Abstract
Purpose of review
This review focuses on studies from the past year that highlight molecular and cellular mechanisms of pancreatic injury arising from acute and chronic pancreatitis.
Recent findings
Factors that induce or ameliorate injury as well as cellular pathways involved have been examined. Causative or sensitizing factors include refluxed bile acids, hypercalcemia, ethanol, hypertriglyceridemia, and acidosis. In addition, the diabetes drug exendin-4 has been associated with pancreatitis, whereas other drugs may reduce pancreatic injury. The intracellular events that influence disease severity are better understood. Cathepsin-L promotes injury through an antiapoptotic effect, rather than by trypsinogen activation. In addition, specific trypsinogen mutations lead to trypsinogen misfolding, endoplasmic reticulum stress, and injury. Endogenous trypsin inhibitors and upregulation of proteins including Bcl-2, fibroblast growth factor 21, and activated protein C can reduce injury. Immune cells, however, have been shown to increase injury via an antiapoptotic effect.
Summary
The current findings are critical to understanding how causative factors initiate downstream cellular events resulting in pancreatic injury. Such knowledge will aid in the development of targeted treatments for pancreatitis. This review will first discuss factors influencing pancreatic injury, and then conclude with studies detailing the cellular mechanisms involved.
Keywords: alcohol, bile acids, calcium, ethanol, Toll-like receptor, trypsinogen
Introduction
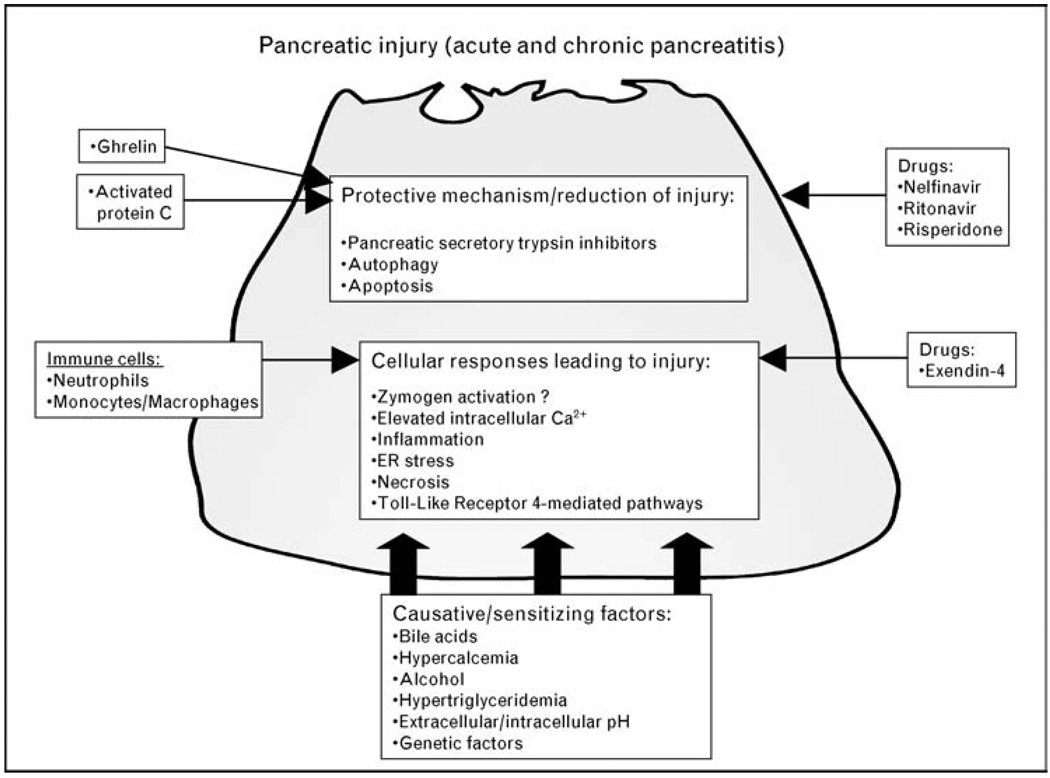
Multiple factors initiate or enhance cellular injury in pancreatitis (Fig. 1). However, beneficial factors have also been identified. In this summary of the past year’s work, we first review general extracellular factors that affect pancreatic injury and then summarize the downstream pathways responsible for these effects.
Figure 1. Mediators of pancreatic injury in acute and chronic pancreatitis.
Multiple factors induce, or sensitize, the pancreatic acinar cell to pancreatitis and cellular injury. Causative/sensitizing factors of pancreatic injury include refluxed bile acids, alcohol, and pH. They largely mediate downstream events such as elevated intracellular Ca2+ via influx through TRPC3 channels and release from IP3 and ryanodine-sensitive Ca2+ stores. Genetic factors, such as mutations in tryspinogen, lead to misfolded proteins and endoplasmic reticulum stress. Immune cells promote necrosis over apoptosis leading to increased disease severity. The diabetes drug exendin-4 facilitates injurious responses, whereas HIV drugs nelfinavir and ritonavir stabilize mitochondria, supporting apoptosis. Some proteins (ghrelin, activated protein C) encourage protective mechanisms leading to reduced injury. IP3, inositol 1,4,5-triphosphate; TRPC3, transient receptor potential isoform 3.
Bile acids
Biliary pancreatitis is the most common cause of pancreatitis [1]. Yet for over a century, it remains unclear how biliary obstruction leads to pancreatitis. Recent work by Perides et al. [2••] suggests that refluxed bile acids bind to a G-protein-coupled, cell surface bile acid receptor expressed on the luminal membrane of acinar cells called Gpbar1. Deletion of Gpbar1 in vivo led to reduced severity of pancreatitis in a mouse model that used retrograde pancreatic duct injection of the bile acid taurolithocholic acid-3-sulfate (TLCS). In vitro, acinar cells from Gpbar1-deficient mice were protected against TLCS-induced generation of pathological Ca2+ transients, intracellular zymogen activation, and cell injury. Interestingly, pancreatic injury from caerulein hyperstimulation in vivo or in vitro was not affected by Gpbar1, suggesting that pathological Gpbar1 activation might be specific to biliary pancreatitis. The overall findings support the notion that biliary pancreatitis could result from bile reflux into the pancreas and that bile acids signal as ligands to receptors on the acinar cell surface.
Aberrant Ca2+ signals
Aberrant intra-acinar Ca2+ signals are critical to the transduction of acinar cell injury [3]. In addition, hypercalcemia is a risk factor for pancreatitis. However, the role of Ca2+ influx in shaping aberrant Ca2+ signals has not been well defined. Kim et al. [4] examined the role of the membrane Ca2+ influx channel transient receptor potential isoform 3 (TRPC3) on acinar cell Ca2+. They showed that acinar cells from mice deficient in TRPC3 had reduced receptor-stimulated Ca2+ influx and a lower sustained Ca2+ transient induced by bile acids or the alcohol metabolite palmitoleic acid ethyl ester (PAEE). Functionally, TRPC3 deficiency prevented the pathological inhibition of amylase secretion and reduced intraacinar zymogen activation, actin depolarization, and pancreatitis severity in vivo. The study underscores the importance of Ca2+ influx in the pathogenesis of pancreatic injury during pancreatitis.
Two recent studies have reported targets of the aberrant acinar cell Ca2+ signal generated during pancreatitis. Shah et al. [5] examined the Ca2+-activated phosphatase, calcineurin. Mice pretreated with the calcineurin inhibitor FK506, or tacrolimus, had markedly reduced intrapancreatic protease activation and pancreatitis severity at both early and late times after in-vivo caerulein hyperstimulation. Heike et al. [6] reported that Ca2+-activated proteases, calpains, are linked to pancreatitis responses. The calpain inhibitor Z-Val-Phe methyl ester reduced cholecystokinin-induced rearrangement of the actin cytoskeleton and degradation of actin-associated proteins. The two Ca2+-dependent pathways could serve as therapeutic targets.
Ethanol metabolites and Ca2+
Ethanol abuse is the most common cause of chronic pancreatitis and the second leading cause of acute disease [1]. Seminal studies by Laposata and Lange [7] demonstrated that the pancreas readily converts ethanol via a nonoxidative pathway into fatty acid ethyl esters (FAEEs). In recent work, Gerasimenko et al. [8•] examined the role of FAEEs on acinar cell Ca2+ signals leading to pathologic zymogen activation. Ca2+ release and trypsin activation induced by PAEE were primarily mediated by release of Ca2+ from the inositol 1,4,5-triphosphate receptor (IP3R) types 2 and 3 that are linked to an acidic, vesicular compartment. The findings suggest that FAEEs pathologically target selective Ca2+-releasing pools within the acinar cell. Del Castillo-Vaquero et al. [9] showed that acute application of ethanol at clinically relevant concentrations (1–50 mmol/l) to isolated acinar cells resulted in Ca2+ influx due to the production of oxidative metabolites of alcohol. Together, the studies implicate a role for alcohol metabolites in acinar cell injury through aberrant Ca2+ signals.
Lipids
Hypertriglyceridemia (serum triglyceride >1000 mg/dl) is an absolute risk factor for pancreatitis, whereas lower levels can predispose to disease [10]. Wang et al. [11] investigated hypertriglyceridemia in a pancreatitis model and found that lipoprotein lipase-deficient mice, rich in circulating serum triglycerides, developed a more severe caerulein-induced pancreatitis than wild-type mice. In addition, increased free fatty acids in the pancreatic microenvironment, due to the hydrolysis of triglycerides by pancreatic lipase, induced pancreatic injury. Interestingly, pancreatic lipase also had a protective effect; it signaled via cyclic GMP to convert pathological sustained Ca2+ signals to physiological Ca2+ oscillations. In work by Siech et al. [12], alcohol administration along with fat, in the form of very-low-density lipoprotein, led to acinar cell injury and apoptosis as well as pancreatic stellate cell (PSC) proliferation. The results of the studies indicate that pancreatic injury can result from exposure of acinar cells to free fatty acids or a combination of ethanol and lipids.
Drugs to treat pancreatitis
Several reports over this year have examined medications that may be protective against pancreatic injury during pancreatitis. Singh et al. [13] examined mechanisms responsible for the protective effects of the protease inhibitors nelfinavir and ritonavir in HIV-infected individuals. They found that the drugs reduced acinar cell injury by preventing mitochondrial membrane depolarization, without affecting the inflammatory response. Yamaguchi et al. [14] showed that the serotonin 5-hydroxytryptamine-2A receptor antagonist risperidone ameliorated severe, necrotic pancreatitis in mice induced by a choline-deficient ethionine-supplemented diet, suggesting a possible therapeutic role for serotonin blockade.
Although much work has been done in studying methods to reduce inflammation after pancreatitis, little is known about ways to enhance recovery or regeneration of the pancreas after injury. Sidhu et al. [15] showed that pharmacologic administration of the endogenous circadian regulator melatonin accelerated pancreatic regeneration in rats recovering from l-arginine-induced pancreatitis. The effect was associated with the activation of PSCs, presumably working to synthesize extracellular matrix components as a platform for the repair process. Babu et al. [16] demonstrated that the naturally occurring antioxidant and anti-inflammatory green tea polyphenols reduced the severity of caerulein-induced acute pancreatitis by preventing nuclear factor-kappa B (NF-κB) activation and reducing oxidative stress.
Drugs causing pancreatitis
Clinical and experimental studies have suggested that drugs used to treat diabetes that increase glucagon-like peptide-1 (GLP-1) levels might be linked to pancreatitis. One study [17] did not demonstrate any sensitizing effect of the GLP-1 agonist, exendin-4, in the caerulein hyperstimulation model in mice. However, two other studies [18,19] that treated rats for up to 3 months with either a GLP-1 agonist or dipeptidyl peptidase 4 inhibitor (which increases GLP-1 levels) found a distinct form of pancreatic injury in some animals. Areas of duct proliferation, fibrosis, and loss of acinar cells reminiscent of chronic pancreatitis were observed. Whether these agents increase the risk of acute pancreatitis or promote the development of chronic pancreatitis are unresolved clinical issues that must be examined prospectively.
pH effects
Experimental and clinical findings have defined a link between a low-pH environment and the onset of acute pancreatitis. An extracellular acid load (pHe), both in vivo and in vitro, enhanced secretagogue-induced zymogen activation and acinar cell injury [20•]. Furthermore, inhibition of the vacuolar ATPase, a proton transporter, blocked low pHe effects on zymogen activation. These studies suggest that an acid challenge sensitizes the pancreas to secretagogue-induced zymogen activation and injury and may increase the risk for the development and severity of acute pancreatitis.
Thus far, we have focused on causative or sensitizing factors of pancreatic injury. In the next section, we explore recent advances in understanding downstream mediators of injury.
Cathepsin-L
The second most abundant lysosomal cysteine proteinase, cathepsin-L (CTSL), cleaves trypsinogen to an inactive form of trypsin [21•]. Surprisingly, however, CTSL−/− mice developed a less severe pancreatitis. The phenotype was attributed to induction of apoptosis, thus implying an antiapoptotic role for CTSL. In addition, the effects of CTSL on disease severity are completely uncoupled from its effects on trypsinogen cleavage.
Trypsinogen
In addition to its proteolytic activation by cathepsins, trypsinogen can undergo autoactivation. Known disease mutations, p.D19A, p.D22G, and p.K23R, in the serine protease 1 (PRSS1) gene, which codes for human cationic trypsinogen, strongly stimulated trypsinogen autoactivation and markedly decreased secretion [22]. An additional study from the same group examined the effect of the p.R116C mutation and found that it induced misfolding of cationic trypsinogen and consequent endoplasmic reticulum stress, unrelated to trypsinogen activation. The latter study [23] suggests that endoplasmic reticulum stress could play a role in chronic pancreatitis.
Endogenous pancreatic secretory trypsin inhibitors (PSTIs) may protect the pancreas from cell damage by inhibiting prematurely activated trypsin. Rodent trypsin inhibitors are highly homologous to human serine protease inhibitor Kazal type 1 (SPINK 1). There are two for rat (PSTI-I and PSTI-II) and one for mouse (SPINK 3). Mutations in the gene encoding PSTIs can lead to chronic pancreatitis. Overexpression of rat PSTI-I in transgenic mice reduced chronic pancreatitis severity [24]. In another study [25] from the same group, SPINK 3−/− mice did not survive beyond 1 week and had elevated levels of pancreatic trypsin. However, mice with either the genotype SPINK 3+/−, or SPINK 3−/− but expressing rat PSTI-I, had a survival rate comparable to that seen for SPINK 3+/+ mice, indicating that a threshold level of expression for PSTI was sufficient to protect against pancreatic injury.
Autophagy
A recognized pathologic pancreatitis response is the accumulation of autophagic vacuoles in the acinar cell. Studies now suggest they have a central role in disease. Fortunato et al. [26•] concluded that an ethanol/lipopolysaccharide (LPS) model of acute pancreatitis involved the depletion of critical lysosomal proteins and inhibition of a late stage of autophagy. Similarly, Mareninova et al. [27••] reported that acute pancreatitis was associated with decreased activity in the autophagic pathway and that autophagic function induced by starvation versus pancreatitis was very different. Together, these studies suggest that decreased autophagic function, especially suppressed degradation of proteins including activated proteases, is central to the pathogenesis of acute pancreatitis.
Mitochondria
Injury and severity in pancreatitis often directly correlate with necrosis and inversely with apoptosis. Necrosis occurs following collapse of the mitochondrial membrane potential and ATP depletion, whereas apoptosis is mediated by cytochrome c release into the cytosol and subsequent caspase activation. Sung et al. [28] recently showed that the prosurvival proteins, Bcl-XL and Bcl-2, protect acinar cells by stabilizing the mitochondria and attenuating necrosis. Induction of oxidative stress and apoptosis with menadione was abrogated when mitochondrial Ca2+ uptake was blocked [24].
Furthermore, Ca2+-elevating secretagogues, cholecystokinin and acetylcholine, transiently raised ATP levels in the cytosol and mitochondria, indicating that acinar cells might need an increase in ATP production to successfully undergo apoptosis and thereby reduce injury [29]. Although factors such as bile acids and nonoxidative ethanol metabolites also increase Ca2+, a concomitant elevation in cytosolic or mitochondrial ATP levels was not observed, suggesting these agents favor necrosis and increased disease severity.
Protein kinase C delta
Protein kinase C (PKC) isoforms are key mediators of acute pancreatitis. Genetic deletion or pharmacologic inhibition of PKC delta reduced activation of NF-κB and zymogens, but did not affect amylase secretion [30]. The targets of PKC delta remain unclear.
Fibroblast growth factor 21
The family of fibroblast growth factors (FGFs) has a diverse range of biological functions. Johnson et al. [31] demonstrated rapid expression of FGF21 during in-vivo models of pancreatitis. Mice lacking the Fgf21 gene in the whole organism revealed increased tissue damage and lethality in two models of acute pancreatitis. The study supports a protective role for FGF21 against cellular damage during inflammation. Further, FGF21 inhibited the early activation of PSCs, thus attenuating fibrosis.
Substance P
Substance P is a proinflammatory mediator that acts through the neurokinin 1 receptor (NRK-1) in acute pancreatitis. Ramnath et al. [32] showed that substance P/NRK-1 induced src family kinase (SFK) activation leading to subsequent activation of chemokines. Treatment of isolated acinar cells with SFK inhibitors or substance P receptor antagonists reduced cell injury, highlighting the detrimental role this pathway plays in acute pancreatitis. In another study, Zhou and Xue [33] showed that administration of ghrelin, a 28-amino acid protein from gastric mucosa involved in growth hormone release, leads to decreased lung injury in a sodium taurocholate model of pancreatitis in rodents. The effect was attributed to a ghrelin-mediated decrease in substance P.
Phosphoinositide 3-kinase/AKT pathway
Using a pharmacological approach, Tamizhselvi et al. [34] showed that hydrogen sulphide activated the phosphoinositide 3-kinase (PI3K)/AKT pathway, thereby reducing levels of inflammatory cytokines in a caerulein-induced mouse model of acute pancreatitis. The studies contrast with previous work demonstrating reduced zymogen activation and pancreatitis severity with PI3K inhibition [35].
Heat shock proteins
Mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MK2) is a member of the MAPK-activated protein kinases and phosphorylates heat shock proteins (HSPs) during experimental pancreatitis, leading to protective effects. Li et al. [36] recently demonstrated, however, that such a protective mechanism might not be applicable in models of severe acute pancreatitis. Following injections of caerulein and LPS in MK2−/− versus wild-type mice, disease severity was reduced in the knockout animals, although HSP25 and HSP60 expression was unaffected. Thus, MK2 participates in early inflammatory responses in acute pancreatitis, independent of the stress proteins.
Activated protein C
The protein C pathway is a major physiological anticoagulant and fibrinolysis system. Protein C is converted to an active serine protease, activated protein C (APC), by thrombin bound to thrombomodulin on the endothelial surface, whereupon it degrades factors involved in coagulation. In a sodium taurocholate rodent model of severe acute pancreatitis, treatment with APC decreased serum tumor necrosis factor-alpha (TNFα), interleukin8, and pancreatic matrix metallopeptidase 9 levels; upregulated pancreatic thrombomodulin and endothelial cell protein C receptor; and reduced disease severity [37].
Innate immunity and immune cells
The Toll-like receptor family (TLR), particularly TLR4, comprises the innate immune response and plays a major role in the early stages of acute pancreatitis. TLR4 initiates a complex signaling response when it interacts with LPS, resulting in a proinflammatory response. Sharif et al. [38] observed the effects of genetic deletion of TLR4 or its coreceptor CD14 in two models of experimental pancreatitis (caerulein and l-arginine-induced). TLR4−/− or CD14−/− mice had less severe acute pancreatitis and reduced lung injury compared with wild type. In another study, Zhou et al. [39] found TNF receptor-associated factor 6 to be a key adaptor of both TLR4-dependent and TLR4-independent signaling pathways and an important factor in acute pancreatitis. Whether the TLR-dependent effects are due to release of mitochondrial DNA warrants further study [40].
Various immune cells can determine the death response in acute pancreatitis. Nakamura et al. [41] showed that neutrophils inhibit apoptosis in pancreatitis by upregulating the ubiquitin ligase, Mdm2, which degrades the tumor promoter p53. Degradation of p53 prevents activation of caspase2, an initiator of apoptosis, and ultimately promotes necrosis and cellular injury. In another study, Kamei et al. [42] explored the role of triggering receptor expressed on myeloid cells-1 (TREM-1), a new receptor of the immunoglobulin superfamily, expressed on neutrophils and monocytes/macrophages. In a model of severe acute pancreatitis, they found that increases in TREM-1 levels corresponded with an increase in disease severity. Blockade of the TREM-1 pathway with its inhibitor LP17 improved hepatic and renal function in severe acute pancreatitis and could provide a potential therapeutic target for pancreatitis-associated organ dysfunction.
Conclusion
In summary, this review has detailed the past year’s progress in identifying mediators, which influence pancreatic injury and understanding their mechanisms at the cellular level. Factors that elevate intracellular Ca2+ signals and initiate proinflammatory responses lead to increases in disease severity. Furthermore, factors that promote antiapoptotic pathways may increase pancreatic injury. Finally, recent strategies that target such downstream events may prove beneficial in ameliorating pancreatitis and reducing cellular and systemic injury.
Acknowledgements
This work was supported by a National Institutes of Health grant (to E.C.T.: R21 DK69702) (to F.S.G.: RO1 DK54021) (to S.Z.H.: RO1 DK083327, R03 DK078707, K12 HD001401), a Veterans Affairs Merit and Senior Career Development Award (to F.S.G.), and a Children’s Digestive Health and Nutrition Young Investigator Award (to S.Z.H.).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 530).
- 1.Lowenfels AB, Sullivan T, Fiorianti J, Maisonneuve P. The epidemiology and impact of pancreatic diseases in the United States. Curr Gastroenterol Rep. 2005;7:90–95. doi: 10.1007/s11894-005-0045-6. [DOI] [PubMed] [Google Scholar]
- 2. Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology. 2010;138:715–725. doi: 10.1053/j.gastro.2009.10.052. Using a novel model of experimental pancreatitis in mice, this study is the first to show that bile acids initiate their effects through a receptor-mediated pathway in acinar cells.
- 3.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 4.Kim MS, Hong JH, Li Q, et al. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah AU, Sarwar A, Orabi AI, et al. Protease activation during in vivo pancreatitis is dependent upon calcineurin activation. Am J Physiol Gastrointest Liver Physiol. 2009;297:G967. doi: 10.1152/ajpgi.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heike W, Saskia H, Frank L, Ludwig J. Calpain-mediated breakdown of cytoskeletal proteins contributes to cholecystokinin-induced damage of rat pancreatic acini. Int J Exp Pathol. 2009;90:387–399. doi: 10.1111/j.1365-2613.2009.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science. 1986;231:497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- 8. Gerasimenko JV, Lur G, Sherwood MW, et al. Pancreatic protease activation by alcohol metabolite depends on Ca2+ release via acid store IP3 receptors. Proc Natl Acad Sci U S A. 2009;106:10758–10763. doi: 10.1073/pnas.0904818106. This study demonstrates the critical role of intracellular Ca2+ release from acidic vesicles in mediating intra-acinar trypsin activation.
- 9.Del Castillo-Vaquero A, Salido GM, Gonzalez A. Increased calcium influx in the presence of ethanol in mouse pancreatic acinar cells. Int J Exp Pathol. 2010;91:114–124. doi: 10.1111/j.1365-2613.2009.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36:54–62. doi: 10.1097/00004836-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Sternfeld L, Yang F, et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 2009;58:422–430. doi: 10.1136/gut.2007.146258. [DOI] [PubMed] [Google Scholar]
- 12.Siech M, Zhou Z, Zhou S, et al. Stimulation of stellate cells by injured acinar cells: a model of acute pancreatitis induced by alcohol and fat (VLDL) Am J Physiol Gastrointest Liver Physiol. 2009;297:G1163–G1171. doi: 10.1152/ajpgi.90468.2008. [DOI] [PubMed] [Google Scholar]
- 13.Singh VP, Bren GD, Algeciras-Schimnich A, et al. Nelfinavir/ritonavir reduces acinar injury but not inflammation during mouse caerulein pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1040–G1046. doi: 10.1152/ajpgi.90642.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi I, Hamada K, Yoshida M, et al. Risperidone attenuates local and systemic inflammatory responses to ameliorate diet-induced severe necrotic pancreatitis in mice: it may provide a new therapy for acute pancreatitis. J Pharmacol Exp Ther. 2009;328:256–262. doi: 10.1124/jpet.108.141895. [DOI] [PubMed] [Google Scholar]
- 15.Sidhu S, Pandhi P, Malhotra S, et al. Melatonin treatment is beneficial in pancreatic repair process after experimental acute pancreatitis. Eur J Pharmacol. 2010;628:282–283. doi: 10.1016/j.ejphar.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 16.Babu BI, Malleo G, Genovese T, et al. Green tea polyphenols ameliorate pancreatic injury in cerulein-induced murine acute pancreatitis. Pancreas. 2009;38:954–967. doi: 10.1097/MPA.0b013e3181b28d11. [DOI] [PubMed] [Google Scholar]
- 17.Koehler JA, Baggio LL, Lamont BJ, et al. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes. 2009;58:2148–2161. doi: 10.2337/db09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matveyenko AV, Dry S, Cox HI, et al. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes. 2009;58:1604–1615. doi: 10.2337/db09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachnani JS, Bulchandani DG, Nookala A, et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia. 2009;53:153–159. doi: 10.1007/s00125-009-1515-4. [DOI] [PubMed] [Google Scholar]
- 20. Bhoomagoud M, Jung T, Atladottir J, et al. Reducing extracellular pH sensitizes the acinar cell to secretagogue-induced pancreatitis responses in rats. Gastroenterology. 2009;137:1083–1092. doi: 10.1053/j.gastro.2009.05.041. This study links metabolic acidosis as a sensitizing factor for acute pancreatitis.
- 21. Wartmann T, Mayerle J, Kahne T, et al. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology. 2010;138:726–737. doi: 10.1053/j.gastro.2009.10.048. Using genetic deletion animals, this study highlights a novel mechanism by which cathepsin-L promotes injury through antiapototic pathways rather than trypsinogen activation.
- 22.Kereszturi E, Sahin-Toth M. Intracellular autoactivation of human cationic trypsinogen mutants causes reduced trypsinogen secretion and acinar cell death. J Biol Chem. 2009;284:33392–33399. doi: 10.1074/jbc.M109.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kereszturi E, Szmola R, Kukor Z, et al. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: a novel disease mechanism. Hum Mutat. 2009;30:575–582. doi: 10.1002/humu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan JD, Romac J, Peng RY, et al. Protection against chronic pancreatitis and pancreatic fibrosis in mice overexpressing pancreatic secretory trypsin inhibitor. Pancreas. 2010;39:e24–e30. doi: 10.1097/MPA.0b013e3181bc45e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romac JM, Ohmuraya M, Bittner C, et al. Transgenic expression of pancreatic secretory trypsin inhibitor-1 rescues SPINK3-deficient mice and restores a normal pancreatic phenotype. Am J Physiol Gastrointest Liver Physiol. 2010;298:G518–G524. doi: 10.1152/ajpgi.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fortunato F, Bürgers H, Bergmann F, et al. Impaired autolysosome formation correlates with lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. 2009;137:350–360. doi: 10.1053/j.gastro.2009.04.003. 360.e1–5. This study shows that alcohol and LPS decrease autophagy.
- 27. Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. This study demonstrates that acute pancreatitis is related to a defect in autophagy that results in decreased protein degradation. The defect may contribute to the elevation of levels of activated proteases observed in the acinar cell during pancreatitis.
- 28.Sung KF, Odinokova IV, Mareninova OA, et al. Prosurvival Bcl-2 proteins stabilize pancreatic mitochondria and protect against necrosis in experimental pancreatitis. Exp Cell Res. 2009;315:1975–1989. doi: 10.1016/j.yexcr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voronina SG, Barrow SL, Simpson AW, et al. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology. 2010;138:1976–1987. doi: 10.1053/j.gastro.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thrower EC, Wang J, Cheriyan S, et al. Protein kinase C delta-mediated processes in cholecystokinin-8-stimulated pancreatic acini. Pancreas. 2009;38:930–935. doi: 10.1097/MPA.0b013e3181b8476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson CL, Weston JY, Chadi SA, et al. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795–1804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 32.Ramnath RD, Sun J, Bhatia M. Involvement of SRC family kinases in substance P-induced chemokine production in mouse pancreatic acinar cells and its significance in acute pancreatitis. J Pharmacol Exp Ther. 2009;329:418–428. doi: 10.1124/jpet.108.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Xue C. Ghrelin attenuates acute pancreatitis-induced lung injury and inhibits substance P expression. Am J Med Sci. 2010;339:49–54. doi: 10.1097/MAJ.0b013e3181b9c3d3. [DOI] [PubMed] [Google Scholar]
- 34.Tamizhselvi R, Sun J, Koh YH, Bhatia M. Effect of hydrogen sulfide on the phosphatidylinositol 3-kinase-protein kinase B pathway and on caerulein-induced cytokine production in isolated mouse pancreatic acinar cells. J Pharmacol Exp Ther. 2009;329:1166–1177. doi: 10.1124/jpet.109.150532. [DOI] [PubMed] [Google Scholar]
- 35.Singh VP, Saluja AK, Bhagat L, et al. Phosphatidylinositol 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest. 2001;108:1387–1395. doi: 10.1172/JCI12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YY, Ochs S, Gao ZR, et al. Regulation of Hsp60 and the role of MK2 in a new model of severe experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G981–G989. doi: 10.1152/ajpgi.00225.2009. [DOI] [PubMed] [Google Scholar]
- 37.Ping C, Yongping Z, Minmin Q, et al. Activated protein C improves the severity of severe acute pancreatitis via up-regulating the expressions of endothelial cell protein C receptor and thrombomodulin. Dig Dis Sci. 2010;55:1599–1609. doi: 10.1007/s10620-009-0909-y. [DOI] [PubMed] [Google Scholar]
- 38.Sharif R, Dawra R, Wasiluk K, et al. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813–819. doi: 10.1136/gut.2008.170423. [DOI] [PubMed] [Google Scholar]
- 39.Zhou XY, Zhou ZG, Ding JL, et al. TRAF6 as the key adaptor of TLR4 signaling pathway is involved in acute pancreatitis. Pancreas. 2010;39:359–366. doi: 10.1097/MPA.0b013e3181bb9073. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura Y, Do JH, Yuan J, et al. Inflammatory cells regulate p53 and caspases in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G92–G100. doi: 10.1152/ajpgi.00324.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamei K, Yasuda T, Ueda T, et al. Role of triggering receptor expressed on myeloid cells-1 in experimental severe acute pancreatitis. J Hepatobiliary Pancreat Surg. 2010;17:305–312. doi: 10.1007/s00534-009-0191-6. [DOI] [PubMed] [Google Scholar]