Abstract
The human Na+/d-glucose cotransporter 2 (hSGLT2) is believed to be responsible for the bulk of glucose reabsorption in the kidney proximal convoluted tubule. Since blocking reabsorption increases urinary glucose excretion, hSGLT2 has become a novel drug target for Type 2 diabetes treatment. Glucose transport by hSGLT2 was studied at 37°C in human embryonic kidney 293T cells using whole cell patch-clamp electrophysiology. We compared hSGLT2 with hSGLT1, the transporter in the straight proximal tubule (S3 segment). hSGLT2 transports with surprisingly similar glucose affinity and lower concentrative power than hSGLT1: Na+/d-glucose cotransport by hSGLT2 was electrogenic with apparent glucose and Na+ affinities of 5 and 25 mM, and a Na+:glucose coupling ratio of 1; hSGLT1 affinities were 2 and 70 mM and coupling ratio of 2. Both proteins showed voltage-dependent steady-state transport; however, unlike hSGLT1, hSGLT2 did not exhibit detectable pre-steady-state currents in response to rapid jumps in membrane voltage. d-Galactose was transported by both proteins, but with very low affinity by hSGLT2 (≥100 vs. 6 mM). β-d-Glucopyranosides were either substrates or blockers. Phlorizin exhibited higher affinity with hSGLT2 (Ki 11 vs. 140 nM) and a lower Off-rate (0.03 vs. 0.2 s−1) compared with hSGLT1. These studies indicate that, in the early proximal tubule, hSGLT2 works at 50% capacity and becomes saturated only when glucose is ≥35 mM. Furthermore, results on hSGLT1 suggest it may play a significant role in the reabsorption of filtered glucose in the late proximal tubule. Our electrophysiological study provides groundwork for a molecular understanding of how hSGLT inhibitors affect renal glucose reabsorption.
Keywords: renal glucose reabsorption, Type 2 diabetes mellitus, human Na+/d-glucose cotransporter 2 inhibitors, patch-clamp electrophysiology
in humans, 180 g/day of glucose is filtered across the kidney glomeruli, 97% of which is reabsorbed in the nephron. Early studies in rat demonstrated that most of the filtered glucose was reabsorbed in the S1 and S2 segments of the proximal tubule (PT) (6). Using isolated perfused segments of rabbit proximal tubules, Barfuss and Schafer (1) reported that the maximum rate of active glucose transport decreased by an order of magnitude from the proximal convoluted tubule to the proximal straight tubule, accompanied by an increase in glucose affinity. Detailed biochemical studies suggested that this active transport was powered by two different sodium-glucose cotransporters (SGLTs) in the brush border membranes: an early, low-affinity (Kmd-glucose ≈6 mM) system with a 1:1 Na+:glucose coupling and a later, high-affinity (Kmd-glucose ≈0.3 mM) system with 2:1 coupling (24–26). Genetic and biochemical studies have led to the proposal that human (h)SGLT2 is the low-affinity transporter in S1/2 segments of the PT and hSGLT1 is the high-affinity transporter in distal segments (S3) (11, 29, 30, 32).
There has been a resurgence of interest in renal glucose transport with the ongoing development of specific hSGLT2 inhibitors to treat patients with diabetes (18). Such inhibitors completely block hSGLT2 activity in vitro (12, 17) but a maximum of ≈50% of glucose reabsorption in vivo (13). Nevertheless, the drugs are effective in lowering blood glucose levels in diabetic patients with the added benefits of weight loss and few adverse events (5, 14). The paucity of side effects is consistent with the benign genetic defect of renal glucose reabsorption known as familial renal glycosuria. This autosomal recessive condition is characterized by elevated urinary glucose excretion in the absence of any other clinical phenotype (21, 22).
Surprisingly, little is known about the basic physiology of hSGLT2 even though it was cloned almost 25 yr ago (29). In this work, we have set out to fully characterize the functional properties of hSGLT2 and hSGLT1 expressed in mammalian cells at 37°C using patch-clamp electrophysiology. Our study provides additional insights into hSGLT2 and 1 biology under physiological conditions and how they interact with inhibitors.
MATERIALS AND METHODS
Cell culture and transfection.
Human embryonic kidney 293T (HEK293T) cells were purchased from the American Type Culture Collection (Manassas, VA). Cells were grown in vented 25-cm2 polystyrene flasks, in Dulbecco's modified Eagle's medium (DMEM, CELLGRO, Manassas, VA) supplemented with 10% fetal bovine serum (Valley Biomedical Products, Winchester, VA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). Cultures were maintained in an incubator at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cells were passaged (1:10) every 4 to 5 days, and before transfection they were seeded to six-well plates or poly-l-lysine-coated 12- or 24-well plates and grown until ≈50–70% confluent, at which time they were transiently transfected. For patch-clamp experiments, HEK293T cells were cotransfected with 1 μg of a vector with hSGLT2 cDNA inserted (PCMV2-hSGLT2) (29) and 0.1 μg of a yellow fluorescent protein (YFP)-encoding vector (which served as a transfection reporter), using the Effectene kit (Qiagen, Germantown, MD), as follows: 90 μl buffer, 8 μl enhancer, and 8 μl Effectene reagent. For hSGLT1 experiments, 1 μg of a single vector containing hSGLT1 cDNA (7) and green fluorescent protein (GFP-IRES-hSGLT) was utilized, with the same quantities of Effectene reagents. Transfection efficiency was generally 40–60%. For radioactive tracer flux experiments, HEK293T cells were transfected using 0.5 μg (per well, 12-well plate) or 0.25 μg (per well, 24-well plate) cDNA, using proportionally adjusted volumes of Effectene reagents as described above.
Radioactive tracer uptakes.
Sugar uptake was measured by performing 50 μM [14C]-α-methyl-d-glucopyranoside (αMDG, GE Healthcare Life Sciences, Piscataway, NJ) uptakes on HEK293T cells in 12- or 24-well plates, 2 days posttransfection. Cells were washed three times (0.5 ml per well per wash) in 37°C 0.9% phosphate-buffered, glucose-free saline [PBS, which contained (in mM) 137 NaCl, 10 Na2HPO4, 1.76 NaH2PO4, 2 KCl, pH 7.4] and subsequently incubated in 50 μM [14C]-αMDG in PBS ± inhibitor/compound for 40 min at 37°C. After incubation, the isotope solution was removed and cells were washed three times in PBS and solubilized in 1% Triton X-100 in PBS. A portion of cell extracts were then assayed using liquid scintillation counting. Uptake was then expressed in picomoles per minute per well, mean ± SE. For each condition, the sample size was n = 3 or 4 wells, and each experiment was repeated at least twice. Protein assays demonstrated no differences, within experimental error, between wells in any given plate.
Whole cell patch-clamp recordings.
Electrophysiological experiments were 2 days posttransfection. Three to fifteen hours before experiments, the cells were washed with glucose-free buffer, trypsinized, mechanically dispersed, and plated to 8 mm or 12 mm poly-l-lysine-coated glass coverslips. Patch-clamp experiments were performed on the stage of a Nikon diaphot epifluorescence microscope (Nikon, Tokyo, Japan). The fluorescence intensity of YFP (or GFP for hSGLT1) was used to select the cells expressing the transporters. The patch pipettes had resistances ≈3–7 MΩ and were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL). Whole cell recordings were performed using an Axopatch 200B amplifier and controlled with pCLAMP10 software (Molecular Devices, Sunnyvale, CA). The extracellular solution contained (in mM) 150 NaCl, 1 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4 (“Na+ buffer”) or 150 cholineCl, 1 CaCl2, 1 MgCl2, pH 7.4 (“choline buffer”). For experiments at 37°C, mannitol (100 mM) was added to the extracellular solution, both as an osmotic control for sugar solutions (which could be 100 mM or higher) and to reduce the noise and improve stability in the whole cell recording mode. The pipette (internal) solution contained (in mM) 145 CsCl, 5 NaCl, 11 EGTA, and 10 HEPES, pH 7.4. For reversal potential (Vr) measurements, the pipette solution contained (in mM) 100 CsCl, 50 NaCl, 11 EGTA, 10 HEPES, pH 7.4, and 30 αMDG. Throughout the course of an experiment, membrane potential (Vm) was held at −60 mV (Vh). For voltage pulse protocols, Vm was stepped, in 20-mV decrements, from Vh to test potentials between +50 and −150 mV. The pulses were applied for 30 ms at 37°C or 100 ms at 22°C before being returned to holding potential. The settling time of the voltage clamp was ≈1 ms. Current was filtered at 2 kHz and digitized at 50 kHz. Glucose-dependent steady-state Na+ currents were filtered at 2 kHz and digitized at 1 kHz using AXOSCOPE10 (Molecular Devices). Temperature was controlled using the SH-27B In-line Solution Heater (Warner). Unless otherwise stated, all experiments were performed at 37°C, and all electrophysiological recordings were at −60 mV.
Steady-state kinetics.
Continuous current records were digitally filtered (1–10 Hz) using pClamp 10.1 software (Molecular Devices). The steady-state sugar-induced currents (Isugar) were obtained by subtracting the baseline current in the presence of Na+ alone from the total current measured in the presence of Na+ and glucose:
| (1) |
Isugar was plotted as a function of external substrate (sugar or Na+) concentration and fitted to the equation:
| (2) |
where Imax is maximal current, [S]o is external substrate concentration, K0.5 is the substrate concentration at half-maximal current (1/2 Imax), and nh is the Hill coefficient.
Determination of stoichiometry.
Na+:glucose stoichiometry for hSGLT1 and 2 was determined from reversal potential measurements using the Gibbs free energy relationship, as described previously in experiments on hSGLT1 in Xenopus laevis oocytes (3):
| (3) |
where [S]i and [S]o are internal and external sugar concentrations, respectively; [Na]o and [Na]i are external and internal sodium; Vr is the reversal potential of Na+/sugar cotransport, i.e., where Isugar is zero; R is gas constant, T is absolute temperature, F is Faraday's constant, and n is the Na+:glucose coupling ratio. In these experiments the sugar used was nonmetabolizable and SGLT-specific, αMDG. The coupling ratio, n, was estimated using two methods. In the first method, under fixed Na+ and αMDG intra- and extracellular concentrations, Vr is determined as the voltage-axis intercept of the current-voltage (I-V) relationship for phlorizin-sensitive hSGLT αMDG current, and Eq. 3 is solved for n; in the second method, Eq. 3 was rearranged to:
| (4) |
[S]i, [Na]o, and [Na]i are maintained constant while only [S]o is varied. The reversal potential Vr is then a linear function of (RT/F)× ln[S]o with slope 1/n:
| (5) |
Phlorizin inhibition.
The effect of phlorizin was first studied by examining the inhibition of steady-state glucose-induced currents as a function of external phlorizin concentration. In these experiments, the glucose concentration used was close to the K0.5 of the sugar and the IC50 was determined by plotting Isugar against [phlorizin]. The IC50 value is approximately twice the inhibitory constant Ki, assuming competitive inhibition (16, 23):
| (6) |
Phlorizin inhibition was further studied by determining the On- and Off-rates for phlorizin inhibition of SGLT according to the pseudo first-order reaction scheme:
| (Scheme I) |
where kOn is the rate constant for phlorizin binding and kOff is the phlorizin release rate constant, and they are related to the half-times:
| (7) |
To determine Off-rate constants, the time courses of phlorizin dissociation from hSGLT2 and hSGLT1 were fitted to single exponential functions to obtain the half-times [t1/2 = ln(2) × τ]. The Ki and kOn were used, given that the ratio kOn/kOff is the dissociation constant Kd, and Kd is the zero substrate limit of the Ki for phlorizin:
and thus:
| (8) |
Ea determination.
The Arrhenius equation was used to estimate the energy of activation (Ea) of hSGLT2:
| (9) |
where I is Iglucose (amperes), A is the preexponential factor, Ea is energy of activation, and R and T have their usual meanings; this is rearranged to
| (10) |
Ea (in kcal/mol) is obtained from the slope of the plot of ln(I) vs. −1/(RT).
Analysis of pre-steady-state currents in response to voltage jump.
hSGLT1 exhibits a pre-steady-state current (or charge movement) with step jumps in membrane voltage in the absence of external sugar. Pre-steady-state currents were extracted from total transient current (It) either by subtracting the bilayer membrane capacitance, or from the difference between the transient currents recorded in the presence or absence of saturating glucose (15). The charge (Q) of the protein was obtained by integrating the pre-steady-state currents over time to give the charge movement (Q), and the charge-voltage (Q-V) data were fitted to the Boltzmann function to estimate Qmax (15).
Statistical analysis.
Data fitting, equation solutions, and t-tests for significance were performed using either SigmaPlot 10.0 (Systat Software, San Jose, CA) or Excel (Microsoft, Redmond, WA).
Reagents.
All reagents were purchased from Fisher Scientific or Sigma and were of the highest purity available.
RESULTS
Radioactive tracer uptakes.
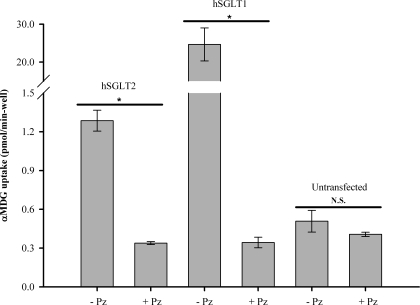
Uptake of 50 μM [14C]-αMDG into HEK293T cells transiently expressing hSGLT2 was threefold higher than that for untransfected cells, a difference eliminated by 100 μM phlorizin (Fig. 1). hSGLT2 phlorizin-sensitive uptake was ≈20-fold lower than that in parallel experiments with hSGLT1. Phlorizin had no significant effect on αMDG uptakes into untransfected cells (P > 0.05, Fig. 1). These results confirm that in our lab, hSGLT2 and hSGLT1 are expressed in HEK293T cells.
Fig. 1.
Radioactive tracer flux experiments in human Na+/d-glucose cotransporter 2 (hSGLT2) and hSGLT1. [14C]-α-methyl-d-glucopyranoside (αMDG, 50 μM) uptake was measured in hSGLT2-, hSGLT1-, and untransfected human embryonic kidney 293T (HEK293T) cells at 37°C. Uptake is expressed as quantity of tracer (pmol) per minute per well; n = 3 wells per bar; error bars are ± SE. Cells transiently expressing either hSGLT2 or hSGLT1 showed significant (P < 0.05) αMDG uptake (−Pz) above background; when SGLT inhibitor phlorizin (Pz, 100 μM) was added, uptake was reduced to background levels (+Pz), as seen in untransfected cells [not significant (N.S.), P > 0.05].
Kinetics.
Patch-clamp techniques were used to determine hSGLT kinetics in HEK293T cells at 37°C and a holding membrane potential (Vm) of −60 mV. Figure 2A shows that, in hSGLT2-expressing cells, the addition of 100 mM d-glucose to the extracellular solution induced a steady inward current of 60 pA, and removal of glucose reversed the current in under 5 s, i.e., within the time of the solution changes. No glucose currents were observed in the absence of external Na+ (Fig. 4B) or in untransfected cells. Steady-state glucose-induced currents were also dependent on Vm. As shown for one cell in Fig. 2B, the hSGLT2 glucose steady-state current approached zero at +50 mV and increased in a supralinear manner as the voltage was varied from +50 to −110 mV. At 22°C, however, the inward Na+/glucose currents were <5 pA over the entire voltage range. This suggested a high activation energy (Ea), so temperature was varied from 22°C to 37°C and steady-state glucose current was fit to the Arrhenius equation (Eqs. 9 and 10), resulting in an estimated Ea of 26 kcal/mol. Both voltage and temperature dependence of steady-state transport was similar to those observed for hSGLT1 (data not shown).
Fig. 2.
Steady-state hSGLT2 Na+/d-glucose cotransport is electrogenic and membrane potential (Vm) and temperature dependent. A: whole cell recordings at 37°C and a Vm of −60 mV in HEK293T cells expressing hSGLT2 revealed inward current upon addition of glucose to the extracellular Na+ solution. Upon returning to Na+-only, current was restored to baseline. In Na+-free buffer (i.e., 150 mM cholineCl), there was no glucose-induced current (Fig. 3B). B: current-voltage (I-V) relationships of the glucose-induced current (□, 22°C; ■, 37°C).
Fig. 4.
Kinetic properties. A: steady-state kinetics of d-glucose transport by hSGLT2 at 37°C and Vm = −60 mV. Glucose-induced currents are plotted against extracellular glucose concentration, and the curves represent the data fit to the Hill equation (Eq. 2) with the Hill coefficient, nh, constrained to 1. Maximal glucose-induced current (Imaxd-glucose) indicates the level of expression in a given cell, whereas the glucose concentration at half-maximal current (K0.5d-glucose) reflects the transporter's apparent affinity for glucose. B: steady-state kinetics of Na+ activation of hSGLT2, 37°C and Vm = −60 mV. Inward, 100 mM glucose-induced current is plotted as a function of extracellular Na+ concentration and the data are fitted to the Hill equation, maximal current (Imax), nh, and apparent Na+ affinity (K0.5Na) values. The statistics for maximal current (ImaxNa) and apparent Na+ affinity (K0.5Na) in B (as in A for sugar) are SEs of the fit. For both substrates, K0.5 values were averaged from multiple cells and compared with those from hSGLT1 (Table 1).
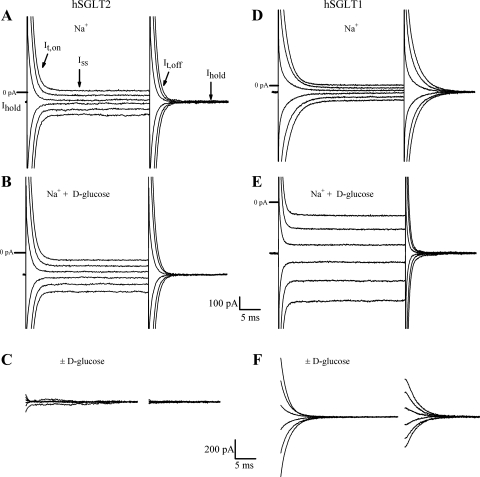
hSGLT1 and hSGLT2 showed differences in the transient current relaxations (It) in response to step jumps in membrane voltage (Fig. 3). In hSGLT2, we observed no clear difference in It between cells exposed to Na+ alone and Na+ plus saturating (50 mM) d-glucose (Fig. 3, A–C): in Na+ alone, the average time constant for It,Off was τ ≈0.9 ms, and in Na+ and d-glucose, τ ≈ 1.0 ms, whereas the steady-state current (at −60 mV) increased by ≈80 pA. Within the settling time of the voltage clamp (≈1 ms), there was no difference in transient currents in the presence and absence of saturating glucose (Fig. 3C). In contrast, hSGLT1 showed an obvious difference in current relaxations in response to step voltage jumps, in the presence and absence of saturating glucose (Fig. 3, D–F). When extracellular buffer contained Na+ alone, and Vt was jumped to the test voltage and back to the holding potential (Vh), a slow transient current was generated. The transient It,Off showed a prolonged relaxation to steady state with time constant τ ≈ 2.5 ms. The addition of a saturating concentration of glucose to the bath generated an inward current of ≈350 pA and abolished the pre-steady-state currents, and the relaxation times of the It,Off curves were similar to those of untransfected cells (τ ≈ 1.2 ms). Na+-glucose difference curves (Fig. 3F) showed slow (τ ≈ 3 ms) transients, characteristic of hSGLT1 pre-steady-state currents previously observed in oocytes. Q-V relationships for hSGLT1 (at 37°C and in 150 mM NaCl buffer) were obtained by integrating the total charge from It,Off as a function of Vm step, giving a maximal charge, Qmax ≈ 5 pC. This value can be used to estimate the number of transporters in the membrane as described previously (33). The apparent valence of the movable charge for the experiment in Fig. 3C was ≈ 1, and the midpoint of the Q-V curve, the voltage at half-maximal charge, V1/2 ≈ −60 mV. These are similar values to those previously obtained studying hSGLT1 in Xenopus oocytes (15). Phlorizin inhibits the hSGLT1 voltage-induced charge movements with a Ki close to that estimated from the inhibition of glucose transport at 22°C (90 vs. 70 nM).
Fig. 3.
Current records in response to step jumps in membrane voltage. Cells were held at the holding membrane potential (Vh = −60 mV). With application of the test voltage pulse, current relaxation showed an initial capacitive spike due to membrane bilayer capacitance followed by decay (It,On) to the steady-state level (Iss). Upon return to Vh, the transient current was in the opposite direction and consisted of the capacitive spike followed by a decay (It,Off) to the baseline holding current value (Ihold). Test voltage pulse duration was 30 ms, starting at +10 mV and ending at −110 mV, in 20-mV decrements. A–C: transient and steady-state current records from a single HEK293T cell expressing hSGLT2 at 37°C, in Na+ alone (A) and Na+ and saturating d-glucose (50 mM) (B), and Na+-d-glucose difference current with the steady-state currents removed (C). D–F: current records for a single HEK293T cell expressing hSGLT1 at 37°C in Na+ alone (D), Na+ and saturating d-glucose (100 mM) (E), and Na+-d-glucose difference current (F). In view of the settling time of the voltage clamp, the transient currents in C and F are shown 1.2 ms after the voltage pulse.
The magnitude of the glucose current increased with the external glucose concentration (Fig. 4A and Eqs. 1 and 2). In this cell the maximal current (Imaxd-glucose) was 39 pA and apparent glucose affinity (K0.5d-glucose) was 4.9 mM. The Imaxd-glucose varied from cell to cell, 20 to 150 pA, whereas the K0.5d-glucose for hSGLT2 was 4.9 ± 0.6 mM (n = 5 cells) and independent of expression level. The Na+ dependence of the hSGLT2 glucose current was also saturable. Figure 4B shows an example with a ImaxNa of 57 pA, a K0.5Na of 19 mM and Hill coefficient (nh) of 0.8. In five cells, the K0.5Na was 25 ± 10 mM and the nh = 0.9 ± 0.2 (Table 1). hSGLT1 glucose kinetics revealed K0.5d-glucose = 1.8 ± 0.2 mM (n = 4 cells), with Imaxd-glucose ranging from ≈300 to 1,000 pA. The K0.5Na for hSGLT1 was 73 ± 19 mM and the nh = 1.5 ± 0.1 (Table 1).
Table 1.
Comparison of kinetic and thermodynamic properties of hSGLT2 and hSGLT1 at 37°C
| hSGLT2 | hSGLT1 | |
|---|---|---|
| K0.5d-glucose, mM | 4.9 ± 0.6 | 1.8 ± 0.2 |
| K0.5αMDG, mM | 6.0 ± 0.9 | 3.8 ± 0.2 |
| K0.5d-galactose, mM | ≥100 | 6.1 ± 0.4 |
| K0.5Na, mM | 25 ± 10 | 73 ± 19 |
| Hill coefficient (nh) | 0.9 ± 0.2 | 1.5 ± 0.1 |
| Na+:glucose coupling (n) | 1:1 | 2:1 |
| Kiphlorizin, nM | 11 ± 0.9 | 140 ± 15 |
| t1/2,Offphlorizin, s | 24 ± 3 | 4 ± 0.5 |
| kOffphlorizin, s−1 | 0.030 ± 0.003 | 0.19 ± 0.02 |
| kOnphlorizin, M/s | 2.7 × 106 | 1.4 × 106 |
Values are means ± SE for n ≥ 3 cells at −60 mV. Rate constant for phlorizin binding (kOnphlorizin) is calculated from the Ki and the phlorizin release rate constant (kOffphlorizin). hSGLT, human Na+/d-glucose cotransporter; αMDG, [14C]-α-methyl-d-glucopyranoside; K0.5, substrate concentration at half-maximal current; t1/2,Off, half-time of recovery of glucose current after removal of phlorizin.
Substrate specificity.
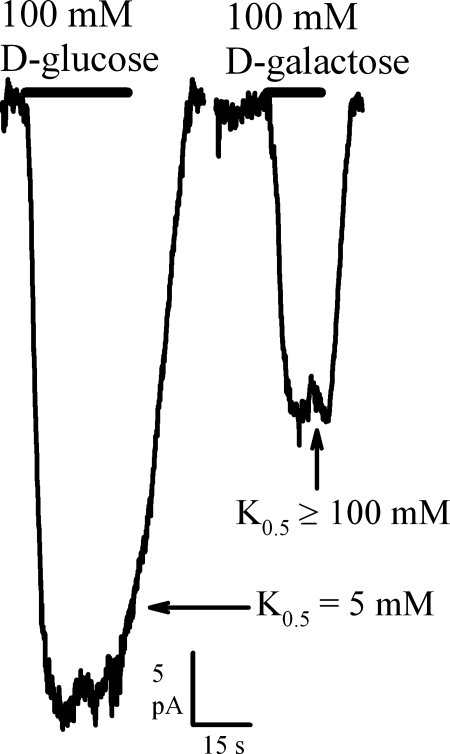
Prior work proposed that hSGLT2 does not transport d-galactose (11). We explored this further by measuring currents produced by d-glucose and d-galactose (Fig. 5). d-galactose at a concentration of 100 mM induced 40 ± 5% (n = 8 cells) of the current due to saturating (100 mM) d-glucose. Addition of d-galactose had no additive effect on the current produced by saturating d-glucose (data not shown), suggesting that the maximum d-galactose current is similar to that for d-glucose, and thus K0.5d-galactose ≥ 100 mM (Table 1). For hSGLT1, the K0.5d-galactose was 6 mM. The kinetics of αMDG transport were K0.5αMDG ≈6 mM for hSGLT2 and ≈4 mM for hSGLT1 (Table 1), with Imax values comparable to those for d-glucose. In contrast, the β-phenyl- and β-indoxyl-d-glucopyranosides had much higher affinities (K0.5 ≈ 0.3 and 0.1 mM) but lower Imax values (30% and 10% of that for d-glucose) (Table 2). Similar hSGLT1 kinetics for β-indoxyl-d-glucopyranoside have been reported, and α-phenyl-d-glucopyranoside did not generate any current in either hSGLT1 or 2 (Table 2).
Fig. 5.
d-Glucose vs. d-galactose as substrates of hSGLT2. Inward current resulting from 100 mM d-glucose vs. 100 mM d-galactose was compared in a single HEK293T cell expressing hSGLT2 at 37°C. K0.5d-glucose and K0.5d-galactose values are presented, based on dose-response studies of both substrates; the K0.5d-galactose value was estimated ≥100 mM.
Table 2.
Estimates for glucopyranoside affinities in hSGLT2 and hSGLT1
| Sugar | hSGLT2 K0.5, mM | hSGLT1 K0.5, mM |
|---|---|---|
| α-Phenyl-d-glucopyranoside | NT | NT |
| β-Phenyl-d-glucopyranoside | 0.3* | 1.6† |
| β-Indoxyl-d-glucopyranoside | 0.14* | 0.06† |
Sugar K0.5 values are means ± SE with n ≥ 3 cells. hSGLT2 experiments were performed at 37°C and hSGLT1 at 22°C. NT, not transported.
Maximal transport is lower for these substrates than for nonglucopyranosides—for β-phenyl-d-glucopyranoside, maximum current (Imax) is ≈30% that of d-glucose; for β-indoxyl-d-glucopyranoside, it is ≈10%.
From experiments in Xenopus oocytes expressing hSGLT1, where maximum transport rate is similarly lower (9, 16).
Na+:glucose coupling ratio.
The concentrative power of a cotransporter is governed by the stoichiometry of substrate and sodium transport. We estimated the Na+-to-glucose coupling ratio (n) using a thermodynamic approach by measuring the reversal potential (Vr) for transport under various glucose and Na+ concentration gradients across the plasma membrane (3). At Vr, cotransport is zero (Iglucose = 0), because the concentration gradients are balanced by the electrical driving force according to a Gibbs free energy relation (Eq. 3). Vr was determined by measuring the sugar currents (obtained in the presence and absence of 100 μM phlorizin) using a pulse protocol (see Fig. 2B). As illustrated in Fig. 6, for hSGLT2 there is a linear relationship between Vr and ln([αMDG]o) if [αMDG]i, [Na+]o, and [Na+]i are constant (Eqs. 4 and 5). The slope gave n = 1 (n = 9 cells). Similar experiments on hSGLT1 gave n = 2 (Table 3).
Fig. 6.
Determination of Na+:glucose coupling ratio for hSGLT2. Reversal potentials (Vr) for hSGLT2 at 37°C with fixed Na+ and αMDG gradients [75 mM extracellular Na+ concentration ([Na+]o), 50 mM intracellular [Na+] ([Na+]i), 30 mM [αMDG]i, and 2, 4, 9, or 16 mM [αMDG]o]. With [Na+]o, [Na+]i, and [αMDG]i constant, Vr is plotted vs. (RT/F) × ln([αMDG]o), where R is gas constant, T is absolute temperature, and F is Faraday's constant. The inverse of the slope is the Na+:glucose coupling ratio, n (Eqs. 4 and 5). As shown in the plot, n = 1.0 ± 0.02 (± error of the fit).
Table 3.
Na+:glucose coupling ratio in hSGLT2 and hSGLT1 at varying external/internal [αMDG] ratios
| hSGLT2 | ||||
|---|---|---|---|---|
| [αMDG]o, mM | 2 | 4 | 9 | 16 |
| n | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.2 | 1.0 ± 0.2 |
| hSGLT1 | ||||
| [αMDG]o, mM | 0.5 | 2 | 4 | 9 |
| n | 2.2 ± 0.9 | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.1 |
Values are means ± SE for n ≥ 3 cells. For hSGLT2, experiments were performed at 37°C, with internal (i) and external (o) components as follows (in mM): [Na+]i =50, [Na+]o =75, [αMDG]i =30 and [αMDG]o is varied. For hSGLT1, [Na+]i =50, [Na+]o =50, [αMDG]i =30, at 22°C (hSGLT1 results were confirmed at 37°C in follow-up experiments; data not shown).
Phlorizin inhibition.
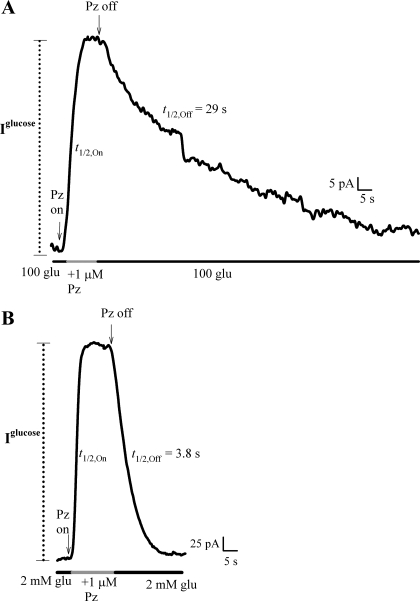
Phlorizin (1 μM) reversibly inhibited the 100 mM glucose current generated by hSGLT2 (Fig. 7A) and the 2 mM glucose current of hSGLT1 (Fig. 7B). To obtain the phlorizin inhibitory constant (Ki), we measured inhibition of the sugar current at half-maximal glucose concentration (i.e., the K0.5, 5 mM for hSGLT2, 2 mM for hSGLT1) as a function of phlorizin concentration, and estimated the Ki from the phlorizin concentration that inhibited sugar current by 50% [IC50; Eq. 6 (16, 23)]. The phlorizin Ki values for hSGLT2 and hSGLT1 were 11 and 140 nM, respectively (Table 1). We explored the mechanism behind this 10-fold difference in Ki. The On- (kOn) and Off- (kOff) rate constants for phlorizin binding were obtained from the half-time (t1/2,Off) of recovery of the glucose current upon removal of the external phlorizin (Fig. 7, Scheme I, and Eq. 7). For the cells in Fig. 7, the t1/2,Off for hSGLT2 was 29 s, and for hSGLT1 it was 3.8 s. These half-times were used to directly calculate kOff. The kOn was determined using its relationship to Ki and kOff (Eq. 8), because the direct measurement of t1/2,On was limited by the solution change half-time (≈0.5 to 3 s). The higher affinity of hSGLT2 for phlorizin than for hSGLT1 was largely due to the faster Off-rate for hSGLT1 than hSGLT2, kOff = 0.2 vs. 0.03 s−1 (Table 1). The calculated kOn values were similar: 2.7 × 106 M/s for hSGLT2 and 1.4 × 106 M/s for hSGLT1.
Fig. 7.
Time course of phlorizin inhibition of hSGLT2 and 1. Continuous current records of the sugar current (100 mM glucose, 60 pA for hSGLT2; 2 mM glucose, 400 pA for hSGLT1) as phlorizin (1 μM) was added or removed from the external solution, in hSGLT2 (A) and hSGLT1 (B). t1/2,On and t1/2,Off are the half-times for the current to reach steady state with phlorizin addition and removal. Upon washout of phlorizin (in the presence of glucose), the current returned to approximately the same level of Iglucose. t1/2,On was estimated to be within the half-time of the solution change: ≈2 s (A) and ≈0.5 s (B). As a result, t1/2,On for phlorizin binding could not be directly determined. For both A and B, t1/2,Off was much slower than the solution change half-time. Also, t1/2,Off by definition does not depend on bath concentrations of either d-glucose or phlorizin. t1/2,Off was used to calculate the kOff (Eq. 7), which along with the Ki was used to determine the theoretical kOn (Eq. 8). In each experiment the t1/2 for solution changes was determined from the current responses upon replacing the external Na+ with choline+.
DISCUSSION
A long-held dogma states that hSGLT2 is a low-affinity, high-capacity transporter in early segments of the proximal tubule (S1, S2) followed by hSGLT1, a high-affinity, low-capacity transporter in the late PT (S3). We were surprised to find that the apparent affinity for d-glucose is similar for the two transporters under physiological conditions (5 mM for hSGLT2 and 2 mM for hSGLT1, Table 1). Although similar values have been observed in αMDG uptakes (12, 19) at 37°C, this is the first time that hSGLT2 and 1 glucose transport kinetics have been measured under voltage clamp and at 37°C. The relative transport capacities of the two isoforms is more difficult to deduce; capacity depends both on copy number and on turnover rates of the two proteins. For hSGLT1, turnover can be determined from the maximum rate of transport (Imax) and the magnitude of pre-steady-state charge movements (Qmax) (15, 33). In hSGLT2 we did not observe pre-steady-state currents (i.e., transient currents) within the speed of the voltage clamp (Fig. 3). Therefore, it is unclear whether the difference in maximal transport rate (Imax) between the two isoforms (10-fold hSGLT1 over hSGLT2) is due to differences in copy number and/or turnover. This subject warrants future study.
For both hSGLT1 and 2, sugar-induced currents were sodium dependent, phlorizin sensitive, and dependent on membrane voltage (Vm). Each isoform's current-voltage (I-V) relationship was nonsaturating and increased with more negative Vm. Activation energies for maximum glucose transport by hSGLT1 and hSGLT2 were similarly high (≈25 kcal/mol), while the temperature dependence of substrate and inhibitor binding (K0.5 and Ki) were relatively low (data not shown; see also Ref. 20).
hSGLT2 and 1 differed in their Na+:glucose stoichiometry, 1 for hSGLT2 and 2 for hSGLT1, and the Hill coefficients determined from Na+ activation kinetics were consistent with these coupling ratios (Table 1). The data for hSGLT2 indicate that, like the bacterial transporter vSGLT of Vibrio parahaemolyticus, there is only one sodium binding site—most likely the Na2 site (4)—where coordinating residues are conserved between the two proteins (31). This raises interesting questions about how two Na+ binding sites in hSGLT1 increase the power of the transport cycle. The measurements of Vr also indicate that both transporters mediate outward sugar transport, i.e., Na+/d-glucose cotransport is readily reversible.
Unlike in hSGLT1, d-galactose is a poor substrate for hSGLT2 with a K0.5 of ≥100 mM (Table 1). Such a difference in selectivity is difficult to explain given the conservation of the predicted sugar-binding sites in the two proteins based on the crystal structure of vSGLT and the amino acid sequence alignment of vSGLT, hSGLT1, and hSGLT2 (4, 31). However, the relatively low overall sequence identity among the isoforms (hSGLT1 100%, hSGLT2 59%, and vSGLT 33%) could result in slight differences in H-bond distances between sugar hydroxyls and binding site residues, giving rise to large differences in sugar selectivity.
hSGLT2 and 1 share similar selectivity for glucopyranosides (phlorizin, α-phenyl-, β-phenyl-, and β-indoxyl-d-glucopyranosides, Tables 1 and 2), i.e., β-glucopyranosides are recognized with high affinity whereas α-phenyl-d-glucopyranoside is not. This indicates that the aglycone must be in the same plane as the pyranose ring to interact with both proteins (8), consistent with the structure of the emerging SGLT2 inhibitors (10, 28).
Our work provides detailed information about how inhibitors block Na+/d-glucose cotransport. We show the affinity of phlorizin for the two transporters (Ki 140 for hSGLT1 vs. 11 nM for hSGLT2) is governed by differences in rate of inhibitor release. As shown in Table 1, the phlorizin Off-rate for hSGLT1 was 10-fold higher than hSGLT2 (0.2 vs. 0.03 s−1). The estimated On-rates were similar (2.7 × 106 M/s for hSGLT2, 1.4 × 106 M/s for hSGLT1) (Table 1). Furthermore, these On-rates were three orders of magnitude lower than expected for diffusion control (≈109 M/s), which implies that phlorizin and the hSGLTs must be in a defined orientation for binding to occur and/or binding occurs in a multistep process, e.g., binding to an outward open conformation followed by occlusion.
In view of the interest in hSGLT inhibitors, what molecular properties account for the 10-fold difference between the phlorizin Ki for hSGLT2 and hSGLT1 (Table 1) (12)? They cannot be strictly due to differences in d-glucose's interaction with the proteins, because apparent affinities are similar (K0.5 = 2–5 mM). Instead, there must be larger differences in the binding energy of the aglycone of phlorizin-phloretin, and this is indeed borne out by the higher (≈10-fold) phloretin affinity of hSGLT2 over hSGLT1 (8, 19). The hSGLT2 and 1 inhibitor binding pockets must have a different architecture as shown by the selectivity of inhibitors for SGLT2 over SGLT1 (28).
This work also can be used to address the relative contributions of hSGLT2 and 1 to glucose reabsorption in the kidney. First, we predict that glucose transport is insensitive to the changes in glomerular filtrate sodium concentration under both physiological and pathophysiological conditions, because the sodium affinity is high (K0.5 of 25 and 70 mM for hSGLT2 and 1; Table 1) relative to its concentration in the proximal tubule (≈140 mM). Next, assuming that the location of hSGLT2 in the human kidney is similar to that for the mouse—the early proximal tubule (27)—and given the d-glucose K0.5 reported here for hSGLT2 (5 mM), it may be inferred that in normal fasting human subjects the brush border transporter works at only 50% capacity, and it would not approach saturation until the blood glucose reaches 35 mM. Over the 5–35 mM range of plasma glucose concentrations, a hSGLT2 competitive inhibitor with a Ki of 1 nM for hSGLT2 and ≈1 μM for hSGLT1 [e.g., dapagliflozin (17)] would, at a plasma concentration of 100 nM, inhibit >90% of glucose reabsorption [using modified Eq. 6 (23)] if hSGLT2 is the primary driver of glucose reuptake. However, only 50–60% inhibition has been reported for dapagliflozin (13), whereas intravenous phlorizin inhibits 100% (2). One explanation is that hSGLT2 drugs such as dapagliflozin are ≈90% bound to plasma proteins (13), thus reducing their effective dose in the proximal tubule. Even so, free dapagliflozin would be present at several times its Ki, greatly reducing the contribution of hSGLT2, while leaving hSGLT1 essentially fully active. This suggests that, far from playing a “cleanup” role, the capacity of hSGLT1 to reabsorb glucose may be >50% of hSGLT2 under normal conditions. hSGLT1 kinetics are consistent with the small amount of glucose lost in urine, because its Na+:glucose coupling ratio gives it 103-fold greater concentrative ability than hSGLT2 (31). Indeed, these kinetic observations may explain why SGLT2-null mice still reabsorb 30–40% of d-glucose from the glomerular filtrate, even though SGLT1 mRNA and protein levels are diminished by ≈40% in these mice (27). These revised roles for hSGLT2 and 1 in glucose reabsorption are important considerations for the design of future inhibitors targeting the renal hSGLTs.
In summary, our study provides new insights into the molecular physiology of hSGLT2 and hSGLT1 in the human kidney and a novel method for evaluating hSGLT inhibitors under physiological conditions.
GRANTS
This work was supported by National Institutes of Health Grant DK-019567 and National Research Service Award DK-082153 (to C. S. Hummel).
DISCLOSURES
E. M. Wright serves on an SGLT2 Inhibitor Advisory Board for Boehringer-Ingelheim, has been a consultant for BMS/AZ, Roche, Merck, and Novartis on SGLT biology, and has been a speaker on SGLT biology at industry-sponsored symposia and workshops at national society meetings.
ACKNOWLEDGMENTS
We thank Dr. Jessica Richardson for assistance with design of hSGLT1- and hSGLT2-containing vectors; we thank also Drs. Baljit Khakh, Yousang Gwack, and Bernard Ribalet for helpful discussions.
Present address of A. A. Voss: Department of Biological Sciences, California State Polytechnic University, Pomona, CA 91768.
REFERENCES
- 1. Barfuss DW, Schafer JA. Differences in active and passive glucose transport along the proximal nephron. Am J Physiol Renal Fluid Electrolyte Physiol 241: F322–F332, 1981 [DOI] [PubMed] [Google Scholar]
- 2. Chasis H, Jolliffe N, Smith HW. The action of phlorizin on the excretion of glucose, xylose, sucrose, creatinine and urea by man. J Clin Invest 12: 1083–1090, 1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen XZ, Coady MJ, Jackson F, Berteloot A, Lapointe JY. Thermodynamic determination of the Na+: glucose coupling ratio for the human SGLT1 cotransporter. Biophys J 69: 2405–2414, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321: 810–814, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33: 2217–2224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frohnert PP, Hohmann B, Zwiebel R, Baumann K. Free flow micropuncture studies of glucose transport in the rat nephron. Pflügers Arch 315: 66–85, 1970 [DOI] [PubMed] [Google Scholar]
- 7. Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature 330: 379–381, 1987 [DOI] [PubMed] [Google Scholar]
- 8. Hirayama BA, Diez-Sampedro A, Wright EM. Common mechanisms of inhibition for the Na+/glucose (hSGLT1) and Na+/Cl−/GABA (hGAT1) cotransporters. Br J Pharmacol 134: 484–495, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirayama BA, Lostao MP, Panayotova-Heiermann M, Loo DD, Turk E, Wright EM. Kinetic and specificity differences between rat, human, and rabbit Na+-glucose cotransporters (SGLT-1). Am J Physiol Gastrointest Liver Physiol 270: G919–G926, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Isaji M. Sodium-glucose cotransporter inhibitors for diabetes. Curr Opin Investig Drugs 8: 285–292, 2007 [PubMed] [Google Scholar]
- 11. Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for d-glucose. J Clin Invest 93: 397–404, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katsuno K, Fujimori Y, Takemura Y, Hiratochi M, Itoh F, Komatsu Y, Fujikura H, Isaji M. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J Pharmacol Exp Ther 320: 323–330, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, Pfister M. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 85: 520–526, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with Type 2 diabetes mellitus. Clin Pharmacol Ther 85: 513–519, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Loo DD, Hazama A, Supplisson S, Turk E, Wright EM. Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci USA 90: 5767–5771, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loo DD, Hirayama BA, Sala-Rabanal M, Wright EM. How drugs interact with transporters: SGLT1 as a model. J Membr Biol 223: 87–106, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, Wu G, Sher PM, Morrison EP, Biller SA, Zahler R, Deshpande PP, Pullockaran A, Hagan DL, Morgan N, Taylor JR, Obermeier MT, Humphreys WG, Khanna A, Discenza L, Robertson JG, Wang A, Han S, Wetterau JR, Janovitz EB, Flint OP, Whaley JM, Washburn WN. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 51: 1145–1149, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Oku A, Ueta K, Arakawa K, Ishihara T, Nawano M, Kuronuma Y, Matsumoto M, Saito A, Tsujihara K, Anai M, Asano T, Kanai Y, Endou H. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes 48: 1794–1800, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Pajor AM, Randolph KM, Kerner SA, Smith CD. Inhibitor binding in the human renal low- and high-affinity Na+/glucose cotransporters. J Pharmacol Exp Ther 324: 985–991, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Parent L, Wright EM. Electrophysiology of the Na+/glucose cotransporter. Soc Gen Physiol Ser 48: 263–281, 1993 [PubMed] [Google Scholar]
- 21. Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich JH, Kemper M, Li Volti S, Neuhaus T, Skovby F, Swift PG, Schaub J, Klaerke D. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 14: 2873–2882, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Scholl-Burgi S, Santer R, Ehrich JH. Long-term outcome of renal glucosuria type 0: the original patient and his natural history. Nephrol Dial Transplant 19: 2394–2396, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems. Wiley, 1975, p. 957 [Google Scholar]
- 24. Turner RJ, Moran A. Further studies of proximal tubular brush border membrane d-glucose transport heterogeneity. J Membr Biol 70: 37–45, 1982 [DOI] [PubMed] [Google Scholar]
- 25. Turner RJ, Moran A. Heterogeneity of sodium-dependent d-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol Renal Fluid Electrolyte Physiol 242: F406–F414, 1982 [DOI] [PubMed] [Google Scholar]
- 26. Turner RJ, Silverman M. Sugar uptake into brush border vesicles from normal human kidney. Proc Natl Acad Sci USA 74: 2825–2829, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Washburn WN. Development of the renal glucose reabsorption inhibitors: a new mechanism for the pharmacotherapy of diabetes mellitus type 2. J Med Chem 52: 1785–1794, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Wells RG, Pajor AM, Kanai Y, Turk E, Wright EM, Hediger MA. Cloning of a human kidney cDNA with similarity to the sodium-glucose cotransporter. Am J Physiol Renal Fluid Electrolyte Physiol 263: F459–F465, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Wright EM. Renal Na+-glucose cotransporters. Am J Physiol Renal Physiol 280: F10–F18, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. doi:10.1152/physrev.00055.2009 [DOI] [PubMed] [Google Scholar]
- 32. You G, Lee WS, Barros EJG, Kanai Y, Huo TL, Khawaja S, Wells RG, Nigam SK, Hediger MA. Molecular characteristics of Na+-coupled glucose transporters in adult and embryonic rat kidney. J Biol Chem 270: 29365–29371, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Zampighi GA, Kreman M, Boorer KJ, Loo DD, Bezanilla F, Chandy G, Hall JE, Wright EM. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J Membr Biol 148: 65–78, 1995 [DOI] [PubMed] [Google Scholar]