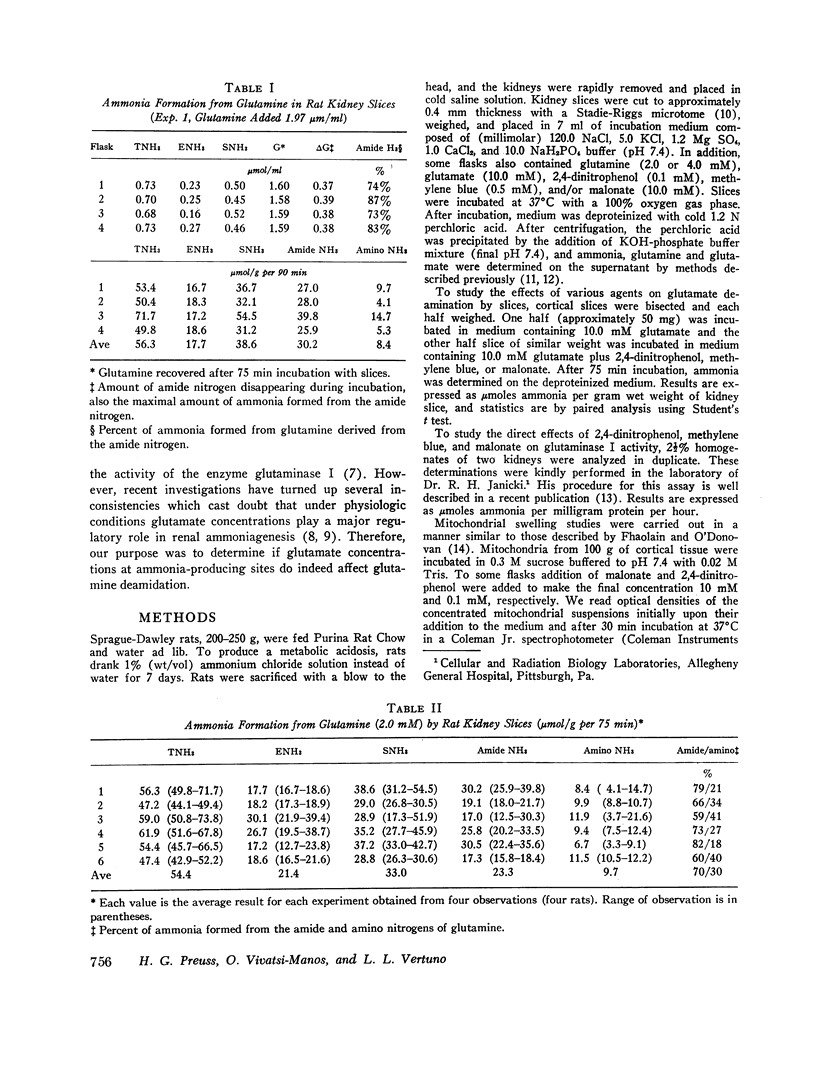
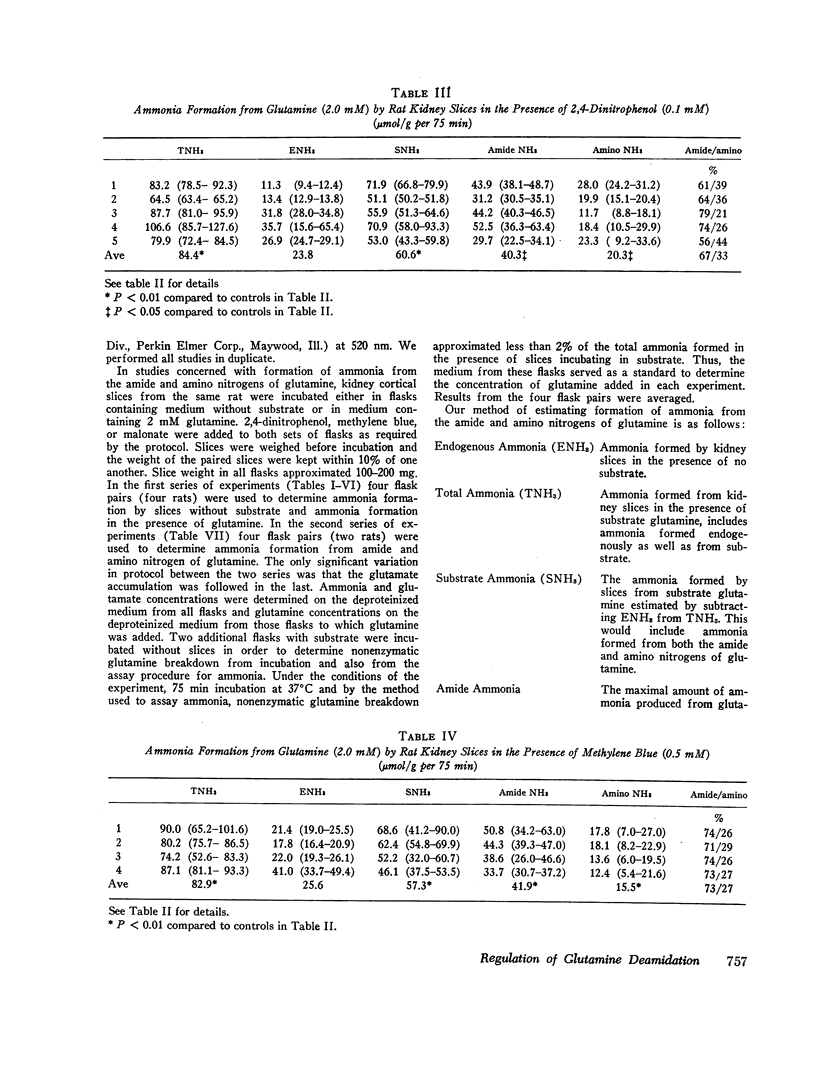
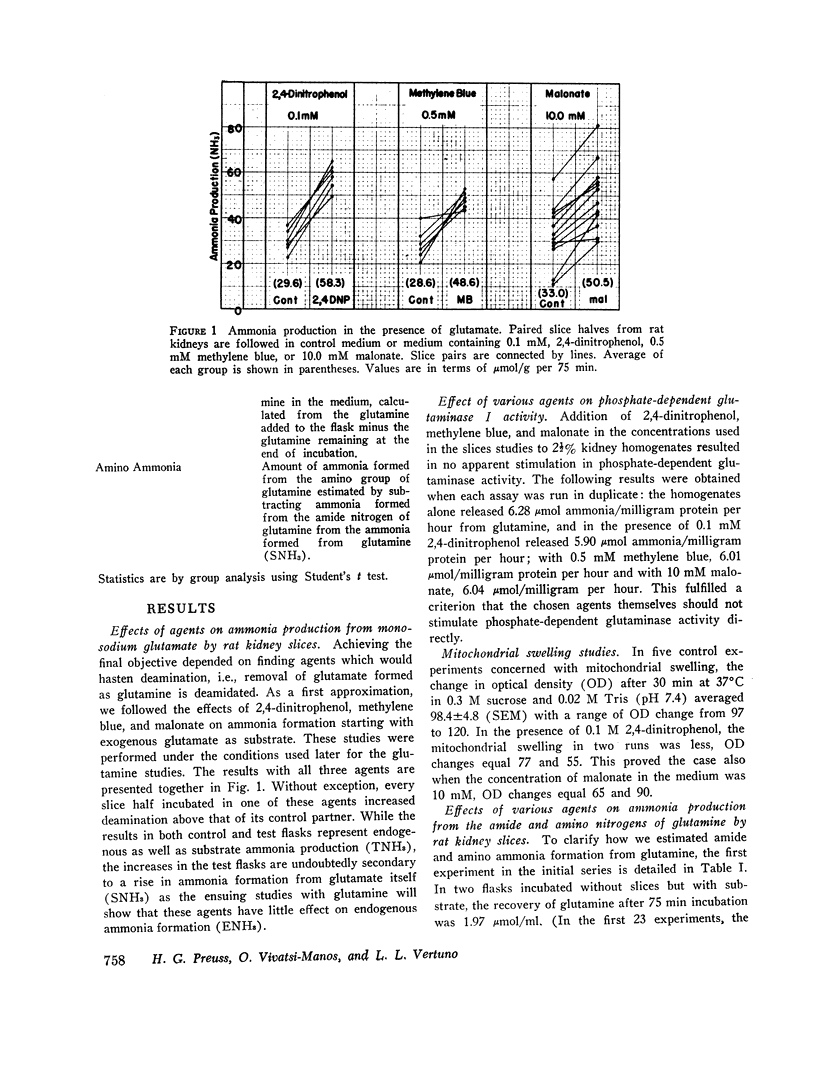
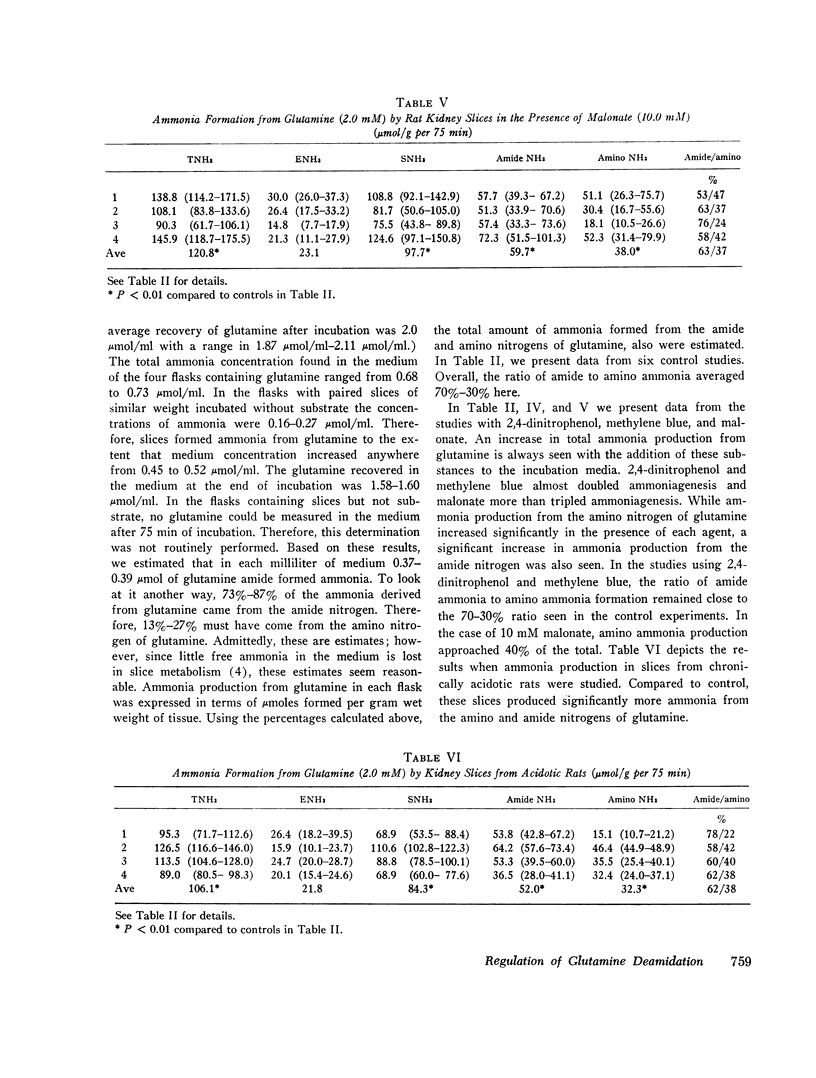
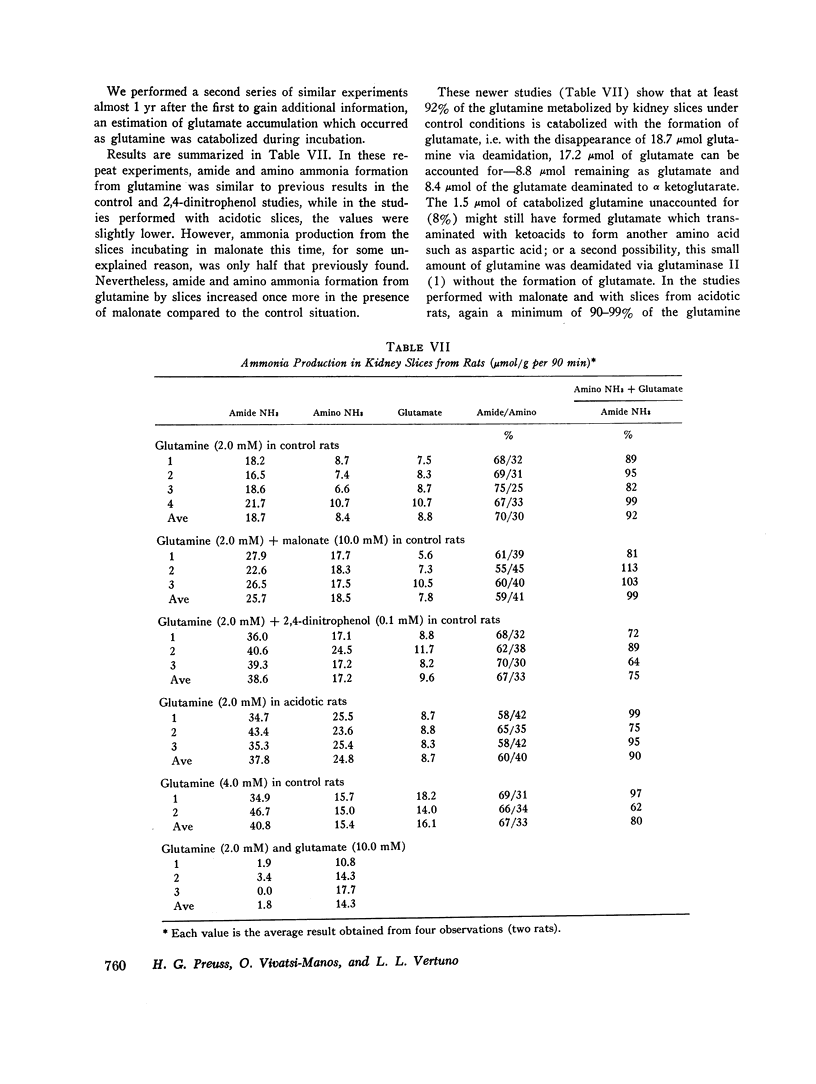
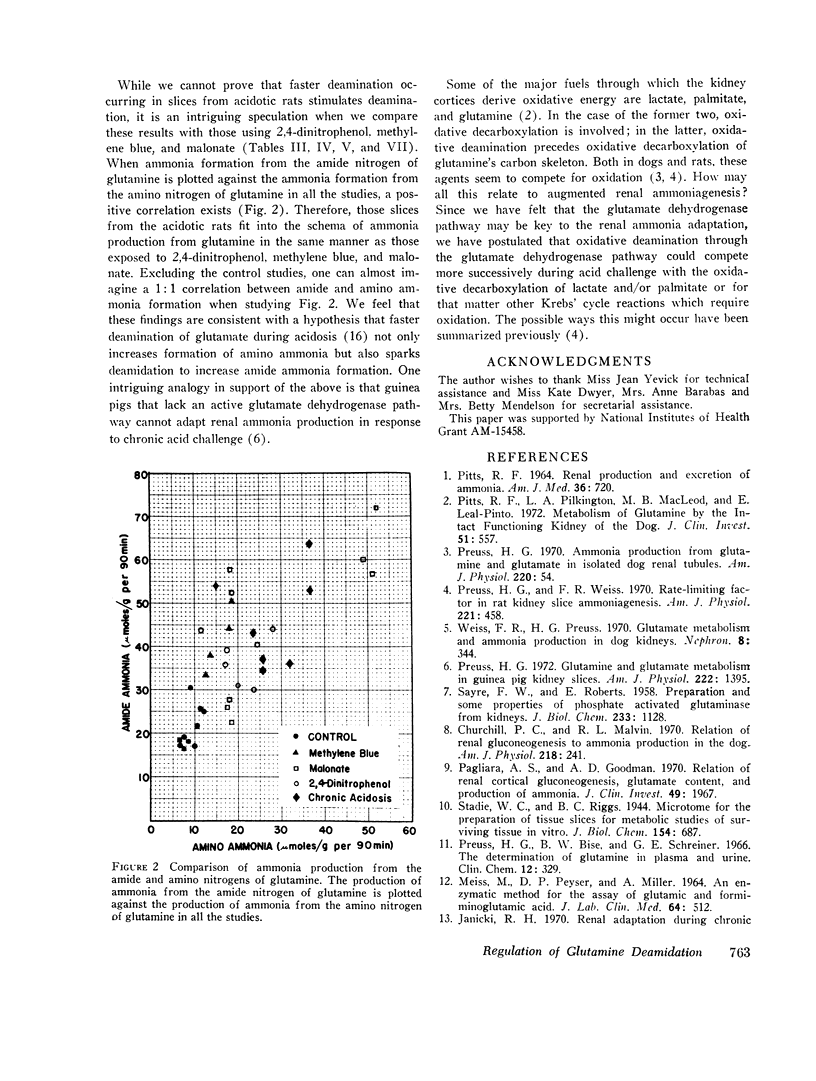
Abstract
Glutamate is known to inhibit the activity of isolated glutaminase I; however, its actual physiologic importance in regulating renal ammoniagenesis has not been established. To determine the regulatory role of glutamate on the metabolism of glutamine by rat kidney slices, we followed the effects on glutamine (2 mM) deamidation of increased removal of glutamate via augmented deamination. Three agents (malonate, 2,4-dinitrophenol, and methylene blue) were known to and shown here to hasten exogenous glutamate deamination. In slices from 10 control rats, 21.5±1.7 (SEM) μmol/g of ammonia were formed from amide nitrogen and 9.3±0.5 (SEM) μmol/g from the amino nitrogen of glutamine in vitro. Over 90% of the glutamine deamidated formed glutamate at one point in its catabolism. After addition of malonate (10 mM), 2,4-dinitrophenol (0.1 mM), or methylene blue (0.5 mM), the production of ammonia from the amino group rose to 29.3±6.0 (SEM) μmol/g, 20.0±1.8 (SEM) μmol/g, and 15.5±4.2 (SEM) μmol/g, respectively; ammonia production from the amide nitrogen rose also, 45.1±7.3 (SEM) μmol/g, 39.7±2.6 (SEM) μmol/g, and 41.9±3.7 (SEM) μmol/g. In the case of the former two, a minimum of 99% and 75% of the glutamine catabolized formed glutamate. Despite increased glutamine catabolism, there was no build up of glutamate in the media. A correlation between the formation of ammonia from the amino and amide nitrogen was apparent. Since none of the three agents selected affected phosphate activated glutaminase I activity directly or appeared to affect glutamine transport, we interpret the increase in deamidation as an expression of deinhibition of glutaminase I activity secondary to lowered glutamate concentrations at the deamidating sites through more rapid removal of glutamate via hastened deamination. Interestingly, slices removed from acidotic rats produced more ammonia from both the amino 29.1±3.8 (SEM) and amide nitrogens 45.9±4.3 (SEM) of glutamine, without a buildup of glutamate in the medium. At least 90% of the glutamine deamidated formed glutamate. A common mechanism is proposed to explain these results and the previous ones.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Churchill P. C., Malvin R. L. Relation of renal gluconeogenesis to ammonia production in the dog. Am J Physiol. 1970 Jan;218(1):241–245. doi: 10.1152/ajplegacy.1970.218.1.241. [DOI] [PubMed] [Google Scholar]
- Fhaolain I. N., O'Donovan D. J. A relationship between rat kidney mitochondrial swelling and glutaminase activation. Am J Physiol. 1971 Jul;221(1):58–63. doi: 10.1152/ajplegacy.1971.221.1.58. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Pathways of glutamine deamination and their control in the rat kidney. Am J Physiol. 1967 Oct;213(4):983–989. doi: 10.1152/ajplegacy.1967.213.4.983. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Hird F. J., Marginson M. A. The formation of ammonia from glutamine and glutamate by mitochondria from rat liver and kidney. Arch Biochem Biophys. 1968 Sep 20;127(1):718–724. doi: 10.1016/0003-9861(68)90282-8. [DOI] [PubMed] [Google Scholar]
- Janicki R. H. Renal adaptation during chronic NH4Cl acidosis in the rat: no role for hyperplasia. Am J Physiol. 1970 Sep;219(3):613–618. doi: 10.1152/ajplegacy.1970.219.3.613. [DOI] [PubMed] [Google Scholar]
- MEISS M., PEYSER D. P., MILLER A. AN ENZYMATIC METHOD FOR THE ASSAY OF GLUTAMIN AND FORMIMINOGLUTAMIC ACID. J Lab Clin Med. 1964 Sep;64:512–518. [PubMed] [Google Scholar]
- PITTS R. F., PILKINGTON L. A., DEHAAS J. C. N15 TRACER STUDIES ON THE ORIGIN OF URINARY AMMONIA IN THE ACIDOTIC DOG, WITH NOTES ON THE ENZYMATIC SYNTHESIS OF LABELED CLUTAMIC ACID AND GLUTAMINES. J Clin Invest. 1965 May;44:731–745. doi: 10.1172/JCI105186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTS R. F. RENAL PRODUCTION AND EXCRETION OF AMMONIA. Am J Med. 1964 May;36:720–742. doi: 10.1016/0002-9343(64)90182-2. [DOI] [PubMed] [Google Scholar]
- Pagliara A. S., Goodman A. D. Relation of renal cortical gluconeogenesis, glutamate content, and production of ammonia. J Clin Invest. 1970 Nov;49(11):1967–1974. doi: 10.1172/JCI106416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts R. F., Pilkington L. A., MacLeod M. B., Leal-Pinto E. Metabolism of glutamine by the intact functioning kidney of the dog. Studies in metabolic acidosis and alkalosis. J Clin Invest. 1972 Mar;51(3):557–565. doi: 10.1172/JCI106844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss H. G., Bise B. B., Schreiner G. E. The determination of glutamine in plasma and urine. Clin Chem. 1966 Jun;12(6):329–337. [PubMed] [Google Scholar]
- Preuss H. G. Glutamine and glutamate metabolism in guinea pig kidney slices. Am J Physiol. 1972 Jun;222(6):1395–1397. doi: 10.1152/ajplegacy.1972.222.6.1395. [DOI] [PubMed] [Google Scholar]
- Preuss H. G., Weiss F. R. Rate-limiting factor in rat kidney slice ammoniagenesis. Am J Physiol. 1971 Aug;221(2):458–464. doi: 10.1152/ajplegacy.1971.221.2.458. [DOI] [PubMed] [Google Scholar]
- SAYRE F. W., ROBERTS E. Preparation and some properties of a phosphateactivated glutaminase from kidneys. J Biol Chem. 1958 Nov;233(5):1128–1134. [PubMed] [Google Scholar]
- TAGGART J. V. Mechanisms of renal tubular transport. Am J Med. 1958 May;24(5):774–784. doi: 10.1016/0002-9343(58)90380-2. [DOI] [PubMed] [Google Scholar]
- TAPLEY D. F. The effect of thyroxine and other substances on the swelling of isolated rat liver mitochondria. J Biol Chem. 1956 Sep;222(1):325–339. [PubMed] [Google Scholar]
- Weiss F. R., Preuss H. G. Glutamate metabolism and ammonia production in dog kidneys. Nephron. 1971;8(4):344–354. doi: 10.1159/000179937. [DOI] [PubMed] [Google Scholar]


