As we entered the decade of the 1980s, liver transplantation was not a therapeutic option considered for many patients with end stage liver disease. Although limited success had been achieved in a few centers with immunosuppression based on azathioprine, steroids, and polyclonal antibody preparations, patient survival rates were approximately 35% at 1 year and less than 20% at 5 years.
By the end of the decade a remarkable change had taken place, and transplantation is now the therapeutic option of choice for many patients with irreversible end stage liver disease. We here review our experience over most of the last decade with liver transplantation under cyclosporine-prednisone induction and maintenance therapy at the University of Pittsburgh.
Materials and Methods
The liver transplantation program at the University of Pittsburgh began in 1981. In the years from 1981 to 1989, 2,395 liver grafts were transplanted after total hepatectomy into 1,819 recipients in the conventional orthotopic approach (Fig 1). Of these, 2029 grafts in 1583 patients were performed using cyclosporine-prednisone induction and maintenance therapy. Since 1984 we have used OKT3 (Orthoclone OKT-3®, Ortho Pharmaceuticals, Raritan, NJ) for treatment of steroid-resistant rejection.
Fig 1.
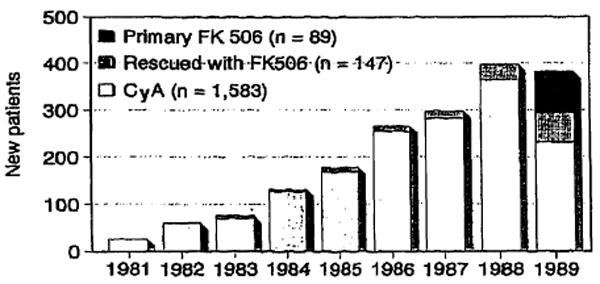
Primary liver transplantations per calendar year at the University of Pittsburgh between 1981 and 1989 including 1583 patients done under cyclosporine-prednisone therapy, 147 patients whose grafts were rescued by conversion to FK 506, and 89 patients treated with primary induction and maintenance with FK 506.
A computer registry has been maintained since 1983 to track outcome in all graft recipients and the records for the complete series of 1,583 patients were reviewed.1 Variables included in the analysis are age at the time of transplantation, ABO blood group, body weight, primary liver disease, graft survival, and patient survival. In addition, since October 1987, patients have been classified for medical urgency according to the required level of medical care.
Descriptive statistics were determined using SPSS/PC+ version 3.1 (SPSS Inc., Chicago, Ill) and survival analysis was performed using BMDP/PC (1988 version, BMDP Statistical Software, Los Angeles, Calif) on an Intel 80386 based microcomputer running MS-DOS 4.01 (Microsoft Corporation, Bellevue, Wash).
Results
As shown in Fig 1, there has been a significant increase in the number of new patients receiving transplants through 1988. In 1989, we began converting to an immunosuppressive protocol based on the new drug FK 506, so the number of patients in the cyclosporine (CyA) based group began to decline for the first time.
Sex
There were 769 (48.6%) males and 814 (51.4%) females in this series. There has been an increase in the proportion of males in the series in the last 2 years (54.9% in 1988 and 54.5% in 1989, Fig 2A). This probably reflects a decrease in the proportion of transplantations performed at this center for cholestatic liver disease, especially for primary biliary cirrhosis, which has a high prevalence in females (Fig 2C).
Fig 2.
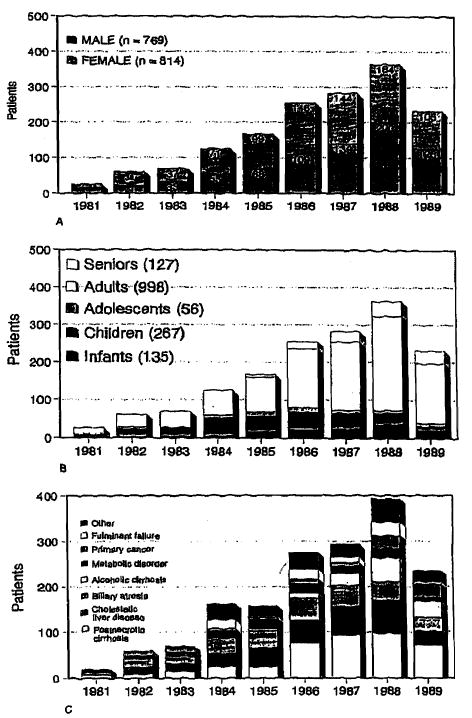
Primary transplantations per calendar year according to (A) sex, (B) age group, and (C) primary liver disease. See Table 1 for definition of age groups.
Age Groups
There were 135 (8.5%) infants (age under 2 years), 267 (16.9%) children (age 2 to 11 years), 56 (3.5%) adolescents (age 12 to 17 years), 998 (63.0%) adults (age 18 to 59 years), and 127 (8.0%) senior (over 60 years) recipients. The proportion of infants in the series has remained between 8% to 10% since 1985 (Fig 2B). Most significant has been the increase in the number and proportion of senior patients accepted in the program from 3.6% in 1985 to 14.7% in 1989 (Fig 2B).
Body weight is related to age but may be a better measure of developmental status in children. Fifty-six (3.5%) of the recipients weighed less than 7 kg and 129 (8.1%) weighed 7 to 11 kg.
Survival rates based on age group and body weight are shown in Table 1. Survival rates for infants are 6% to 10% lower at 1 year and 5% to 8% lower at 5 years than for other age groups. Pediatric recipients under 12 kg body weight have a 10% to 20% higher mortality than larger patients.
Table 1. Percent Patient Survival According to Age and Weight at Time of Transplantation*.
| Age (years) | N | 1 month | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | |
|---|---|---|---|---|---|---|---|---|---|
| Infant | under 2 | 135 | 81.5 | 66.7 | 63.6 | 61.7 | 60.4 | 58.3 | 55.4 |
| Child | 2 to 11 | 267 | 87.3 | 76.0 | 73.8 | 71.4 | 70.5 | 68.8 | 68.1 |
| Adolescent | 12 to 17 | 56 | 82.1 | 71.4 | 69.6 | 63.6 | 63.6 | 60.6 | 60.6 |
| Adult | 18 to 59 | 998 | 89.7 | 76.3 | 73.7 | 69.0 | 66.4 | 64.2 | 62.8 |
| Senior | over 60 | 127 | 86.6 | 71.7 | 69.3 | 66.0 | 61.9 | 61.9 | |
| Under 7 kg | 56 | 78.6 | 64.3 | 56.9 | 52.2 | 48.6 | 48.6 | 41.6 | |
| 7 to 11 kg | 129 | 83.0 | 65.1 | 64.3 | 63.4 | 62.3 | 60.8 | 60.8 | |
| 12 to 19 kg | 149 | 89.3 | 77.9 | 74.5. | 71.6 | 71.6 | 68.8 | 67.7 | |
| 20 to 39 kg | 94 | 89.4 | 83.0 | 81.9 | 80.8 | 78.0 | 76.1 | 76.1 | |
| 40 kg or more | 973 | 90.0 | 77.5 | 75.0 | 70.6 | 68.3 | 65.9 | 63.8 |
Life table method.
Blood Groups
Patient survival based on recipient ABO blood groups are summarized in Table 2. We have not observed a difference in survival based solely on recipient blood group.
Table 2. Percent Patient Survival Based on Recipient ABO Blood Group*.
| N | 1 month | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | |
|---|---|---|---|---|---|---|---|---|
| O | 696.0 | 76.0 | 74.0 | 72.5 | 69.1 | 66.3 | 64.5 | 62.9 |
| A | 625.0 | 87.8 | 74.2 | 72.5 | 68.3 | 67.0 | 65.9 | 64.6 |
| B | 196.0 | 87.2 | 74.0 | 72.4 | 67.5 | 66.6 | 61.7 | 61.7 |
| AB | 66.0 | 87.9 | 71.2 | 69.6 | 63.9 | 58.6 | 55.0 | 55.0 |
Life table method.
Primary Liver Disease
The most common indication for liver replacement in this series is postnecrotic cirrhosis (432 patients, 27.3%) followed by cholestatic liver disease including primary biliary cirrhosis and primary sclerosing cholangitis (324, 20.5%), biliary atresia (265, 16.7%), alcoholic cirrhosis (134, 8.5%), inborn errors of metabolism (132, 8.3%), primary hepatobiliary cancer (105, 6.6%), and fulminant hepatic failure (75, 4.7%).
Patient survival based on primary liver disease is summarized in Table 3. The highest patient survival rates are seen in patients receiving liver grafts for inborn errors of metabolism, cholestatic liver disorders, postnecrotic cirrhosis (excluding HBsAg+ patients), and alcoholic cirrhosis.
Table 3. Percent Patient Survival Based on Primary Liver Disease*.
| N | 1 month | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | |
|---|---|---|---|---|---|---|---|---|
| Cholestatic disorders | 324 | 92.9 | 80.3 | 77.8 | 75.7 | 74.3 | 72.2 | 71.1 |
| Postnecrotic cirrhosis† | 359 | 88.0 | 77.4 | 76.0 | 73.0 | 70.7 | 69.6 | 67.4 |
| Alcoholic cirrhosis | 134 | 90.3 | 82.1 | 80.6 | 75.1 | 72.7 | 72.7 | |
| Biliary atresia | 265 | 84.2 | 71.7 | 69.4 | 67.6 | 66.6 | 63.8 | 62.8 |
| Neonatal hepatitis | 7 | 85.7 | 71.4 | 71.4 | 51.0 | 51.0 | 51.0 | 51.0 |
| Metabolic disorders | 132 | 88.6 | 75.8 | 75.0 | 75.0 | 75.0 | 73.8 | 73.8 |
| Fulminant failure | 75 | 78.7 | 61.3 | 60.0 | 53.8 | 49.0 | 49.0 | 49.0 |
| Chronic hepatitis B | 73 | 86.3 | 63.0 | 58.8 | 48.6 | 48.6 | 48.6 | 48.6 |
| Primary cancer | 105 | 94.3 | 72.4 | 63.7 | 43.7 | 33.0 | 26.4 | 26.4 |
| Autoimmune hepatitis | 42 | 85.7 | 83.3 | 83.3 | 80.4 | 80.4 | 80.4 | |
| Budd-Chiari syndrome | 24 | 79.2 | 66.7 | 66.7 | 66.7 | 54.6 | 54.6 | 54.6 |
| Secondary biliary cirrhosis | 25 | 68.0 | 56.0 | 51.9 | 51.9 | 51.9 | 51.9 | 51.9 |
Life Table method.
Excluding HBsAg+.
Since 1985 there has been a significant increase in the proportion of patients receiving liver grafts at this center for alcoholic cirrhosis (from 3.0% in 1985 to 15.6% in 1989, Fig 1C). Patient follow-up is maintained by a skilled team of nurse clinicians who keep up regular contact with patients, their families, and community physicians. We recently surveyed 121 surviving patients who received grafts for alcoholic cirrhosis or for liver disease in which alcoholism was believed to be a significant contributing factor. Only 10 patients (8.3%) were known for certain to be actively drinking and 6 (5.0%) more patients were suspected to have resumed drinking. Thus, 105 patients (86.8%) were believed to be abstinent. This is consistent with the high survival rates seen after transplantation for alcoholic cirrhosis (80.6% at 1 year with 109 patients at risk and 75.1% at 2 years with 44 patients at risk).
Lower survival rates are seen in patients receiving grafts for fulminant hepatic failure, chronic hepatitis B, and primary cancer. An interesting exception is the high survival for the 15 patients who received transplants for fulminant hepatitis B (80% survival at 1 year and at 5 years, Table 4).
Table 4. Percent Patient Survival After Liver Transplantation for Acute or Chronic Viral Hepatitis*.
| N | 1 month | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | |
|---|---|---|---|---|---|---|---|---|
| Acute non-A, non-B | 32 | 84.4 | 68.8 | 68.8 | 58.7 | 50.0 | 50.0 | 50.0 |
| Chronic non-A, non-B | 292 | 88.0 | 77.1 | 75.0 | 72.1 | 69.5 | 68.7 | 65.8 |
| Acute hepatitis B | 15 | 86.7 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 |
| Chronic hepatitis B | 73 | 86.3 | 63.0 | 58.8 | 48.6 | 48.6 | 48.6 | 48.6 |
Life table method.
Medical Urgency
We recently summarized the experience in this center since the adoption of a national system for liver allocation in the United States, which places a heavy emphasis on medical urgency as measured by the level of medical support required by the patient waiting for a transplant.2 Fig 3 presents a life table survival plot adapted from this report for 812 patients who received grafts under the UNOS point system between October 1987 and December 1989. There is a strong relationship (P < .001) between medical condition of the patient classified in this manner and mortality after transplantation.
Fig 3.
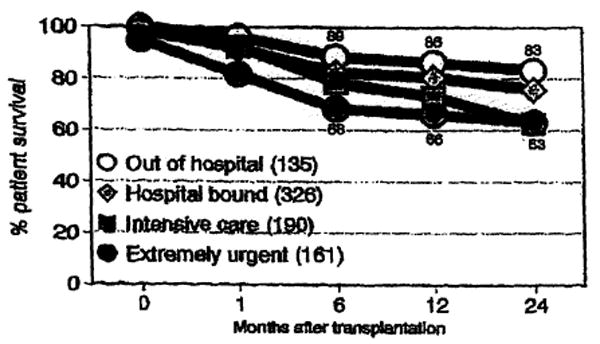
Actuarial (life table) patient survival rates based on the level of medical care required by the recipient at the time of transplantation.
Retransplantation
Hepatic retransplantation in this series of patients will be the subject of a future report. In brief, of the 1583 patients in this series, as of June 30, 1990, 1216 received only one graft and 367 (24.0%) had required at least one retransplantation. The primary nonfunction rate for the series of first grafts is 6.6%. The highest incidence of retransplantation is in patients who received their first graft for biliary atresia (28.0%) or for fulminant hepatic failure (27.5%). Lowest retransplantation rates are in patients receiving transplants for alcoholic cirrhosis (13.5%) and primary biliary cirrhosis (18.4%).
Discussion
The experience reported here from this single large center and the accumulating experience in the multicenter registries reported elsewhere in these proceedings,3 demonstrate the efficacy of liver transplantation for many forms of end stage liver disease. By 1985, it seemed evident from the first years of experience at this center with cyclosporine-prednisone induction and maintenance therapy, that survival rates after liver transplantation approaching 75% to 80% at 1 year and 65% to 70% could be achieved for many patients.4 These initial expectations are confirmed with this extended experience in nearly 1600 patients. Certainly, for postnecrotic cirrhosis (excluding HBV carriers), cholestatic liver disease, and inborn errors of metabolism, these results have stood the test of time.
High success rates have been achieved in the patients given a transplant for fulminant hepatitis B, as these patients develop antibodies and remain immune from reinfection. Although most of the HBsAg+ patients transplanted for postnecrotic cirrhosis have remained virus carriers and many patients have developed detectable infection in the graft, there is still cause for optimism here, too. Active and passive immunization and long-term therapy with interferon may result in seroconversion of HBsAg+ patients and we believe that significant further improvement in patient survival can and will be achieved.5,6
Although survival after liver transplantation for infants and low body weight children is lower than for older or larger patients, improvement here is also beginning to be achieved. Improved methods of organ preservation with UW solution, which is believed to protect the microcirculation of the liver, has reduced the incidence of arterial thrombotic complications and thus the need for retransplantation. Increasing use of reduced liver grafts for small children increases the availability of organs for these patients and can help reduce the longer waiting times and consequent deterioration in their condition, Finally, recognition of the added technical difficulty that results from prior surgery on infants and small children needing liver transplantation should encourage discretion in the use of alternative, less-effective surgical procedures.
Liver transplantation for alcoholic cirrhosis has been controversial.7 Our initial experience with this group of patients led us to adopt an aggressive approach, and the number of patients with this disease treated at our center has been increasing yearly. The extended results reported here support continuation of this policy.
Survival after liver transplantation is related to the patient's condition at the time of transplantation. We have been able to achieve nearly 90% 1-year survival in patients who are able to be transplanted before they become dependent upon in-hospital care, compared to only 65% to 70% survival in patients in the intensive care unit at the time of transplantation. This emphasizes the excellent results that can be achieved if patients have timely access to transplantation. Although efforts to ease the donor shortage must continue, it is equally important that patients who are potential candidates for liver replacement be referred to the transplant center before they have deteriorated to the point where the prospects for survival have significantly diminished. Liver transplantation is no longer an option to be taken out of desperation.
We continue to experience high recurrence rates after liver transplantation for hepatobiliary cancer. Additional treatment with adjuvant chemotherapy or radiotherapy in the preoperative, intraoperative, and postoperative phases of care are being studied in a effort to improve results.
As we enter the decade of the 1990s, new therapeutic agents such as FK 506, rapamycin, deoxyspergualin, and other agents may find widespread application in clinical transplantation. The results achieved during the last decade with cyclosporine-prednisone therapy have provided a new standard against which results with these new agents will be compared.
Acknowledgments
This work was supported by research grants from the Veterans Administration and project grant DK 29961 from the National institutes of Health, Bethesda, Maryland.
References
- 1.Markus BH, Mitchell S, Gordon RD, et al. Transplant Proc. 1988;20:385. [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon RD, Hartner CM, Casavilla A, et al. Transplantation. in press. [Google Scholar]
- 3.Gordon RD, Bismuth H. Transplant Proc. 1991;23 this issue. [PubMed] [Google Scholar]
- 4.Iwatsuki S, Starzl TE, Todo S, et al. Transplant Proc. 1988;20:498. [PMC free article] [PubMed] [Google Scholar]
- 5.Lauchart W, Muller R, Pichlmayr R. Transplant Proc. 1987;19:2387. [PubMed] [Google Scholar]
- 6.Ferla G, Colledan M, Doglia M, et al. Transplant Proc. 1988;20(Suppl 1):566. [PubMed] [Google Scholar]
- 7.Starzl TE, Van Thiel D, Tzakis A, et al. JAMA. 1988;260:2542. [PMC free article] [PubMed] [Google Scholar]


