Abstract
In response to everyday life stress, some individuals readily develop reproductive dysfunction (i.e., they are stress sensitive), whereas others are more stress resilient. When exposed to mild combined psychosocial plus metabolic stress (change in social environment plus reduced diet), female cynomolgus monkeys can be categorized as stress sensitive (SS; they rapidly become anovulatory in response to stress), medium stress resilient (MSR; they slowly become anovulatory in response to prolonged stress), or highly stress resilient (HSR; they maintain normal menstrual cycles in response to stress). Previously, we reported that monkeys that develop abnormal menstrual cycles following exposure to mild combined stress (MSR + SS) have increased plasma cortisol levels the day they move to a novel room and start a reduced diet compared with HSR monkeys. In this study, we examined whether there is a similar acute effect of mild combined stress on the reproductive axis specifically in the combined group of MSR + SS animals by measuring LH pulse frequency and whether treatment with a CRH-R1 antagonist can prevent a stress-induced suppression of LH pulse frequency presumably by inhibiting activity of the HPA axis. Animals that developed abnormal menstrual cycles in response to stress (MSR + SS monkeys) suppressed LH pulse frequency in response to stress exposure. Pretreatment with 10 mg/kg iv antalarmin prevented the stress-induced suppression of LH secretion in these animals without the stress-induced increase in cortisol secretion being blocked. We conclude that CRH, acting via nonneuroendocrine mechanisms to regulate neurotransmitter systems other than the HPA axis, plays a role in causing stress-induced reproductive impairment in stress-sensitive individuals.
Keywords: antalarmin, corticotropin-releasing hormone, corticotropin-releasing hormone receptor 1, cortisol, reproduction
stress-induced reproductive dysfunction is a common cause of infertility in women (36). Our laboratory has developed a nonhuman primate model of stress-induced reproductive dysfunction in which female cynomolgus macaques that have 28-day menstrual cycles, like women, are exposed to a combination of mild psychosocial stress plus a moderate metabolic stress of reduced calorie intake, either with or without exercise (4–6, 12–14, 16, 17, 28, 52), based on clinical descriptions of the stressor (psychogenic and metabolic) levels in women seeking treatment for stress-induced amenorrhea (2, 25, 40). Using this model, previous work in our laboratory has shown that monkeys differ in their sensitivity to stress-induced reproductive dysfunction (4, 12, 13, 52), with some monkeys showing no impairment of reproductive function following exposure to mild combined stress (i.e., they are “stress resilient”) and others showing marked sensitivity of the reproductive axis to stress (i.e., they are “stress sensitive”). Specifically, when exposed to mild combined psychosocial and metabolic stress (change in social environment plus reduced diet), monkeys can be categorized as stress sensitive (SS; they rapidly become anovulatory in response to stress), medium stress resilient (MSR; they slowly become anovulatory in response to prolonged stress), or highly stress resilient (HSR; they maintain normal menstrual cycles in response to stress) (4–6, 12–14, 16, 17, 28, 52). SS monkeys also show decreased estradiol concentrations in the follicular phase and decreased progesterone concentrations in the luteal phase of a normal menstrual cycle prior to any stress exposure (4), indicating the reproductive axis is less robustly active than in more stress-resilient monkeys even in the absence of stress.
The underlying cause of stress-induced reproductive dysfunction in women is thought to be reduced central hypothalamic gonadotropin-releasing hormone (GnRH) drive to the reproductive axis (36). This is based primarily on the widespread observation that women with this disorder have decreased LH pulse frequency (2, 3, 8, 21, 22, 25, 32, 34, 40, 47, 49). Elevated activity of the hypothalamic-pituitary-adrenal (HPA) axis, as regulated by central corticotropin-releasing hormone (CRH) neurons, has been implicated in several studies of women with stress-induced reproductive dysfunction (2, 7, 11, 33, 35, 41). In the companion article to this study (28a), we reported that although baseline activity of the HPA axis does not differ between monkeys with different sensitivities to stress-induced reproductive dysfunction, MSR + SS animals do have an increase in daytime cortisol secretion following acute exposure to a mild psychosocial plus metabolic stress paradigm. Furthermore, we have reported that the most SS monkeys have an increase in CRH mRNA in the caudal region of the paraventricular nucleus compared with more stress-resilient monkeys (17). CRH neurons project to numerous places in the brain to regulate stress-related behaviors and other processes (38). CRH signaling is mediated through the two CRH receptor (CRH-R) subtypes CRH-R1 and CRH-R2, which are thought to activate and terminate the stress response, respectively (20). Since it appears that the CRH-R1 receptor is integral in mounting a physiological response to stress (20), blocking the CRH-R1 receptor with a specific antagonist would be expected to attenuate the normal activation of the adrenal axis in response to acute stress. Thus, in this study we tested whether exposure to the combined stress of move plus moderate diet led to an acute suppression of reproductive axis activity (measured by examining pulsatile LH secretion) and whether a selective CRH-R1 antagonist could prevent the suppression of pulsatile LH secretion in MSR + SS monkeys.
Antalarmin (a generous gift from the National Institutes of Health), originally developed by Chen (19), is a small lipophilic nonpeptide compound that has been shown to penetrate the blood-brain barrier (27, 29) and selectively block CRH-R1 receptors. Antalarmin is related to the widely used CP 154,526, with the addition of a methyl group to improve lipophilicity and penetration of the blood-brain barrier (51). The lipophilic nature of this drug is believed to result in wide distribution to various brain regions involved in the regulation of stress-related behavior and neuroendocrine function (27). Some studies have reported that antalarmin blocks stress-induced ACTH and cortisol release (9, 51) and stress-induced behaviors (23, 43) in the rodent. In the nonhuman primate, Habib et al. (27) reported that orally administered antalarmin (20 mg/kg po) attenuated social stress-induced increases in ACTH and cortisol and stress-related behaviors in male rhesus monkeys, a finding recently corroborated in the marmoset (24). In contrast, intravenously administered antalarmin was reported to reduce CRH-induced elevations in ACTH, but not cortisol, when given to rhesus monkeys at doses of 1.0, 3.2, and 10 mg/kg iv (10). In this study, we tested the ability of intravenous antalarmin to prevent the acute stress-induced suppression of pulsatile LH secretion in the combined group of MSR + SS monkeys.
METHODS AND MATERIALS
Animals
Fifteen adult female cynomolgus monkeys (Macaca fascicularis) were utilized for this set of experiments and were from the same group of animals utilized in the companion article to this study examining activity of the HPA axis in response to mild psychosocial and metabolic stress (28a). Monkeys were housed at the Oregon National Primate Research Center (ONPRC) in individual stainless steel cages (32 × 24 × 27 in.) in a temperature-controlled room (23 ± 2°C) with lights on for 12 h/day (0700–1900). Animals were fed two meals a day consisting of four high-protein monkey chow biscuits (no. 5047, jumbo biscuits; Ralston Purina, St. Louis, MO) at 0930 and 1530, and a supplement of one-quarter piece of fresh fruit was provided with the afternoon meal. Animals had their vaginal area swabbed daily to check for menses. The first day of menses was designated as day 1 of a menstrual cycle. Food intake, measured just before the next meal was fed, was recorded for each meal, and weight was measured weekly. All protocols and procedures were reviewed and approved by the Institutional Animal Care and Use Committee at ONPRC.
Catheterization
Each monkey had a chronic indwelling venous catheter placed in a subclavian vein under isofluorane anesthesia (VedCo, St. Joseph, MO). At surgery, the tip of the catheter was positioned in the right atrium of the heart, and the free end was routed subcutaneously to the back and threaded through a small cutaneous incision between the scapulae. To protect the catheter line, each animal wore a fitted nylon jacket connected to a 36-inch flexible metal tether that attached to a swivel mounted to the top of the monkey's cage (15). Silastic tubing extended from the top of the swivel through a hole in the wall to a sampling syringe and stopcock in the adjacent room. This catheter system allowed for blood sampling and intravenous infusions without sedating or disturbing the animal while allowing the monkey free range of motion within the cage. Catheter lines were kept patent with a constant infusion of physiological saline (Baxter Healthcare, Deerfield, IL) containing heparin sodium (4 IU/ml) at a rate of ∼100 ml/day. Weekly inspections of catheter systems and replacement of a sterile dressing covering the catheter exit site, to keep the exit site aseptic and prevent infection, were performed under Ketaset (10 mg/kg iv ketamine hydrochloride; Wyeth, Madison, NJ). Ketamine was never administered within 24 h preceding an experimental procedure.
Overall Study Design
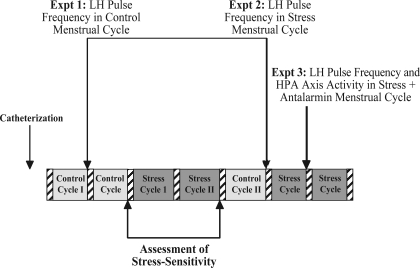
Prior to the studies presented here and in the companion article to this study (28a), monkeys had been maintained in a stable social environment on their standard diet and had shown at least one normal menstrual cycle (control cycle 1), indicted by a peak luteal phase of progesterone of 2 ng/ml or higher (indicating ovulation), and a normal cycle length (25–38 days; Refs. 53 and 54). The timeline of the experiments presented in this study is shown in Fig. 1. Experiment 1 (LH pulse frequency in a control nonstressed menstrual cycle, as described below) was conducted prior to assessment of stress-sensitivity and immediately following control cycle 1. Following completion of experiment 1, the animals underwent categorization for stress sensitivity of the reproductive axis, with the mild combined stress paradigm lasting for a two-cycle duration (stress cycles 1 and 2), as described below. When each monkey had exhibited at least one normal menstrual cycle following exposure to the mild combined stress paradigm (control cycle 2), experiment 2 or experiment 3 was conducted during the next cycle (experiments 2 and 3 were counterbalanced for order effects). Between experiments 2 and 3, each monkey had to show at least one normal menstrual cycle before progressing on to the next planned experiment.
Fig. 1.
Schematic diagram of the timeline of experiments detailed in this study. Hatched bars indicate menstrual periods. Expt, experiment; HPA, hypothalamic-pituitary-adrenal.
Assessment of Stress Sensitivity
For each monkey, sensitivity of the reproductive axis to stress was categorized by assessing changes in menstrual cycle length, ovulation, and reproductive hormone secretion when monkeys were exposed to a mild psychosocial and metabolic stressor, as described previously (4–6, 12–14, 16, 17, 28, 52). This study was performed after each monkey had been living in its home cage surrounded by familiar monkeys for several months. To provide a standardized mild psychosocial stress, monkeys were moved on the first day of their menstrual cycle from their home cage to a single cage in a novel room, where they were surrounded by unfamiliar monkeys. As a metabolic stress, each animal's available caloric intake was reduced by 20%. Blood samples (0.6 ml/sample) were taken every other day to assess reproductive steroid hormone concentrations using the blood collection protocol described in experiment 1. Monkeys that mensed within 38 days subsequent to the initiation of stress were moved for a second stress cycle and remained on 20% lower calorie intake (53, 54).
Animals were categorized as HSR if they presented a normal ovulatory menstrual cycle [25–38 days in length, peak E2 >200 pg/ml in follicular phase, peak P4 >2 ng/ml in luteal phase (53, 54)] in stress cycle 1 and again in stress cycle 2. MSR animals were defined as those animals that presented a normal ovulatory menstrual cycle in response to stress cycle 1 but failed to mense by day 38 of stress cycle 2. Animals that immediately suppressed normal menstrual cyclicity upon exposure to stress (i.e., they failed to ovulate and mense within 60 days of stress cycle 1) were categorized as SS. Animals that exhibited disrupted menstrual cycles during this characterization of stress sensitivity (i.e., MSR and SS monkeys) were allowed to recover normal menstrual cyclicity before further experiments were resumed. For the experiments presented here, a combined group of MSR + SS animals and an HSR group were studied in response to antalarmin treatment.
Experimental Protocols
Experiment 1: characterization of LH pulse frequency in a control menstrual cycle.
On day 5 of a control, nonstressed menstrual cycle immediately following control cycle 1, blood samples (0.6 ml/sample) were collected into heparinized syringes every 15 min via the remote sampling system for 8 h beginning at 0900 and continuing until 1700. Progesterone was checked in the luteal phase of the preceding cycle to ensure that each animal was having a normal ovulatory menstrual cycle prior to this experiment (in control cycle 1). Samples were collected into heparinized syringes and placed in an empty sterile plastic centrifuge tube on ice. Samples were immediately centrifuged at 3,000 rpm for 15 min at 4°C, and plasma was pipetted into a plastic O-ring storage vial (containing 20 μl of a solution composed of equal volumes of 30% sodium citrate and 1,000 IU/ml sodium heparin to prevent clotting of plasma proteins) and stored at −20°C until assayed. Red blood cells were sterilely resuspended and reinfused through the remote catheter system. Hematocrit was checked at intervals during the study and remained in the normal physiological range. This experiment was conducted prior to the categorization of stress sensitivity to accurately characterize LH pulse frequency prior to any exposure to stress.
Experiment 2: characterization of LH pulse frequency following acute exposure to mild combined stress.
On day 1 of another menstrual cycle, each animal was moved at 0930 to a cage in a novel room, and its available caloric intake was reduced by 20%. One animal presented normal ovulatory menstrual cycles during the control period (control cycle 1) but failed to resume normal menstrual cycles following exposure to the mild combined stress paradigm within a period of 6 mo, and therefore, experiment 2 was scheduled after it was determined that the steroid hormones E2 and P4 were low (<50 pg/ml and <0.2 ng/ml, respectively), simulating an early follicular phase. Similar plasma levels of estrogen and progesterone were observed in all monkeys that were sampled on day 1 of a normal ovulatory menstrual cycle, and therefore, these monkeys were included in all analyses. Of note, mean plasma concentrations of estrogen and progesterone did not differ for measurements made on day 1 vs. day 5 in experiments 1 and 2. Beginning at 1000, blood samples (0.6 ml/sample) were collected for assessment of plasma LH concentration into heparinized syringes every 15 min via the remote sampling system, continuing until 1800 that day. Blood samples were collected and processed in an identical manner to that described in experiment 1. Upon completion of this experiment, the animal's diet was returned to normal, and it was kept in the new room to resume normal menstrual cyclicity.
Experiment 3: characteracterization of LH pulse frequency and HPA axis activity following acute exposure to mild combined stress with antalarmin pretreatment.
On day 1 of another menstrual cycle, each monkey was again moved to a single cage in a novel room and placed on a reduced diet (20% reduction in available caloric intake) at 0930. The order of experiments 2 and 3 were randomized such that some animals were studied in the stress-alone condition (experiment 2) first, and others were studied in the stress-plus-antalarmin pretreatment condition first (experiment 3) to control for order effects. In experiment 3, animals were pretreated with 10 mg/kg iv antalarmin 15 min prior to the move to the novel room and receipt of the reduced-calorie morning meal. Intravenous antalarmin solution was dissolved in ethanol-cremophor-sterile water (5:5:90, vol/vol/vol; Refs. 10 and 27) and administered through the iv catheter at 9:15 AM. Beginning at 1000, blood samples (0.6 ml/sample) were collected into heparinized syringes every 15 min via the remote sampling system, continuing until 1800. Also beginning at 1000 and continuing every 2 h until 1800, blood samples (0.4 ml/sample) for ACTH and cortisol were collected and processed to measure the ability of a CRH-R1 antagonist to prevent the increase in cortisol secretion in response to mild combined stress, as was observed in MSR + SS monkeys in the companion article to this study (28a). Samples to be assayed for cortisol were collected and processed in a manner identical to those assayed for LH. Samples to be assayed for ACTH were collected into sterile plastic centrifuge tubes on ice containing 20 μl of EDTA to prevent protein degradation. Samples were immediately centrifuged at 3,000 rpm for 15 min at 4°C, and plasma was pipetted into a plastic O-ring storage vial (containing 10 μl of aprotinin), flash-frozen in a dry ice-ethanol bath, and stored at −20°C until assayed. At 1400, a second identical dose of antalarmin was administered based on literature reporting the half-life of antalarmin to be 7.82 h (27). The use of this dosage is based on previous literature (10) and pilot studies in our laboratory indicating that the 10 mg/kg iv dose was most effective in suppressing the stress-induced rise in ACTH and cortisol.
Assays
Plasma LH was measured by RIA at the University of Pittsburgh assay core, as described previously (53, 54), using recombinant cynomolgus monkey LH (National Hormone and Peptide Program, Harbor-University of California Los Angeles Medical Center, Torrance, CA) as a standard. The sensitivity of the LH assays was 0.063 ng/ml, and the intra- and interassay coefficients of variation for the LH assays used in these studies, as calculated from three low-, medium-, and high-concentration serum pool controls run in each assay, were 3.84 and 15.36%, respectively. LH pulses were identified using the Pulsar algorithm developed by Merriam and Wachter (42), using the following G values: G(1): 4.40; G(2): 2.60; G(3): 1.92; G(4): 1.46; and G(5): 1.13. For all Pulsar analyses, values below the level of assay detectability were assigned the minimal detectable LH concentration in the assay. The rise above baseline in each Pulsar-defined pulse was used in quantifying pulse amplitude. Pulse frequency was calculated as the number of Pulsar-defined pulses in 8 h. Mean LH concentrations were calculated by taking the mean of all LH values collected over the 8-h sampling period.
Plasma ACTH and cortisol, as well as E2 and P4, were measured with an Immulite 2000 machine (Siemens Healthcare Diagnostics, Deerfield, IL) in the Endocrine Services Laboratory core facility at ONPRC. The ONPRC assay core has validated the usage of the Immulite 2000 for monkey serum hormones, including cortisol (5, 6), E2, and P4 (30). The Immulite 2000 has been validated independently for monkey ACTH measurements (50). The sensitivity of estradiol assays is 20 pg/ml, 0.2 ng/ml for progesterone, 10 ng/ml (1 μg/dl) for cortisol, and 5 pg/ml for ACTH. For all hormone assays, values below the level of assay detectability were assigned the minimal detectable concentration in the assay. All quality control samples and validations, provided by the company, were analyzed each time before use for hormonal measurements in samples. The Immulite 2000 runs three quality control (QC) serum pools daily, and thus no specific intra-assay QC data are available. The interassay coefficient of variation, reflecting variability in daily QC results over a period of 1.5 yr in which these assays were run, was as follows: ACTH 7.7%, cortisol 8.1%, estradiol 8.5%, and progesterone 9.4%.
Statistical analyses.
There are substantial individual differences in the “normal” frequency of pulsatile LH release from the pituitary in both human and nonhuman primates (22), which combined with the low sample size of a nonhuman primate study would lower the statistical power available to detect significant differences using parametric tests. Therefore, for all analysis of LH pulse frequency, Fisher's exact test was used, assigning a criterion of less than or greater than 4 pulses/8 h for nonparametric analysis based on work from Pohl et al. (46) finding a pulse frequency of <4 pulses/8 h as failing to induce ovulation in the nonhuman primate. One HSR and one SS monkey failed to complete experiment 3. Thus, these monkeys were excluded from within-subjects comparisons across the experimental series.
Plasma ACTH and cortisol response to mild combined stress with antalarmin pretreatment (experiment 3) was compared with ACTH and cortisol data in experiments 1 and 4 in in the companion article to this study with these same monkeys (24-h ACTH and cortisol in a nonstressed control menstrual cycle and in a cycle following exposure to mild combined stress, respectively) (28a). Samples were collected using the same procedures as in the companion article. Comparisons of ACTH and cortisol levels in control, stressed, and stress plus antalamin conditions were made using a mixed design repeated-measures ANOVA (repeated-measures design with between-groups comparison). Differences between groups were considered significant if P ≤ 0.05. All statistical analyses were performed with SPSS version 15.0 statistical software (SPSS, Chicago, IL).
RESULTS
Six animals were categorized as HSR, five animals were categorized as MSR, and four animals were categorized as SS.
Experiments 1 and 2: Effects of Acute Stress on LH Pulse Frequency
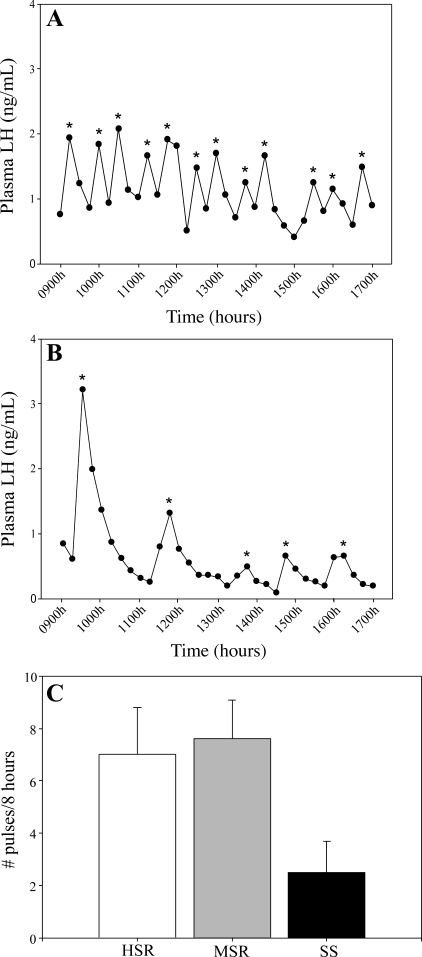
In a normal, nonstressed menstrual cycle (experiment 1), SS monkeys (2.5 ± 1.2 pulses/8 h; Fig. 2) showed a trend toward having lower LH pulse frequency than both MSR (7.6 ± 1.5 pulses/8 h, P = 0.06) and HSR monkeys (7.0 ± 1.8 pulses/8 h, P = 0.08). There were no group differences in either mean LH (F2,12 = 0.01, P = 0.99) or LH interpulse interval (F2,12 = 1.28, P = 0.31). Following acute exposure to mild combined stress (experiment 2), HSR monkeys (8.4 ± 1.4 pulses/8 h) had higher LH pulse frequency than both MSR (1.4 ± 1.2 pulses/8 h, P = 0.00) and SS monkeys (2.0 ± 1.2 pulses/8 h, P = 0.01). There were no group differences in either mean LH (F2,11 = 1.56, P = 0.25) or LH interpulse interval (F2,11 = 2.76, P = 0.10).
Fig. 2.
Representative LH pulse patterns in 1 highly stress-resilient (HSR; A) and 1 stress-sensitive (SS; B) monkey over an 8-h period in the early follicular phase of a nonstressed, control menstrual cycle. *Pulses defined by PULSAR algorithm. C: mean LH pulse frequency measured in the early follicular phase of a nonstressed, control menstrual cycle in HSR, medium stress-resilient (MSR), and SS monkeys.
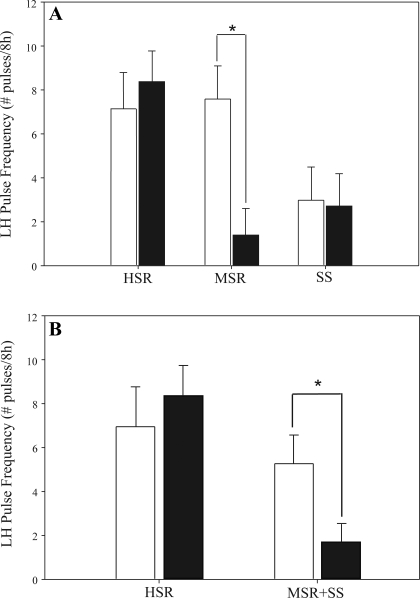
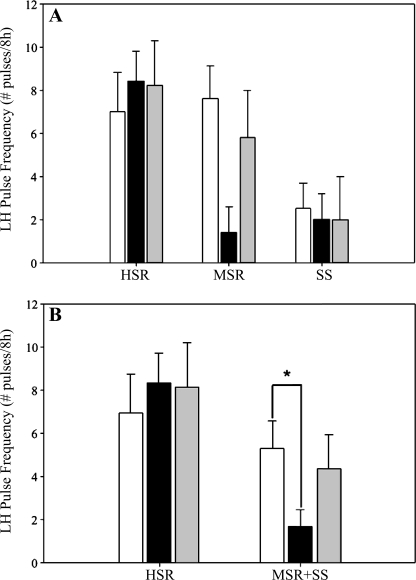
When LH pulse frequency was compared within a group of monkeys during the control and stress cycles, HSR monkeys maintained a normal LH pulse frequency whether measured in a control, nonstressed menstrual cycle or in response to acute mild combined stress (control cycle: 7.00 ± 1.81 pulses/8 h; stress cycle: 8.40 ± 1.36 pulses/8 h; P = 0.58; Fig. 3A). SS monkeys maintained a low pulse frequency whether measured in a control, nonstressed menstrual cycle or in response to acute mild combined stress (control cycle: 2.50 ± 1.29 pulses/8 h; stress cycle: 2.00 ± 1.23 pulses/8 h; P = 0.50; Fig. 3A). However, MSR monkeys showed high LH pulse frequency in a control cycle (7.60 ± 1.50 pulses/8 h; Fig. 3A) but a significant suppression of LH pulse frequency when exposed to acute mild combined stress (1.40 ± 1.19 pulses/8 h, P = 0.02). When animals that became anovulatory in response to mild combined stress (MSR + SS) were grouped [as in the companion article to this study (28a)], this group showed a significant suppression of LH pulse frequency (control cycle: 5.33 ± 1.29 pulses/8 h; stress cycle: 1.67 ± 0.79 pulses/8 h; P = 0.03; Fig. 3B) in response to mild combined stress compared with the control cycle.
Fig. 3.
LH pulse frequency measured during daytime hours (1000–1800) both in the early follicular phase of a control, nonstressed menstrual cycle (open bars) and on the same day in a cycle with mild combined stress (move + diet) in HSR, MSR, and SS monkeys (A) and in HSR vs. animals that became anovulatory in response to mild combined stress (MSR + SS; B). *P < 0.05.
Experiment 3: Effect of Antalarmin on the HPA Axis and LH Pulse Frequency Response to Acute Stress
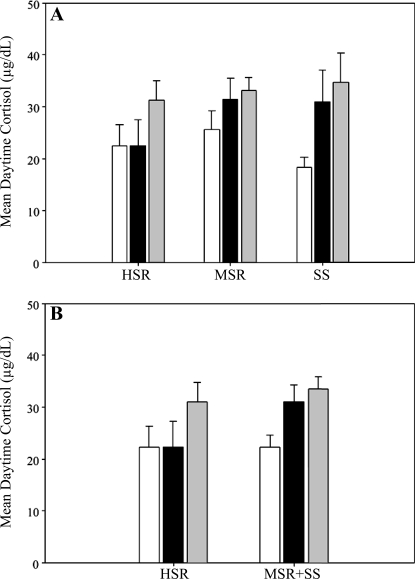
Pretreatment with 10 mg/kg iv antalarmin did not suppress ACTH or cortisol in any group [control cycle and stress cycle cortisol data taken from the companion article to this study (28a); Fig. 4]. In contrast, pretreatment with antalarmin prior to exposure to mild combined stress prevented the stress-induced suppression of LH pulse frequency in the MSR group (control cycle: 7.60 ± 1.50 pulses/8 h, stress + antalarmin cycle: 5.80 ± 2.20 pulses/8 h, P = 0.22; Fig. 5A), but there was not a similar effect of antalarmin in the HSR or SS groups. Antalarmin also prevented the stress-induced suppression of LH pulse frequency in the combined group of animals that became anovulatory in response to mild combined stress (MSR + SS; control cycle: 5.33 ± 1.29 pulses/8 h; stress + antalarmin cycle: 4.38 ± 1.63 pulses/8 h; P = 0.25; Fig. 5B).
Fig. 4.
Mean levels of plasma cortisol measured during daytime hours (1000–1800) both in the early follicular phase of a control, nonstressed menstrual cycle (open bars) and in the same phase of a cycle with (gray bars) and without (black bars) antalarmin pretreatment prior to exposure to mild combined stress (move + diet) in HSR, MSR, and SS monkeys (A) and in HSR vs. animals that became anovulatory in response to MSR + SS (B). Control and stress cycle data reprinted from Fig. 5, A and B, of the companion article to this study (28a).
Fig. 5.
LH pulse frequency measured during daytime hours (1000–1800) both in the early follicular phase of a control, nonstressed menstrual cycle (open bars) and on the same day in a cycle with (gray bars) and without (black bars) pretreatment with antalarmin prior to exposure to MSR + SS (move + diet) in HSR, MSR, and SS monkeys (A) and in HSR vs. animals that became anovulatory in response to MSR + SS (B). *P < 0.05.
DISCUSSION
In this study, we report that in a nonstressed, control menstrual cycle, the most SS monkeys that quickly develop stress-induced amenorrhea have decreased LH pulse frequency compared with more stress-resilient animals, indicating that these individuals have decreased central drive to the reproductive axis even in the absence of overt stress. We also found that there is an acute effect of stress on the reproductive axis, as measured by acute suppression of LH pulse frequency following exposure to mild psychosocial and metabolic stress in some, but not all, monkeys. HSR animals showed no suppression of pulsatile LH secretion when exposed to acute stress. In contrast, acute exposure to mild combined stress caused MSR monkeys to suppress the frequency of pulsatile LH secretion to levels typical of SS monkeys. Interestingly, acute stress exposure did not lead to a further suppression of LH pulse frequency in SS monkeys that show LH secretory impairment even in nonstressed conditions. And when considered together, monkeys in which stress can lead to anovulation (i.e., MSR + SS animals) showed a significant impairment of pulsatile LH secretion when acutely stressed. Importantly, pretreatment with the specific CRH-R1 antagonist antalarmin prior to exposure to mild combined stress was able to prevent acute stress-induced suppression of LH pulse secretion without suppressing activity of the HPA axis in MSR + SS animals. Because antalarmin prevented a stress-induced suppression of LH pulse frequency without preventing stress-induced suppression of ACTH or cortisol secretion, these findings suggest that reproductive effects of antalarmin are likely to act through nonneuroendocrine mechanisms (i.e., not through suppression of the HPA axis) to regulate other neurotransmitter systems that modulate the activity of the reproductive axis.
The reported effects of antalarmin on reproductive function have been extremely limited. Antalarmin infusion into the amniotic sac, fetus, and jugular vein of pregnant ewes can delay preterm birth onset by suppressing the increase in fetal CRH normally associated with triggering parturition (18). Interestingly, similar to our findings in this study, maternal cortisol did not differ between vehicle- and antalarmin-treated ewes in that study. However, the reproductive effects of antalarmin may not be all beneficial. Makrigiannakis et al. (39) reported that female rats treated with antalarmin showed a 70% decrease in implanted embryos in early pregnancy. This increase in early miscarriage with CRH-R1 receptor antagonism is thought to occur by inhibiting the apoptosis of activated T lymphocytes, a process that is normally induced by CRH in the uterus, thus protecting the fetus from T lymphocyte attack and maintaining early pregnancy (31). Although we report here that treatment with antalarmin is able to restore LH pulse frequency following stress exposure, the full reproductive effects at each level of the hypothalamic-pituitary-gonadal (HPG) axis and resulting outcomes for successful menstrual cyclicity, fertility, establishment, and maintenance of pregnancy remain to be fully studied.
The reported effects of antalarmin on HPA axis activation and stress-induced behaviors in rodents and primates are inconsistent. Some studies have reported that antalarmin blocks stress-induced cortisol and ACTH release (9, 51), whereas others have found no effect (55). Interestingly, several groups have reported a blockade of stress-induced behaviors but not a blockade of activity of the HPA axis (23, 43), and still others report that any effect of antalarmin on behavior or endocrine hormone secretion is dependent on both the strain (26) and behavioral measurement used (44). Research has been more limited in primate species. Habib et al. (27) reported that orally administered antalarmin (20 mg/kg po) attenuated social stress-induced increases in ACTH, cortisol, and stress-related behaviors in male rhesus monkeys, a finding recently corroborated in the marmoset (24). However, Ayala et al. (1) found that orally administered antalarmin did not attenuate stress-induced elevations in ACTH or cortisol but did reduce stress-related behaviors. Intravenously administered antalarmin was also reported to reduce CRH-induced elevations in ACTH, but not cortisol, at doses of 1.0, 3.2, and 10 mg/kg iv (10). From these findings it is clear that the effects of antalarmin are dependent upon a variety of factors, including species, strain, dose, route of administration, and experimental procedure. It appears that such differences may exist even between various primate species. In this study, we found that treatment with 10 mg/kg iv antalarmin did not suppress the stress-induced increase in daytime cortisol reported in the companion article to this study (28a). This finding was quite unexpected given that data from our pilot studies with antalarmin had indicated that intravenously administered antalarmin at a dose of 10 mg/kg iv did suppress both ACTH and cortisol in an acute psychological stress paradigm. It is likely that the different stressors used (exposure to a leather catch glove was used in the pilot study, and mild diet plus move to novel room was used in this set of experiments) made a substantial difference in the effects of antalarmin on ACTH and cortisol secretion. In the case of the pilot study, leather catch glove exposure was designed to imitate hand catching (48). This is a significant stressor and was intentionally used to provide a condition in which it would be possible to detect suppression of stress-induced ACTH and cortisol by antalarmin. However, the combined stress of reduced diet plus move to a novel room used in this study is a much milder stressor leading to a smaller increase in ACTH and cortisol and may have contributed to the differential findings. Nevertheless, the finding that antalarmin was able to partially prevent the stress-induced suppression of LH pulse frequency despite a lack of an effect on either stress-induced ACTH or cortisol release indicates that the increased cortisol response to mild combined stress observed in MSR + SS monkeys [as reported in the companion article to this study (28a)] is not causal to the acute stress-induced suppression of LH pulse frequency in these same monkeys.
It is also interesting that female monkeys with different sensitivities to stress-induced reproductive dysfunction had differences in frequency of pulsatile LH secretion in a nonstressed condition as well as different responses of the central drive to the reproductive axis to stress. Monkeys that are resilient to stress-induced reproductive dysfunction have an LH pulse frequency typical of the frequency usually associated with the early follicular phase [i.e., ∼1 pulse/h (45)]. In HSR monkeys, the circhoral release of LH was maintained even in response to acute mild combined stress. In contrast, SS monkeys had a slower LH pulse frequency, and therefore presumably decreased frequency of central GnRH release, even in the absence of overt stress. As expected, SS monkeys that had fewer than three pulses in the 8-h sampling period (i.e., 2 of the 4 SS monkeys) did not ovulate following experiment 1 in the nonstressed menstrual cycle following control cycle 1. This is in agreement with previous literature finding that a minimum pulse frequency threshold of three pulses in an 8-h period is necessary to induce ovulation in female monkeys (46). Interestingly, exposure to acute mild combined stress did not further suppress LH pulse frequency in SS animals, possibly because of an already compromised GnRH pulse generator operating suboptimally in normal, nonstressed conditions. MSR monkeys that only lose menstrual cyclicity in response to prolonged exposure to stress had a circhoral LH pulse frequency in normal, nonstressed conditions but rapidly suppressed the frequency of pulsatile LH secretion in response to acute mild combined stress. This stress-induced suppression of pulsatile LH could reflect increased inhibitory input to the reproductive axis via increased GABA, CRH, or opioidergic mechanisms, decreased stimulatory input through serotonin or glutamatergic mechanisms, or both, since differences in several of these pathways have been reported in SS monkeys (5, 6, 16, 17).
Previous work utilizing this animal model has characterized the secretion of reproductive steroid hormones in SS vs. stress-resilient monkeys and reported similar findings to what has been observed in women with stress-induced reproductive dysfunction vs. normally cycling women (4, 47). Monkeys that are highly resilient to stress-induced reproductive dysfunction, like eumenorrheic women, develop normal preovulatory levels of circulating estradiol, even during menstrual cycles in which they were exposed to everyday life stresses (4). Our current finding that these highly stress-resilient monkeys have normal LH pulse frequency even in response to acute mild combined stress corroborates this and more fully characterizes the response of the HPG axis to stress as being highly resilient in these individuals. Previous studies in our laboratory also have shown that SS monkeys have low but preovulatory levels of estradiol that rapidly suppresses in response to mild combined stress, resulting in a loss of menstrual cyclicity (4). Our finding that monkeys sensitive to stress-induced reproductive dysfunction have decreased central drive to the HPG axis even in the absence of overt stress may account for the lower estradiol secretion. In this study we did not find that exposure to everyday life stresses further suppresses LH pulse frequency in these monkeys, which we attribute to the already slow pulsatile secretion of LH in normal conditions. However, it is possible that in a larger sample of SS monkeys a further suppression of LH secretion might be observed if the majority of animals completely suppressed LH secretion to nondetectable levels when exposed to stress.
MSR monkeys that have been defined in this study and in previous studies by our laboratory as losing reproductive function only in response to prolonged exposure to everyday life stress (4) have a LH pulse pattern that resembles HSR monkeys in a normal, nonstressed menstrual cycle and SS monkeys in a menstrual cycle in which they were exposed to acute mild combined stress. This characterization of pituitary secretion of LH in control and stressed menstrual cycles also supports previously observed steroid hormone activity in MSR individuals (4). Medium stress-resilient monkeys typically have peak estradiol and progesterone levels comparable with those of highly stress-resilient monkeys in normal, nonstressed conditions as well as in response to short-term (1 menstrual cycle's length) exposure to mild combined stress. However, with repeated exposure to mild combined stresses (i.e., in a second menstrual cycle), they suppress secretion of both estradiol and progesterone to levels similar to what is observed in SS monkeys. Our finding that the medium stress-resilient monkeys have normal LH pulse frequency in a control, nonstressed follicular phase but rapidly suppress LH secretion to frequencies similar to that of SS monkeys corroborates these previous findings. It may be that medium stress-resilient monkeys are acutely affected in the early follicular phase by everyday life stresses but are able to recover throughout the remainder of the cycle to ovulate and have a normal cycle, unless this stress is prolonged. This acute response to mild combined stress and subsequent recovery is in contrast to that of SS animals that appear to not be able to recover from the effects of everyday life stresses and fail to have an ovulatory menstrual cycle.
The findings reported herein further support the validity of this nonhuman primate model of stress-induced reproductive dysfunction by indicating that reproductive disruption in SS monkeys occurs at least at the level of the pituitary. Furthermore, although we did report in the companion article to this this study (28a) that MSR and SS monkeys show an elevation of daytime cortisol in response to mild combined stress, but not in baseline nonstressed conditions or in acute reactivity to a single psychosocial stressor, our finding in this study that treatment with a specific CRH-R1 antagonist is able to prevent the acute stress-induced suppression of LH pulse frequency without blocking the aforementioned increase in daytime cortisol following stress further indicates that elevated HPA axis activity is not a primary neural mechanism underlying sensitivity to stress-induced reproductive dysfunction.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant U54-HD-18185 and Grant RR-00163.
DISCLOSURES
S. M. Herod, C. R. Pohl, and J. L. Cameron all reported no biomedical financial interests or potential conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to Jon Reyes, Diana Takahashi, Paul Loprinzi, Amanda Bulechowsky, Whitney McGee, the Endocrine Services Laboratory at ONPRC, and the University of Pittsburgh assay core for their technical expertise. We also appreciate the Division of Animal Resources at ONPRC for their knowledgeable assistance.
Present address of S. M. Herod: Department of Biology and Chemistry, Azusa Pacific University, Azusa, CA 91702.
REFERENCES
- 1. Ayala AR, Pushkas J, Higley JD, Ronsaville D, Gold PW, Chrousos GP, Pacak K, Calis KA, Gerald M, Lindell S, Rice KC, Cizza G. Behavioral, adrenal, and sympathetic responses to long-term administration of an oral corticotropin-releasing hormone receptor antagonist in a primate stress paradigm. J Clin Endocrinol Metab 89: 5729–5737, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril 67: 1024–1030, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, Yen SSC. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 68: 301–308, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol 38: 199–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril 83(1): 148–55, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bethea CL, Streicher JM, Mirkes S, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience 132: 151–166, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Biller BM, Federoff HJ, Koenig JI, Klibanski A. Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab 70: 311–317, 1990 [DOI] [PubMed] [Google Scholar]
- 8. Bomba M, Gambera A, Bonini L, Peroni M, Neri F, Scagliola P, Nacinovich R. Endocrine profiles and neuropsychologic correlates of functional hypothalamic amenorrhea in adolescents. Fertil Steril 87: 876–885, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Bornstein SR, Webster EL, Torpy DJ, Richman SJ, Mitsiades N, Igel M, Lewis DB, Rice KC, Joost HG, Tsokos M, Chrousos GP. Chronic effect of a nonpeptide corticotropin-releasing hormone type 1 receptor antagonist on pituitary-adrenal function, body weight, and metabolic regulation. Endocrinology 139: 1546–1555, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Broadbear JH, Winger G, Rivier JE, Rice KC, Woods JH. Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys. Neuropsychopharmacology 29: 1112–1121, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 91: 1561–1565, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Cameron JL. Stress and behaviorally induced reproductive dysfunction in primates. Semin Reprod Endocrinol 15: 37–45, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Cameron JL. Reproductive dysfunction in primates, behaviorally induced. In: Encyclopedia of Stress, edited by Fink G. New York: Academic, 2000, p. 366–372 [Google Scholar]
- 14. Cameron JL, Bridges MW, Graham RE, Bench L, Berga SL, Matthews K. Basal heartrate predicts development of reproductive dysfunction in response to psychological stress (Abstract PI-76). The Endocrine Society Program and Abstracts 80th Annual Meeting, New Orleans, LA, June 24–27, 1998 [Google Scholar]
- 15. Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta). Endocrinology 128: 1532–1540, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic expression of serotonin 1A, 2A and 2C receptor and GAD67 mRNA in female cynomolgus monkeys with different sensitivity to stress. Brain Res 1142: 1–12, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Centeno ML, Sanchez RL, Reddy AP, Cameron JL, Bethea CL. Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology 86: 277–288, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Chan EC, Falconer J, Madsen G, Rice KC, Webster EL, Chrousos GP, Smith R. A corticotropin-releasing hormone type I receptor antagonist delays parturition in sheep. Endocrinology 139: 3357–3360, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Chen YP. International Patent No. WO94/13676, June 23, 1994 [Google Scholar]
- 20. Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides 22: 733–741, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Couzinet B, Young J, Brailly S, Le Bouc Y, Chanson P, Schaison G. Functional hypothalamic amenorrhoea: a partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol (Oxf) 50: 229–235, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Crowley WF, Jr, Filicori M, Spratt DI, Santoro NF. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res 41: 473–531, 1985 [DOI] [PubMed] [Google Scholar]
- 23. Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong ML, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology 140: 79–86, 1999 [DOI] [PubMed] [Google Scholar]
- 24. French JA, Fite JE, Jensen H, Oparowski K, Rukstalis MR, Fix H, Jones B, Maxwell H, Pacer M, Power ML, Schulkin J. Treatment with the CRH-1 antagonist antalarmin reduces behavioral and endocrine responses to social stressors in marmosets (Callithrix kuhlii). Am J Primatol 69: 877–889, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril 60: 486–492, 1993 [PubMed] [Google Scholar]
- 26. Grakalic I, Schindler CW, Baumann MH, Rice KC, Riley AL. Effects of stress modulation on morphine-induced conditioned place preferences and plasma corticosterone levels in Fischer, Lewis, and Sprague-Dawley rat strains. Psychopharmacology (Berl) 189: 277–286, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci USA 97: 6079–6084, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herod SM, Centeno ML, Bethea CL, Cameron JL. Evidence for a non-neuroendocrine role of corticotropin-releasing hormone (CRH) in female cynomolgus monkeys sensitive to stress-induced reproductive dysfunction (Abstract No. 283.3). Program of the 38th Annual Meeting of the Society for Neuroscience, Washington, DC, 2008 [Google Scholar]
- 28a. Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab (First published October 19, 2010). doi: 10.1152/ajpendo.00224.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsin LW, Tian X, Webster EL, Coop A, Caldwell TM, Jacobson AE, Chrousos GP, Gold PW, Habib KE, Ayala A, Eckelman WC, Contoreggi C, Rice KC. CHR-R1 receptor binding and lipophilicity of pyrrolopyrimidines, potential nonpeptide corticotropin-releasing hormone type 1 receptor antagonists. Bioorg Med Chem 10: 175–183, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Jensen JT, Zelinski MB, Stanley JE, Fanton JW, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception 77: 303–307, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Reproductive functions of corticotropin-releasing hormone. Research and potential clinical utility of antalarmins (CRH receptor type 1 antagonists). Am J Reprod Immunol 51: 269–274, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Khoury SA, Reame NE, Kelch RP, Marshall JC. Diurnal patterns of pulsatile luteinizing hormone secretion in hypothalamic amenorrhea: reproducibility and responses to opiate blockade and an alpha 2-adrenergic agonist. J Clin Endocrinol Metab 64: 755–762, 1986 [DOI] [PubMed] [Google Scholar]
- 33. Kondoh Y, Uemura T, Murase M, Yokoi N, Ishikawa M, Hirahara F. A longitudinal study of disturbances of the hypothalamic-pituitary-adrenal axis in women with progestin-negative functional hypothalamic amenorrhea. Fertil Steril 7: 748–752, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 83: 25–32, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Lindahl MS, Olovsson M, Nyberg S, Thorsen K, Olsson T, Poromaa IS. Increased cortisol responsivity to adrenocorticotropic hormone and low plasma levels of interleukin-1 receptor antagonist in women with functional hypothalamic amenorrhea. Fertil Steril 87: 136–142, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Liu JH. Hypothalamic amenorrhea: clinical perspectives, pathophysiology, and management. Am J Obstet Gynecol 163: 1732–1736, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Luton J, Thiebolt P, Valcke J, Mahoudeau JA, Bricaire H. Reversible gonadotropin deficiency in male Cushing's disease. J Clin Endocrinol Metab 45: 488–495, 1977 [DOI] [PubMed] [Google Scholar]
- 38. Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav 73: 147–158, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, Rice KC, Gravanis A, Chrousos GP. Corticotroopin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol 2: 1018–1024, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalmic amenorrhea. Fertil Steril 76: 310–316, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Meczekalski B, Tonetti A, Monteleone P, Bernardi F, Luisi S, Stomati M, Luisi M, Petraglia F, Genazzani AR. Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotropin-releasing hormone test. Eur J Endocrinol 142: 280–285, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol Endocrinol Metab 243: E310–E318, 1982 [DOI] [PubMed] [Google Scholar]
- 43. Myers DA, Gibson M, Schulkin J, Greenwood Van-Meerveld B. Corticosterone implants to the amygdala and type 1 CRH receptor regulation: effects on behavior and colonic sensitivity. Behav Brain Res 161: 39–44, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Nielsen DM, Carey GJ, Gold LH. Antidepressant-like activity of corticotropin-releasing factor type-1 receptor antagonists in mice. Eur J Pharmacol 499: 135–146, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Pohl CR, Knobil E. The role of the central nervous system in the control of ovarian function in higher primates. Annu Rev Physiol 44: 583–593, 1982 [DOI] [PubMed] [Google Scholar]
- 46. Pohl CR, Richardson DW, Hutchison JS, Germak JA, Knobil E. Hypophysiotropic signal frequency and the functioning of the pituitary-ovarian system in the rhesus monkey. Endocrinology 112: 2076–2080, 1983 [DOI] [PubMed] [Google Scholar]
- 47. Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab 61: 851–858, 1985 [DOI] [PubMed] [Google Scholar]
- 48. Rogers CJ, Brissette-Storkus CS, Chambers WH, Cameron JL. Acute stress impairs NK cell adhesion and cytotoxicity through CD2, but not LFA-1. J Neuroimmunol 99: 230–241, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 66: 733–739, 1988 [DOI] [PubMed] [Google Scholar]
- 50. Vogeser M, Engelhardt D, Jacob K. Comparison of two automated adrenocorticotropic hormone assays. Clin Chem 46: 1998–2000, 2000 [PubMed] [Google Scholar]
- 51. Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology 137: 5747–5750, 1996 [DOI] [PubMed] [Google Scholar]
- 52. Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab 293: E270–E276, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Nosbisch C, Cameron JL. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology 142: 2381–2389, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab 86: 5184–5193, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Wong ML, Webster EL, Spokes H, Phu P, Ehrhart-Bornstein M, Bornstein S, Park CS, Rice KC, Chrousos GP, Licinio J, Gold PW. Chronic administration of the non-peptide CRH type 1 receptor antagonist antalarmin does not blunt hypothalamic-pituitary-adrenal axis responses to acute immobilization stress. Life Sci 65: PL53–PL58, 1999 [DOI] [PubMed] [Google Scholar]