Abstract
Electrical stimulation is an indispensible tool in studying electrically excitable tissues in neurobiology and neuroendocrinology. In this work, the consequences of high-intensity electrical stimulation on the release of catecholamines from adrenal gland slices were examined with fast-scan cyclic voltammetry at carbon fiber microelectrodes. A biphasic signal, consisting of a fast and slow phase, was observed when electrical stimulations typically used in tissue slices (10 Hz, 350 μA biphasic, 2.0 ms/phase pulse width) were applied to bipolar tungsten-stimulating electrodes. This signal was found to be stimulation dependent, and the slow phase of the signal was abolished when smaller (≤250 μA) and shorter (1 ms/phase) stimulations were used. The slow phase of the biphasic signal was found to be tetrodotoxin and hexamethonium independent, while the fast phase was greatly reduced using these pharmacological agents. Two different types of calcium responses were observed, where the fast phase was abolished by perfusion with a low-calcium buffer while both the fast and slow phases could be modulated when Ca2+ was completely excluded from the solution using EGTA. Perfusion with nifedipine resulted in the reduction of the slow catecholamine release to 29% of the original signal, while the fast phase was only decreased to 74% of predrug values. From these results, it was determined that high-intensity stimulations of the adrenal medulla result in depolarizing not only the splanchnic nerves, but also the chromaffin cells themselves resulting in a biphasic catecholamine release.
Keywords: carbon fiber, exocytosis, catecholamine release, diffusion
electrical stimulation is frequently used to excite neuronal tissue and evoke exocytosis. However, selectivity is always a concern because the stimulus can cause exocytosis from afferent terminals within the tissue as well as depolarizing cell bodies within the tissue (26). An example that was characterized several years ago is the electrical stimulation of the intact adrenal gland. Wakade and Wakade (50) showed that an electrical stimulation could evoke catecholamine release both by directly depolarizing the chromaffin cells as well as by depolarizing the splanchnic nerve that causes acetylcholine release that in turn triggers catecholamine exocytosis from chromaffin cells. These investigators were able to distinguish these two contributions by the select use of pharmacological agents and well as by adjusting the stimulation parameters.
Release of catecholamines from isolated chromaffin cells has been extensively investigated in recent years by electrochemical and patch-clamp techniques. Electrochemistry with carbon fiber microelectrodes can resolve each exocytotic event by oxidizing the packet of released catecholamines. Patch clamp probes exocytosis by measuring capacitance changes as the area of the cell membrane is increased by exocytotic events (35, 45, 46, 52). More recently, these techniques have been used in slices of the adrenal gland to understand the processes that occur in a more intact preparation (31, 47). Arroyo et al. (4) showed that amperometric recordings in adrenal slices can resolve exocytotic events that are identical to those measured at the single cell level. Petrovic et al. (37) used both amperometry and cyclic voltammetry to probe the pharmacology of catecholamine release within adrenal slices. Cyclic voltammetry is an electrochemical method that allows the identification of the species detected, allowing norepinephrine and epinephrine to be distinguished. Both electrochemical studies in intact slices demonstrated that electrically evoked release was caused by activation of the splanchnic nerve because hexamethonium (Hex), a nicotinic receptor antagonist, blocked the evoked release.
In this work, we reinvestigate the dual release pathway identified many years ago by Wakade and Wakade (50). Patch-clamp recordings have shown that chromaffin cells become permeable to Ca2+ when their membranes are depolarized and that Ca2+ plays an integral role in the process of exocytosis (5, 33, 34, 42). In addition, chromaffin cells are capable of firing action potentials that can be decreased in frequency by tetrodotoxin (TTX), a Na+ channel blocker, and that is dependent on K+ channels for repolarization (22, 32). Here, we use a method with high spatial and temporal resolution, fast-scan cyclic voltammetry (FSCV), as well as more modern pharmacology probing the roles of specific Ca2+ channels to resolve the originally claimed dual mechanism of electrically evoked catecholamine release with contribution both from splanchnic nerve stimulation and direct chromaffin cell depolarization. These studies reveal that the chromaffin cell is more difficult to depolarize than the splanchnic nerve.
MATERIALS AND METHODS
Animals.
Wild-type C57BL/6J female mice, 4–8 wk old, were used in this work (stock no. 000664, Jackson Laboratories, Bar Harbor, ME). Upon receipt, mice were housed at the University of North Carolina Husbandry Facility with ad libitum access to food and water and under closely controlled environmental conditions (temperature, humidity, and 12:12-h light-dark cycles). Live animal handling procedures and experimental protocols were approved by the University of North Carolina Institutional Animal Care and Use Committee.
Adrenal slice methods.
Adrenal gland slicing procedures were adapted from literature descriptions with slight modification (6, 31, 37). Mice were anesthetized under anhydrous ethyl ether (Fisher Scientific) and decapitated. A midline abdominal incision allowed for access to the adrenal glands. Glands were placed into ice-cold and 95% O2-5% CO2 saturated low Ca2+ bicarbonate-buffered saline (BBS), containing (in mM) 0.1 CaCl2, 125 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 3 MgCl2, 10 glucose, and 10 HEPES, and its pH was adjusted to 7.4. Excess fat was removed from the glands, and the glands were embedded in a 3% agarose (wt/vol) in low-Ca2+ BBS (Promega). Once cooled, agarose blocks containing the glands were secured onto a Teflon specimen holder that was used to orient the largest base of the gland parallel to the slicing blade. The glands were cut into slices of ∼200 μm in thickness using a vibratome slicer filled with low-Ca2+ BBS (Campden Instruments, World Precision Instruments, Leics, UK).
Slices were immediately placed into a perfusion chamber (open diamond bath-heated chamber, RC-22, Warner Instruments, Hamden, CT) and secured with a stainless steel Lycra thread anchor (1.5 mm-2.0 mm thread spacing, Warner Instruments). The chamber was continually perfused with 95% O2-5% CO2 saturated BBS, at a rate of 1–2 ml/min with the same composition as the low-Ca2+ BBS, except with 2 mM CaCl2 and 1 mM MgCl2 at pH 7.4. To ensure physiological conditions and to minimize slice perturbations, the temperature of the perfusion chamber was kept at 37°C using an automatic temperature controller (Warner Instruments). All of the experiments were completed within a 10-h post mortem period.
Fast-scan cyclic voltammetry.
Carbon fiber elliptical microelectrodes were fabricated as previously described (9, 20). Before use, carbon fiber microelectrodes were soaked in an activated carbon/2-propanol mixture for a minimum of 30 min to remove surface debris (38). Microelectrodes were then backfilled with 4 M CH3COOK/0.15 M NaCl and mounted onto a potentiostat headstage. A stainless-steel wire was inserted into the microelectrode to make electrical contact. Experiments were conducted inside a grounded Faraday's cage. Catecholamine release was monitored with an Axopatch 200B patch-clamp amplifier in voltage-clamp mode and β = 0.1 configuration (Axon Instruments, Molecular Devices, Union City, CA). The signal was filtered with a built-in analog low-pass Bessel filter (80 dB/decade) at 5 kHz. The potentiostat was controlled via LABVIEW software, TarHeel CV (ESA, Chelmsford, MA). Measured currents were digitized using a National Instruments PCI-MIO-16XE-10 card and monitored as a function of the applied potential. The waveform for cyclic voltammetry was a potential sweep from −400 mV to +1,000 mV and back to −400 mV at a scan rate of 600 V/s applied at a 10-Hz repetition rate. Between scans, the electrode potential was held at −400 mV allowing for catecholamine adsorption onto a carbon fiber surface, a procedure that increases the sensitivity to catecholamines (17).
Electrical stimulation of slices employed two tungsten microelectrodes glued together with their tips separated by ∼250 μm (FHC, Bowdoin, ME). The stimulating electrode was positioned on the slice surface under a ×40 water immersion objective Fluor WD 2.0 on an upright microscope (Nikon Eclipse E600FN, Lewisville, TX). Microelectrodes were positioned with a multimanipulator controller (1-μm display resolution, MPC-200-ROE, Sutter Instruments). To avoid recordings from a damaged tissue layer, microelectrodes were lowered ∼30 μm below the slice surface (21, 37). To evoke catecholamine release, trains of initially positive biphasic pulses (10 pulse, 10 Hz) were applied to the stimulating electrodes between scans using a National Instruments PCI-6711 card, controlled by Tar Heel CV to ensure no overlap between cyclic voltammetry scans for data collection and stimulation pulses. Slices were equilibrated for ∼2 h before data collection. During the equilibration period, slices were electrically stimulated every 3–5 min. These data were used to establish a baseline level of catecholamine release.
Voltammetric data analysis employed the TarHeel CV data analysis package (ESA). Digital background subtraction was used to remove the background current to reveal the cyclic voltammogram of the species detected. Sets of cyclic voltammograms were displayed as color plots (27). Each color plot displays a set of cyclic voltammograms that were sequentially acquired in time (abscissa) while the applied potential is shown as the ordinate. The currents are shown in color. To follow the time course of concentration changes, the current at +600 mV (the peak current for catecholamine oxidation) was extracted from the color plots. Since the electrochemical signals recorded in the adrenal gland are a mixture of epinephrine, and norepinephrine (37), electrodes were post calibrated in a flow injection system with 5 μM epinephrine, norepinephrine, and a 50:50 mixture of 2.5 μM of each catecholamine. Concentrations measured during application of pharmacological agents are reported as values normalized to currents recorded before drug application.
Pharmacology.
Pharmacological agents employed herein include thapsigargin (THG), TTX, Hex, 4-diphenylacetoxyl-N-methylpiperidine (4-DAMP), ethylene glycol tetraacetic acid (EGTA), atropine, and nifedipine. All were from Sigma-Aldrich (St. Louis, MO) and were used as received. Drugs were dissolved in low-Ca2+ or recording BBS and superfused through a slice chamber at a rate of ∼2 ml/min.
Statistical analysis.
Statistical analysis was done in GraphPad Prism (GraphPad Software, San Diego, CA). Student's t-test (unpaired) was used to test for significant differences between data sets. Data were considered significantly different at the 95% confidence level; n is nnumber of slices, unless noted otherwise.
RESULTS
Characteristics of evoked catecholamine release.
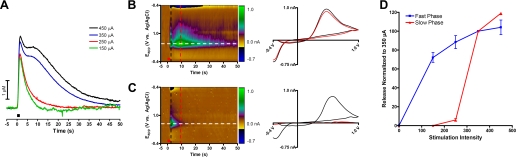
In prior work we showed that local electrical stimulation of adrenal medullary slices with stimulus trains consisting of 10 biphasic pulses, 1 ms/phase, 150 μA at 10 Hz results in catecholamine release that increases during the stimulation and then decreases 1–2 s after the stimulation is terminated (37). Furthermore, we showed that the pharmacological manipulation during such stimulations was consistent with activation of the splanchnic nerve causing release of acetylcholine. We refer to this as the fast phase of catecholamine release. However, quite different results were obtained with an identical stimulation except with a greater amplitude (n = 3, Fig. 1A). With stimulus amplitude of 450 μA, catecholamine release is still evoked, but it lasts over a longer time frame. A maximum corresponding to the fast phase is observed shortly (1–2 s) after the stimulation is terminated, and it is followed by a broad, larger increase that maximizes ∼8–10 s after the stimulation. We refer to this second feature as the slow phase of release. As the stimulus amplitude was diminished, the slow phase diminished more rapidly than the fast phase. Similar behavior was seen within chromaffin-cell clusters throughout the adrenal medulla. In some instances, the slow phase was larger in amplitude than the fast phase and overwhelmed it. The cyclic voltammograms (Fig. 1, B and C, encoded as color plots) show that catecholamine release is the primary contributor to both phases. The maximum amplitude of each phase as a function of stimulus intensity is shown in Fig. 1D.
Fig. 1.
Effect of stimulation intensity on biphasic release. A: representative electrically stimulated catecholamine release with decreasing intensity (350–150 μA) with 2.0 ms/phase pulse width. Bar represents time of stimulation. B: color plot of catecholamine release from a 450-μA stimulation. Cyclic voltammograms at 2 s (black, fast phase) and 9 s (red, slow phase) are shown on the right as indicated by the dashed lines. Eapp, applied potential. C: color plot of catecholamine release from a 150-μA stimulation. Cyclic voltammograms at 2 s (black, fast phase) and 9 s (red, slow phase) are shown on the right as indicated by the dashed lines. D: simulation intensity data were summarized for the fast and slow phase as the amount of release relative to that collected with a 350-μA stimulation (n = 3).
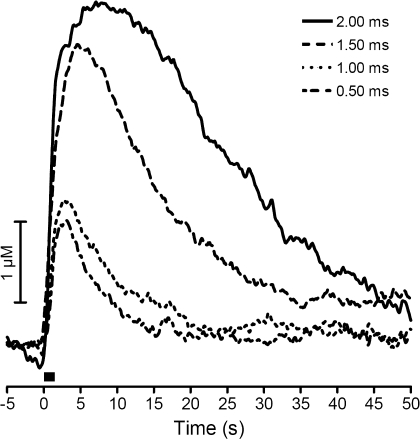
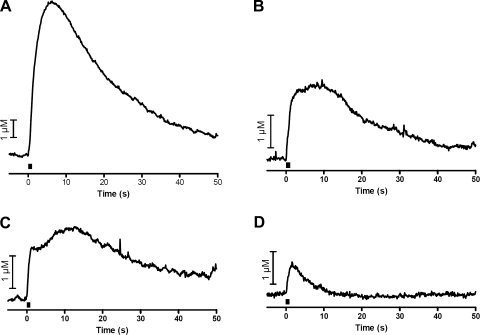
To probe further the effects of electrical stimulation parameters on catecholamine release, various stimulation pulse widths were applied to the slice using a 350-μA stimulation amplitude. Stimulating pulse widths (per phase) of 2.0 ms, 1.5 ms, 1.0 ms, and 0.5 ms were employed (pulse widths were delivered in random order). The slow phase was not present with stimulation widths of ≤1 ms per phase (n = 5, Fig. 2). The remaining experiments described here were designed to probe the origin of the slow release phase and used relatively large stimulations (350 μA and 2.0 ms/phase width).
Fig. 2.
Effect of stimulation width on biphasic release. Representative electrically stimulated catecholamine release at an intensity of 350 μA with decreasing pulse width (2.0–0.5 ms/phase) is shown. Bar represents time of stimulation.
Modulation of catecholamine release by ions.
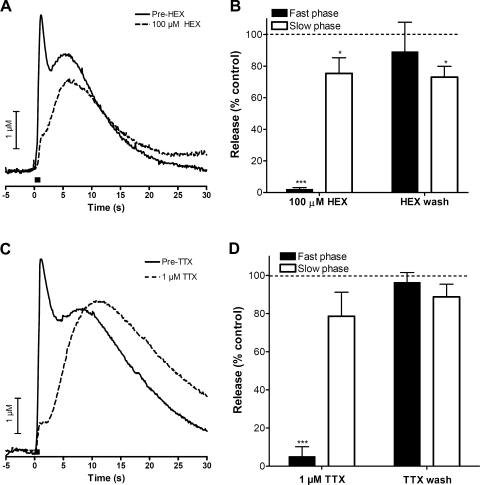
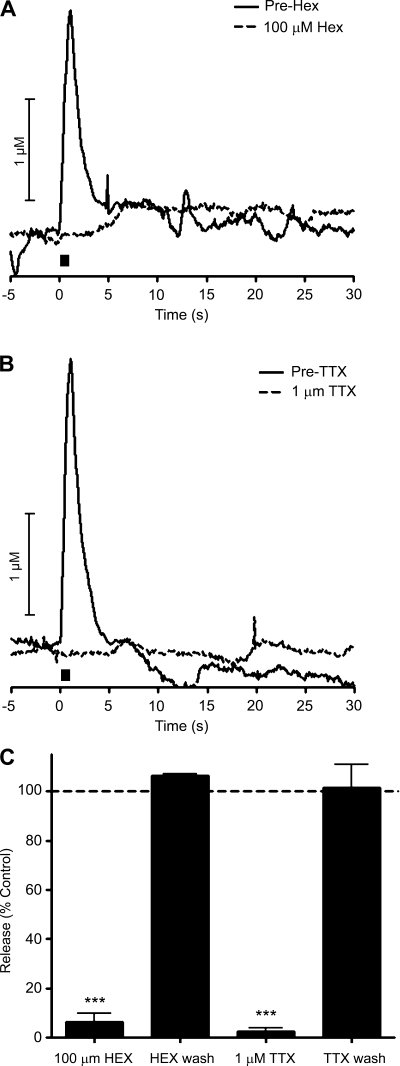
We investigated the role of cholinergic receptors on the slow phase of catecholamine release. As previously shown, the fast phase was diminished in the presence of 100 μM Hex, a nicotinic acetylcholine receptor blocker. In contrast, the slow phase was only marginally affected by Hex (representative traces are shown in Fig. 3A and average maximal amplitudes are shown in Fig. 3B; n = 5). Previous work has shown that splanchnic nerve-dependent catecholamine release measured by FSCV is independent of muscarinic receptors (37). Perfusion with 1.0 μM 4-DAMP (n = 3, data not shown), a selective muscarinic receptor antagonist, also did not affect the slow phase of electrically stimulated release. Furthermore, perfusion with 0.5 μM atropine (n = 3, data not shown), a high-affinity muscarinic receptor antagonist, had no effect on the biphasic release. The inability of cholinergic antagonists to affect the slow phase of catecholamine release indicates that acetylcholine released from the splanchnic nerve is unnecessary for this type of release.
Fig. 3.
Modulation of catecholamine release by ions. A: representative trace for the perfusion of 100 μM hexamethonium (Hex) before and after the application of drug. B: summary of effects of Hex on fast and slow catecholamine release (n = 5). C: representative trace for the perfusion of 1 μM tetrodotoxin (TTX) before and after the application of drug. D: summary of effects of TTX on fast and slow catecholamine release (n = 5). Bar in A and C represents time of stimulation. *P < 0.05, ***P < 0.001.
To evaluate the involvement of Na+-dependent action potentials in slow phase catecholamine release, 1 μM TTX, an inhibitor of voltage-gated Na+ channels (10), was perfused through the slice. The slow phase of the electrically stimulated release was found to be much less sensitive to TTX than the fast phase, which was abolished almost immediately upon perfusion as previously reported (representative example m Fig. 3C). A summary of the effects of TTX on the maximum amplitude of the two phases is shown in Fig. 3D (n = 5).
Biphasic signal dependence on intra- and extracellular Ca2+ concentration.
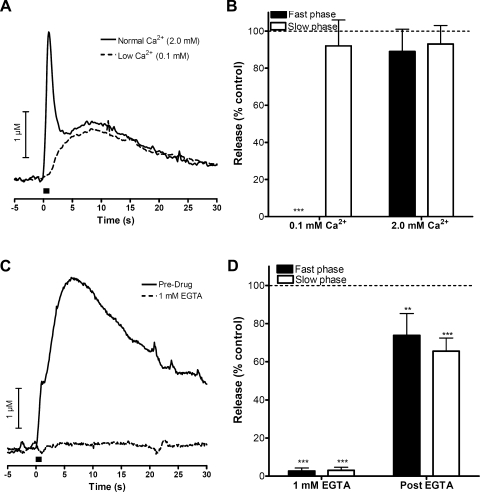
To explore the Ca2+ concentration ([Ca2+]) dependence of the slow phase of electrically stimulated release, the slices were perfused with BBS containing 0.1 mM Ca2+. In this media, the fast phase release component was eliminated while the slow phase remained intact (representative example shown in Fig. 4A). Data from multiple trials are summarized in Fig. 4B (n = 3). When the slice was perfused with BBS containing 0 mM Ca2+ and 1 mM EGTA, both the fast and slow release was abolished within 15 min (representative example in Fig. 4C). Both phases partially recovered when extracellular Ca2+ levels were returned to their normal level (2 mM). These data are summarized in Fig. 4D (n = 5). Thus, while the slow phase does not require concentrations of physiological levels of extracellular Ca2+, it does require some Ca2+ for release to occur.
Fig. 4.
Biphasic signal dependence on extracellular Ca2+. A: representative experiment for perfusion with a low extracellular calcium (0.1 mM) buffer before and after application of low-Ca2+ buffer. B: summary of effects of perfusion of low-calcium buffer on fast and slow catecholamine release (n = 3). C: representative experiment where extracellular calcium was completely removed from the perfusion buffer using 1 mM EGTA before and after application of drug. D: summary of effects of EGTA on fast and slow catecholamine release (n = 5). Bar in A and C represents time of stimulation. **P < 0.01, ***P < 0.001.
To test the possibility that the slow phase requires release of cytoplasmic Ca2+ levels from intracellular stores such as endoplasmic reticulum, the slices were perfused with THG. THG is a pharmacological agent that increases cytosolic Ca2+ levels by binding to sarco(endo)plasmic reticulum Ca2+-ATPase enzymes and preventing Ca2+ from being pumped into the sarcoplasmic and endoplasmic reticula (29). Perfusion of 1 μM THG dissolved in low-[Ca2+] BBS buffer for 1 h did not have an effect on either the fast or the slow components of electrically stimulated release (n = 4, data not shown).
Effect of L-type Ca2+ channel blocker on slow release.
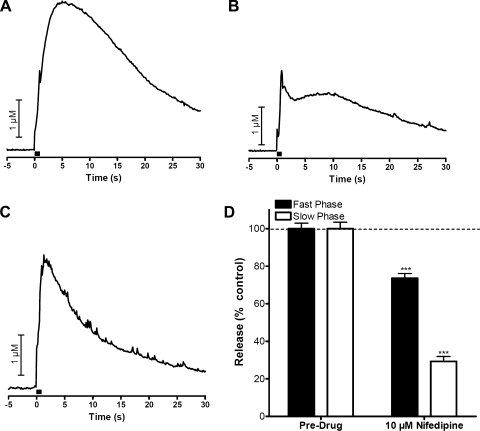
It has been shown that L-type voltage-gated Ca2+ channels (VGCCs) are located on chromaffin cells but not on the splanchnic nerve and that they play a key role in the homeostatic mechanism of catecholamine release (1, 25, 41). To evaluate the role of these channels in the different phases of release, we perfused 10 μM nifedipine, an L-type Ca2+ channel blocker, through the slice. The maximal amplitude of the slow phase was decreased to 29% of its original value by this perfusion while 74% of the fast phase remained. Figure 5A shows biphasic release before nifedipine is put on board, while Fig. 5B is the observed release 10 min after nifedipine application, and, finally, Fig. 5C shows that the slow phase was completely abolished by nifedipine 20 min after nifedipine had been put on board. A summary of the effects of nifedipine on the biphasic response is shown in Fig. 5D (n = 5).
Fig. 5.
Role of L-type Ca2+ channels on biphasic release. A: biphasic release before the application of drug. B: release 10 min into perfusion with 10 μM nifedipine. C: release 20 min into perfusion with 10 μM nifedipine. D: summary of effects of nifedipine on fast and slow catecholamine release after at least 15 min of incubation (n = 5). Bar in A–C represents time of stimulation. ***P < 0.001.
Biphasic signal dependence on the distance between working and stimulating electrodes.
To probe the effect of the location of the stimulating electrode relative to the carbon fiber microelectrode, the stimulating electrode was moved away from the working electrode in 50-μm increments and release was evoked (Fig. 6). At increasing distances, both the fast and slow phases decrease in intensity. The slow phase is diminished to a greater degree, however, allowing the fast phase to predominate at greater separations of the stimulating electrode and the carbon fiber microelectrode. At the greatest distances between the electrodes, where only the fast phase was present (200 μm), perfusion with 100 μM Hex (Fig. 7A) or 1 μM TTX (Fig. 7B) reversibly eliminated the stimulated release. These data are summarized in Fig. 7C (n = 3, P < 0.001 for each drug).
Fig. 6.
Biphasic signal dependence on the distance between the working and stimulating electrodes using a 350-μA, 1 ms/phase stimulation (10 Hz, 10 pulses). A: 0 μm. B: 50 μm. C: 100 μm. D: 200 μm. Bar represents time of stimulation.
Fig. 7.
Modulation of remotely stimulated release by ions. A: representative experiment showing the effect of 100 μM Hex with 200 μm between the stimulating and working electrode. B: representative experiment showing the effect of 1 μM TTX with 200 μm between the stimulating and working electrode. C: summary of effects of perfusion of Hex (n = 3) and TTX (n = 3) as well as the recovery of fast release when the stimulating electrode is 200 μm from the working electrode. Bar in A and B represents time of stimulation.***P < 0.001.
DISCUSSION
Prior work in the perfused adrenal gland demonstrated that electrical stimulation could evoke catecholamine release by two mechanisms: indirectly by depolarization of the splanchnic nerve or directly by depolarization of the chromaffin cells themselves (48, 50). Catecholamine release arising from splanchnic nerve stimulation occurs because released acetylcholine from the splanchnic nerve activates nicotinic receptors on chromaffin cells. The prior work demonstrated that direct depolarization of chromaffin cells required higher stimulation intensity than was required to activate the splanchnic nerve. The present work in which release is detected with a microsensor inserted directly into slices containing the adrenal medulla supports the prior findings and provides additional information concerning the temporal and spatial dependence of the two mechanisms that cause catecholamine release. The fast phase of release clearly originates from splanchnic nerve stimulation because it is blocked by a nicotinic antagonist. The slow phase of release is unaffected by nicotinic antagonists, but it is inhibited by an L-type calcium channel antagonist. This demonstrates that the slow phase of release originates from direct depolarization of chromaffin cells because acetylcholine release from the splanchnic nerve is known to be unaffected by L-type calcium channel antagonists, whereas their presence inhibits catecholamine release from electrically excited adrenal glands (1, 41).
Evidence that the splanchnic nerve is not involved in the slow phase of catecholamine release also comes from the experiments with TTX incubation. Consistent with our prior work, TTX blocked the splanchnic-nerve dependent component of release of catecholamines, demonstrating the need for action potential propagation supported by voltage-dependent Na+ channels (19, 51). Chromaffin cells are drastically different, however, because they do not require the opening of Na+ for catecholamine release. Rather, direct depolarization of the chromaffin cell can cause release due to opening of VGCCs (16, 43). This is an important distinction because it illustrates the different mechanisms in play during high-intensity electrical stimulation and the resulting biphasic release.
The splanchnic nerve consists of fine fibers closely associated with Schwann cells that innervate the chromaffin cells (12, 44). As such, they would be expected to be depolarized by milder electrical stimulations than the chromaffin cells themselves. The splanchnic nerve can be depolarized over large distances (over 200 μm) due to the morphology of its innervation throughout the gland while still being susceptible to pharmacological manipulations (12, 18, 44). This propagation of the stimulation through the splanchnic nerve network allows release to be evoked from chromaffin cells throughout the tissue including those directly adjacent to the microelectrode. Release adjacent to the microelectrode would appear rapidly, leading to the fast appearance of released catecholamines. While chromaffin cells themselves are electrically excitable (22), their lack of myelination and their larger size requires that they receive a greater stimulus intensity to cause exocytosis (28). Thus, the chromaffin cells most likely to be directly depolarized by the stimulation are those that are closest to the stimulating electrode. For detection, the released catecholamines from direct chromaffin cell stimulation must diffuse to the detecting microelectrode, a time-dependent process. Consistent with this interpretation, the amplitude of the slow phase diminished more rapidly than the fast phase when the stimulating electrode was moved further away from the carbon fiber microelectrode.
We also considered other mechanisms that could be the origin of the slow phase of release. One is the interplay of intracellular [Ca2+] and muscarinic cholinergic receptors. At isolated chromaffin cells, muscarinic receptor activation has been shown to cause release of Ca2+ from intracellular stores (11, 39, 49). Exocytosis, when evoked by mobilization of intracellular Ca2+ stores, is temporally delayed in comparison to endogenous acetylcholine induced response (30). However, the slow phase did not display any dependence on THG, a pharmacological agent that causes release of Ca2+ from intracellular stores. Furthermore, the slow phase was insensitive to both atropine and 4-DAMP, a group of highly selective muscarinic receptor blockers (2). Taken together, the negative findings indicate that the slow phase of catecholamine release does not originate from mobilization of intracellular Ca2+ via activation of muscarinic receptors. Electroporation was also considered as a mechanism contributing to the slow phase of release. High electric fields are known to cause transient pores in cell membranes (15, 36, 40). Electroporation was achieved at single cells stimulated with microelectrodes as evidenced by entry of fluorescent dye (40). However, no evidence for electroporation was detected within slices using the tungsten-stimulating electrodes with the stimulation parameters used to observe the slow (or fast) phases of catecholamine release. It requires very large electric fields to cause electroporation of cells (at least 1 kV/cm). Given the intensity of the stimulation (2.45 V to achieve 350 μA) as well as the distance between the stimulating electrodes (100 μm), electric fields no larger than 250 V/cm were exposed to chromaffin cells within a slice. Furthermore, when electric fields close to 1 kV/cm were applied to the tungsten-stimulating electrodes, bubbles were observed at the electrode surface, indicating that electroporation using electrodes with a tungsten surface is not feasible because the electric fields required to cause electroporation also cause the breakdown of water at the electrode and would cause visible tissue damage.
Owing to the crucial contribution of Ca2+ signaling in the secretion of catecholamines (7, 8, 14), it is important to pay close attention to the types of VGCCs that are present on the cell being studied, as well as the different roles each plays in the multistep process of release. It has been shown that while N and P/Q VGCCs are functionally coupled with exocytosis on chromaffin cells and are believed to be spatially related to one another (3, 13), while L-type VGCCs, which take a longer time than P/Q and N VGCCs to inactivate, are known to regulate the autorhythmicity of chromaffin cell action potentials and could play a key role in the homeostatic mechanism of catecholamine release (25). However, L-type VGCCs are not present on splanchnic nerves but do play a role in direct stimulation of chromaffin cells by high K+ and therefore are an excellent candidate for investigating the difference between the two mechanisms (1, 23, 41). These properties of L-type VGCCs support the data observed here where the slow phase, not observed in stimulations exciting only the splanchnic nerve, is slower in onset and due to chromaffin cell excitation near the stimulating electrodes. Similar to results previously observed, the nicotinic acetylcholine receptor-mediated release decreased ∼25% in the presence of nifedipine, while the slow phase was no longer distinguishable, leaving only the signal due to the decay of the fast phase (1). Similar to earlier work, the fast phase was highly dependent on extracellular calcium, further corroborating the splanchnic origin of this signal. As shown by Wakade and Wakade (50), completely depleting the extracellular buffer of calcium using EGTA abolishes catecholamine release elicited by direct depolarization of the chromaffin cells. While other calcium channels on the chromaffin cells could be contributing to the signal from direct chromaffin cell stimulation, it is apparent that the L-type channels play a key role in the entry of Ca2+ into the chromaffin cells during this process.
In this study, the high temporal and spatial resolution of FSCV at carbon fiber microelectrodes allowed us to distinguish between direct depolarization of chromaffin cells in murine adrenal slices and depolarization of splanchnic nerves that innervate them. While whole gland stimulations (24, 48, 50) established this difference, in this work we were able to temporally and spatially resolve the two components. It is well known in the literature that care must be taken to select appropriate parameters for electrical stimulation of living tissue to study mechanisms of release (26). The present work shows that lower currents and smaller stimulation pulse widths are the proper choice when studying secretion from murine adrenal slices since they trigger the physiological mechanisms which lead to catecholamine release.
GRANTS
This work was funded by National Institutes of Health grant NS-38879.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Akiyama T, Yamazaki T, Mori H, Sunagawa K. Effects of Ca2+ channel antagonists on acetylcholine and catecholamine releases in the in vivo rat adrenal medulla. Am J Physiol Regul Integr Comp Physiol 287: R161–R166, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Alamo L, Garcia AG, Borges R. Electrically-evoked catecholamine release from cat adrenals. Role of cholinergic receptors. Biochem Pharmacol 42: 973–978, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Alvarez YD, Ibanez LI, Uchitel OD, Marengo FD. P/Q Ca2+ channels are functionally coupled to exocytosis of the immediately releasable pool in mouse chromaffin cells. Cell Calcium 43: 155–164, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Arroyo G, Fuentealba J, Sevane-Fernandez N, Aldea M, Garcia AG, Albillos A. Amperometric study of the kinetics of exocytosis in mouse adrenal slice chromaffin cells: physiological and methodological insights. J Neurophysiol 96: 1196–1202, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Augustine GJ, Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol 450: 247–271, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbara JG, Poncer JC, McKinney RA, Takeda K. An adrenal slice preparation for the study of chromaffin cells and their cholinergic innervation. J Neurosci Methods 80: 181–189, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Burgoyne RD. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta 1071: 174–202, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Burgoyne RD, Morgan A, Robinson I, Pender N, Cheek TR. Exocytosis in adrenal chromaffin cells. J Anat 183: 309–314, 1993 [PMC free article] [PubMed] [Google Scholar]
- 9. Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal Chem 68: 3180–3186, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol 20: 15–43, 1980 [DOI] [PubMed] [Google Scholar]
- 11. Cheek TR, O'Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells. Distinct nicotinic and muscarinic patterns. FEBS Lett 247: 429–434, 1989 [DOI] [PubMed] [Google Scholar]
- 12. De Diego AM. Electrophysiological and morphological features underlying neurotransmission efficacy at the splanchnic nerve-chromaffin cell synapse of bovine adrenal medulla. Am J Physiol Cell Physiol 298: C397–C405, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Fox AP, Cahill AL, Currie KP, Grabner C, Harkins AB, Herring B, Hurley JH, Xie Z. N- and P/Q-type Ca2+ channels in adrenal chromaffin cells. Acta Physiol (Oxf) 192: 247–261, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev 86: 1093–1131, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand 177: 437–447, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Gerber SH, Haunstetter A, Kruger C, Kaufmann A, Nobiling R, Haass M. Role of [Na+]i and [Ca2+]i in nicotine-induced norepinephrine release from bovine adrenal chromaffin cells. Am J Physiol Cell Physiol 269: C572–C581, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 128: 1413–1419, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Kajiwara R, Sand O, Kidokoro Y, Barish ME, Iijima T. Functional organization of chromaffin cells and cholinergic synaptic transmission in rat adrenal medulla. Jpn J Physiol 47: 449–464, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Kao CY, Fuhrman FA. Pharmacological studies on tarichatoxin, a potent neurotoxin. J Pharmacol Exp Ther 140: 31–40, 1963 [PubMed] [Google Scholar]
- 20. Kawagoe KT, Zimmerman JB, Wightman RM. Principles of voltammetry and microelectrode surface states. J Neurosci Methods 48: 225–240, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Kennedy RT, Jones SR, Wightman RM. Simultaneous measurement of oxygen and dopamine: coupling of oxygen consumption and neurotransmission. Neuroscience 47: 603–612, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol 307: 199–216, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez MG, Shukla R, Garcia AG, Wakade AR. A dihydropyridine-resistant component in the rat adrenal secretory response to splanchnic nerve stimulation. J Neurochem 58: 2139–2144, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Malhotra RK, Wakade AR. Non-cholinergic component of rat splanchnic nerves predominates at low neuronal activity and is eliminated by naloxone. J Physiol 383: 639–652, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marcantoni A, Carabelli V, Comunanza V, Hoddah H, Carbone E. Calcium channels in chromaffin cells: focus on L and T types. Acta Physiol (Oxf) 192: 233–246, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 141: 171–198, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Michael D, Travis ER, Wightman RM. Color images for fast-scan CV measurements in biological systems. Anal Chem 70: 586A–592A, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Millar J, Stamford JA, Kruk ZL, Wightman RM. Electrochemical, pharmacological and electrophysiological evidence of rapid dopamine release and removal in the rat caudate nucleus following electrical stimulation of the median forebrain bundle. Eur J Pharmacol 109: 341–348, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Miranda-Ferreira R, de Pascual R, Caricati-Neto A, Gandia L, Jurkiewicz A, Garcia AG. Role of the endoplasmic reticulum and mitochondria on quantal catecholamine release from chromaffin cells of control and hypertensive rats. J Pharmacol Exp Ther 329: 231–240, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Misbahuddin M, Isosaki M, Houchi H, Oka M. Muscarinic receptor-mediated increase in cytoplasmic free Ca2+ in isolated bovine adrenal medullary cells. Effects of TMB-8 and phorbol ester TPA. FEBS Lett 190: 25–28, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Moser T, Neher E. Rapid exocytosis in single chromaffin cells recorded from mouse adrenal slices. J Neurosci 17: 2314–2323, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nassar-Gentina V, Pollard HB, Rojas E. Electrical activity in chromaffin cells of intact mouse adrenal gland. Am J Physiol Cell Physiol 254: C675–C683, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20: 389–399, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Neher E, Augustine GJ. Calcium gradients and buffers in bovine chromaffin cells. J Physiol 450: 273–301, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA 79: 6712–6716, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Olofsson J, Nolkrantz K, Ryttsen F, Lambie BA, Weber SG, Orwar O. Single-cell electroporation. Curr Opin Biotechnol 14: 29–34, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Petrovic J, Walsh PL, Thornley KT, Miller CE, Wightman RM. Real-time monitoring of chemical transmission in slices of the murine adrenal gland. Endocrinology 151: 1773–1783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ranganathan S, Kuo TC, McCreery RL. Facile preparation of active glassy carbon electrodes with activated carbon and organic solvents. Anal Chem 71: 3574–3580, 1999 [Google Scholar]
- 39. Role LW, Perlman RL. Both nicotinic and muscarinic receptors mediate catecholamine secretion by isolated guinea-pig chromaffin cells. Neuroscience 10: 979–985, 1983 [DOI] [PubMed] [Google Scholar]
- 40. Ryttsen F, Farre C, Brennan C, Weber SG, Nolkrantz K, Jardemark K, Chiu DT, Orwar O. Characterization of single-cell electroporation by using patch-clamp and fluorescence microscopy. Biophys J 79: 1993–2001, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shukla R, Wakade AR. Functional aspects of calcium channels of splanchnic neurons and chromaffin cells of the rat adrenal medulla. J Neurochem 56: 753–758, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron 20: 1243–1253, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Sontag JM, Sanderson P, Klepper M, Aunis D, Takeda K, Bader MF. Modulation of secretion by dopamine involves decreases in calcium and nicotinic currents in bovine chromaffin cells. J Physiol 427: 495–517, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tomlinson A, Coupland RE. The innervation of the adrenal gland. IV. Innervation of the rat adrenal medulla from birth to old age. A descriptive and quantitative morphometric and biochemical study of the innervation of chromaffin cells and adrenal medullary neurons in Wistar rats. J Anat 169: 209–236, 1990 [PMC free article] [PubMed] [Google Scholar]
- 45. Travis ER, Wightman RM. Spatio-temporal resolution of exocytosis from individual cells. Annu Rev Biophys Biomol Struct 27: 77–103, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Troyer KP, Wightman RM. Temporal separation of vesicle release from vesicle fusion during exocytosis. J Biol Chem 277: 29101–29107, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron 23: 607–615, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol 313: 463–480, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wakade AR, Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience 10: 973–978, 1983 [DOI] [PubMed] [Google Scholar]
- 50. Wakade AR, Wakade TD. Secretion of catecholamines from adrenal gland by a single electrical shock: electronic depolarization of medullary cell membrane. Proc Natl Acad Sci USA 79: 3071–3074, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Watanabe A, Tasaki I, Singer I, Lerman L. Effects of tetrodotoxin on excitability of squid giant axons in sodium-free media. Science 155: 95–97, 1967 [DOI] [PubMed] [Google Scholar]
- 52. Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA 88: 10754–10758, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]