Abstract
Stress-induced reproductive dysfunction is a relatively common cause of infertility in women. In response to everyday life stress, some individuals readily develop reproductive dysfunction (i.e., they are stress sensitive), whereas others are more stress resilient. Female cynomolgus monkeys, when exposed to mild combined psychosocial and metabolic stress (change in social environment + 20% reduced calorie diet), can be categorized as stress sensitive (SS; they rapidly become anovulatory in response to stress), medium stress resilient (MSR; they slowly become anovulatory in response to prolonged stress), or highly stress resilient (HSR; they maintain normal menstrual cycles in response to stress). In this study, we examined whether increased sensitivity to stress-induced reproductive dysfunction is associated with elevated adrenal axis activity by measuring 1) the diurnal release of ACTH and cortisol, 2) ACTH and cortisol in response to an acute psychological stress, 3) the percent suppression of cortisol in response to dexamethasone negative feedback, 4) the diurnal release of ACTH and cortisol following exposure to mild psychosocial and metabolic stress, 5) the concentration of cortisol in hair, and 6) adrenal weight. SS monkeys (n = 5) did not differ from MSR (n = 5) or HSR (n = 7) monkeys in any measurement of baseline HPA axis activity or the integrated measurements of chronic HPA axis activity. However, MSR + SS monkeys (n = 10) did secrete more cortisol than HSR monkeys during the daytime hours (1000–1800) following exposure to a novel social environment and reduced diet. We conclude that increased activity of the HPA axis is unlikely to be the primary mechanism causing increased sensitivity to stress-induced reproductive dysfunction.
Keywords: cortisol, corticotropin-releasing hormone, amenorrhea, menstrual cycle
stress-induced amenorrhea is a common cause of infertility (33) thought to be caused by a combined effect of mild psychosocial stress and metabolic stress acting to suppress reproductive function (3, 24, 35). Elevation of hypothalamic-pituitary-adrenal (HPA) axis activity has been suggested as a potential neural mechanism underlying the etiology of stress-induced amenorrhea (sometimes called functional hypothalamic amenorrhea) (3, 4), since cortisol has been shown to be elevated in this patient population (3, 8, 31, 32, 36, 47), although this has not been reported in all studies of this disorder (20). There are several different mechanisms that may underlie elevated activity of the HPA axis in stress-induced amenorrhea. These include elevated basal activity of the HPA axis, elevated response of the HPA axis to stress, and/or decreased sensitivity of the central neural drive to the HPA axis to glucocorticoid negative feedback.
Cortisol secretion in women with stress-induced amenorrhea has been studied over the 24-h day, and some studies show elevations in cortisol compared with women with normal menstrual cycles (3, 8, 32, 47). However, there is controversy regarding the timing of the elevation in cortisol during the diurnal cycle. Some investigators have reported elevated cortisol levels in women with stress-induced amenorrhea during only the nighttime hours (3, 8), during only the daytime hours (47), or during both the day and night (32). It may also be important that these studies have been performed in a clinical setting and may, therefore, not be representative of baseline activity of the HPA axis. Studies examining the response of the HPA axis to acute psychosocial stress in women with stress-induced amenorrhea are lacking. There are several studies that have reported that adrenocorticotropic hormone (ACTH) responses to acute corticotropin-releasing hormone (CRH) challenge are lower in women with stress-induced amenorrhea compared with normal women (31, 36), which may be indicative of more chronic activation of the HPA axis leading to a chronic increase in cortisol negative feedback. However, there have also been reports that CRH responsiveness is no different (8) than in healthy control women. Few studies have examined sensitivity to glucocorticoid negative feedback in stress-induced amenorrhea. However, Lindahl et al. (32) reported that women with stress-induced amenorrhea do not have decreased sensitivity to glucocorticoid negative feedback, as measured by a dexamethasone suppression test (16). Thus, overall, there is some evidence that there is increased activity of the HPA axis in women with stress-induced amenorrhea compared with women who display normal menstrual cycles, but the evidence to date is not strong enough to indicate that increased activation of the HPA axis plays a causal role leading to stress-induced amenorrhea.
Our laboratory has developed a nonhuman primate model of sensitivity to stress-induced amenorrhea in which female cynomolgus macaques that have 28-day menstrual cycles like women are exposed to a combination of mild psychosocial stress plus a mild metabolic stress of reduced diet with or without exercise (5–7, 12, 13, 17, 18, 26, 27, 51). Using this model, previous work has shown that monkeys differ in their sensitivity to stress-induced reproductive dysfunction such that some monkeys readily lose reproductive function with exposure to mild combined stress (i.e., they are “stress sensitive”), whereas others are more stress resilient. In this study, we tested the hypothesis that female cynomolgus monkeys showing increased sensitivity to stress-induced reproductive dysfunction, like women with stress-induced amenorrhea, would have greater activation of the HPA axis by one or more of the specific mechanisms discussed above.
MATERIALS AND METHODS
Animals
Seventeen adult female cynomolgus monkeys (Macaca fascicularis), 7–10 yr of age, were housed in individual stainless steel cages in a temperature-controlled room (23 ± 2°C) with lights on for 12 h/day (0700–1900). Animals were fed two meals a day consisting of four high-protein monkey chow biscuits (no. 5047, jumbo biscuits; Ralston Purina, St. Louis, MO) at 0930 and 1530, and a supplement of one-quarter piece of fresh fruit was provided with each afternoon meal. Animals had their vaginal area swabbed daily with a cotton-tipped swab to detect menstrual bleeding. The first day of menses was designated as day 1 of a menstrual cycle. Food intake was measured at each meal, and weight was measured weekly. All protocols and procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Oregon National Primate Research Center (ONPRC).
Catheterization
Each monkey had a chronic indwelling venous catheter placed in a subclavian vein, using previously described methods (15, 25). At surgery, the tip of the catheter was positioned in the right atrium of the heart, and the free end of the catheter was routed subcutaneously to the back and threaded through a small cutaneous incision between the scapulae. To protect the catheter line, each animal wore a fitted nylon jacket that was connected to a 36-in. flexible metal tether that attached to a swivel mounted on the top of the monkey's cage. Silastic tubing extended from the top of the swivel through a hole in the wall to a sampling syringe and stopcock attached to a saline infusion system in the adjacent room. This catheter system allowed for blood sampling and intravenous infusions without the animal being sedated or disturbed while allowing the monkey free range of motion within the cage. Catheter lines were kept patent with a constant infusion of physiological saline (Baxter Healthcare, Deerfield, IL) containing heparin sodium (4 IU/ml) at a rate of ∼100 ml/day. Weekly inspections of catheter systems and replacement of a sterile dressing covering the catheter exit site were performed under Ketaset (ketamine hydrochloride, 10 mg/kg iv) to keep the exit site aseptic and prevent infection. Ketamine was never administered within 24 h preceding an experimental procedure. Following catheterization surgery, animals were allowed enough of an adaptation period to the jacket/swivel/tether system to resume normal menstrual cyclicity before any experimental procedure was performed.
Overall Study Design
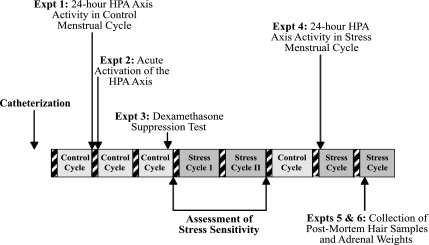
Prior to the studies presented herein, monkeys had been maintained in a stable social environment on their standard diet and had shown at least one normal ovulatory menstrual cycle (control cycle 1) determined by a luteal phase progesterone concentration of 2 ng/ml or higher (indicating ovulation) and normal cycle length (25–38 days) (51, 52). Figure 1 depicts the timeline of the experiments conducted in this study. Before each experiment, each monkey had to show at least one normal menstrual cycle before progressing on to the next planned experiment. For example, if the monkey had a normal menstrual cycle following experiments 1 and 2, then experiment 3 was conducted in the luteal phase of the following cycle. If the animal failed to exhibit a normal cycle following experiments 1 and 2, then experiment 3 was postponed until after the animal showed a normal menstrual cycle. Following completion of experiments 1–3, the animals underwent characterization of sensitivity of the reproductive axis to impairment during a mild combined stress paradigm lasting for a two-cycle duration (stress cycles 1 and 2), as described below. Once each monkey exhibited at least one normal menstrual cycle following exposure to the mild combined stress paradigm (control cycle 2), experiment 4 was conducted on day 1 of the next cycle during exposure to psychosocial and metabolic stress. Data for experiments 5 and 6 were collected at the time the animals were euthanized on day 4 of the next cycle during exposure to psychosocial and metabolic stress.
Fig. 1.
Schematic diagram of the timeline of experiments detailed in this study. Hatched bars indicate menstrual periods. Expt, experiment; HPA, hypothalamic-pituitary-adrenal.
Characterization of Stress Sensitivity of the Reproductive Axis
Monkeys were studied for two menstrual cycles to evaluate the sensitivity of the reproductive axis to stress. Characterization of sensitivity of the reproductive axis to stress was performed after completion of HPA axis function. Following completion of experiments 1–3, animals were exposed to a combined psychosocial and metabolic stress, and the response of the reproductive axis to this stress was determined to categorize each monkey as “highly stress resilient” (HSR), “medium stress resilient” (MSR), or “stress sensitive” (SS) (50–52). On day 1 of the first “stress” menstrual cycle, animals were moved to a new cage in a novel housing room surrounded by unfamiliar monkeys. Simultaneously, they were placed on a moderate diet that had 20% fewer calories than their average daily intake in the preceding cycle. Blood samples (0.6 ml/sample) were taken every other day using sterile techniques to measure reproductive steroid hormone levels. Monkeys that mensed within 25–38 days subsequent to the initiation of stress were moved for a second stress cycle to a different novel room and remained on a reduced-calorie intake diet (51, 52). As established by previous studies (50), animals that showed a suppression of reproductive function in the first stress cycle and did not show menses within 38 days were categorized as SS. Animals that did not show suppression of reproductive function in response to either stress cycle 1 or 2 were categorized as HSR. Some animals had a normal cycle for the first stress cycle but suppressed normal menstrual cyclicity in the second stress cycle. These animals were categorized as MSR.
Experimental Protocols
Activity of the HPA axis in each monkey was assessed in six ways. Baseline activity of the HPA axis over a normal day was evaluated by measuring plasma ACTH and cortisol levels every 2 h for 24 h. Responsiveness of the HPA axis to acute stress in a control, nonstressed cycle was evaluated by measuring ACTH and cortisol responsiveness to an acute, standardized psychological stress. The sensitivity of the HPA axis to negative feedback was evaluated by measuring the ability of dexamethasone to inhibit cortisol release. Diurnal activity of the HPA axis was again measured in response to a mild combined psychosocial and metabolic stress by measuring ACTH and cortisol every 2 h during the first day of stress exposure. Concentration of cortisol in a sample of hair and adrenal weights were evaluated as integrative measurements of HPA axis activity.
Experiment 1: characterization of baseline diurnal ACTH and cortisol secretion.
To measure baseline activity of the HPA axis, plasma ACTH and cortisol levels were measured over a normal 24-h period in the early follicular phase of the menstrual cycle between days 2 and 4 of the cycle. Blood samples (0.4 ml/sample) for ACTH and cortisol were collected into separate syringes via the remote catheter system at 2-h intervals from 0800 until 1000 the following day. Samples to be assayed for cortisol were collected into heparinized syringes and placed in an empty sterile plastic centrifuge tube on ice. Blood samples to be assayed for ACTH were collected into nonheparinized syringes and placed in sterile plastic centrifuge tubes containing 20 μl of 28.8 mg/ml EDTA and kept on ice. Both samples were immediately centrifuged at 3,000 rpm for 15 min at 4°C. Plasma samples were pipetted into plastic O-ring storage vials (for cortisol the vials contained 20 μl of a solution composed of equal volumes of 30% sodium citrate and 1,000 IU/ml sodium heparin to prevent clotting of plasma proteins; for ACTH the vials contained 10 μl of 500 KIU/ml aprotinin to prevent peptide degradation). Samples were stored at −20°C until assays were performed. Red blood cells from cortisol samples only (not containing EDTA) were resuspended in sterile heparinized saline and reinfused through the catheter system. Hematocrit was checked at intervals during the study and remained in the normal physiological range throughout the sampling period.
Experiment 2: HPA axis activation to acute stress.
To characterize the intensity and duration of HPA axis activation in response to a standardized acute stressor, leather “catch gloves” were presented to each monkey in a standardized protocol (25, 42). These gloves are typically used to hand-catch unanesthetized monkeys, and our laboratory has previously reported that this paradigm is a significant psychological stressor, inducing anxiety-related behaviors such as open-mouth threats or vocalizations and elevating plasma ACTH and cortisol (25, 42). This study was performed on the day after completion of experiment 1. Because plasma concentrations of ACTH and cortisol fluctuate over a normal day, experiment 2 was always conducted at 1600. Performing experiment 2 at a consistent time in the afternoon, when ACTH and cortisol levels are usually lower than in the morning hours, faciliatated observation of stress-induced increases in ACTH and cortisol and limited variability due to diurnal fluctuations in hormone secretion. A baseline blood sample for ACTH and cortisol (0.4 ml/sample) was collected via the remote catheter system at 1600. Following the collection of a baseline sample, a person unfamiliar to the monkey (a female who was not the animal's primary caretaker, wearing a red laboratory jacket) entered the room and stood next to the monkey's cage, presenting the leather catch gloves to the monkey at 1615, 1630, and 1645 for a 2-min duration at each entry. The person with the catch gloves on manipulated the lock on the cage, touched the tops and sides of the cage, and pretended to initiate capture of the animal but did not open the cage. Following the last entry of the person wearing catch gloves, the door to the room was shut for the evening. Blood samples for ACTH and cortisol (0.4 ml/sample) were then collected at 1700, 1800, and 1900, using the same procedures detailed for experiment 1.
Experiment 3: dexamethasone suppression of the HPA axis.
To evaluate individual differences in the sensitivity of the HPA axis to glucocorticoid negative feedback, animals were given a dexamethasone suppression test (16) in the luteal phase on day 17 of a menstrual cycle. A baseline blood sample for cortisol (0.6 ml/sample) was collected at 0800, and the synthetic glucocorticoid dexamethasone sodium phosphate (150 μg/kg; Henry Schein, Melville, NY) was administered to each monkey at 1700 that evening (16). Blood samples (0.6 ml/sample) for cortisol and dexamethasone were collected prior to infusion and at 5, 15, 30, and 60 min and at 2, 6, and 15 h after dexamethasone administration to calculate percent suppression of cortisol. Individual clearance rates of dexamethasone in each animal were also calculated.
Experiment 4: characterization of diurnal ACTH and cortisol secretion following exposure to mild combined stress.
To measure 24-h activity of the HPA axis to mild psychosocial and metabolic stress, plasma ACTH and cortisol levels were measured over a 24-h period on day 1 of a menstrual cycle with exposure to mild psychosocial and diet stress. At the beginning of the day, the study animal was moved to a single cage in a novel room at 0930 and placed on a 20% reduced-calorie diet. Blood samples (0.4 ml/sample) for ACTH and cortisol were collected into separate syringes via the remote catheter system at 2-h intervals from 1000 until 1000 the following day, using procedures identical to those in experiment 1. Due to initial concerns regarding the volume of blood collected from these monkeys over the entire experimental period, some animals were only sampled for ACTH and cortisol every 4 h. Mean daytime (1000–1800) and nighttime (2200–0600) ACTH and cortisol values were calculated and compared. Upon completion of the 24-h experimental period, animals remained in the novel room and were returned to a full diet to resume normal menstrual cycles.
Experiment 5: measurement of hair cortisol.
After completion of all physiological experiments, each animal was moved on the 1st day of its final menstrual cycle to a novel room and placed on a 20% reduced-calorie diet. On the 4th day of their cycle, each animal was sedated with ketamine in their home cage, transported to the necropsy suite, and given an overdose of pentobarbital sodium (25 mg/kg iv) for euthanasia. Monkeys were euthanized according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Following administration of pentobarbital sodium, hair was carefully shaved from the nape of the neck between the cisterna magna and scapular bones. Procedures for collection, treatment of hair samples, and measurement of hair cortisol were followed as established by Davenport et al. (21). Briefly, ∼250 mg of hair was washed twice with isopropanol by gentle inversion, dried for 6 days, and ground to a fine powder with a ball mill grinder. Methanol (1.0 ml) was added to ∼50 mg of hair powder, and samples were rotated gently overnight to extract cortisol from the powder. The samples were then centrifuged for 30 s, and 600 μl of the methanol extract was transferred to a new tube. After evaporation of the solvent, the dried extracts were reconstituted with 400 μl of assay buffer and then analyzed in duplicate by enzyme immunoassay, following the manufacturer's instructions (Salimetrics, State College, PA).
Experiment 6: adrenal weights.
Following administration of pentobarbital sodium and collection of hair, monkeys were exsanguinated by severance of the descending aorta. The left ventricle of the heart was cannulated, and the head of each animal was first perfused with 1 liter of saline. Left and right adrenal glands, along with other tissues, were quickly removed during the saline infusion period, weighed, flash-frozen on isopentane, and stored at −20°C for future studies. Immediately following infusion of 1 liter of saline, perfusion continued with 7 liters of 4% paraformaldehyde in 3.8% borate, pH 9.5, after which the brain was removed and processed for other studies.
Hormone Assays
All plasma assays were performed by the ONPRC Endocrine Services Laboratory (ESL). Plasma cortisol and ACTH, as well as estrogen and progesterone, were measured using an Immulite 2000 (Siemens Healthcare Diagnostics, Deerfield, IL), an automatic clinical platform using polystyrene beads coated with hormone-specific antibodies as the solid phase and chemiluminescent quantifications. The ESL core has validated the use of the Immulite 2000 for several hormones in the rhesus monkey, including cortisol, ACTH, estrogen, and progesterone. Validation and comparison between the Immulite ACTH assay and the Nichols Advantage ACTH assay have been published (49). Both assays yield similar results for measuring ACTH in human samples, including linearity (r2 > 0.99), range (10–1,250 pg/ml for Immulite), and consistency in sample values (Pearson's, r = 0.98). Validation of the Immulite cortisol assay was performed by direct comparison of monkey serum samples analyzed coordinately by the Immulite 2000 and a Roche Elecsys 2010 analyzer (also a chemiluminescence-based clinical platform by F. Hoffmann-La Roche, Basel, Switzerland) that had previously been validated for monkey cortisol measurements (6). The comparison involved 109 monkey samples with cortisol levels ranging from 20 to 550 ng/ml. The coefficient of correlation between the two platforms was 0.95. Sequential samples taken over 24 h showed a distinct elevation in cortisol levels in the morning measured by both assay platforms. The validation for estrogen and progesterone was a direct comparison of monkey serum samples analyzed coordinately by an Immulite 2000 and a Roche Elecsys 2010 analyzer that had been validated for monkey steroid measurements (28). The comparison involved 105 monkey samples with estradiol levels ranging from 10 to 400 pg/ml. The coefficient of correlation between the two platforms was 0.93. The progesterone values in 62 monkey samples ranged from 0.2 to 9 ng/ml; the coefficient of correlation between the two platforms was 0.95. The sensitivity of estrogen assays by the Immulite 2000 was 20 pg/ml, and for progesterone it was 0.2 ng/ml. All quality control samples and validations provided by the Immulite 2000 were analyzed each time before hormonal measurements in samples were made. As with many validated clinical platforms, the Immulite 2000 runs three quality control (QC) serum pools daily, and as such, no specific intra-assay QC data are available. The interassay coefficient of variation, reflecting variability in daily QC results over a period of 1.5 yr in which these assays were run, was as follows: ACTH, 7.7%; cortisol, 8.1%; estradiol, 8.5%; progesterone, 9.4%.
For dexamethasone clearance calculations, plasma dexamethasone concentration was assayed with an ELISA assay from Neogen (Lexington, KY). This assay was originally a forensic assay, and thus it was designed to provide a simple positive or negative evaluation of whether dexamethasone was present in the biological fluid of interest. The ESL core prepared dexamethasone standards from crystalline steroid (Steraloids, Wilton, NH) by preparing the steroid in redistilled ethanol (1 mg/ml) and then making serial dilutions to create a five-point standard curve ranging from 0.05 to 100 ng/ml. Standards and 10–20 μl of serum were incubated in individual wells of a 96-well plastic plate for 2 h. Color developed after washing for 30 min, and optical density was measured at 650 nm wavelength. Data were processed using the Softmax analysis program (Molecular Devices, Sunnyvale, CA) in a single assay to provide the data reported. The intra-assay coefficient of variation was 11.8%.
Statistical Analyses
Group differences for ACTH and cortisol concentrations across time (experiments 1, 2, and 4), as well as left and right adrenal weights (experiment 6), were determined by mixed-design repeated-measures ANOVA (repeated-measures design with between-group comparisons). Percent suppression of cortisol due to dexamethasone negative feedback (experiment 3) and concentration of cortisol in hair (experiment 5) were determined by one-way ANOVA with Fisher's least significant difference post hoc analyses. Chi-squared analysis was used to determine differences in distribution of flattened diurnal cortisol patterns between different stress-sensitivity groups in experiment 1. A 24-h cortisol pattern was considered flattened if the peak/nadir ratio was <2, a clinical criteria often used as a gross measurement of abnormal diurnal cortisol activity (39). Differences between groups were considered significant if P ≤ 0.05. Pearson's r correlation analysis was used to evaluate associations between integrated 24-h cortisol area under the curve and concentration of cortisol in hair, adrenal weights, and thymus weights. All statistical analyses were performed with SPSS version 15.0 statistical software (SPSS, Chicago, IL).
RESULTS
Seven animals were categorized as HSR, five animals were categorized as MSR, and five animals were categorized as SS.
Experiment 1: Characterization of Baseline Diurnal ACTH and Cortisol Secretion
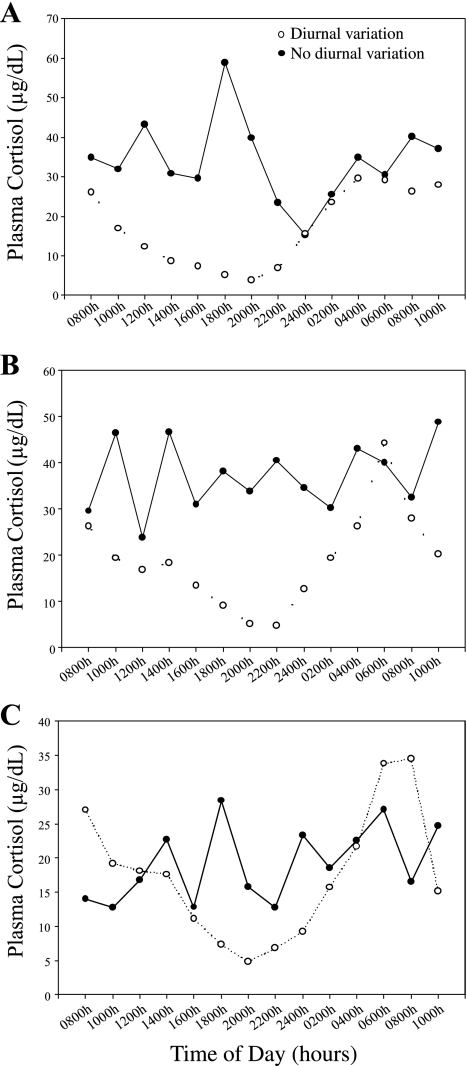
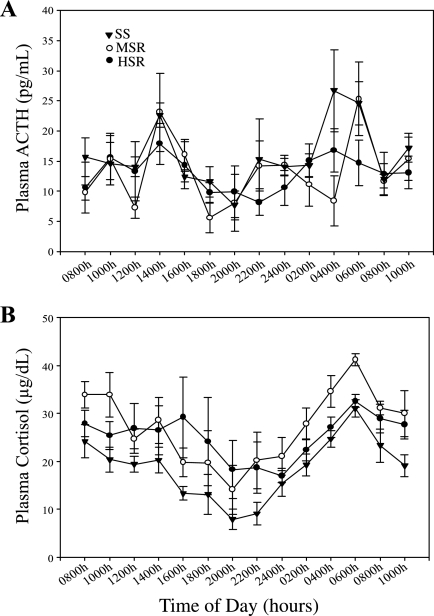
Most animals showed a normal diurnal pattern of plasma cortisol release over the 24-h sampling period, but some failed to show the typical nighttime suppression of HPA axis activity. Normal (peak/nadir ratio >2) and flattened (peak/nadir ratio <2) plasma cortisol release patterns occurred in all three stress sensitivity groups (HSR, MSR, and SS; Fig. 2), and there was no difference in the distribution of flattened cycles between stress sensitivity groups (χ2 = 1.71, P = 0.42). There was no significant interaction of the within-group variable of time of day and the between-group variable of stress sensitivity group for either plasma concentrations of ACTH (F2,12 = 1.08, P = 0.37) or cortisol (F2,10 = 0.76, P = 0.50). There was a significant main effect of time of day for plasma cortisol concentrations (F1,12 = 6.78, P = 0.03), but only a trend for ACTH (F1,12 = 4.46, P = 0.06). However, there was not a main effect of stress sensitivity group on plasma concentrations of ACTH (F2,12 = 0.05, P = 0.95; Fig. 3A) or cortisol (F2,10 = 1.09, P = 0.37; Fig. 3B) over the 24-h period. In addition, there was no difference in the mean ± SE 24-h integrated plasma ACTH (SS: 409.31 ± 49.79 pg·ml−1·24 h−1; MSR: 370.89 ± 44.27 pg·ml−1·24 h−1; HSR: 362.65 ± 41.71 pg·ml−1·24 h−1; P = 0.75) or cortisol (SS: 478.41 ± 35.91 μg·dl−1·24 h−1; MSR: 697.42 ± 79.25 μg·dl−1·24 h−1; HSR: 628.37 ± 78.14 μg·dl−1·24 h−1; P = 0.15) levels between stress sensitivity groups. There were also no group differences in the mean daytime (1000–1800) or nighttime (2200–0600) plasma ACTH or cortisol levels (data not shown).
Fig. 2.
Two representative monkeys each from the highly stress-resilient (HSR; A), medium stress-resilient (MSR; B), and stress-sensitive (SS; C) groups exhibiting either normal diurnal patterns of cortisol secretion (○, dashed line) or patterns of cortisol secretion lacking diurnal variation (●, solid line).
Fig. 3.
Plasma ACTH (A) and plasma cortisol release (B) over a normal day for SS (▾), MSR (○), or HSR (●) monkeys.
An alternative method of analysis was undertaken to categorize animals according to the pattern of HPA axis activity. Using established clinical criteria (39), peak/nadir cortisol levels (0600–2000) were calculated. Ratios greater than two were considered an index of a normal diurnal rhythm in plasma cortisol secretion, whereas peak/nadir ratios less than two were considered an index of hypersecretion of cortisol. There were no differences in the propensity of different groups of monkeys (HSR, MSR, and SS) to show particular patterns of cortisol secretion [normal patterns: 4/12 HSR, 4/12 MSR, 4/12 SS; abnormal patterns: 3/5 HSR, 1/5 MSR, 1/5 SS (χ2 = 1.04, P = 0.60; data not shown)].
Experiment 2: HPA Axis Activation to Acute Stress
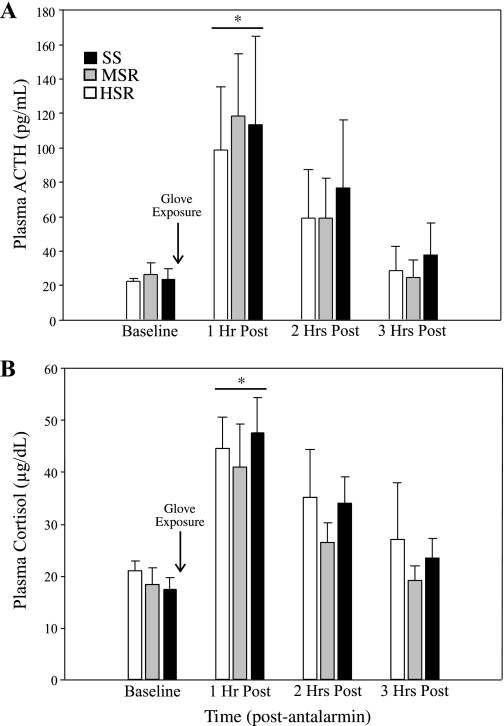
There was not a significant interaction of time by stress sensitivity group for either ACTH (F2,13 = 0.29, P = 0.75) or cortisol (F2,13 = 0.27, P = 0.77) release in response to acute psychological stress. There was a main effect of time for both ACTH (F1,13 = 15.68, P < 0.01) and cortisol (F1,13 = 17.26, P < 0.01) release. The acute stress of leather glove presentation significantly increased ACTH (baseline: 22.46 ± 2.44 pg/ml; 1 h post: 108.23 ± 20.29 pg/ml; t = −4.04, P < 0.01; Fig. 4A) and cortisol (baseline: 18.81 ± 1.38 μg/dl; 1 h post: 44.59 ± 3.83 μg/dl; t = −7.10, P < 0.01; Fig. 4B) for all animals. There was also no difference in the mean ± SE integrated plasma ACTH (SS: 199 ± 79.17 pg·ml−1·3 h−1; MSR: 203.05 ± 63.29 pg·ml−1·3 h−1; HSR: 168.51 ± 62.21 pg·ml−1·3 h−1; P = 0.92) or cortisol (SS: 102.23 ± 10.55 μg·dl−1·3 h−1; MSR: 86.42 ± 13.31 μg·dl−1·3 h−1; HSR: 103.44 ± 16.96 μg·dl−1·3 h−1; P = 0.69) levels between stress sensitivity groups.
Fig. 4.
Plasma ACTH (A) and plasma cortisol release (B) at baseline and at 1, 2, or 3 h following exposure to an acute psychological stress (i.e., glove exposure, as indicated by arrow) in SS (black bars), MSR (gray bars), or HSR (open bars) monkeys. *Significant difference from baseline hormone levels, P < 0.01.
Experiment 3: Dexamethasone Suppression of the HPA Axis
Percent suppression of cortisol was calculated by comparing 0800 baseline levels to plasma samples collected at 0800 the following day, 15 h after dexamethasone treatment. There was no difference in the percent suppression of cortisol with dexamethasone treatment between HSR (69.6 ± 6.4%), MSR (72.2 ± 14.5%), and SS (55.4 ± 15.9%, P = 0.60) monkeys. Clearance rates of dexamethasone were not different between the groups (HSR: 11.19 ± 0.81 ml/min; MSR: 10.88 ± 0.76 ml/min; SS: 11.09 ± 0.92 ml/min; P = 0.97), and there was no correlation between individual clearance rates of dexamethasone and concentration of plasma cortisol collected 15 h postdexamethasone (r = 0.24, P = 0.39; data not shown).
Experiment 4: Characterization of Diurnal ACTH and Cortisol Secretion Following Exposure to Mild Combined Stress
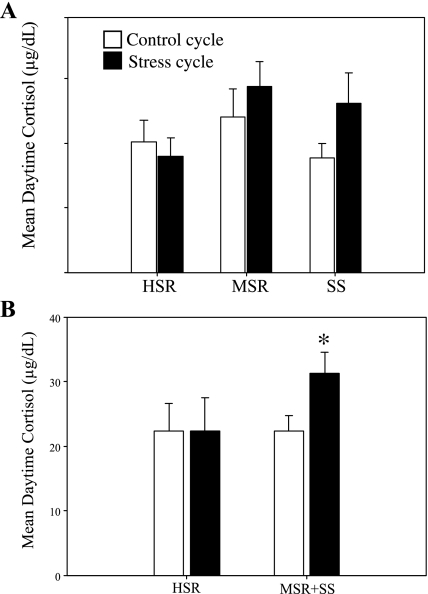
There was a main effect of condition (F1,11 = 5.22, P = 0.04) but not stress sensitivity group (F2,11 = 0.714, P = 0.51) on mean daytime cortisol levels when monkeys were exposed to a mild combined stress. HSR monkeys did not differ between a control, nonstressed cycle and a cycle in which they were exposed to mild combined stress (control: 22.06 ± 5.15 μg/dl; stress: 22.44 ± 5.08 μg/dl; t = −0.06, P = 0.95). In contrast, both MSR (control: 25.60 ± 3.56 μg/dl; stress: 31.44 ± 4.01 μg/dl; t = −1.89, P = 0.12) and SS monkeys (control: 18.35 ± 1.87 μg/dl; stress: 30.95 ± 6.13 μg/dl; t = −2.83, P = 0.06) showed a trend toward increased circulating cortisol when the control and stressed cycles (experiment 1 vs. experiment 4) were compared. This increase was specific to the daytime hours (1000–1800; Fig. 5A). There was no main effect of either condition (F1,10 = 0.00, P = 1.00) or stress sensitivity group (F2,10 = 0.48, P = 0.63) in mean nighttime cortisol levels. Because both MSR and SS groups appeared to have increased cortisol activation in response to the move plus diet, and because both groups showed a physiological effect of stress on the reproductive axis (i.e., both groups showed at least one anovulatory menstrual cycle when stressed), the groups were combined (n = 9) to make a single comparison with the HSR group (n = 5; Fig. 5B). Animals that became anovulatory in response to stress (MSR + SS) had significantly higher mean daytime cortisol on the day they were moved to a new room and put on a mild diet compared with a control menstrual cycle (control: 22.38 ± 2.39 μg/dl; stress: 31.22 ± 3.27 μg/dl; t = −3.26, P = 0.01), whereas the HSR animals that remained ovulatory had similar daytime cortisol levels when exposed to control vs. stress conditions (Fig. 5B). There were no interaction or main effects of condition (control cycle vs. stressed cycle) or stress sensitivity grouping for ACTH concentration over the 24-h experimental period (data not shown).
Fig. 5.
Mean levels of plasma cortisol measured during daytime hours (1000–1800) both in the early follicular phase of a control, nonstressed menstrual cycle (open bars) and in the same phase of a cycle with exposure to mild combined stress (move + diet; closed bars) in HSR, MSR, and SS monkeys (A) and in in HSR vs. animals that became anovulatory in response to mild combined stress (MSR + SS) (B). *Significant difference, P = 0.01.
Experiments 5 and 6: Measurement of Hair Cortisol and Adrenal Weights
The concentration of cortisol found in hair collected at the end of all experimental protocols did not differ between HSR (93.19 ± 9.71 pg/mg), MSR (113.36 ± 10.61 pg/mg), or SS (100.33 ± 20.71 pg/mg) animals (F = 0.68, P = 0.52). Adrenal weights did not differ between stress sensitivity groups with respect to left (HSR: 0.38 ± 0.02 g; MSR: 0.36 ± 0.04 g; SS: 0.41 ± 0.01 g; P = 0.50) or right (HSR: 0.29 ± 0.02 g; MSR: 0.30 ± 0.02 g; SS: 0.34 ± 0.02 g; P = 0.29) adrenal weights. Integrated cortisol levels measured in experiment 1 did not correlate with left adrenal weight (r = −0.01, P = 0.96), right adrenal weight (r = −0.16, P = 0.55), or concentration of cortisol found in hair (r = 0.26, P = 0.35). Likewise, hair cortisol concentrations were not correlated with either left (r = 0.05, P = 0.87) or right (r = 0.10, P = 0.73) adrenal weights. Thymus weight at euthanization also did not correlate with integrated cortisol (r = 0.17,P = 0.60), left adrenal weight (r = −0.16, P = 0.61), or right adrenal weight (r = 0.05, P = 0.87). There were also no correlations with integrated cortisol or hair cortisol concentrations when left and right adrenal weights were combined as a single adrenal weight per animal (data not shown). However, there was a significant correlation between thymus weight and concentration of cortisol in hair (r = −0.59, P = 0.04).
DISCUSSION
In five different physiological measurements designed to characterize baseline activity and response to an acute stress by the HPA axis in control, nonstressed conditions, we did not find any evidence that activity of the HPA axis is associated with sensitivity to stress-induced reproductive dysfunction. However, monkeys that develop stress-induced menstrual cycle disturbances (i.e., MSR and SS monkeys) did secrete more cortisol, but not ACTH, compared with HSR monkeys in a sixth specific condition of acute exposure to mild combined psychosocial and metabolic stress. The increase in cortisol secretion was specific to the daytime when the monkeys could see other novel animals in the room. These findings indicate that increased activation of the HPA axis occurs only in very limited circumstances in animals that are more sensitive to stress-induced reproductive dysfunction compared with more stress-resilient individuals.
Women with stress-induced amenorrhea report more stress in their lives compared with eumenorrheic women (10, 35). Interestingly, we found in this study that SS female monkeys were more stressed by mild psychosocial plus metabolic stress. Thus, SS monkeys were more likely to experience stress. Although SS monkeys did not have elevated baseline cortisol levels, they did show elevated cortisol in response to mild psychosocial plus metabolic stress. Perhaps the same occurs in women who experience stress-induced amenorrhea, and this accounts for the variability that has been reported in cortisol levels in women with stress-induced amenorrhea. Our findings would support the hypothesis that individuals with stress-induced amenorrhea do not have elevated baseline cortisol levels, but they are rather more likely to experience stress and thus have a higher probability of having elevated stress-induced cortisol when studies are performed.
This study utilized a relatively new measurement of integrated chronic HPA axis activity by measuring the concentration of cortisol found in hair (21). Hair cortisol concentrations have been shown to correlate significantly with integrated 24-h urinary cortisol levels (44) and self-reported measurements of perceived stress (29). However, in this study, we did not find a correlation between concentration of cortisol in hair and any measurement of HPA axis activity. It is possible that concentrations of cortisol in biological fluids such as plasma, saliva, or urine, which exhibit diurnal and environmentally induced fluctuations, may show greater variance than cortisol measurements in hair, leading to nonsignificant correlations between hair cortisol and these measurements. Interestingly, we did find a significant inverse correlation between hair cortisol levels and postmortem weight of the thymus gland, consistent with previous data indicating that exposure to chronic stress or high levels of exogenous glucocorticoids can cause thymic atrophy (37). This a posteriori finding provides evidence that hair cortisol is a good integrated measurement of chronic stress load, whereas moment-to-moment variation in ACTH and cortisol secretion may not provide ideal integrated measurements of HPA axis activity.
The majority of the evidence presented herein found that activity of the HPA axis was not associated with sensitivity to stress-induced reproductive dysfunction. This finding does not support the hypothesis that SS monkeys have elevated chronic basal activity of the HPA axis. We had explored this hypothesis in part because we had reported previously that both MSR and SS monkeys have increased CRH gene expression in the caudal paraventricular nucleus (PVN), amygdala, and thalamus compared with HSR monkeys (18). Both glucocorticoids and CRH itself have been shown to be capable of suppressing reproductive axis activity in some circumstances. Glucocorticoids have been shown to suppress activity of the reproductive axis only if the elevated circulating levels are high in concentration and chronic in nature, a finding replicated in rodents (1, 2, 30, 40, 45), monkeys (23, 38, 43), and humans (9, 34). The levels of elevated cortisol concentrations that have been reported in women with stress-induced amenorrhea (3, 8, 31, 32, 36) are only moderately elevated and are not in the high range known to be directly suppressive to activity of the reproductive axis. Moreover, in the one circumstance in which we found differences in cortisol activation to mild psychosocial and metabolic stress in SS + MSR vs. HSR monkeys, this was also a very small physiological difference.
On the other hand, elevated CRH has been shown to more acutely suppress activity of the reproductive axis (25, 41, 53, 54). Interestingly, the adrenal glands and endogenous cortisol are not required for acute CRH-induced suppression of the hypothalamic-pituitary-gonadal axis (54). Furthermore, it has also been reported that short-term ACTH infusions do not interfere with normal pulsatile LH secretion in the macaque (53). Thus, our findings in the present study do not rule out a role for CRH acting as a neurotransmitter rather than a neuroendocrine hormone in causing sensitivity of the reproductive axis to stress. Increased CRH gene expression in the caudal PVN may be acting in a nonneuroendocrine manner to regulate other neurotransmitter systems that mediate function of the reproductive axis, including norepinephrine, dopamine, serotonin, γ-aminobutyric acid, and glutamate (22, 48).
The serotonergic system is at least one neurotransmitter network that appears to show marked physiological differences between female monkeys sensitive and resilient to stress-induced reproductive dysfunction. SS monkeys have suppressed physiological release of serotonin (6), fewer serotonergic cells, and low expression of a number of genes in the serotonin pathway, including FEV1, TPH2, SERT, MAO-A, and MAO-B, in the dorsal raphe nucleus (5, 7). Moreover, treatment with a selective serotonin reuptake inhibitor, citalopram, increases ovarian steroid hormone secretion in SS monkeys (14). The CRH system is a regulator of serotonergic neurons in the dorsal raphe nucleus (19). Thus, it is possible that in SS monkeys elevated CRH may be leading to the suppression of serotonin neurons in the raphe nucleus. In fact, studies in the rat indicate that the caudal PVN does not project to the median eminence to stimulate ACTH and cortisol release in the HPA axis but rather projects to the limbic system and brainstem regions (45). Preliminary data from our laboratory suggest that SS monkeys indeed have greater immunocytochemical staining of CRH fibers in the dorsal raphe nucleus compared with more stress-resilient monkeys (26). Thus, increased activity of CRH neurons in the caudal PVN may be linked to sensitivity of the reproductive axis to stress not through increased activation of the HPA axis but by inhibiting serotonin neuronal activity in the raphe nucleus.
To further explore the link between elevated CRH gene expression and the suppression of reproductive function in SS individuals, our laboratory tested the ability of antalarmin, a specific CRH receptor 1 antagonist, to improve stress-induced reproductive dysfunction as detailed in the companion article to this study (27a). In that study, treatment with intravenously administered antalarmin prior to stress exposure prevented the stress-induced suppression of LH pulse frequency without blocking the stress-induced increase in ACTH and cortisol release in MSR + SS animals, thereby showing that the HPA axis is not involved in mediating sensitivity to stress-induced reproductive dysfunction, although nonneuroendocrine CRH neurons may play a role in this process.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant U54 HD18185 and Grant RR-00163.
DISCLOSURES
S. M. Herod, A. M. Dettmer, M. A. Novak, J. S. Meyer, and J. L. Cameron all reported no biomedical financial interests or potential conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to Jon Reyes, Diana Takahashi, Paul Loprinzi, Amanda Bulechowsky, and the Endocrine Services Laboratory at ONPRC for their technical expertise. We also appreciate the Division of Animal Resources at ONPRC and the University of Pittsburgh for their knowledgeable assistance.
Present address of S. M. Herod: Department of Biology and Chemistry, Azusa Pacific University, Azusa, CA 91702. Present address of A. M. Dettmer: Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA 15213.
REFERENCES
- 1. Baldwin DM. The effect of glucocorticoids on estrogen-dependent luteinizing hormone release in the ovariectoized rat and on gonadotropin secretion in the intact female rat. Endocrinology 105: 120–128, 1979 [DOI] [PubMed] [Google Scholar]
- 2. Baldwin DM, Sawyer CH. Effects of dexamethasone on LH release and ovulation in the cyclic rat. Endocrinology 94: 1397–1403, 1974 [DOI] [PubMed] [Google Scholar]
- 3. Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril 67: 1024–1030, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Berga SL, Loucks-Daniels TL, Adler LJ, Chrousos GP, Cameron JL, Matthews KA, Marcus MD. Cerebrospinal fluid levels of corticotrophin-releasing hormone in women with functional hypothalamic amenorrhea. Am J Obstet Gynecol 182: 776–784, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol 38: 199–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril 83: 148–155, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Bethea CL, Streicher JM, Mirkes S, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience 132: 151–166, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Biller BM, Federoff HJ, Koenig JI, Klibanski A. Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab 70: 311–317, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Boccuzzi G, Angeli A, Bisbocci D, Fonzo D, Gaisano GP, Ceresa F. Effect of synthetic luteinizing hormone releasing hormone (LH-RH) on the release of gonadotropins in Cushing's disease. J Clin Endocrinol Metab 40: 892–895, 1975 [DOI] [PubMed] [Google Scholar]
- 10. Bomba M, Gambera A, Bonini L, Peroni M, Neri F, Scagliola P, Nacinovich R. Endocrine profiles and neuropsychologic correlatese of functional hypothalamic amenorrhea in adolescents. Fertil Steril 87: 876–885, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 91: 1561–1565, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Cameron JL. Reproductive dysfunction in primates, behaviorally induced. In: Encyclopedia of Stress, edited by Fink G. New York: Academic, 2000, p. 366–372 [Google Scholar]
- 13. Cameron JL. Stress and behaviorally induced reproductive dysfunction in primates. Semin Reprod Endocrinol 15: 37–45, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Cameron JL, Bytheway JA, Guay S, Bethea CL, Kerr D, Rockcastle N, Perel JM, Axelson JD. Treatment with a serotonin reuptake inhibitor increases reproductive hormone secretion in stress sensitive monkeys (Abstract No. 192.18). Society for Neuroscience Annual Meeting, San Diego, CA, 2004 [Google Scholar]
- 15. Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta). Endocrinology 128: 1532–1540, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James NM, Kronfol Z, Lohr N, Steiner M, deVigne JP, Young E. A specific laboratory test for the diagnosis of melancholia: standardization, validation, and clinical utility. Arch Gen Psychiatry 38: 15–22, 1981 [DOI] [PubMed] [Google Scholar]
- 17. Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic gonadotrophin-releasing hormone expression in female monkeys with different sensitivity to stress. J Neuroendocrinol 19: 594–604, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Centeno ML, Sanchez RL, Reddy AP, Cameron JL, Bethea CL. Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology 86: 277–288, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci 22: 959–969, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Couzinet B, Young J, Brailly S, Le Bouc Y, Chanson P, Schaison G. Functional hypothalamic amenorrhoea: a partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol (Oxf) 50: 229–235, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147: 255–261, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Dobson H, Ghuman S, Prabhakar S, Smith R. A conceptual model of the influence of stress on female reproduction. Reproduction 125: 151–163, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod 33: 423–431, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril 60: 486–492, 1993 [PubMed] [Google Scholar]
- 25. Helmreich DL, Mattern LG, Cameron JL. Lack of a role of the hypothalamic-pituitary-adrenal axis in fasting-induced suppression of LH secretion in adult male rhesus monkeys (Macaca mulatta). Endocrinology 132: 2427–2437, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Herod SM, Centeno ML, Bethea CL, Cameron JL. Evidence for a non-neuroendocrine role of corticotropin-releasing hormone (CRH) in female cynomolgus monkeys sensitive to stress-induced reproductive dysfunction (Abstract No. 283.3). Program of the 38th Annual Meeting of the Society for Neuroscience, Washington, DC, 2008 [Google Scholar]
- 27. Herod SM, Pohl CR, Cameron JL. Antalarmin prevents the stress-induced suppression of LH pulse frequency without blocking activation of the HPA axis (Program No. 338.4). Program of the Endocrine Society's 90th Annual Meeting, San Francisco, CA, 2008 [Google Scholar]
- 27a. Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab (September 7, 2010). doi:10.1152/ajpendo.00224.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jensen JT, Zelinski MB, Stanley JE, Fanton JW, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception 77: 303–307, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med 30: 103–107, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kamel F, Kubajak CL. Modulation of gonadotropin secretion by corticosterone: interaction with gonadal steroids and mechanism of action. Endocrinology 121: 561–568, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Kondoh Y, Uemura T, Murase M, Yokoi N, Ishikawa M, Hirahara F. A longitudinal study of disturbances of the hypothalamic-pituitary-adrenal axis in women with progestin-negative functional hypothalamic amenorrhea. Fertil Steril 76: 748–752, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Lindahl MS, Olovsson M, Nyberg S, Thorsen K, Olsson T, Poromaa IS. Increased cortisol responsivity to adrenocorticotropic hormone and low plasma levels of interleukin-1 receptor antagonist in women with functional hypothalamic amenorrhea. Fertil Steril 87: 136–142, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Liu JH. Hypothalamic amenorrhea: clinical perspectives, pathophysiology, and management. Am J Obstet Gynecol 163: 1732–1736, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Luton J, Thiebolt P, Valcke J, Mahoudeau JA, Bricaire H. Reversible gonadotropin deficiency in male Cushing's disease. J Clin Endocrinol Metab 45: 488–495, 1977 [DOI] [PubMed] [Google Scholar]
- 35. Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril 76: 310–316, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Meczekalski B, Tonetti A, Monteleone P, Bernardi F, Luisi S, Stomati M, Luisi M, Petraglia F, Genazzani AR. Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotropin-releasing hormone test. Eur J Endocrinol 142: 280–285, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Myers LP, Fan R, Zheng Q, Pruett SB. Sodium methyldithiocarbamate causes thymic atrophy by an indirect mechanism of corticosterone up-regulation. J Immunotoxicol 2: 97–106, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Plant TM. Ontogeny of gonadotropin secretion in the rhesus macaque (Macaca mulatta). In: Neuroendocrine Aspects of Reproduction, edited by Norman RL. New York: Academic, 1983, p. 133–147 [Google Scholar]
- 39. Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing's syndrome. J Clin Endocrinol Metab 83: 2681–2686, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Ringstrom SJ, Schwartz NB. Cortisol suppresses the LH, but not the FSH, response to gonadotropin-releasing hormone after orchidectomy. Endocrinology 116: 472–474, 1985 [DOI] [PubMed] [Google Scholar]
- 41. Rivier C, Rivier J, Vale W. Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science 231: 607–609, 1986 [DOI] [PubMed] [Google Scholar]
- 42. Rogers CJ, Brissette-Storkus CS, Chambers WH, Cameron JL. Acute stress impairs NK cell adhesion and cytotoxicity through CD2, but not LFA-1. J Neuroimmunol 99: 230–241, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Sapolsky RM. Stress-induced suppression of testicular function in the wild baboon: role of glucocorticoids. Endocrinology 116: 2273–2278, 1985 [DOI] [PubMed] [Google Scholar]
- 44. Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 30: 183–191, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205: 260–272, 1982 [DOI] [PubMed] [Google Scholar]
- 46. Smith ER, Johnson J, Weick RF, Levine S, Davidson JM. Inhibition of the reproductive system in immature rats by intracerebral implantation of cortisol. Neuroendocrinology 8: 94–106, 1971 [DOI] [PubMed] [Google Scholar]
- 47. Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic-amenorrhea. J Clin Endocrinol Metab 66: 733–739, 1988 [DOI] [PubMed] [Google Scholar]
- 48. Tilbrook AJ, Turner AI, Clarke IJ. Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress 5: 83–100, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Vogeser M, Engelhardt D, Jacob K. Comparison of two automated adrenocorticotropic hormone assays. Clin Chem 46: 1998–2000, 2000 [PubMed] [Google Scholar]
- 50. Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab 293: E270–E276, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt D, Nosbisch C, Cameron JL. Induction of menstrual cycle disturbances in cynomolgus monkeys during strenuous exercise training: longitudinal changes in reproductive hormones and menstrual cyclicity. Endocrinology 142: 2381–2389, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab 86: 5184–5193, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Xiao EN, Ferin M. The inhibitory action of corticotropin-releasing hormone on gonadotropin secretion in the ovariectomized rhesus monkey is not mediated by adrenocorticotropic hormone. Biol Reprod 38: 763–767, 1988 [DOI] [PubMed] [Google Scholar]
- 54. Xiao E, Luckhaus J, Niemann W, Ferin M. Acute inhibition of gonadotropin secretion by corticotropin-releasing hormone in the primate: are the adrenal glands involved? Endocrinology 124: 1632–1637, 1989 [DOI] [PubMed] [Google Scholar]