Abstract
Studies suggest that there are two distinct pools of proteinase-activated receptor-2 (PAR2) present in intestinal epithelial cells: an apical pool accessible from the lumen, and a basolateral pool accessible from the interstitial space and blood. Although introduction of PAR2 agonists such as 2-furoyl-LIGRL-O-NH2 (2fAP) to the intestinal lumen can activate PAR2, the presence of accessible apical PAR2 has not been definitively shown. Furthermore, some studies have suggested that basolateral PAR2 responses in the intestinal epithelium are mediated indirectly by neuropeptides released from enteric nerve fibers, rather than by intestinal PAR2 itself. Here we identified accessible pools of both apical and basolateral PAR2 in cultured Caco2-BBe monolayers and in mouse ileum. Activation of basolateral PAR2 transiently increased short-circuit current by activating electrogenic Cl− secretion, promoted dephosphorylation of the actin filament-severing protein, cofilin, and activated the transcription factor, AP-1, whereas apical PAR2 did not. In contrast, both pools of PAR2 activated extracellular signal-regulated kinase 1/2 (ERK1/2) via temporally and mechanistically distinct pathways. Apical PAR2 promoted a rapid, biphasic PLCβ/Ca2+/PKC-dependent ERK1/2 activation, resulting in nuclear localization, whereas basolateral PAR2 promoted delayed ERK1/2 activation which was predominantly restricted to the cytosol, involving both PLCβ/Ca2+ and β-arrestin-dependent pathways. These results suggest that the outcome of PAR2 activation is dependent on the specific receptor pool that is activated, allowing for fine-tuning of the physiological responses to different agonists.
Keywords: arrestin, extracellular signal-regulated kinase 1/2, signal transduction, cofilin
proteinase-activated receptor-2 (PAR2) is a G protein-coupled receptor (GPCR) that is activated via cleavage of its NH2 terminus by serine proteinases including trypsin, mast cell tryptase, kallikreins, membrane-type serine proteinase 1, and the tissue factor VIIa-Xa complex (5, 14, 17, 29, 37, 48). Proteolytic cleavage of the NH2-terminal domain exposes a tethered ligand that binds and activates the receptor itself. PAR2 is reported to trigger a wide variety of cellular responses (e.g., proliferation, chemotaxis, ion transport, epithelial barrier function, and tumor cell metastasis) in a cell type-specific manner (30, 40). In vitro, peptides corresponding to the tethered ligand (SLIGRL/KV-NH2) and synthetic analogs of tethered ligand peptides, such as 2-furoyl-LIGRL-O-NH2 (2fAP) can activate PAR2 and trigger downstream signals in the absence of proteolytic cleavage (2, 30, 43). Previous studies have established that activation of PAR2 can evoke both pro- and antiinflammatory responses in the intestine, including vasodilation, smooth muscle relaxation, cytokine upregulation, immune cell recruitment, and increased nociception and hyperalgesia (7, 8, 12, 23, 31–33).
Here we examined the roles of apical and basolateral PAR2 in intestinal epithelial cells. Studies that used PAR2-specific antibodies or conducted intracolonic administration of fluorescently conjugated 2fAP suggested that intestinal epithelial cells (both in vivo and in various cell culture lines) express PAR2 on both the apical and basolateral surfaces (14, 30, 37). Furthermore, expression of basolateral PAR2 at the perijunctional margin of human colonic mucosa was reported to be increased in patients with Crohn's disease, and its expression correlated with a neutrophil-mediated decrease in transepithelial resistance (12). However, the accessibility of each receptor pool to agonists and the corresponding downstream signaling and physiological responses, to either basolateral or apical receptor activation, have not yet been clarified. Studies suggest that basolateral PAR2 can be activated by mast cell tryptase or proteinases released by recruited leukocytes (14, 33, 50), while apical receptor can be activated by trypsin (either pancreatic or secreted by the enterocytes) or by luminal proteinases released by invading pathogens (12, 18, 28, 33, 34, 37). Many of these studies suggest that inflammatory responses in the intestine can be induced by luminal introduction of PAR2-activating peptides and by serosal exposure to mast cell tryptase. While the specific cellular responses such as ion secretion and changes in transepithelial electrical resistance (RT) have been reported in response to basolaterally administered agonist in cultured cells (19, 41, 45, 51–53), little is known about signaling via apical PAR2. Furthermore, the possibility that there are distinctions in signaling between the apical and basolateral receptor populations has not yet been addressed. This is a particularly pertinent question given the recent evidence that PAR2 is capable of signaling through multiple independent pathways, involving G protein-dependent and β-arrestin-dependent events, leading to a diverse array of physiological responses (44, 54, 55, 57, 58). Here we examine the following: 1) whether apical and basolateral PAR2 are accessible to labeled 2fAP in polarized intestinal epithelial monolayers (Caco2-BBe); 2) whether there are temporal and mechanistic distinctions in apical and basolateral PAR2 signaling; and 3) whether either pool uses β-arrestin-dependent signaling pathways.
MATERIAL AND METHODS
All chemicals were from Sigma unless otherwise stated. Antibodies and final dilutions for Western blot (WB) and immunofluorescence (IF) analysis were as follows: from BD Biosciences, mouse monoclonal anti-cofilin (1:1,000 for WB) and mouse monoclonal anti-zona occludin 1 (ZO-1) (1:500 IF); from Cell Signaling, rabbit anti-phospho (Ser3)-cofilin (1:1,000 for WB); rabbit anti-phospho (Thr202/Tyr204)-ERK (1:1,000 for WB, 1:500 for IF); and mouse anti-ERK1/2 (1:1,000 for WB). Rabbit anti-PAR2 9717 (1:300 for IF), raised against a peptide corresponding to the COOH terminus of mouse PAR2 (SVKTSY) and characterized previously (14), was obtained from the Center for Ulcer Research and Education (University of California Los Angeles). Alexa Fluor 488-labeled phalloidin (1:20 IF) was from Invitrogen. Activating peptides 2fAP (2-furoyl-LIGRL-ornithine-NH2), rhodamine-labeled 2fAP and reverse 2fAP (2-furoyl-LRGIL-ornithine-NH2) were synthesized by Genemed (South San Francisco, CA). Pharmacological inhibitors were used as follows: Ca2+ chelator BAPTA-AM (Sigma) was prepared in dimethyl formamide, diluted to 10 mM in DMSO and used at 30 μM final concentration; PLC inhibitor U73122 (Tocris) was solubilized in chloroform, diluted to 100 mM in DMSO immediately before use at 1 μM final concentration; PKC inhibitor GF109203X (Tocris) was solubilized at 25 mM in DMSO and used at 1 μM final concentration. Dominant-negative β-arrestin-1, corresponding to amino acids 319–418, was a gift from Dr. Robert Lefkowitz (Duke University Medical School, Durham, NC).
Transfection and cell lines.
A human intestinal colon carcinoma cell line, selected for brush border formation (Caco2-BBe), was obtained from Dr. David Lo (University of California, Riverside) and grown in advanced Dulbecco's modified Eagle's medium, 15 mM HEPES, and 10% fetal calf serum. Transient transfections were performed on 95% confluent cells using FuGENE6 (Roche), and experiments were performed between 48 and 72 h after transfection. For other experiments, Caco2-BBe cells stably transfected with a luciferase reporter construct in which luciferase transcription is controlled by three AP-1 binding sites and a TATA box cloned upstream from the luciferase gene (42). To accomplish this, the AP-1-luciferase reporter was cotransfected into Caco2-BBe cells along with pRSVneo. The transfected cells were cultured in medium containing 400 μg/ml G418, and neomycin-resistant clones were pooled to create a cell line stably transfected with the reporter. The transfection and assays of luciferase activity were performed as described previously (39).
PAR2 labeling with rhodamine-2fAP.
Caco2-BBe cells were seeded on permeable supports (Costar Snapwell, 24 well, 0.4 μm pore) at 2 × 104 cells per well and grown for 10–14 days to confluence. After the monolayers attained a resistance of 750 Ω/cm2 (Epithelial Voltohmmeter, WPI, Sarasota, FL), they were exposed to 1 μM rhodamine-labeled 2fAP or RAP at 4°C for 1 h, or at 4°C for 1 h then 37°C for 1 h. The cells were rinsed three times with ice-cold sterile phosphate-buffered saline solution (PBS) to remove unbound label, and then fixed in neutral buffered formalin (Fisher) on ice for 1 h. Fixed cells were labeled with Alexa Fluor 488-phalloidin.
Protein analysis and Western blotting.
Caco2-BBe cells were seeded on permeable supports (Costar Snapwell, 6 well, 0.4 μm pore) at 5 × 104 cells per well, then starved of serum for 24 h. Where indicated, inhibitors were added to both apical and basolateral compartments at the concentrations indicated above for 10 min. Vehicle controls were preincubated with DMSO alone. Monolayers were exposed to 1 μM 2fAP on the apical or basolateral surface for 0–60 min at 37°C, then solubilized in Laemmli sample buffer [62.5 mM Tris·HCl (pH 6.8), 2% (wt/vol) sodium dodecyl sulfate, 50 mM dithiothreitol, and 0.01% (wt/vol) bromophenol blue]. Protein was analyzed by SDS-PAGE (15% for cofilin, 10% for ERK1/2) followed by WB. Blots were imaged using the LICOR Odyssey imaging system, and LICOR software was used to calculate integrated intensities of bands. Images of WB were assembled using Adobe Photoshop 5.0 and Adobe Illustrator CS4. Some gels were spliced to eliminate blank lanes or lanes containing samples unrelated to the figure.
Electrical measurements.
The short-circuit current (Isc) and RT across confluent Caco2-BBe monolayers grown on permeable supports (Costar Snapwell, 6 well, 0.4 μm pore) were measured using a conventional Ussing chamber technique, as described previously (3). Snapwell inserts were incubated in chambers (EM-CSYS-2, Physiologic Instruments) containing Tyrode solution (140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1.25 mM CaCl2, 12 mM glucose, and 10 mM Na-HEPES, pH 7.4) mixed continuously by gas (100% O2) lift. Chambers were maintained at 37°C by heated water jackets. Once the resting Isc stabilized (10 min), the changes in Isc and RT after sequential addition of 1 μM of 2fAP to the luminal and serosal compartments were recorded.
Microscopy.
Confluent Caco2-BBe monolayers were fixed and imaged as described previously (26). Serial sections were taken on Zeiss LSM-510 using ×63 or ×100 objectives. Three-dimensional reconstructions of the images were done using Imaris 6.3 software.
Immunolabeling of PAR2 in ileum.
Four-month-old female wild-type C57Bl6 mice (Jackson Labs) and PAR2−/− mice in a C57Bl6 background (from Dr. Robin Plevin, University of Strathclyde, Glasgow, UK) were used for immunofluorescent labeling. All animal protocols were approved by the Institutional Animal Care and Use Committee of University of California, Riverside. Mice were euthanized, and short segments of mouse ileum were fixed in ice-cold formalin for 5 h, infiltrated with cryoprotectant (30% sucrose in PBS) overnight, and frozen in OTC medium (Triangle Biomedical Sciences) at −35°C. Sections of 10 μm thickness were cut on a cryostat microtome (Microm) and mounted on polylysine-coated glass slides (Fisher Superfrost Plus). Sections were incubated sequentially with blocking solution (1 h), primary antibody (overnight at 4°C), and secondary antibodies conjugated to Alexa Fluor-488 and/or -546 (Molecular Probes). Antibody-9717 against mouse PAR2 proved suitable for use with undenatured sections but not with sections subjected to antigen unmasking with either heat or SDS. When colabeling with mouse monoclonal antibody against ZO-1, endogenous mouse IgG was blocked by preincubation in goat anti-mouse IgG for 1 h. Confocal images were acquired with a Zeiss LSM-510 microscope and assembled using Adobe Photoshop.
Barbed end labeling.
Caco2 monolayers plated on collagen-coated Transwell inserts, or mouse ileum sections were prepared as described above and treated with 2fAP for 5 min. 2fAP administration was restricted to the basolateral surface of Caco2 monolayers as described above. It was not possible to restrict 2fAP administration to the basolateral surface of mouse ileum, so tissue sections were bathed in 2fAP containing solution. Media were immediately exchanged for actin monomer buffer [20 mM HEPES, pH 7.5, 138 mM KCl, 4 mM MgCl2, 3 mM EGTA, 0.2 mg/ml of saponin, 1 mM ATP, 1% BSA, and 0.3 μM rhodamine-labeled G-actin (from Cytoskeleton, Boulder, CO)], and cells were incubated for 1 min to allow incorporation of labeled monomers into cells. Cells/tissue were washed in 0.1 M glycine-PBS for 10 min, then fixed, stained, and imaged as described in Microscopy.
Data and statistical analysis.
All graphs and statistical analysis were performed using Prism or Microsoft Excel 2003. All experiments were performed a minimum of three times. Phosphoprotein levels were normalized to total protein levels before calculation of fold change with respect to untreated controls. One-way ANOVA and two-tailed Tukey t-tests were used to determine statistical significance of and significant differences between values under different conditions.
RESULTS
Expression of accessible apical and basolateral PAR2 in polarized Caco2-BBe monolayers.
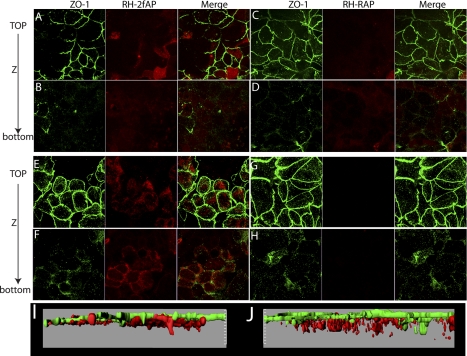
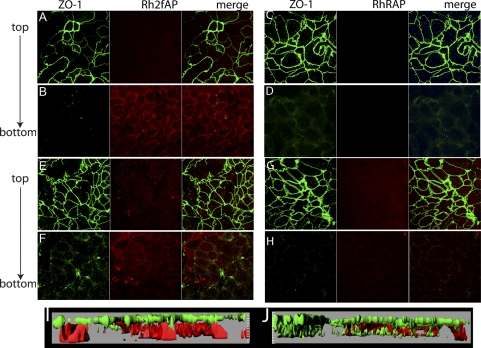
Serine proteinases released by neutrophils and mast cells can activate PAR2 in the intestinal epithelium evoking inflammation, and administration of PAR2 agonists to the serosal side of epithelial monolayers promotes Ca2+ mobilization and ion secretion (15, 19, 33), suggesting that basolateral PAR2 is also accessible to agonists in the interstitial space and blood. Studies have also shown that PAR2 activating peptides administered intracolonically can bind PAR2 and trigger inflammatory responses (7, 8, 30), suggesting apical PAR2 is expressed on the cell surface and accessible to peptide agonist. However, it is formally possible that process of administering the peptide compromised the epithelium, allowing access of peptide agonists to the serosa. Thus, we examined the surface accessibility of apical and basolateral PAR2 in polarized colonic epithelial cell monolayers (Caco2-BBe) grown on permeabilized supports. The integrity of the cell monolayer was confirmed by measuring RT; only monolayers with RT > 750 Ω/cm2 were used in subsequent experiments. Rhodamine-labeled PAR2 agonist peptide (2-furoyl-LIGRL, Rh-2fAP) was administered to either the apical or the basolateral compartment for 1 h, and cells were held at 0°C to prevent receptor trafficking, after which they were fixed and stained with antibody to ZO-1 to mark tight junctions. Confocal fluorescence microscopy revealed that apically administered Rh-2fAP bound the surface of the epithelial monolayers (Fig. 1, A and B), whereas an inactive control peptide (2-furoyl-LRGIL, Rh-RAP) did not, indicating that the signal is not due to nonspecific binding of labeled peptides (Fig. 1, C and D). No significant Rh-2fAP was detected in the lower chamber after apical administration, as determined by measurement of absorbance of media in a microplate reader (absorbance = 546 nm), confirming that no leakage across the monolayers occurred. Three-dimensional reconstruction of Z sections revealed that apical PAR2 was localized in the same plane as the tight junctions, consistent with their presence on the apical surface (Fig. 1I). Since PAR2 agonist peptides promote removal of receptor from the surface by clathrin-mediated receptor endocytosis (22), we next investigated whether the labeled peptide was internalized along with PAR2. After monolayers were warmed to 37°C, Rh-2fAP (Fig. 1, E and F), but not Rh-RAP (Fig. 1, G and H), was observed in vesicles below the region demarcated by the ZO-1 (Fig. 1J). Basolaterally administered Rh-2fAP labeled PAR2 along the basolateral membrane, a pool of receptor not labeled by apically applied agonist (Fig. 2, A–D, I). When cells were warmed to 37°C, bound Rh-2fAP peptide was internalized (Fig. 2, E–H, J). Interestingly, when the cells were warmed to 37°C in the presence of Rh-2fAP, ZO-1 redistributed from the tight junctions to an intracellular pool below the apical plane (Table 1). This effect on ZO-1 was not observed with the inactive control peptide (Fig. 2). We conclude that both apical and basolateral pools of PAR2 exist, and both are expressed on the cell surface as they are both accessible to agonist. Furthermore, while basolateral PAR2 activation promotes redistribution of tight junction proteins from the membrane to the cytoplasm, apical administration does not.
Fig. 1.
Labeling of apical proteinase-activated receptor-2 (PAR2) with rhodamine-2-furoyl-LIGRL-O-NH2 (Rh-2fAP) in polarized Caco2-BBe monolayers. A–D: Caco2-BBe monolayers were labeled with apically administered Rh-2fAP (A and B) or a rhodamine-labeled negative control peptide, 2-furoyl-LRGIL (Rh-RAP) (C and D). Cells were fixed and stained with antibody for tight junction protein zona occludin-1 (ZO-1) to demarcate the apical surface. E–H: to monitor internalization, Rh-2fAP (E and F) and Rh-RAP (G and H)-labeled cells were warmed to 37°C for 1 h. I and J: three-dimensional reconstruction of the microscopy images via Imaris software further shows labeling of apical PAR2 with Rh-2fAP on the apical membrane surface (I) and PAR2-induced internalization at 37°C (J).
Fig. 2.
Labeling of basolateral PAR2 with Rh-2fAP in polarized Caco2-BBe cells. A–D: Caco2-BBe monolayers were labeled with basolaterally applied Rh-2fAP (A and B) and Rh-RAP (C and D) for 1 h at 4°C, fixed, and stained with anti-ZO-1 as described for Fig. 1. E–H: internalization of bound Rh-2fAP was monitored by warming cells, treated with Rh-2fAP (E and F) or Rh-RAP (G and H), to 37°C for 1 h. I and J: three-dimensional reconstruction of images of cells on ice (I) and at 37°C (J), via Imaris software.
Table 1.
Redistribution of zona occludin-1 by PAR2
| Treatment | ZO-1 Redistribution∗ |
|---|---|
| RAP apical | 492 ± 51 |
| 2fAP apical | 364 ± 52 |
| RAP basolateral | 498 ± 92 |
| 2fAP basolateral | 955 ± 100†‡ |
Values are means ± SE. Caco2-Bbe monolayers, treated with rhodamine 2-furoyl-LIGRL-O-NH2 (2fAP) and maintained on ice or warmed to 37°C for 1 h, as described in Figs. 1 and 2, were analyzed by confocal microscopy, and three-dimensional images were reconstructed using Imaris software. The numbers of zona occludin-1 (ZO-1) fluorescent vesicles, >50 nM, were quantified, and the numbers of vesicles located 5 μm below the apical surface are presented. Measurements were taken from 10 images from 2 independent experiments. PAR2, proteinase-activated receptor 2; RAP, 2-furoyl-LRGIL.
Number of ZO-1-positive fluorescent spheres ≥5 μm below apical surface.
Significantly greater than RAP treated, P = 0.01;
significantly greater than 2fAP apical, P = 0.005.
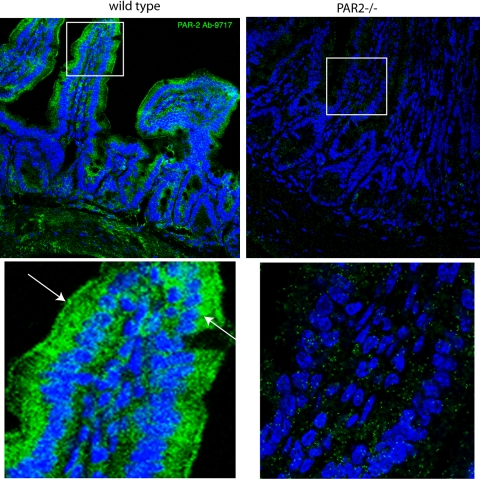
These experiments using cultured cells suggest that PAR2 could be present at both the apical and basolateral surfaces in vivo. To evaluate this possibility, we examined the distribution of PAR2 in mouse terminal ileum by confocal immunofluorescence microscopy. We observed labeling primarily at the brush border of villus enterocytes, and weaker labeling of the lateral membrane and submucosal nerve plexi (Fig. 3, left), confirming previous reports (16). No such labeling was observed in identically processed segments of ileum from PAR2 knockout mice, confirming the specificity of antibody-9717 (Fig. 3, right).
Fig. 3.
Labeling of PAR2 in proximal ileum. Isolated mouse proximal ileum from wild-type mice (left) and PAR2−/− mice (right) was fixed, sectioned, and stained with PAR2 antibody 9717 (green) and TOPRO3 (blue) for detection of cell nuclei, and phalloidin (red) for detection of cell cytoskeleton and microvilli. Bottom: higher magnifications of boxed regions at top. (Arrows indicate apical and basolateral PAR2.)
Basolateral PAR2 activation evokes electrogenic Cl− secretion in Caco2-BBe.
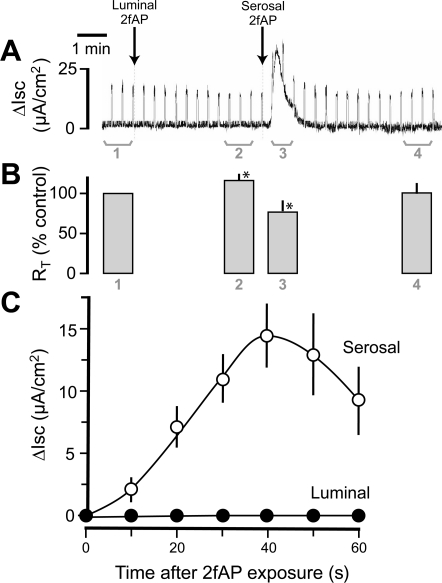
Studies on intestinal mucosa have demonstrated that basolateral, but not apical, activation of PAR2 promotes Cl− secretion in distal colonic mucosa and cultured cell lines (19, 51). We first confirmed this result in Caco2-BBe monolayers. As shown in Fig. 4, serosal addition of 2fAP evoked a transient Isc response, whereas luminal addition did not. The Isc response triggered by basolateral PAR2 peaked after 40 s and averaged 14.5 ± 2.50 μA/cm2 (Fig. 4C). Luminal 2fAP elicited a small but significant increase in RT after a brief (∼1.5 min) delay, whereas serosal 2fAP produced a reduction in RT that was (as expected) temporally correlated with the induced secretory current (Fig. 4B). We conclude that, in contrast to basolateral PAR2, apical PAR2 is not coupled to the Cl− secretory mechanism.
Fig. 4.
Activation of basolateral PAR2 induces an increase in short-circuit current (ΔIsc) and a decrease in transepithelial resistance (RT). Caco2-BBe monolayers were grown to confluence on Snapwell filters with 0.4-μm pores, mounted in a Ussing chamber, and allowed to equilibrate for 30 min. A change in current was evoked every 20 s with a 2-mV bipolar pulse to monitor transepithelial resistance. Short-circuit current was recorded after addition of 2fAP (1 μM), first to the apical (luminal) side, which generated no response, and then to the basolateral (serosal) side. A: representative tracing of the change in short-circuit current over time. B: transepithelial resistance was calculated for the bracketed regions in A, and mean values from 3 independent experiments were determined: 1, resting values; 2, values after apical PAR2 addition; 3, values after basolateral PAR2 addition; 4, values after transient short-circuit changes returned to baseline. *P < 0.05. C: graph depicting mean ± SE ΔIsc over time after either serosal (○) or luminal (●) 2fAP treatment from 3 separate experiments.
Apical and basolateral PAR2 promote temporally and mechanistically distinct pathways of ERK1/2 activation.
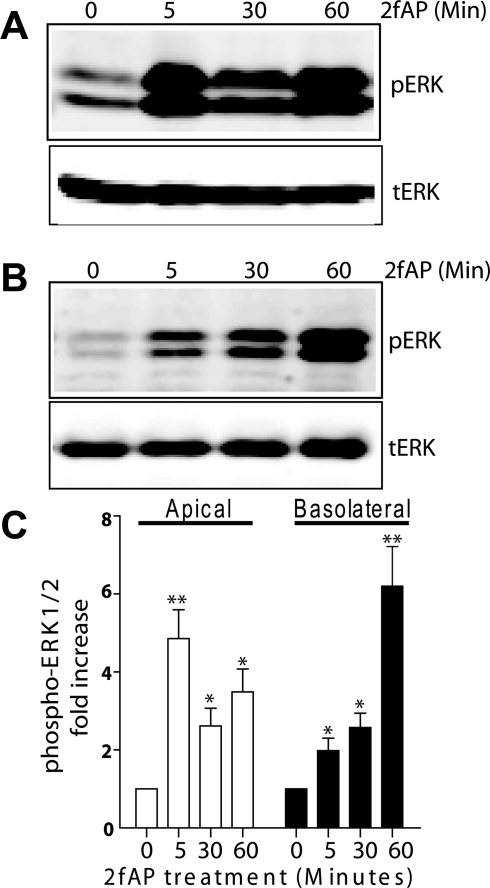
Studies dissecting the molecular mechanism of PAR2-stimulated ion transport in colonic epithelial cells revealed a complex mechanism involving Gαq/Ca2+-dependent activation of selective PKC isoforms, transactivation of EGF receptor (EGFR), ERK1/2 activation leading to prostaglandin E2 (PGE2) release and subsequent activation of the Gαs/cAMP-coupled PGE2 receptor (51–53). EGFR transactivation and subsequent activation of ERK1/2 is a mechanism shared by other Ca2+-dependent Cl− secretory pathways in the intestine (36) and PAR2 is capable of activating ERK1/2 by multiple mechanisms, leading to distinct cellular responses (21, 25, 33, 38, 44). Whether all of these pathways exist sequentially or represent independent mechanisms for promoting Cl− secretion is not clear. We first investigated whether both receptor pools were capable of activating ERK1/2 and whether they utilized the same signaling pathways to do so. After introduction of 2fAP to the apical or basolateral side of Caco2-BBe monolayers grown on permeabilized supports (Fig. 5, A and B, respectively), we observed a temporal difference in the activation of ERK1/2, as determined by Western blot analysis with anti-phospho-ERK. Apical administration of 2fAP promoted a biphasic increase in ERK1/2 phosphorylation, peaking at 5 min (4.86 ± 0.70-fold over baseline), decreasing at 30 min, and peaking again at 60 min (3.49 ± 0.36-fold over baseline). In contrast, basolateral 2fAP promoted a slow and prolonged ERK1/2 activation reaching 6.20 ± 0.96-fold change over baseline by 60 min. We conclude that both receptor pools are capable of signaling to the ERK1/2 pathway.
Fig. 5.
Temporally distinct ERK1/2 activation by apical and basolateral PAR2. A and B: polarized Caco2-BBe monolayers were treated with 2fAP at either the apical (A) or the basolateral (B) surface for 0–60 min. Representative Western blot analyses performed with anti-phospho-ERK1/2 (pERK, top) and anti-total ERK2 (tERK, bottom) and imaged using the LICOR-Odyssey 2 color Infrared Imaging system are shown. Integrated intensities of each band were determined, pERK levels were normalized to tERK, and fold changes in normalized pERK in treated versus untreated samples were determined. C: bar graph depicting mean ± SE fold increase over baseline in normalized pERK. Baseline is defined as normalized pERK from untreated cells. Statistically significant increases in ERK1/2 activation were determined by ANOVA and differences between experimental groups were determined by two-tailed t-tests: *P < 0.04, **P < 0.005, n = 4.
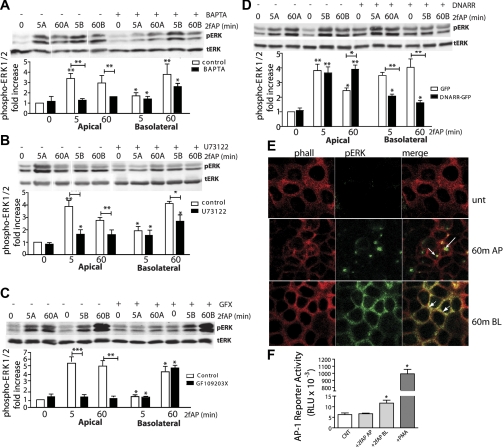
To determine which signaling pathways were utilized by each receptor pool, we repeated ERK1/2 phosphorylation assays in the presence of inhibitors the classical Gαq signaling pathway which results in activation of PLCβ, generation of inositol triphosphate and diacylglycerol, mobilization of intracellular Ca2+, and activation of classical PKC isoforms. We used three different inhibitors of this pathway: PLCβ inhibitor U73122, calcium chelator BAPTA-AM, and the broad-spectrum PKC inhibitor GF109203X. ERK1/2 activation by apical PAR2 was abolished by BAPTA-AM, U73122, and GF109203X. In contrast, basolateral ERK1/2 activation was insensitive to GF109203X inhibition but was inhibited by BAPTA-AM and U73122 by ∼50% (Fig. 6, A–C). Thus, both basolateral and apical PAR2 can activate ERK1/2 through Gαq/Ca2+-mediated pathways, but only the apical PAR2 pathway requires PKC and relies more heavily on the Ca2+ and PLCβ. PAR2 can also promote ERK1/2 activation via G protein-independent pathways involving β-arrestins (21, 38, 47). To examine the contribution of β-arrestins to basolateral and apical PAR2 signaling, cells were transfected with a dominant-negative mutant of β-arrestin-1 consisting of the clathrin-binding domain (amino acids 319–418). Transfection of dominant-negative β-arrestin (DNarr) inhibited ERK1/2 activation by basolaterally, but not apically, administered 2fAP, suggesting that only the basolateral PAR2 pool required input from this pathway (Fig. 6D). A small increase in baseline pERK was also observed in resting DNarr-transfected cells, which may be due to the ability of β-arrestins to promote desensitization and internalization of many GPCRs present in epithelial cells, some of which may contribute to the baseline levels of ERK1/2. We conclude that PAR2 uses multiple mechanisms for ERK1/2 activation in intestinal epithelial cells, and that the apical PAR2 pool requires PLCβ, Ca2+, and PKC, while the basolateral PAR2 requires PLCβ, Ca2+, and β-arrestins.
Fig. 6.
Distinct mechanisms of ERK1/2 activation by apical and basolateral PAR2. A–C: polarized Caco2-BBe monolayers were treated with 2fAP at either the apical or the basolateral surface for 0–60 min after pretreatment with vehicle (DMSO) or with the following inhibitors: Ca2+-chelating agent BAPTA-AM (A), PLCβ inhibitor U73122 (B), or PKC inhibitor GF109203X (C). Top: representative Western blots. Bottom: bar graphs depicting mean ± SE fold change over baseline in normalized phospho-ERK. Apical administration is indicated by “A” and basolateral administration is indicated by “B” after the time for each blot. Baseline is defined as normalized pERK observed in vehicle-pretreated cells without 2fAP addition. D: monolayers were transfected with green fluorescent protein (GFP)-tagged dominant-negative β-arrestin-1 (DNARR-GFP) or GFP alone, and PAR2-stimulated ERK1/2 phosphorylation was determined as described above. Bar graph depicts mean ± SE fold increase in ERK1/2 phosphorylation over baseline, which is defined as that observed in untreated, GFP-transfected cells. For all graphs, statistically significant increases in ERK1/2 phosphorylation over baseline or between bracketed groups (inhibitor vs. control treated) were determined by two-tailed t-tests: *P < 0.05, **P < 0.02, ***P < 0.005, n = 4. Lanes in A and B were spliced to remove blank lanes (A) and duplicate samples (B) as indicated by white spaces. E: monolayers were untreated (Unt) or treated with apically (AP) and basolaterally (BL) administered 2fAP for 60 min, fixed, and stained with anti-pERK and phalloidin. Arrows indicate nuclear pERK. Arrowheads indicate membrane pERK. F: Caco2-BBe cells were cultured until they were confluent monolayers on Snapwell supports in serum-containing medium. Cells were washed with PBS and transferred to serum-free medium for 16 h. The cells were then treated with PBS vehicle (control; CNT), 1 μM 2fAP apical, 1 μM 2fAP basolateral, or 500 nM phorbol 12-myristate 13-acetate (PMA) apical and basolateral. Six hours later, cell extracts were prepared and assayed for luciferase activity, expressed in relative light units (RLU). Each bar is the mean ± SE of results obtained with 4 different Snapwells. *Significantly higher than control, as determined by two-tailed t-tests, P < 0.05.
Previous studies have demonstrated that β-arrestin-dependent ERK1/2 activation by PAR2 and other GPCRs results in sequestration of the activated ERK1/2 at the membrane, where it plays a role in actin cytoskeletal reorganization, rather than transcriptional and proliferative effects associated with nuclear ERK1/2 localization (20, 21, 49, 56). Thus, we predicted that differential activation of ERK1/2 observed with apical versus basolateral PAR2 activation might also be associated with different localization patterns. Caco2-BBe monolayers were treated with 2fAP, administered either apically or basolaterally, for 60 min, fixed, and stained with anti-phospho-ERK1/2 and phalloidin (to visualize cell membrane). Upon apical PAR2 activation, phosphorylated ERK1/2 was predominantly nuclear, while after basolateral addition, phosphorylated ERK1/2 was mostly retained at the membrane and diffusely localized throughout the cytoplasm (Fig. 6E). Thus, consistent with our previous observations, β-arrestin-dependent activation of ERK1/2 was associated with cytosolic/membrane sequestration. To test the possibility that basolateral and apical PAR2 activation might differentially affect transcription, we examined the effect of 2fAP on activation of the transcription factor AP-1 in monolayers of Caco2-BBe, stably transfected with a reporter construct in which luciferase transcription is controlled by a promoter element consisting of three AP-1 binding sites and a TATA box (42). AP-1 is activated by several signaling pathways including ERK1/2 and JNK (46). Interestingly, activation of basolateral but not apical PAR2 resulted in induction of the AP-1-luciferase reporter (Fig. 6F). Thus, the transcriptional effects resulting from ERK1/2 activation are likely to differ depending on whether apical or basolateral PAR2 is activated.
Basolateral PAR2 activation results in activation of cofilin, while apical PAR2 activation does not.
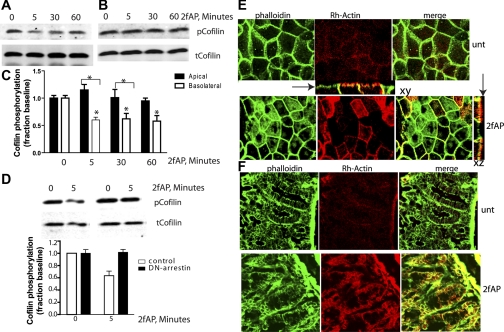
One of the major pathways activated by β-arrestins downstream of PAR2 is the dephosphorylation and activation of the actin filament-severing protein, cofilin (57, 58). Cofilin promotes rapid actin reorganization by severing existing actin filaments and creating free barbed ends to which actin monomers can spontaneously add. Since only basolateral PAR2 required β-arrestins for ERK1/2 activation, we examined whether it also resulted in cofilin dephosphorylation. Consistent with the findings above, basolaterally administered 2fAP induced cofilin dephosphorylation after 5 and 60 min (Fig. 7, A and C) while apical 2fAP did not (Fig. 7, B and C). As was previously demonstrated, PAR2-stimulated cofilin activation was β-arrestin dependent, as transfection of dominant-negative β-arrestin blocked cofilin dephosphorylation in response to basolateral PAR2 activation (Fig. 7D). A major effect of cofilin actin filament-severing activity is the creation of actin free barbed ends, which promotes remodeling of the actin cytoskeleton. Barbed end formation can be monitored in cells by observing incorporation of labeled actin monomers into lightly permeabilized cells after agonist treatment. Actin monomers will not incorporate into stable filaments; thus they can be used to monitor sites of filament severing. Consistent with cofilin activation, we observe that actin monomers incorporate into cells upon basolateral, but not apical, PAR2 activation in both cultured Caco2-BBe monolayers (Fig. 7E). Similar barbed incorporation, colocalizing with the tight junction marker ZO-1, was observed upon 2fAP treatment in isolated intestinal mucosa (Fig. 7F). We conclude that basolateral PAR2 is capable of triggering β-arrestin-dependent and G protein-dependent signals, while apical PAR2 does not trigger β-arrestin-dependent signaling.
Fig. 7.
Basolateral PAR2 promotes cofilin phosphorylation. A and B: Caco2-BBe monolayers were treated as described in Fig. 4, and Western blots were analyzed with anti-phospho-cofilin (pcofilin, top) an anti-total-cofilin (tcofilin, bottom). Representative Western blots from cells activated with basolateral (A) or apical (B) 2fAP are shown. C: fold change over untreated was determined as described for phospho-ERK, and mean ± SE fold change over baseline is depicted in the bar graphs. Statistically significant results between treated and untreated cells are indicated or between apical or basolateral phospho-cofilin levels (*P < 0.05). D: monolayers were transfected with GFP-tagged dominant-negative β-arrestin-1 corresponding to amino acids 319–418, and cofilin dephosphorylation was determined as described above. Samples were run in triplicate, and images were spliced to remove repeated samples. E: Caco-2BBe monolayers were treated with basolateral 2fAP for 5 min, after which cells were lightly permeabilized and free actin barbed ends were labeled with rhodamine-actin monomers (red). Cells were fixed and stained with phalloidin (green) to highlight all cellular actin filaments, and images were collected with a Zeiss LSM510 confocal microscope, ×100 objective. Insets: side views of barbed end labeling at tight junctions are shown. Arrows indicate apical surface. F: intestinal mucosa was treated with or without 2fAP for 5 min, after which rhodamine actin labeling was performed as described in D. Tissue was fixed, frozen sections (10 μm each) were prepared and stained with phalloidin, and images were collected as described above.
DISCUSSION
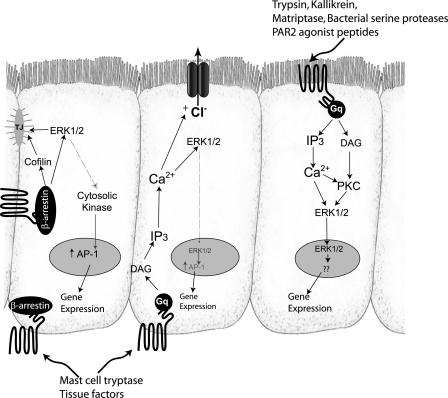
Studies on PAR2 in the intestine have suggested important roles for both apically and basolaterally accessible receptor. For example, luminal introduction of 2fAP to mice colon triggered inflammation, while upon injury and pathogen invasion, mast cells release tryptase to induce inflammation, which presumably involves basolateral and serosal PAR2 (8, 12, 13, 23, 24, 30, 32, 35). The studies described here demonstrate the existence of spatially and temporally distinct signaling pathways in colonic epithelial cells by apical and basolateral pools of PAR2. Both pools of receptor are accessible to agonist activation and promote activation of ERK1/2; however, the mechanisms of ERK1/2 activation differ between apical and basolateral receptors, with only basolateral PAR2 utilizing a β-arrestin-dependent signaling component. Furthermore, only basolateral agonist promoted activation of the actin filament-severing protein, cofilin, and generation of free actin barbed ends, and redistribution of ZO-1 from the membrane, all processes that might be involved in modulation of tight junctions (Fig. 8). The use of different signaling mechanisms by apically and basolaterally exposed PAR2 may allow for a distinct set of cellular events in response to serine proteinases present in the lumen or those released from immune cells present in the serosa at the basolateral surface.
Fig. 8.
Model for PAR2 signaling from apical and basolateral surfaces. Apical and basolateral PAR2 would be accessible to different proteolytic agonists, leading to distinct responses. Activation of both PAR2 pools leads to activation of traditional Gαq (which leads to phosphatidylinositol 4,5-bisphosphate hydrolysis into inositol triphosphate and diacylglycerol), mobilization of intracellular Ca2+, and activation of PKC. ERK1/2 activation by basolateral PAR2 is mediated by both the Gαq-dependent pathway and a β-arrestin-dependent pathway but is independent of PKC; the majority of ERK1/2 activated by this pathway does not translocate to the nucleus. PAR2 activated at the apical surface activates ERK1/2 and requires typical Gαq-dependent signals such as Ca2+ and PKC, but it does not require β-arrestins. Basolateral but not apical PAR2 is capable of promoting AP-1 activation, which may occur indirectly via phosphorylation of a cytoplasmic protein kinase, such as p90Rsk. Transcriptional targets of apical PAR2 have not yet been identified but likely involve nuclear ERK1/2. Basolateral but not apical PAR2 stimulated short-circuit current changes, which have been reported to involve Ca2+, ERK1/2, and transactivation of EGF receptor. Basolateral but not apical PAR2 also activates cofilin via a β-arrestin-dependent-pathway, leading to generation of free actin barbed ends at tight junctions (TJ).
The precise role of epithelial PAR2 in intestinal inflammation is still unclear, and the identification of separate signaling pathways by different pools of receptor is important for the ultimate understanding of PAR2-induced inflammation. Intestinal epithelial cells are the first line of defense against the luminal pathogens, providing a barrier against their invasion of the underlying serosal layer. Factors that affect epithelial barrier function can involve multiple components, including alterations in ion and water secretion, modification of tight junction proteins, and reorganization of actin filaments. Studies have also suggested PAR2 can lower intestinal epithelial integrity by promoting tight junction reorganization, decreasing RT, and increasing Cl− secretion (6–8, 19, 33, 52, 53).
Intestinal Cl− secretion is regulated by a complex interplay of neurocrine, paracrine, endocrine, and inflammatory signals to provide for optimal luminal fluidity, convective mixing, and flushing in defense against pathogenic intruders (4). Transient Cl− secretory responses are triggered by agonists (such as acetylcholine and PAR2) that evoke a mobilization of intracellular Ca2+ (36, 51), whereas sustained Cl− secretion is observed in response to agonists (such as vasoactive intestinal peptide) that promote the accumulation of intracellular cAMP (39). PAR2-induced Cl− secretion was shown here and by others to require activation of basolateral receptors (19, 51), which is somewhat puzzling because this process is reported to involve some of the same Gαq-dependent events elicited by apical receptors. One explanation is that the mechanisms leading to changes in Isc involve interaction of PAR2 with receptors or effectors present only at the basolateral surface. For example, many reports have suggested that intestinal Cl− secretion involves transactivation of EGFR and subsequent activation of ERK1/2, and others have suggested involvement of secreted factors such as PGE2 and substance P (51). Thus, there is likely an additional layer of complexity beyond the differences in Gαq versus β-arrestin-dependent signaling described here, and differences in the response to Gαq-dependent signals downstream of apical and basolateral receptors may explain why apical PAR2 does not promote short-circuit current changes.
In the studies described here, 2fAP added at the basolateral surface led to a mild reduction in transepithelial resistance and redistribution of ZO-1 from the tight junctions, events that might be associated with disruption of barrier function. Only basolateral PAR2 activated the actin filament-severing protein cofilin and promoted actin assembly at tight junctions, processes previously shown to be β-arrestin dependent (57, 58). However, because both apical and basolateral pools of PAR2 are able to promote activation of ERK1/2, and apical PAR2 is a more potent activator of early ERK1/2 phosphorylation, this difference is not due to one pool simply being less accessible to activation. Both cofilin activity and ERK1/2-dependent myosin light-chain kinase (MLCK) phosphorylation, have been shown to be important for redistribution of tight junction proteins. Furthermore β-arrestins have been suggested to mediate PAR2-induced MLCK-phosphorylation (6, 33). The data shown here suggest that activation of basolateral PAR2 might play a greater role in tight junction reorganization (Fig. 8). Thus, it will be interesting to determine whether this pathway is involved in the regulation of epithelial integrity.
Another distinction between the Gαq/Ca2+ and β-arrestin-dependent pathways of ERK1/2 activation by PAR2 is that the Gαq pathway is associated with gene expression and proliferation, while the β-arrestin-dependent pathway is associated with actin reorganization and chemotaxis (21, 25, 26). Indeed, we observe a primarily nuclear ERK1/2 localization in response to apical PAR2 activation, while basolaterally activated ERK1/2 was sequestered in the cytoplasm and at the cell membrane. Apical PAR2 might be exposed to luminal proteases upon damage to the protective mucus layer or released from invading pathogens, and activation of ERK1/2 might generate a transcriptional response appropriate to combating the injury or infection. In contrast, basolateral PAR2 may respond primarily to proteases present in the bloodstream or released from invading immune and mast cells to promote cytoskeletal reorganization and gene expression/proliferative responses. The two pools of receptors may also promote a distinct set of transcriptional responses. In favor of this hypothesis, basolateral but not apical PAR2 activation resulted in activation of the AP-1 transcription factor. Although cytoplasmic/membrane sequestration of ERK1/2 by β-arrestins is typically associated with a decrease in transcription of genes involved in cell cycle progression, some cytosolic targets of ERK1/2, such as the protein kinase p90Rsk, can translocate to the nucleus to elicit proliferative responses such as AP-1 activation (9–11). These responses are also associated with prolonged ERK1/2 activation such as elicited by basolateral receptor (11). β-Arrestin-dependent phosphorylation of p90Rsk by ERK1/2 has been demonstrated for the angiotensin II type 1A receptor (1). Furthermore, the small amount of nuclear ERK1/2 (depicted in Fig. 8, middle cell) may also contribute to the observed activation of AP-1 by phosphorylating Elk-1 (27).
There are also reports of 2fAP applied to the lumen of the intestine increasing bacterial translocation. We have observed that luminal activation of PAR2, as well as activation of apical PAR2 in cultured monolayers, increases bacterial uptake into epithelial cells (C. Lau and K. A. DeFea, unpublished observations), which may be another mechanism by which apical PAR2 contributes to PAR2-induced inflammation. Future studies addressing the possible differences in gene expression profiles in response to apical versus basolateral PAR2 activation as well as elucidation of other downstream effects of apical PAR2 activation are needed to help answer this question.
GRANTS
This work was supported by National Institutes of Health Grant 1R01GM066151 (to K. A. DeFea).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Robin Plevin (University of Strathclyde, Glasgow, UK) and KOWA Pharmaceuticals (Tokyo, Japan) for the PAR2−/− mice. We thank Center for Ulcer Research and Education (University of California Los Angeles) for the antibody to PAR2 (9717) and Dr. David Carter (University of California Riverside Institute for Integrative Genome Biology) for assistance with three-dimensional reconstruction of microscopic images.
REFERENCES
- 1. Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. β-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem 284: 8855–8865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al Ani B, Saifeddine M, Hollenberg MD. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can J Physiol Pharmacol 73: 1203–1207, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bajwa PJ, Alioua A, Lee JW, Straus DS, Toro L, Lytle C. Fenofibrate inhibits intestinal Cl secretion by blocking basolateral KCNQ1 K+ channels. Am J Physiol Gastrointest Liver Physiol 293: G1288–G1299, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Barrett KE. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol Cell Physiol 265: C859–C868, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA 97: 5255–5260, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cenac N, Chin AC, Garcia-Villar R, Salvador-Cartier C, Ferrier L, Vergnolle N, Buret AG, Fioramonti J, Bueno L. PAR2 activation alters colonic paracellular permeability in mice via IFN-γ-dependent and -independent pathways. J Physiol 558: 913–925, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol 161: 1903–1915, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, Coelho AM, Fioramonti J, Bueno L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol 170: 4296, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Chen RH, Abate C, Blenis J. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci USA 90: 10952–10956, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen RH, Chung J, Blenis J. Regulation of pp90rsk phosphorylation and S6 phosphotransferase activity in Swiss 3T3 cells by growth factor, phorbol ester-, and cyclic AMP-mediated signal transduction. Mol Cell Biol 11: 1861–1867, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen RH, Tung R, Abate C, Blenis J. Cytoplasmic to nuclear signal transduction by mitogen-activated protein kinase and 90 kDa ribosomal S6 kinase. Biochem Soc Trans 21: 895–900, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol 181: 5702–5710, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coelho AM, Ossovskaya V, Bunnett NW. Proteinase-activated receptor-2: physiological and pathophysiological roles. Curr Med Chem Cardiovasc Hematol Agents 1: 61–72, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, Payan DG, Bunnett NW. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest 100: 1383–1393, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corvera CU, Dery O, McConalogue K, Gamp P, Thoma M, Al Ani B, Caughey GH, Hollenberg MD, Bunnett NW. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J Physiol 517: 741–756, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cottrell GS, Amadesi S, Pikios S, Camerer E, Willardsen JA, Murphy BR, Caughey GH, Wolters PJ, Coughlin SR, Peterson A, Knecht W, Pothoulakis C, Bunnett NW, Grady EF. Protease-activated receptor 2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis. Gastroenterology 132: 2422–2437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cottrell GS, Coelho AM, Bunnett NW. Protease-activated receptors: the role of cell-surface proteolysis in signalling. Essays Biochem 38: 169–183, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Cottrell GS, Amadesi S, Grady EF, Bunnett NW. Trypsin IV, a novel agonist of protease-activated receptors 2 and 4. J Biol Chem 279: 13532–13539, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Cuffe JE, Bertog M, Velazquez-Rocha S, Dery O, Bunnett N, Korbmacher C. Basolateral PAR-2 receptors mediate KCl secretion and inhibition of Na+ absorption in the mouse distal colon. J Physiol 539: 209–222, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeFea KA. Stop that cell! β-Arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol 69: 535–560, 2007 [DOI] [PubMed] [Google Scholar]
- 21. DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148: 1267–1281, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dery O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and beta-arrestin-1 tagged with green fluorescent protein: beta-arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem 274: 18524–18535, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA 98: 13936–13941, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fyfe M, Bergström M, Aspengren S, Peterson A. PAR-2 activation in intestinal epithelial cells potentiates interleukin-1β-induced chemokine secretion via MAP kinase signaling pathways. Cytokine 31: 358–367, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem 278: 34418–34426, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Ge L, Shenoy SK, Lefkowitz RJ, DeFea K. Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J Biol Chem 279: 55419–55424, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Godeny MD, Sayeski PP. ERK1/2 regulates ANG II-dependent cell proliferation via cytoplasmic activation of RSK2 and nuclear activation of elk1. Am J Physiol Cell Physiol 291: C1308–C1317, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Hansen KK, Sherman PM, Cellars L, Andrade-Gordon P, Pan Z, Baruch A, Wallace JL, Hollenberg MD, Vergnolle N. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci USA 102: 8363–8368, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hollenberg MD, Oikonomopoulou K, Hansen KK, Saifeddine M, Ramachandran R, Diamandis EP. Kallikreins and proteinase-mediated signaling: proteinase-activated receptors (PARs) and the pathophysiology of inflammatory diseases and cancer. Biol Chem 389: 643–651, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Hollenberg MD, Renaux B, Hyun E, Houle S, Vergnolle N, Saifeddine M, Ramachandran R. Derivatized 2-furoyl-LIGRLO-amide, a versatile and selective probe for proteinase-activated receptor 2: binding and visualization. J Pharmacol Exp Ther 326: 453–462, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Hoogerwerf WA, Zou L, Shenoy M, Sun D, Micci MA, Lee-Hellmich H, Xiao SY, Winston JH, Pasricha PJ. The proteinase-activated receptor 2 is involved in nociception. J Neurosci 21: 9036–9042, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hyun E, Andrade-Gordon P, Steinhoff M, Vergnolle N. Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut 57: 1222–1229, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, Coelho AM, Singh P, Grady EF, Perdue M, Bunnett NW. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem 280: 31936–31948, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Kajikawa H, Yoshida N, Katada K, Hirayama F, Handa O, Kokura S, Naito Y, Yoshikawa T. Helicobacter pylori Activates gastric epithelial cells to produce interleukin-8 via protease-activated receptor 2. Digestion 76: 248–255, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Kawabata A, Kuroda R. [PAR (protease-activated receptor) as a novel target for development of gastric mucosal cytoprotective drugs]. Nippon Yakurigaku Zasshi 120: 85P–87P, 2002 [PubMed] [Google Scholar]
- 36. Keely SJ, Uribe JM, Barrett KE. Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. J Biol Chem 273: 27111–27117, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bohm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci USA 94: 8884–8889, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar P, Lau CS, Mathur M, Wang P, DeFea K. Differential effects of β-arrestins on protease-activated-receptor-2 desensitization, internalizaiton and MAPK signaling. Am J Physiol Cell Physiol 293: C346–C367, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Lee JW, Wang P, Kattah MG, Youssef S, Steinman L, DeFea K, Straus DS. Differential regulation of chemokines by IL-17 in colonic epithelial cells. J Immunol 181: 6536–6545, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev 53: 245–282, 2001 [PubMed] [Google Scholar]
- 41. Mall M, Gonska T, Thomas J, Hirtz S, Schreiber R, Kunzelmann K. Activation of ion secretion via proteinase-activated receptor-2 in human colon. Am J Physiol Gastrointest Liver Physiol 282: G200–G210, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Marten NW, Hsiang CH, Yu L, Stollenwerk NS, Straus DS. Functional activity of hepatocyte nuclear factor-1 is specifically decreased in amino acid-limited hepatoma cells. Biochim Biophys Acta 1447: 160–174, 1999 [DOI] [PubMed] [Google Scholar]
- 43. McGuire JJ, Saifeddine M, Triggle CR, Sun K, Hollenberg MD. 2-Furoyl-LIGRLO-amide: a potent and selective proteinase-activated receptor 2 (PAR-2) agonist. J Pharmacol Exp Ther 309: 1124–1131, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Ramachandran R, Mihara K, Mathur M, Rochdi MD, Bouvier M, DeFea K, Hollenberg MD. Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol Pharmacol 76: 791–801, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sato S, Ito Y, Kondo M, Ohashi T, Ito S, Nakayama S, Shimokata K, Kume H. Ion transport regulated by protease-activated receptor 2 in human airway Calu-3 epithelia. Br J Pharmacol 146: 397–407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, Morris DR, Trejo J. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharmacol 67: 78–87, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem 275: 26333–26342, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem 278: 6258, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Tremaine WJ, Brzezinski A, Katz JA, Wolf DC, Fleming TJ, Mordenti J, Strenkoski-Nix LC, Kurth MC. Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC 2059): an open-label pilot study. Aliment Pharmacol Ther 16: 407–413, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Van der Merwe JQ, Hollenberg MD, MacNaughton WK. EGF receptor transactivation and MAP kinase mediate proteinase-activated receptor-2-induced chloride secretion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 294: G441–G451, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Van der Merwe JQ, Moreau F, MacNaughton WK. Protease-activated receptor-2 stimulates intestinal epithelial chloride transport through activation of PLC and selective PKC isoforms. Am J Physiol Gastrointest Liver Physiol 296: G1258–G1266, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Van der Merwe JQ, Ohland CL, Hirota CL, MacNaughton WK. Prostaglandin E2 derived from cyclooxygenases 1 and 2 mediates intestinal epithelial ion transport stimulated by the activation of protease-activated receptor 2. J Pharmacol Exp Ther 329: 747–752, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Wang P, DeFea K. Protease-activated-receptor-2 simultaneously directs beta-arrestin-dependent inhibition and Gαq-dependent activation of PI3K. Biochemistry 45: 9374–9385, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Wang P, Kumar P, Wang C, DeFea K. Differential regulation of class IA phosphoinositide 3-kinase catalytic subunits p110 alpha and beta by protease-activated-receptor 2 and beta-arrestins. Biochem J 428: 221–230, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100: 10782–10787, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem 282: 20634–20646, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Zoudilova M, Min J, Richards HL, Carter D, Huang T, DeFea KA. Beta-arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J Biol Chem 285: 14318–14329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]