Abstract
Type 2 diabetes (T2DM) is characterized by hyperglycemia, dyslipidemia, and increased inflammation. Previously, we showed that high glucose (HG) induces Toll-like receptor (TLR) expression, activity, and inflammation via NF-κB followed by cytokine release in vitro and in vivo. Here, we determined how HG-induced inflammation is affected by free fatty acids (FFA) in human monocytes. THP-1 monocytic cells, CD14+ human monocytes, and transiently transfected HEK293 cells were exposed to various FFA (0–500 μM) and glucose (5–20 mM) for evaluation of TLR2, TLR4, NF-κB, IL-1β, monocyte chemoattractant protein-1 (MCP-1), and superoxide release. In THP-1 cells, palmitate increased cellular TLR2 and TLR4 expression, generated reactive oxygen species (ROS), and increased NF-κB activity, IL-1β, and MCP-1 release in a dose- and time-dependent manner. Similar data were observed with stearate and FFA mixture but not with oleate. Conversely, NADPH oxidase inhibitor treatment repressed glucose- and palmitate-stimulated ROS generation and NF-κB activity and decreased IL-1β and MCP-1 expression. Silencing TLR2, TLR4, and p47phox with small inhibitory RNAs (siRNAs) significantly reduced superoxide release, NF-κB activity, IL-1β, and MCP-1 secretion in HG and palmitate-treated THP-1 cells. Moreover, data from transient transfection experiments suggest that TLR6 is required for TLR2 and MD2 for TLR4 to augment inflammation in FFA- and glucose-exposed cells. These findings were confirmed with human monocytes. We conclude that FFA exacerbates HG-induced TLR expression and activity in monocytic cells with excess superoxide release, enhanced NF-κB activity, and induced proinflammatory factor release.
Keywords: palmitate, hyperglycemia, Toll-like receptor 2, Toll-like receptor 4
the levels of plasma free fatty acids (FFA) and glucose are elevated in obesity and type 2 diabetes (T2DM) and are implicated in the excess cardiovascular disease (CVD) risk associated with diabetes (15). Cell culture and animal studies have established chronic inflammation in the development and progression of insulin resistance (IR) and T2DM (29). In parallel, epidemiologic studies showed elevated plasma levels of C-reactive protein (CRP), serum amyloid A, cytokines, and chemokines as potential mediators of inflammation and predictors of CVD in T2DM patients (28). Monocytes and macrophages are pivotal cells orchestrating the development of IR and CVD (4). They are closely linked to the chronic inflammatory processes that underline IR, T2DM, and atherosclerosis (27). Proinflammatory factors derived from them have deleterious effects in the development and progression of IR and diabetes (27). Thus the mechanisms by which these metabolic abnormalities (dyslipidemia and hyperglycemia) contribute to systemic inflammation are essential for understanding of the pathophysiology of IR, T2DM, and CVD.
Activation of the innate immune system via Toll-like receptors (TLRs) is implicated in a plethora of inflammatory diseases (8, 33, 36, 41). TLRs are evolutionarily conserved pattern recognition receptors expressed on several cell types including monocytes (36). Among the TLRs, TLR2 and TLR4 play a critical role in the pathogenesis of IR, diabetes, and atherosclerosis in both clinical and experimental conditions (8, 33, 41). Shi et al. (33) demonstrated TLR4 as a molecular link between FFA, inflammation, and the innate immune system. We recently showed (9, 10) that hyperglycemia induces TLR2/4 in human monocytes with concomitant inflammation via NF-κB activation and further provided proof of concept using monocytes from T2DM patients. Studies by Schwartz et al. (31), Coll et al. (6), and others (3) have indicated that increased concentrations of saturated fatty acids (SFA) lead to the activation of TLR2 and TLR4, potentially inducing inflammation. Therefore, elevated plasma FFA and glucose concentrations may facilitate the initiation and progression of IR and T2DM (5, 34) through TLR and contribute to the development of CVD (24). Thus it is important to address the combined effects of FFA and glucose on TLR-mediated inflammation to elucidate the mechanisms of chronic inflammation seen in IR, T2DM, and CVD. We now describe the novel finding that FFA in the presence of high glucose (HG) exacerbate TLR2 and TLR4 expression, which results in NADPH oxidase-dependent superoxide (O2−) production in human monocytes, and that this sequence of events is required for the inflammatory response to FFA + HG in monocytic cells. Collectively, our findings suggest that FFA and HG in the systemic circulation of obese and T2DM patients may cause excessive TLR activation resulting in NADPH oxidase-dependent proinflammatory response via NF-κB.
MATERIALS AND METHODS
Reagents.
The THP-1 human monocytic cell line was obtained from American Type Culture Collection. d-Glucose and mannitol were from Sigma. Apocynin (15 and 30 μM) and diphenyleneiodonium chloride (DPI; 5 and 10 μM) were from Calbiochem. Purified LPS was obtained from List Biological Laboratory. Pam3CSK4 (TLR2 ligand) was purchased from Invivogen. Macrophage-activating lipopeptide-2 (MALP-2) was from Alexis. Mouse pDisplay-HA-TLR4, 2, 1, and 6 were obtained from Lynn Hajjar (University of Washington). NF-κB(2x)-luciferase reporter construct was provided by Frank Mercurio (Signal Pharmaceuticals). pRSV-β-galactosidase plasmid was from Jongdae Lee (Univ. of California, San Diego). Anti-human TLR2 and TLR4 antibodies and isotype-matched IgG controls were obtained from eBioscience. Prevalidated p47phox (NM_000265), TLR2 (NM_003264), and TLR4 (NM_138554) small inhibitory RNAs (siRNAs) and siPORT amine reagent were purchased from Ambion. The concentrations of the various inhibitors and siRNAs used in the present study were reported previously (9, 10).
Fatty acid preparation.
Palmitate, stearate, and oleate fatty acids were obtained from NuChek. Low-endotoxin bovine serum albumin (BSA, FFA free) was purchased from Sigma. FFA were dissolved in 0.1 M NaOH at 70°C and then complexed with 10% BSA at 55°C for 10 min such that a final FFA concentration of 500 μM was achieved as described previously (7, 23). After filtration, the concentration of albumin-bound FFA was measured with the NEFA-C kit from Wako. Stock solutions of 5 mM FFA with 10% BSA and 10% BSA control solutions were prepared fresh before experiments. The mixtures of FFA used in some confirmatory experiments were obtained from NuChek (3). FFA-BSA complexes (molar ratio 2.4:1) and all the reagents were tested for LPS contamination with the Limulus amebocyte lysate (LAL) assay, and the average endotoxin level was <100 EU/ml consistently in all the experiments, as this low LPS concentration does not interfere with TLR2/4 measurement (9, 10).
Cell culture and treatments.
THP-1 cells were subcultured in endotoxin-free RPMI with 5.5 mM glucose as described previously (10). After 2 days in culture, cells (1 × 106 cells/ml) were exposed to FFA-BSA (10–500 μM) in the presence or absence of glucose (5–15 mM) as indicated, with BSA alone serving as control. LPS (170 ng/ml), Pam3CSK4 (170 ng/ml), and MALP-2 (1 ng/ml) were used as positive controls with polymyxin B (10 μg/ml) pretreatment in all experiments as described previously (9, 10). In addition, cell viability was determined by the Trypan blue exclusion method and was >92% in all experiments. In pharmacological inhibitor studies, cells were pretreated for 2 h with indicated agents, followed by 24 h of FFA-BSA treatment with and without HG (15 mM) (10). After treatments, cells were collected and RNA was isolated for RT-PCR. Conditioned medium was used for ELISA assays. Human monocytes (CD14+) were isolated from blood obtained from three healthy volunteers per experiment, to minimize variations in the data collected, as described previously (9). All human protocols were approved by the University of California, Davis Institutional Review Board, and informed consent was obtained from study subjects.
Fluorescence-activated cell sorter analysis of TLR2 and TLR4.
TLR2 and 4 expressions were determined by flow cytometry as described previously (9, 10). Briefly, after treatment with FFA-BSA and HG, cells were incubated with anti-human TLR2/4 antibodies or IgG isotype controls and were analyzed with a BD FACS Array Bioanalyzer. Results are expressed as mean fluorescence intensity (MFI)/105 cells. The intra- and interassay coefficients of variation (CVs) were determined to be <10%.
RNA extraction and RT-PCR.
RNA was isolated from the cells with TRI reagent (Invitrogen, Carlsbad, CA). RT-PCR was performed with TLR2, TLR4, p47phox, and 18S RNA primer probe sets purchased from SA Bioscience. Data are presented as fold induction of transcripts for TLR gene normalized to 18S in cells treated with FFA+HG (9, 10).
Enzyme-linked immunosorbent assay.
IL-1β and monocyte chemoattractant protein-1 (MCP-1) were measured in the conditioned media by enzyme-linked immunosorbent assay (ELISA) (R&D Systems), as reported previously (10). The intra- and interassay CVs were between 7% and 10% for both assays.
siRNA transfection.
All assays were performed with THP-1 cells as described previously (10), with suitable vehicle and scrambled siRNA controls and subsequently treated with FFA-BSA + HG (15 mM) for 24 h. Transfection rates of 70% of cells were accepted for all the experiments. Knockdown efficiency of the siRNAs is indicated via 1) flow cytometric analysis of TLR2 and TLR4, 2) real-time PCR of the target gene, and 3) measurement of the end product (cytokines) of the target gene.
NF-κB transcription factor activity assay.
NF-κB p65 DNA binding activity in the nuclear extracts of FFA-BSA+HG-treated cells were determined with the nonradioactive TransAM transcription factor assay, as described previously (9, 10). The intra- and interassay CVs for the assay were <10%.
Luciferase reporter gene assays for TLR2 and TLR4.
Assays were performed as described previously (10, 22). Briefly, 293T cells (which lack TLR2/4 receptors) were cotransfected with TLR4 and MD2, or TLR2 and TLR1/TLR6 expression plasmids, a luciferase plasmid containing NF-κB(2x)-binding site and a β-galactosidase plasmid, and corresponding empty vectors as controls with SuperFect transfection reagent (Qiagen), as described previously (10, 22). Transfected cells were treated with FFA-BSA in HG or synthetic TLR ligands for 18 h before lysis. Luciferase and β-galactosidase enzyme activities were determined (10, 22).
Superoxide assay.
O2− production was measured by the superoxide dismutase (SOD)-inhibitable reduction of cytochrome c, as described previously (38). Results were expressed as nanomoles per minute per milligram of protein (38).
Statistical analysis.
Results of the experimental studies are reported as the means ± SD of four separate experiments. Differences were analyzed by ANOVA with appropriate post hoc analyses. A probability value of P < 0.05 was considered significant. All statistical analyses were performed with GraphPad Prism Software.
RESULTS
TRL2 and TLR4 mRNA and protein expression are significantly increased in human monocytic cells with FFA-BSA+HG.
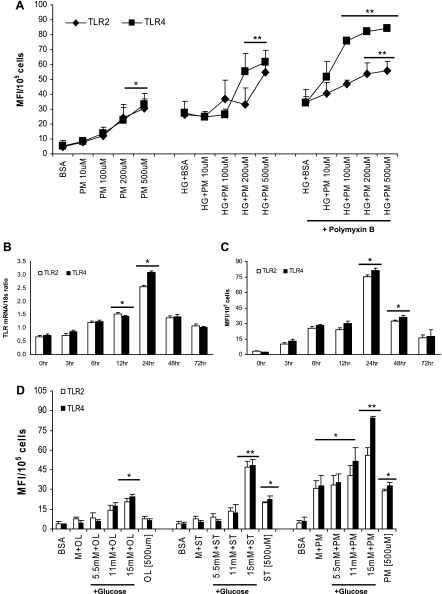
To extend and add to our previous observation that incubation of monocytic cells with HG for 24 h induces TLR2 and TLR4 expression and functional activity (10), we first examined the effect of increasing palmitate-BSA (PM-B; 10–500 μM) + HG (15 mM) concentrations on both TLR2 and TLR4 surface expression in monocytic cells. PM-B+HG significantly increased both TLR2 and TLR4 in a dose-dependent manner, with maximum response observed at 500 μM PM-B (Fig. 1A) compared with BSA- or HG+BSA-treated cells. Analysis of the time-response effects of 500 μM PM-B in the presence of HG using RT-PCR showed that both TLR2 and TLR4 mRNA levels were significantly increased by PM-B+HG (5- to 6-fold, P < 0.001) after 24 h compared with control cells and returned to basal levels by 72 h (Fig. 1B). Next, we examined cell surface expression of TLR2 and TLR4, using flow cytometry in monocytic cells. TLR2 and TLR4 protein levels corroborated mRNA levels with PM-B+HG treatment (Fig. 1C). Because of the maximal increase in TLR2 and TLR4 expression occurring at 24 h of incubation with 500 μM PM-B+HG, all subsequent experiments were conducted for this duration and with this concentration. The present cell culture studies are based on concentrations of PM (500 μM) found in diabetes patients, because exaggerated and prolonged postprandial hyperlipidemia is an important characteristic of diabetic dyslipidemia, in addition to persistent hyperglycemia, reinforcing the clinical relevance of these experiments. Secreted levels of IL-1β (PM-B+HG 13 ± 3 vs. PM-B 6 ± 2.5 pg/ml; P < 0.05) and MCP-1 (PM-B+HG 122 ± 13 vs. PM-B 41 ± 6 pg/ml; P < 0.01) in the conditioned media coincided with maximal TLR2 and TLR4 expression in PM-B+HG-exposed cells.
Fig. 1.
A: dose response of palmitate (PM)-bovine serum albumin (BSA) in high glucose (HG). Toll-like receptor (TLR)2 and TLR4 surface protein expression was measured in THP-1 cells exposed to increasing PM-BSA concentrations in 15 mM glucose for 24 h by flow cytometry as described in materials and methods. Values are expressed as mean fluorescence intensity units (MFI)/105 cells. *P < 0.005 vs. BSA control; **P < 0.05 vs. HG+BSA. B: time course of PM-BSA in HG. TLR2 and TLR4 mRNA expression ratios in THP-1 cells exposed to PM-BSA with 15 mM glucose and treated for indicated times were determined by RT-PCR as described in materials and methods. 18S mRNA was used as the housekeeping gene. Values are expressed as mean ± SD ratio. *P < 0.01 vs. 0 h. C: time course of PM-BSA in HG. TLR2 and TLR4 surface expression in THP-1 cells exposed to PM-BSA with 15 mM glucose and treated for indicated times were determined by flow cytometry as described in materials and methods. Values are means ± SD. *P < 0.05 vs. 0 h. D: dose response of glucose with 3 different free fatty acid (FFA)-BSA complexes. TLR2 and TLR4 surface protein expression was measured in THP-1 cells exposed to 500 μM FFA-BSA complexes with increasing glucose concentrations for 24 h by flow cytometry as described in materials and methods. Values are means ± SD. *P < 0.05 vs. BSA control or mannitol (9.5 mM); **P < 0.05 vs. 5.5 mM+ST/PM OL, oleate; ST, stearate.
In parallel, we examined the effect of increasing glucose concentration, using 500 μM FFA-BSA. Significant additive increase in TLR2 and TLR4 expression was observed at 15 mM glucose (Fig. 1D) with stearate and PM-B. Oleate-BSA did not show appreciable effects. Therefore, we chose to use a 15 mM glucose concentration in all the experiments with PM-B because this closely relates to the physiological levels of glucose and FFA in controlled diabetic patients. Addition of mannitol (9.5 mM) to PM-B had no enhancing response, ruling out osmotic effects.
Because FFA are soluble in aqueous solutions only when coupled to BSA (18), we used a BSA-alone control in our experiments and observed that this did not result in the induction of TLR2 or TLR4 expression, confirming the specificity of the response. Furthermore, to rule out the contaminating endotoxin (ligand for TLR4), we used two methods: 1) pretreatment of cells with polymyxin B (as shown in Fig. 1A, this pretreatment had no effect on the TLR response) and 2) consistently measuring endotoxin concentrations of all the reagents used and eliminating reagents with >100 EU/ml from further use.
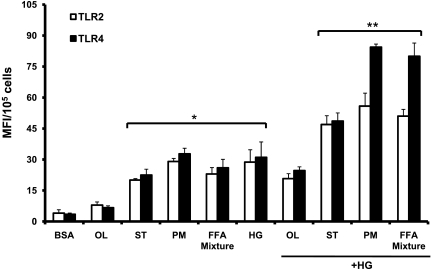
Given that 88% of circulating FFA comprise oleate (C18:1), palmitate (C16:0), and stearate (C18:0) at a ratio of 1.6:1:0.5 in humans (16), we examined their inflammatory effects in the presence of HG. Stearate-BSA and FFA mixture-BSA significantly induced TLR2 and TLR4 expression, and this was further enhanced in the presence of HG in THP-1 cells (Fig. 2) compared with BSA- or HG-treated cells. In contrast, effects of oleate on TLR2/4 were not significant, in line with earlier studies (3) (Fig. 2).
Fig. 2.
Effect of different FFA-BSA complexes in HG. TLR2 and TLR4 surface protein expression was measured in THP-1 cells exposed to 500 μM FFA-BSA complexes with HG (15 mM) for 24 h by flow cytometry as described in materials and methods. Values are means ± SD. *P < 0.001 vs. BSA control; **P < 0.05 vs. HG.
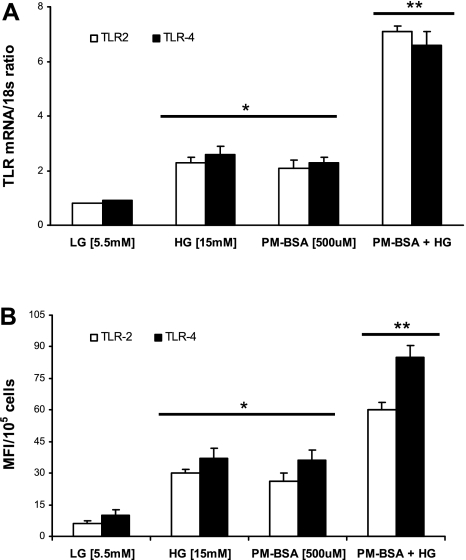
Additionally, we confirmed the THP-1 monocytic TLR2 and TLR4 activation with PM-B+HG, using primary human monocytes (n = 12 healthy volunteers) and RT-PCR and flow cytometry. TLR2 mRNA (3.4-fold ↑, P < 0.05) and TLR4 mRNA (2-fold ↑, P < 0.05) were significantly increased with PM-B+HG compared with HG- or PM-treated cells (Fig. 3A). Similarly, TLR2 and TLR4 protein expression were also enhanced with PM-B+HG compared with HG- or PM-treated cells (Fig. 3B).
Fig. 3.
Effect of PM-BSA in HG on human monocytes. A: TLR2 and TLR4 mRNA expression ratios in human monocytes exposed to PM-BSA with 15 mM glucose were determined by RT-PCR as described in materials and methods. 18S mRNA was used as the housekeeping gene. Values are expressed as mean ± SD ratio. *P < 0.005, **P < 0.05 vs. HG or PM-BSA. B: TLR2 and TLR4 surface protein expression was measured in human monocytes exposed to 500 μM PM-BSA in 15 mM glucose for 24 h by flow cytometry as described in materials and methods. Values are expressed as means ± SD. *P < 0.05 vs. low glucose (LG); **P < 0.05 vs. HG or PM+BSA.
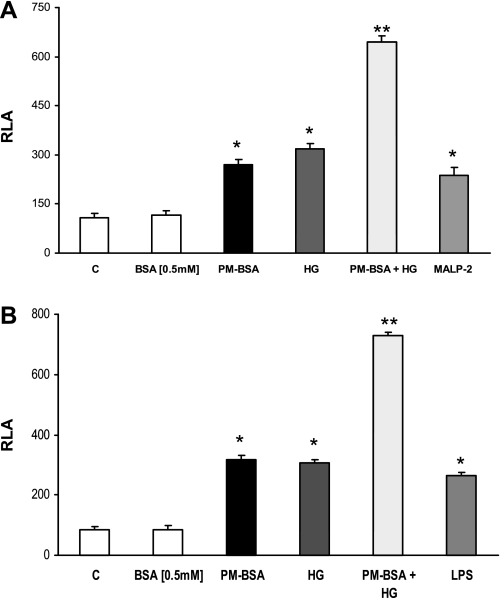
To further confirm PM-B+HG-induced TLR2 and TLR4 expression, we used reporter-based cotransfection assays (10, 21, 22). Recently, it has been shown that TLR2 dimerizes with TLR1 or TLR6 and results in receptor activation and inflammation upon SFA challenge. To determine whether TLR1 or TLR6 is required for the activation of TLR2 by PM-B+HG, 293T cells were cotransfected with murine TLR2 and either TLR1 or TLR6. PM-B+HG activated NF-κB when TLR2 was cotransfected with TLR6 (Fig. 4A) and not TLR1 (data not shown) compared with HG. PM-B+HG induced significant NF-κB transactivation in TLR4-MD2-cotransfected 293T cells compared with HG (Fig. 4B). Purified LPS, Pam3CSK4, and MALP-2 were used as positive controls (Fig. 4). These results demonstrate that PM-B+HG-enhanced HG-induced TLR4 and TLR2 receptor activity and engagement requires MD2 and TLR6 as coreceptors, respectively, resulting in NF-κB activation, further confirming our data.
Fig. 4.
Ectopic expression of TLR2, 4, or 6 or MD2 alone in 293T cells confirm the excess responsiveness of PM in HG. A: 293T cells were cotransfected with NF-κB(2x)-luciferase reporter plasmid and the expression plasmid of TLR2 and TLR6 as described in materials and methods. Relative luciferase activity (RLA) was determined by normalization with β-galactosidase activity. Values are means ± SD. *P < 0.05 vs. BSA; **P < 0.01 vs. FFA-BSA or HG. MALP-2, macrophage-activating lipopeptide-2. B: 293T cells were cotransfected with NF-κB(2x)-luciferase reporter plasmid and the expression plasmid of TLR4 and MD2 as described in materials and methods. RLA was determined by normalization with β-galactosidase activity. Values are means ± SD. *P < 0.05 vs. BSA; **P < 0.01 vs. PM-BSA or HG.
Effect of PM-B+HG on superoxide production in THP-1 cells: role of TLR2, TLR4, and p47phox.
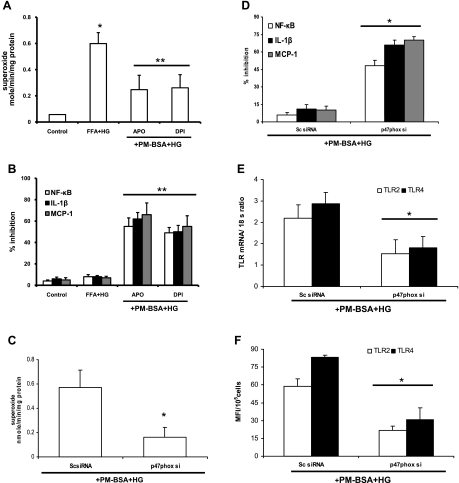
NADPH oxidase is considered the most important enzyme required for reactive oxygen species (ROS) generation in phagocytic cells. Previously, we (10) and others (23) have shown that HG and FFA-induced TLR2/4 expression facilitates monocyte NADPH oxidase production via p47phox, a key component needed for ROS generation. However, the combined effects of glucose and FFA on O2− production have not been examined. Therefore, we examined the role of NADPH oxidase in TLR2- and TLR4-modulated O2− production and inflammation under PM-B+HG with two strategies: 1) pharmacological inhibitors apocynin and DPI and 2) siRNAs. Cells were pretreated with apocynin (15 and 30 μM) or DPI (5 and 10 μM) for 2 h, followed by exposure to PM-B+HG for 24 h. Both inhibitors (at 30 μM and 10 μM) significantly blocked PM-B+HG-stimulated O2− production in THP-1 cells compared with PM-B+HG (Fig. 5A). Moreover, apocynin and DPI pretreatment significantly decreased PM-B+HG-induced NF-κB, IL-1β, and MCP-1 in THP-1 cells (% inhibition compared with PM-B+HG; Fig. 5B).
Fig. 5.
Role of NADPH oxidase. A: effect of NADPH oxidase inhibitors [apocynin (Apo) and diphenyleneiodonium (DPI)] on superoxide release by THP-1 cells. Cells pretreated with inhibitors were exposed to PM-BSA+HG, and superoxide release was measured as described in materials and methods. Values (in nmol·min−1·mg protein−1) are expressed as means ± SD. *P < 0.05 vs. PM-BSA+HG. B: effect of NADPH oxidase inhibitors (apocynin and DPI) on NF-κB activity, IL-1β, and monocyte chemoattractant protein-1 (MCP-1) release by THP-1 cells. Cells pretreated with inhibitors were exposed to PM-BSA+HG, and NF-κB activity, IL-1β, and MCP-1 release were measured as described in materials and methods. Values are expressed as mean ± SD % inhibition of control. **P < 0.01 vs. PM-BSA+HG. C: inhibition of NADPH oxidase subunit p47phox with small inhibitory RNAs (siRNAs) affects PM-BSA+HG-induced superoxide release by THP-1 cells as described in materials and methods. Values (in nmol·min−1·mg protein−1) are expressed as means ± SD. *P < 0.05 vs. scrambled control (Sc)-siRNA. D: inhibition of NADPH oxidase subunit p47phox with siRNAs affects PM-BSA+HG-induced NF-κB activity, IL-1β, and MCP-1 release by THP-1 cells as described in materials and methods. Values are expressed as mean ± SD % inhibition of control. *P < 0.05 vs. Sc-siRNA. E: inhibition of NADPH oxidase subunit p47phox with siRNAs affects PM-BSA+HG-induced TLR2 and TLR4 mRNA expression in THP-1 cells as described in materials and methods. Values are expressed as mean ± SD expressed mRNA/18S. *P < 0.005 vs. Sc-siRNA. F: inhibition of NADPH oxidase subunit p47phox with siRNAs affects PM-BSA+HG-induced TLR2 and TLR4 surface expression in THP-1 cells as described in materials and methods. Values are expressed as means ± SD. *P < 0.05 vs. Sc-siRNA.
Because p47phox is the most common subunit of NADPH oxidase involved under hyperglycemia and hyperlipidemia conditions, we hypothesized that PM-B+HG-mediated NF-κB activation and O2− production are p47phox dependent. To test this hypothesis, we selectively reduced expression of p47phox with appropriate scrambled p47phox siRNA (Sc-siRNA; as a negative control) in THP-1 cells using siRNA. As expected, treatment with p47phox siRNA, but not Sc-siRNA, reduced O2− production with PM-B+HG stimulation (Fig. 5C). Moreover, PM-B+HG-mediated increases in NF-κB, IL-1β, and MCP-1 were reduced after p47phox siRNA treatment (% inhibition compared with PM-B+HG; Fig. 5D), whereas scrambled control siRNA had minimal effect. In addition, p47phox knockdown in THP-1 cells treated with PM-B+HG showed significantly decreased TLR2/4 mRNA (Fig. 5E) and protein (Fig. 5F) levels compared with Sc-siRNA-transfected cells.
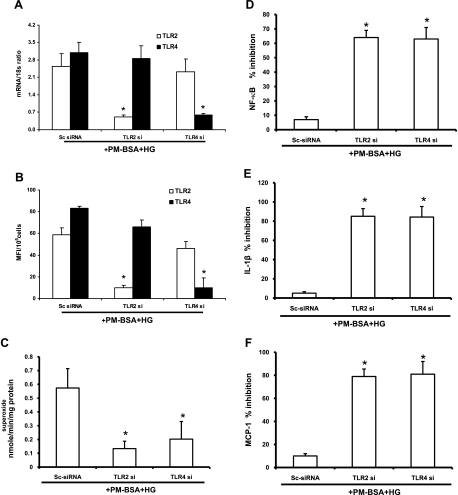
In a second set of experiments, we determined whether TLR2/4 is necessary for the effects of PM-B+HG on O2− production, NF-κB, IL-1β, and MCP-1. THP-1 cells were transfected with TLR2/4 siRNA and exposed to PM-B+HG for 24 h. TLR2 and TLR4 surface expression was measured by flow cytometry and mRNA via RT-PCR analysis. TLR2 knockdown resulted in significant inhibition of TLR2 mRNA (79% ↓, P < 0.001) and protein (83% ↓, P < 0.05), with no change in TLR4 expression (Fig. 6, A and B). In TLR4-transfected cells, significant decrease in TLR4 mRNA (80% ↓, P < 0.05) and protein (88% ↓, P < 0.05) expression was observed after PM-B+HG treatment compared with scrambled controls, with minimal change in TLR2 (Fig. 6, A and B). Furthermore, PM-B+HG-mediated increases in O2− production (Fig. 6C), NF-κB, IL-1β, and MCP-1 were reduced with both TLR2 and TLR4 siRNA treatment (% inhibition is depicted in Fig. 6, D–F) compared with scrambled control siRNA. Taken together, our results suggest that PM-B+HG amplifies the proinflammatory response through TLR2/4-mediated O2− production and activation of NF-κB with IL-1β and MCP-1 release in monocytes. Furthermore, these inflammatory effects may be attenuated by blocking TLR2, TLR4, and O2− production.
Fig. 6.
A: inhibition of TLR2 and TLR4 with siRNAs affects PM-BSA+HG-induced TLR2 and TLR4 mRNA expression in THP-1 cells as described in materials and methods. Values are expressed as mean ± SD expressed mRNA/18S. *P < 0.05 vs. Sc-siRNA. B: inhibition of TLR2 and TLR4 with siRNAs affects PM-BSA+HG induced TLR2 and TLR4 surface expression in THP-1 cells as described in materials and methods. Values are expressed as means ± SD. *P < 0.005 vs. Sc-siRNA. C: inhibition of TLR2 and TLR4 with siRNAs affects PM-BSA+HG-induced superoxide release by THP-1 cells as described in materials and methods. Values (in nmol·min−1·mg protein−1) are expressed as means ± SD. *P < 0.02 vs. Sc-siRNA. D: inhibition of TLR2 and TLR4 with siRNAs affects PM-BSA+HG-induced NF-κB activity in THP-1 cells as described in materials and methods. Values are expressed as mean ± SD % inhibition of control. *P < 0.001 vs. Sc-siRNA. E: inhibition of TLR2 and TLR4 with siRNAs affects PM-BSA+HG-induced IL-1β release by THP-1 cells as described in materials and methods. Values are expressed as mean ± SD % inhibition of control. *P < 0.05 vs. Sc-siRNA. F: inhibition of TLR2 and TLR4 with siRNAs affects PM-BSA+HG-induced MCP-1 release by THP-1 cells as described in materials and methods. Values are expressed as mean ± SD % inhibition of control. *P < 0.05 vs. Sc-siRNA.
DISCUSSION
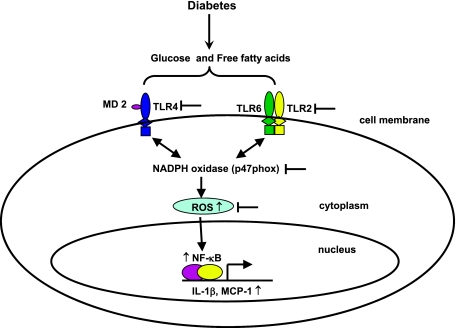
Our data suggest that SFA amplify HG-induced expression of TLR2 and TLR4, O2− release, NF-κB activity, and proinflammatory factors in human monocytic cells. They extend our previous observations on the effects of glucose excess on these innate immune receptors and proinflammatory factors (10) by showing that FFA enhance the effect of glucose on TLR2 and TLR4 expression. Moreover, FFA stimulate TLR2 and TLR4 expression via a pathway that is both ROS- and NF-κB dependent, similar to findings observed previously with glucose excess (10), and is suppressible by exposure to specific NADPH oxidase inhibitors. Our findings also implicate both TLR2 and TLR4 in FFA-stimulated expression of proinflammatory factors. Finally, it appears that specific TLR cofactors (TLR6, MD2) associated with glucose excess TLR2/4 induction are involved in FFA-enhanced TLR expression as well (Fig. 7).
Fig. 7.
Schematic representation of how glucose and palmitate induce TLR activation and inflammation in monocytes. According to the data presented, palmitate in HG amplifies TLR2 and TLR4 expression and function via increased NF-κB activation and cytokine production. In parallel, both HG and palmitate promote production of superoxide via p47phox, which by themselves induce inflammation. Inhibition of TLR expression and NADPH oxidase (p47phox) with siRNA and specific chemical inhibitors significantly attenuates HG- and palmitate-amplified TLR-mediated inflammation in monocytes.
There are few studies showing the combined effects of physiologically relevant concentrations of glucose and FFA in human monocytes. Lamharzi et al. (20) showed that FFA and glucose stimulate macrophage proliferation involving glucose-dependent oxidation of LDL. de Kreutzenberg et al. (12) showed that PM+HG play a significant role in SIRT-1 expression in vitro and in T2DM and metabolic syndrome subjects. Okuyama et al. (26) demonstrated potentiating cytotoxic effects of PM+HG in HIT-T15 β-cells via overproduction of O2−. Also, there are studies demonstrating the harmful effects of excess glucose and FFA in a variety of cells that include pancreatic β-cells (43), cardiomyocytes (40), adipocytes (42), myocytes (1), and vascular cells (17) through a number of signaling pathways. Thus it is important to understand how these cells sense the increased metabolic stress within the cell. Therefore, we examined the direct effect of palmitate and HG on the sentinel sensors of innate immune cells, namely, TLRs. In addition, there are no studies examining their combined effect on innate immune responses through TLR2 and TLR4. Our data suggest that the addition of palmitate to HG significantly increased O2− production, NF-κB activation, IL-1β, and MCP-1 release compared with HG alone. NF-κB activity and cytokines returned near to basal level by treatment with TLR2, TLR4, or p47phox siRNAs. Partial recovery from palmitate and HG-induced inflammation via TLR2 and TLR4 inhibition suggests the presence of alternate NF-κB inflammatory pathways. Recent data indicate that peroxisome proliferator-activated receptor (PPAR)γ activation may be related to TLR activation supporting this process (30).
Interestingly, saturated and monounsaturated fatty acids differ significantly in their contribution to inflammation (21, 22). Thus it is generally thought that SFA induce inflammation (22) whereas monounsaturated fatty acids increase insulin sensitivity in diabetic patients (22) and healthy subjects (39). However, the precise mechanisms are still unknown. Recent studies have demonstrated a link between SFA and IR via TLRs (33). Moreover, our group showed increased TLR2 and TLR4 expression and activity in T2DM patients (9). In view of these interesting findings, we examined the enhancing effects of oleate, palmitate, and stearate on TLR2 and TLR4 expression in HG. While palmitate and stearate significantly amplified TLR expression, NF-κB, and inflammatory factors in HG, oleate had no effect. At this point, cellular mechanisms for oleate's negative effects on TLRs are unclear and will be examined in future studies. As shown by cell viability data, oleate was not cytotoxic. Our findings are in line with earlier reports that stearate and palmitate, but not oleate, increased TLR expression and cytokine production in islets (3). In future studies, we will investigate whether docosahexaenoic acid (C22:6 n–3) and eicosapentaenoic acid [the major n–3 polyunsaturated fatty acids (PUFAs)] (22) ameliorate HG and palmitate-induced TLR-mediated inflammatory effects in vitro and in T2DM patients. Moreover, our results should be interpreted carefully as some of the experimental conditions are not entirely physiologically relevant in normal conditions.
NADPH oxidase is accepted as a key enzyme involved in ROS generation in monocyte-macrophages. Tripathy et al. (37) showed that infusion of FFA causes an increase in ROS generation, NF-κB p65-dependent activity, and plasma inflammatory cytokine levels in healthy humans, linking FFA and an increase in NF-κB, a key step to the induction of inflammation. Our cell culture experiments provide mechanistic details in support of earlier studies in humans demonstrating the innate immune (TLR2/4), oxidative (p47phox, ROS), and inflammatory (NF-κB) effects of high-fat and high-carbohydrate meals (13, 14, 25). We showed previously (10) that p47phox is an essential component of monocyte NADPH oxidase-mediated ROS generation via TLRs under HG. Therefore, to test the role of PM+HG-induced O2− overproduction and inflammation, we treated THP-1 cells with apocynin and DPI, inhibitors of NADPH oxidase activity, both of which reduced PM+HG-induced O2− overproduction, in line with earlier studies (10, 38). Next, we asked the question whether p47phox is involved in the amplification of palmitate response in excess glucose. Using p47phox (cytosolic) siRNAs, we measured O2− release. Data suggest that exacerbated O2− release with FFA and HG is ameliorated with p47phox inhibition, while p47phox Sc-siRNA had no effect.
Engagement of both TLR2 and TLR4 by FFA in excess glucose conditions leads to the activation of a common inflammatory gene activation mediated by NF-κB. In the present study, NF-κB activation coincided with TLR2/4 expression, suggesting that they are linked. Palmitate- and stearate-activated TLR2 and TLR4 expression were associated with NF-κB activation; oleate that had no effect on TLR2/4 expression also had no effect on NF-κB activation and proinflammatory factor release stimulated by HG, palmitate, or both. These data are in line with other studies (35, 2, 19) showing stimulatory effects of SFA on NF-κB activity. Moreover, silencing TLR2 or TLR4 with siRNAs significantly abrogates NF-κB activity and inflammatory mediator release, consistent with previous observations suggesting that the TLR2/4 pathway can be activated by SFA (22, 32, 39).
Our results have important implications for understanding the combined effects of hyperglycemia and dyslipidemia commonly observed in obesity and T2DM and the mechanisms underlying subclinical inflammation in vivo, in that the levels of glucose and FFA could potentially result in different degrees of TLR activation, and thereby proinflammatory factors in monocytes. This in turn could affect systemic inflammation, an important determinant of IR in adipose tissue and skeletal muscle. Thus excess of glucose and SFA, collectively, are likely to exacerbate TLR-induced proinflammatory factors and may have deleterious metabolic consequences in obesity and T2DM. Conversely, inhibition of TLR-mediated inflammatory pathways might actually benefit these patients. Interestingly, recent findings in mouse models were consistent with our in vitro observations. Thus deficiency of TLR2 protects mice from high-fat diet-induced obesity by regulating basal and insulin-induced glucose uptake in adipocytes (11). Absence of TLR4 was associated with reduced IR in diet-induced obese mice (33), and neutralization of TLR2 with antisense oligonucleotide attenuated IR in high-fat diet-fed mice (4). In summary, the results reported here demonstrate that the stimulatory effects of hyperglycemia are enhanced with SFA by engaging TLR2 and TLR4 receptors and inducing O2− release, NF-κB activation, and proinflammatory factor release, thus contributing to systemic inflammation evident in T2DM.
GRANTS
This work was supported by ADA-JF-07-16 to M. R. Dasu and National Institutes of Health K24-AT-00596 to I. Jialal.
DISCLOSURES
Authors declare no conflicts of interest that might bias this work.
ACKNOWLEDGMENTS
We thank Drs. Sridevi Devaraj and Daniel Hwang, University of California, Davis, for helpful discussions.
REFERENCES
- 1. Aas V, Rokling-Andersen M, Wensaas AJ, Thoresen GH, Kase ET, Rustan AC. Lipid metabolism in human skeletal muscle cells: effects of palmitate and chronic hyperglycaemia. Acta Physiol Scand 183: 31–41, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J Nutr 135: 1841–1846, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Böni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150: 5218–5229, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DM, Velloso LA, Carvalheira JB. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol 199: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Chait A, Bornfeldt KE. Diabetes and atherosclerosis: is there a role for hyperglycemia? J Lipid Res 50, Suppl: S335–S339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coll T, Palomer X, Blanco-Vaca F, Escolà-Gil JC, Sánchez RM, Laguna JC, Vázquez-Carrera M. Cyclooxygenase 2 inhibition exacerbates palmitate-induced inflammation and insulin resistance in skeletal muscle cells. Endocrinology 151: 537–548, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Cousin SP, Hügl SR, Wrede CE, Kajio H, Myers MG, Jr, Rhodes CJ. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology 142: 229–240, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J Lipid Res 50, Suppl: S340–S345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33: 861–868, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces Toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57: 3090–3098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis JE, Braucher DR, Walker-Daniels J, Spurlock ME. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem (April 28, 2010). doi:10.1016/j.jnutbio.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 12. de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP, Avogaro A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes 59: 1006–1015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A, Dandona P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 33: 991–997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 32: 2281–2287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339: 229–234, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Hagenfeldt L, Wahren J, Pernow B, Räf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest 51: 2324–2330, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939–1945, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kragh-Hansen U. Molecular aspects of ligand binding to serum albumin. Pharmacol Rev 33: 17–53, 1981 [PubMed] [Google Scholar]
- 19. Laine PS, Schwartz EA, Wang Y, Zhang WY, Karnik SK, Musi N, Reaven PD. Palmitic acid induces IP-10 expression in human macrophages via NF-kappaB activation. Biochem Biophys Res Commun 358: 150–155, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, Bornfeldt KE. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes 53: 3217–3225, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Lee JY, Hwang DH. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells 21: 174–185, 2006 [PubMed] [Google Scholar]
- 22. Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res 44: 479–486, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol 29: 1370–1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet 371: 1800–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 85: 2970–2973, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Okuyama R, Fujiwara T, Ohsumi J. High glucose potentiates palmitate-induced NO-mediated cytotoxicity through generation of superoxide in clonal beta-cell HIT-T15. FEBS Lett 545: 219–223, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72: 219–246, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327–334, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J 23: 831–834, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Saitoh Y, Chun-ping C, Noma K, Ueno H, Mizuta M, Nakazato M. Pioglitazone attenuates fatty acid-induced oxidative stress and apoptosis in pancreatic beta-cells. Diabetes Obes Metab 10: 564–573, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol 30: 802–808, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281: 26865–26875, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci USA 103: 16454–16459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 27: 84–91, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 21: 335–376, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52: 2882–2887, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Venugopal SK, Devaraj S, Yang T, Jialal I. Alpha-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-alpha. Diabetes 51: 3049–3054, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nälsén C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH, KANWU Study. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia 44: 312–319, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Wang X, McLennan SV, Allen TJ, Tsoutsman T, Semsarian C, Twigg SM. Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. Am J Physiol Cell Physiol 297: C1490–C1500, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Wong FS, Wen L. Toll-like receptors and diabetes. Ann NY Acad Sci 1150: 123–132, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Yeop Han C, Kargi AY, Omer M, Chan CK, Wabitsch M, O'Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes 59: 386–396, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest 93: 870–876, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]