute pancreatitis. Qualitatively similar results were observed in patients with ARF-D (Figure 2).
Figure 1.
Multivariable-adjusted odds ratio for death among black patients with ARF (A) and without ARF (B), stratified by selected concomitant procedures and diagnoses. Multivariable models adjusted for age, gender, need for mechanical ventilation, and D-CI. The reference group is white patients with the corresponding diagnoses and/or procedure. Point estimates are represented by the symbol and 95% confidence intervals by horizontal lines. Values <1 denote lower in-hospital mortality for black patients. Results are stratum specific (i.e., represent differences in odds for death for black versus white patients for a given procedure or diagnosis) and are not comparable across strata. P values for the interaction term (ARF * race) were as follows: CABG <0.001, catheter/PCI <0.001, AMI <0.001, CHF 0.72, CKD 0.31, pneumonia <0.001, acute pancreatitis 0.09, sepsis <0.001, gastrointestinal (GI) hemorrhage 0.25, and acute hepatic failure 0.86.
Figure 2.
Multivariable-adjusted odds ratio for death among black patients with ARF-D and selected concomitant procedures and diagnoses. Multivariable models adjusted for age, gender, need for mechanical ventilation, and D-CI. The reference group is white patients with the corresponding diagnoses and/or procedure. Point estimates are represented by the symbol and 95% confidence intervals by horizontal lines. Values <1 denote lower in-hospital mortality for black patients. Results are stratum specific (i.e., represent differences in odds for death for black versus white patients with ARF-D for a given procedure or diagnosis) and are not comparable across strata.
Missing Race Data
A total of 154,792 discharges with ARF were missing race information. Compared with the 399,928 black and white patients with ARF, those with missing race information were more likely to be from the Midwest (missing 47.3 versus black/white 15.0%), less likely to be from the northeast (4.6 versus 29.8%), and more likely to be discharged from a rural hospital (18.3 versus 9.5%). The age-standardized in-hospital mortality was 17.9% for patients with missing race information and 19.7% in black or white patients included in our analyses.
DISCUSSION
In a large sample of hospital discharges from 2000 to 2003 including 26 states across the United States, we found that black patients with ARF and ARF-D had significantly lower in-hospital mortality than white patients with ARF and ARF-D. Our findings persisted after adjustment for age, gender, and comorbidity.
These findings were consistently observed across hospital types, geographic region, hospital volume of ARF-D, age and gender, and some but not all concomitant diagnoses and procedures. In no diagnosis or procedure stratum did we find that black patients with ARF or ARF-D had significantly higher in-hospital mortality than white patients. These findings are paradoxical in light of the overwhelming evidence of racial disparities in access to care and the quality of medical care that black Americans receive. For example, black patients are admitted to the intensive care unit with a higher severity of illness17 but receive fewer resource-intensive interventions than white patients.18
What could account for the lower in-hospital mortality of black patients with ARF and ARF-D? Differences in case mix were evident between black and white patients with ARF. For example, CABG, CHF, and AMI all were more common among white patients with ARF than black patients. However, differences in case mix alone could not account for our findings in stratified analyses. We found lower in-hospital mortality for black patients with ARF and several associated diagnoses and procedures, such as pneumonia, CHF, sepsis, AMI, CABG, gastrointestinal hemorrhage, and cardiac catheterization and percutaneous coronary intervention (PCI).
We also found that the relation between race and mortality differed by whether ARF developed (i.e., effect modification) in a number of diagnoses and procedures. Black patients who underwent CABG or had AMI had higher in-hospital mortality without ARF (and overall, as shown previously)2,19–21 but lower in-hospital mortality when ARF developed.
Differences in LOS can confound studies examining in-hospital mortality. For example, Jencks et al.22 found that an apparent survival advantage in patients who were hospitalized in California versus New York was due to significantly shorter LOS in California. We were unable to ascertain 30-d mortality because the Nationwide Inpatient Sample (NIS) does not permit individual patient-level analyses after discharge. However, differences in LOS are an unlikely explanation for our findings, because black and white patients with ARF and ARF-D had comparable LOS. More frequent discharge to skilled nursing facilities can also bias in-hospital mortality comparisons. In our study, white patients were more likely than black patients to be discharged to skilled nursing facilities, which would tend to lower the observed in-hospital mortality for white patients more than for black patients.
Several clinical factors could partially explain the findings observed. We found that black patients were on average 8 yr younger than their white counterparts with ARF and ARF-D. Black patients were also >9 yr younger than their white counterparts without ARF. Older age is a well-established risk factor for ARF23–26; despite the younger age of the black population, the risk for ARF was comparable and in fact slightly higher than that for white patients. It is noteworthy that a younger age at disease onset has also been observed in ESRD. The association between younger age and ARF and ESRD among black patients could reflect an increased susceptibility to ARF and progressive CKD. A higher risk for developing ARF among black patients has been found in other studies. In a study of hospitalized Medicare beneficiaries, Xue et al.27 found that ARF was 1.5-fold more common among black than white patients. In two studies using clinically detailed databases, Bridges et al.21 and Thakar et al.28 found that black patients have a significantly higher risk for ARF after CABG than white patients. A comparison of patients by race after ARF was not reported in the study by Bridges et al..21 In the study by Thakar et al.,28 the authors found lower in-hospital mortality among black patients with ARF, but this difference was not statistically significant; however, only 849 black patients were included, approximately 5% (n = 42) of whom developed ARF, suggesting that the study was underpowered to detect mortality differences in ARF according to race. In addition, the investigators did not address the possibility of effect modification by race, which we have shown to be present in CABG- and ARF-related mortality.
If a more modest toxic or metabolic insult were required to cause ARF in black patients—perhaps as a result of lower nephron number associated with lower birth weight,29 higher prevalence (or increased severity) of diabetes,30 or other social and/or biologic determinants—then the population of black patients with ARF could have less severe associated (nonrenal) disease than white patients with ARF. Unmeasured confounding by severity of illness could explain higher mortality among white patients; however, on the basis of a similar mean D-CI and a higher fraction of black patients with D-CI ≥6, it does not seem that black patients were healthier than white patients overall. Moreover, the consistency of the findings across underlying disease and procedure strata suggests the possibility of a biologic basis for the observations. The consistency of our findings across the spectrum of age suggests that the marked age difference among white and black patients did not account for the observed mortality difference with ARF.
It is interesting to note that a larger fraction of black patients with ARF received dialysis than white patients with ARF. This finding contrasts with the pattern observed for other resource-intensive procedures, such as total hip replacement4 and cardiac catheterization.2,3 It is unclear why black patients with ARF were more likely to undergo dialysis than white patients with ARF. Possible explanations include greater severity of ARF in black patients, higher creatinine values in black than white patients (which could lower the threshold for physicians to initiate dialysis in the setting of ARF), younger age of black patients with ARF, and differing coding practices by race. The finding that black patients with ARF were more commonly discharged from hospitals with high volume of ARF-D did not account for the higher likelihood of dialysis, because black patients with ARF were more likely to undergo dialysis in every quartile of hospital volume of ARF-D. Similarly, hospital volume of ARF-D was unlikely to account for the racial differences in mortality for ARF, because the mortality differences were present in every quartile of hospital volume of ARF-D.
Some studies have demonstrated racial differences in biologic processes that are central in the pathophysiology of ARF, such as abnormalities in endothelial function,31,32 oxidative stress,33 and inflammation.34,35 Whether these differences could account for the paradoxical findings by ARF status deserves further investigation. Furthermore, the parallel findings of improved survival for black patients with ARF and ESRD and emerging evidence of a similar pattern in CKD36 suggest that the response to uremia and to the provision of dialysis may vary by race. Although biologic differences may be evident, investigators should be cautioned that race is also a social construct and that observed differences in clinical outcomes across races may have social or economic underpinnings.37
This study has several strengths, including the large sample size, multiregional representation, and inclusion of all payer types. We had the capacity to examine the major study question across multiple strata of underlying diagnoses and procedures and demonstrated the consistency of the main findings. Limitations of this study include the lack of laboratory and other detailed clinical data during the course of ARF; potential for misclassification (likely nondifferential38) of race in hospital discharge abstracts; lack of information on causes of death or postdischarge vital status; inability to track individual patients to account for readmissions; and inability to determine the extent of recovery after ARF, including the likelihood of requiring maintenance dialysis.
Information on race was missing in approximately one quarter of the total data set, the majority (82%) from 11 states that did not provide race information on hospital discharge abstracts. Still, discharges from every region in the United States were included in the final data set, enhancing the generalizability of our findings. We found lower in-hospital mortality among patients with ARF and no recorded race information. The racial makeup of patients without race information is of course unknown; the survival benefit observed for blacks with ARF could have been overestimated if the majority of discharges with missing race information were white. Conversely, we could have underestimated the survival benefit for black patients with ARF depending on the racial distribution of hospitalized patients in the 11 states cited. The states that did not provide race information had slightly lower overall black populations (11.4% of total) than states that did report race (12.5%).39 However, the consistency of our findings across regions of the United States suggests that missing data were not a major source of bias.
The reliance on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes may also be problematic, particularly for ARF. We recently demonstrated that ICD-9-CM codes have low sensitivity (35.4%) but high specificity (97.7%) for ARF using conventional diagnostic criteria; the sensitivity, specificity, and predictive values of ICD-9-CM codes for ARF-D, conversely, each exceeded 90%.40 An apparent increased risk for ARF among black patients (and potentially lower in-hospital mortality) could be observed if black patients were more likely to be coded as having ARF, but in the three-hospital study in which ICD-9-CM codes were compared with serum creatinine– based definitions of ARF, we found no significant difference in the sensitivity of the ICD-9-CM code for ARF among black compared with white patients (39.2 versus 35.5%; P = 0.12).40 Furthermore, our findings of significant mortality differences between black and white patients with ARF-D, for which ICD-9-CM codes are reliable, support the validity of our conclusions. Although we do not believe that coding practices could explain the large and consistent findings here, we do not have individual patient-level clinical data to review from NIS.
Black patients with ARF and ARF-D have lower overall in-hospital mortality than white patients. Potential explanations for this finding, such as differences in susceptibility to kidney injury, severity of illness, or the response to uremia, deserve further investigation.
CONCISE METHODS
Data Source
We analyzed data from the 2000 to 2003 NIS, the largest all-payer administrative database in the United States. The NIS captures patient- and hospital-level data from a stratified probability sample of hospitals from states participating in the Healthcare Cost and Utilization Project. The NIS provides data on patient demographics, hospital characteristics, in-hospital mortality, disposition, LOS, and up to 15 diagnosis and 15 procedure codes based on the ICD-9-CM. The database has been used for a variety of clinical investigations, including previous studies examining racial differences in the outcomes of hospitalized individuals.19,41
Patient Population
The NIS 2000 to 2003 contains information on 30,735,429 discharges. We analyzed data from discharges of patients who were at least 18 yr of age and had race reported as black or white (n = 15,820,871). Eleven of the 38 participating states (GA, IL, KY, ME, MN, NE, NV, OH, OR, WA, and WV) in the NIS did not report race information and were therefore excluded from the analyses. Data from these states accounted for 82% of all discharges missing race information. We identified patients with ARF by the presence of any of the following ICD-9-CM codes: 584.5 (ARF, with lesion of tubular necrosis), 584.6 (ARF, with lesion of cortical necrosis), 584.7 (ARF, with lesion of renal medullary necrosis), 584.8 (ARF, with other specified pathologic lesions), and 584.9 (ARF, unspecified). To avoid inclusion of patients who were admitted for initiation of hemodialysis for ESRD, we excluded 4393 discharges with procedure codes for arteriovenous fistula or graft creation (39.27, 39.42, 39.43, and 39.93), leaving 399,928 with ARF. We identified the subset of patients with ARF-D (n = 39,264) by the additional presence of the procedure code for hemodialysis (39.95), which includes continuous renal replacement therapies such as continuous venovenous hemofiltration and hemodiafiltration. We also identified patients with the following diagnoses and procedures commonly associated with ARF (see Appendix 1 for ICD-9-CM codes): CHF, AMI, pneumonia, sepsis, CKD, acute pancreatitis, acute hepatic failure, gastrointestinal hemorrhage, CABG, and cardiac catheterization and PCI. More than 99.5% of discharges had complete information for multivariable logistic regression models.
Appendix.
ICD-9-CM codes for procedures and diagnoses
| Parameter | ICD-9-CM Code(s) |
|---|---|
| Procedure | |
| hemodialysis | 39.95 |
| mechanical ventilation | 93.90, 93.92, 96.01, 96.04, 96.05, 96.70, 96.71, 96.72 |
| CABG | 36.1× |
| cardiac catheterization/PCI | 88.53 to 88.57, 36.01, 36.02, 36.05, 36.06, 36.07, 36.09 |
| Diagnosis | |
| acute renal failure | 584.× |
| sepsis | 038.×, 112.5, 112.81, 020.2, 790.7, 785.59 |
| AMI | 410.× |
| CHF | 428.× |
| pneumonia | 480.×, 481, 482.×, 483.×, 484.×, 485, 486 |
| CKD | 585 |
| acute pancreatitis | 577.0 |
| acute hepatic failure | 570 |
| GI hemorrhage | 578.× |
| diabetes | 250.× |
To determine a proxy for the activity and experience with dialysis for ARF, we divided hospitals into quartiles according to the annual number of discharges with ARF-D. The median number of ARF-D discharges per year for hospitals included in this analysis was 36 (interquartile range 16 to 67).
Statistical Analyses
All analyses were performed using survey procedures in SAS version 9.1 (SAS Institute, Cary, NC) or SUDAAN version 9.0 (Research Triangle Institute, Research Triangle Park, NC) to account for the complex survey design of the NIS. Frequencies, means, and medians were calculated with the use of sampling weights. Given the large size of the data set, extremely small differences between groups may be statistically significant, and for that reason, P values are not presented.
To compare in-hospital mortality between white and black patients with ARF, we fit a series of logistic regression models. First, we fit unadjusted logistic regression models to estimate the odds for death for patients according to race. Then we fit age- (10-yr intervals) and gender-adjusted models and multivariable models, the latter incorporating the D-CI and the presence or absence of mechanical ventilation to adjust for comorbidity and severity of illness. The D-CI is the sum of the weighted number of comorbid conditions based on 17 diagnostic categories identified from ICD-9-CM diagnosis codes. Comorbid conditions represented in the D-CI include AMI, CHF, CKD, diabetes, liver disease, and cancer, among others.42 We compared in-hospital mortality between white and black patients without ARF, with ARF, and with ARF-D in the following diagnostic or procedure strata: Sepsis, CKD, pneumonia, acute hepatic failure, acute pancreatitis, gastrointestinal hemorrhage, AMI, CABG, and cardiac catheterization and PCI. Effect modification by the presence of ARF was tested by entering the multiplicative interaction term (ARF * race) into multivariable models. Generalized estimating equations for logistic regression models were used to account for the complex survey design of the NIS and to adjust for clustering of patient outcomes within hospitals.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Mortality patterns—United States, 1997. MMWR Morb Mortal Wkly Rep. 1999;48:664–668. [PubMed] [Google Scholar]
- 2.Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, Manhapra A, Mallik S, Krumholz HM. National Registry of Myocardial Infarction Investigators: Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353:671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA. 1994;271:1175–1180. [PubMed] [Google Scholar]
- 4.Hoaglund FT, Oishi CS, Gialamas GG. Extreme variations in racial rates of total hip arthroplasty for primary coxarthrosis: A population-based study in San Francisco. Ann Rheum Dis. 1995;54:107–110. doi: 10.1136/ard.54.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns RB, McCarthy EP, Freund KM, Marwill SL, Shwartz M, Ash A, Moskowitz MA. Black women receive less mammography even with similar use of primary care. Ann Intern Med. 1996;125:173–182. doi: 10.7326/0003-4819-125-3-199608010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ayanian JZ, Weissman JS, Chasan-Taber S, Epstein AM. Quality of care by race and gender for congestive heart failure and pneumonia. Med Care. 1999;37:1260–1269. doi: 10.1097/00005650-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Frankenfield DL, Rocco MV, Frederick PR, Pugh J, McClellan WM, Owen WF., Jr Racial/ethnic analysis of selected intermediate outcomes for hemodialysis patients: Results from the 1997 ESRD Core Indicators Project. Am J Kidney Dis. 1999;34:721–730. doi: 10.1016/s0272-6386(99)70399-9. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM. Racial disparities in access to renal transplantation: Clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343:1537–1544. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–1669. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 10.Reddan D, Klassen P, Frankenfield DL, Szczech L, Schwab S, Coladonato J, Rocco M, Lowrie EG, Owen WF., Jr National ESRD CPM Work Group: National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol. 2002;13:2117–2124. doi: 10.1097/01.asn.0000022422.79790.a8. [DOI] [PubMed] [Google Scholar]
- 11.Bleyer AJ, Tell GS, Evans GW, Ettinger WH, Jr, Burkart JM. Survival of patients undergoing renal replacement therapy in one center with special emphasis on racial differences. Am J Kidney Dis. 1996;28:72–81. doi: 10.1016/s0272-6386(96)90133-x. [DOI] [PubMed] [Google Scholar]
- 12.Bloembergen WE, Port FK, Mauger EA, Wolfe RA. Causes of death in dialysis patients: Racial and gender differences. J Am Soc Nephrol. 1994;5:1231–1242. doi: 10.1681/ASN.V551231. [DOI] [PubMed] [Google Scholar]
- 13.Cowie CC, Port FK, Rust KF, Harris MI. Differences in survival between black and white patients with diabetic end-stage renal disease. Diabetes Care. 1994;17:681–687. doi: 10.2337/diacare.17.7.681. [DOI] [PubMed] [Google Scholar]
- 14.Mesler DE, McCarthy EP, Byrne-Logan S, Ash AS, Moskowitz MA. Does the survival advantage of nonwhite dialysis patients persist after case mix adjustment? Am J Med. 1999;106:300–306. doi: 10.1016/s0002-9343(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 15.Pei YP, Greenwood CM, Chery AL, Wu GG. Racial differences in survival of patients on dialysis. Kidney Int. 2000;58:1293–1299. doi: 10.1046/j.1523-1755.2000.00285.x. [DOI] [PubMed] [Google Scholar]
- 16.US Renal Data System. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. 2004
- 17.Williams JF, Zimmerman JE, Wagner DP, Hawkins M, Knaus WA. African-American and white patients admitted to the intensive care unit: Is there a difference in therapy and outcome? Crit Care Med. 1995;23:626–636. doi: 10.1097/00003246-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, Califf RM, Vidaillet H, Davis RB, Muhlbaier LH, Connors AF. Race, resource use, and survival in seriously ill hospitalized adults. The SUPPORT Investigators. J Gen Intern Med. 1996;11:387–396. doi: 10.1007/BF02600183. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47:417–424. doi: 10.1016/j.jacc.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 20.Konety SH, Vaughan Sarrazin MS, Rosenthal GE. Patient and hospital differences underlying racial variation in outcomes after coronary artery bypass graft surgery. Circulation. 2005;111:1210–1216. doi: 10.1161/01.CIR.0000157728.49918.9F. [DOI] [PubMed] [Google Scholar]
- 21.Bridges CR, Edwards FH, Peterson ED, Coombs LP. The effect of race on coronary bypass operative mortality. J Am Coll Cardiol. 2000;36:1870–1876. doi: 10.1016/s0735-1097(00)00956-6. [DOI] [PubMed] [Google Scholar]
- 22.Jencks SF, Williams DK, Kay TL. Assessing hospital-associated deaths from discharge data. The role of length of stay and comorbidities. JAMA. 1988;260:2240–2246. [PubMed] [Google Scholar]
- 23.Yegenaga I, Hoste E, Van Biesen W, Vanholder R, Benoit D, Kantarci G, Dhondt A, Colardyn F, Lameire N. Clinical characteristics of patients developing ARF due to sepsis/systemic inflammatory response syndrome: Results of a prospective study. Am J Kidney Dis. 2004;43:817–824. doi: 10.1053/j.ajkd.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 24.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, Daley J. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 25.Moore RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med. 1984;100:352–357. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- 26.Mehran R, Aymong ED, Nikolsky E, Lasicx Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 27.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 28.Thakar CV, Liangos O, Yared JP, Nelson D, Piedmonte MR, Hariachar S, Paganini EP. ARF after open-heart surgery: Influence of gender and race. Am J Kidney Dis. 2003;41:742–751. doi: 10.1016/s0272-6386(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 29.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int. 2005 Suppl 97:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S, Malarcher AM, Giles WH, Myers G. Racial, ethnic and socioeconomic disparities in the clustering of cardiovascular disease risk factors. Ethn Dis. 2004;14:43–48. [PubMed] [Google Scholar]
- 31.Kahn DF, Duffy SJ, Tomasian D, Holbrook M, Rescorl L, Russell J, Gokce N, Loscalzo J, Vita JA. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40:195–201. doi: 10.1161/01.hyp.0000024571.69634.ed. [DOI] [PubMed] [Google Scholar]
- 32.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: Predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 33.Zitouni K, Nourooz-Zadeh J, Harry D, Kerry SM, Betteridge DJ, Cappuccio FP, Earle KA. Race-specific differences in antioxidant enzyme activity in patients with type 2 diabetes: A potential association with the risk of developing nephropathy. Diabetes Care. 2005;28:1698–1703. doi: 10.2337/diacare.28.7.1698. [DOI] [PubMed] [Google Scholar]
- 34.Crosse K, Umeadi OG, Anania FA, Laurin J, Papadimitriou J, Drachenberg C, Howell CD. Racial differences in liver inflammation and fibrosis related to chronic hepatitis C. Clin Gastroenterol Hepatol. 2004;2:463–468. doi: 10.1016/s1542-3565(04)00162-4. [DOI] [PubMed] [Google Scholar]
- 35.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 36.Smith GL, Shlipak MG, Havranek EP, Masoudi FA, McClellan WM, Foody JM, Rathore SS, Krumholz HM. Race and renal impairment in heart failure: Mortality in blacks versus whites. Circulation. 2005;111:1270–1277. doi: 10.1161/01.CIR.0000158131.78881.D5. [DOI] [PubMed] [Google Scholar]
- 37.Foster MW, Sharp RR. Beyond race: Towards a whole-genome perspective on human populations and genetic variation. Nat Rev Genet. 2004;5:790–796. doi: 10.1038/nrg1452. [DOI] [PubMed] [Google Scholar]
- 38.Blustein J. The reliability of racial classifications in hospital discharge abstract data. Am J Public Health. 1994;84:1018–1021. doi: 10.2105/ajph.84.6.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Census Bureau. Census 2000 Summary File 1, GCT-P6. Washington, DC,: US Census Bureau; Race and Hispanic or Latino.
- 40.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 41.Bateman BT, Schumacher HC, Boden-Albala B, Berman MF, Mohr JP, Sacco RL, Pile-Spellman J. Factors associated with in-hospital mortality after administration of thrombolysis in acute ischemic stroke patients: An analysis of the nationwide inpatient sample 1999 to 2002. Stroke. 2006;37:440–446. doi: 10.1161/01.STR.0000199851.24668.f1. [published erratum appears in Stroke 38: 451, 2007] [DOI] [PubMed] [Google Scholar]
- 42.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]