Abstract
As insulin's movement from plasma to muscle interstitium is rate limiting for its metabolic action, defining the regulation of this movement is critical. Here, we address whether caveolin-1 is required for the first step of insulin's transendothelial transport, its uptake by vascular endothelial cells (ECs), and whether IL-6 and TNFα affect insulin uptake or caveolin-1 expression. Uptake of FITC-labeled insulin was measured using confocal microscopy in control bovine aortic ECs (bAECs), in bAECs in which caveolin-1 was either knocked down or overexpressed, in murine ECs from caveolin-1−/− mice and in bAECs exposed to inflammatory cytokines. Knockdown of caveolin-1 expression in bAECs using specific caveolin-1 siRNA reduced caveolin-1 mRNA and protein expression by ∼70%, and reduced FITC-insulin uptake by 67% (P < 0.05 for each). Over-expression of caveolin-1 increased insulin uptake (P < 0.05). Caveolin-1-null mouse aortic ECs did not take up insulin and re-expression of caveolin-1 by transfecting these cells with FLAG-tagged caveolin-1 DNA rescued FITC-insulin uptake. Knockdown of caveolin-1 significantly reduced both insulin receptor protein level and insulin-stimulated Akt1 phosphorylation. Knockdown of caveolin-1 also inhibited insulin-induced caveolin-1 and IGF-1 receptor translocation to the plasma membrane. Compared with controls, IL-6 or TNFα (20 ng/ml for 24 h) inhibited FITC-insulin uptake as well as the expression of caveolin-1 mRNA and protein (P < 0.05 for each). IL-6 or TNFα also significantly reduced plasma membrane-associated caveolin-1. Thus, we conclude that insulin uptake by ECs requires expression of caveolin-1 supporting a role for caveolae mediating insulin uptake. Proinflammatory cytokines may inhibit insulin uptake, at least in part, by inhibiting caveolin-1 expression.
Keywords: caveolae, proinflammatory cytokine, insulin uptake, endothelial cells
for insulin to stimulate skeletal muscle glucose metabolism, it must first access and then traverse muscle's vascular endothelium. Work from several laboratories has shown that insulin delivery from plasma to the interstitial fluid compartment of skeletal muscle is a rate-limiting step in insulin's peripheral action (23, 51). Muscle blood flow (3), flow distribution(46), and the transendothelial insulin transport (TET) (19, 47) all contribute to insulin delivery. Insulin's action to increase muscle blood flow and recruit microvasculature indicates that insulin promotes its own delivery. Insulin delivery to muscle interstitium is delayed in insulin-resistant subjects, suggesting that vascular insulin resistance contributes to muscle metabolic insulin resistance (24, 43). Although insulin's vasodilatory effects occur via enhanced nitric oxide production (3, 46), the pathways involved in transendothelial insulin transport and the factors that might contribute to it being slowed by insulin resistance are not clear. Insulin uptake, the first step of TET, is an insulin receptor-mediated process and closely related to subsequent transendothelial transport (39, 47, 49). We and others (39, 47) have previously shown that filipin, a detergent that disrupts lipid rafts, reduces insulin uptake, consistent with involvement of caveolae in insulin transendothelial transport. However, this cholesterol interacting detergent also affects non-caveolae lipid rafts, and other nonspecific effects cannot be excluded.
Insulin resistance is closely associated with low-grade chronic “inflammation” characterized by abnormal cytokine production (33). Tumor necrosis factor-α (TNFα) induces vascular insulin resistance both in vivo (53) and in vitro (20) by interfering with vascular endothelial cell (EC) insulin signaling.
Caveolin-1, a 21-kDa integral membrane protein, is required for caveolae formation (36). Downregulation of caveolin-1 expression has been reported to significantly reduce albumin uptake by ECs (41). However, the impact of altering caveolin-1 expression on insulin transport has not been identified.
In the present study, we sought to determine the involvement of caveolin-1 in insulin uptake by ECs and test whether experimental insulin resistance, produced by IL-6 or TNFα affected caveolin-1 abundance and insulin uptake. TNFα was chosen because of its demonstrated effect to produce vascular insulin resistance (see above). IL-6 was examined because in muscle it is induced by exercise and its effect on insulin sensitivity is controversial (5), with studies showing beneficial metabolic effects (30) and studies linking it to insulin resistance, obesity, and type 2 diabetes (10, 31, 32). The effect of IL-6 on insulin uptake had not previously been examined.
MATERIALS AND METHODS
Cell culture.
Bovine aorta ECs (bAECs; BioWhittaker, Walkersville, MD; passage nos. 2–8) and caveolin-1−/− mouse aortic ECs (MAECs; passage nos. 5–7; a gift from Dr. Martin A. Schwartz, University of Virginia) were grown in EGMMV and EGM-2 medium, respectively in eight-well slide chambers for immunocytochemical staining or in six-well plates for Western blot (see below).
Small interfering RNA design and transfection.
A specific small interfering RNA (siRNA) duplex was designed as described previously (50) and purchased from Dharmacon (Lafayette, CO) along with a scrambled control siRNA (siCONTROL Non-Targeting siRNA #3). BAECs were seeded and transfected with siRNA duplex to a final concentration of 30 nM (or as noted) using oligofectamine (Invitrogen, Carlsbad, CA) when cells reached 30–50% confluence. Forty-eight hours after transfection, cells were serum starved followed by treatment with 50 nM insulin for 30 min. Cells were then either fixed with cold methanol for 10 min at −20°C before immunocytochemical staining (see below) or washed twice with ice-cold 1× PBS solution and lysed in ice-cold lysis buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% NP40, 0.25% sodium deoxycholate, 1 mM EGTA, 1 mM sodium orthovanadate, 1 mM NaF, 1 μg/mL aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 mM phenylmethylsulfonyl fluoride). Cell lysates were centrifuged for 10 min at 4°C (14,000 g), and the supernatants were used for Western blotting (see below).
DNA constructs and transfection.
A plasmid encoding FLAG-tagged wild-type caveolin-1 was obtained from Dr. Martin A. Schwartz (8). The plasmid DNA for transfections was purified by EndoFree Plasmid Maxi kit (Qiagen). The proper orientation and sequence were verified by sequencing. The caveolin-1−/− MAECs were grown to ∼70% confluence in eight-well slide chambers and then transfected with 0.4 μg of total DNA using Lipofectamin2000 according to the manufacturer's instructions (Invitrogen). Forty-eight hours after transfection, cells were serum starved for 6 h followed by incubation with 50 nM FITC-insulin for 30 min, as described above.
Proinflammatory cytokine treatment.
The bAECs were incubated in serum-free basal medium for 24 h in the presence or absence of TNFα (20 ng/ml) or IL-6 (20 ng/ml) (37) (Sigma-Aldrich, St. Louis, MO) and then treated with either 50 nM FITC-insulin (Molecular Probes, Eugene, OR) or regular insulin (Humulin R, Eli Lilly, Indianapolis, IN) for 30 min at 37°C as described above.
Western blotting.
Equal protein-containing cell lysates were diluted with 6× sample buffer (375 mM TrisHCl, pH 6.8, 12% SDS, 60% glycerol, 300 mM DTT, 0.06% bromophenol blue), and boiled for 5 min. The loaded samples were then electrophoresed on polyacrylamide gels and transferred to nitrocellulose membranes. After being blocked with 5% low-fat milk in Tris-buffered saline plus Tween 20, membranes were incubated with antibody against caveolin-1 (Santa Cruz Biotechnology, Santa Cruz, CA), or insulin receptor (IR)β, or IGF-IRβ (Santa Cruz Biotechnology), phospho-Akt1 (Ser473; Upstate Cell Signaling, Lake Placid, NY), or total Akt, phospho-ERK1/2 (Thr202/Tyr204), or total ERK1/2 (Cell Signaling Technology, Beverly, MA) or β-actin or GAPDH (Sigma-Aldrich), respectively, overnight at 4°C. This was followed by incubation with a species-specific secondary antibody coupled to horseradish peroxidase (Amersham Life Sciences, Piscataway, NJ), and the blots were developed using an enhanced chemiluminescence Western blotting kit (Amersham Life Sciences). The developed films were scanned using a densitometer (Molecular Dynamics, Piscataway, NJ) and quantified using ImageQuant 5.0 software. In experiments examining overexpression of caveolin-1 in bAECs, simultaneous detection of caveolin-1 and FLAG was carried out using an Odyssey LI-COR infrared imaging system (LI-COR Biosciences) following the manufacturer's protocols. The primary antibodies used were a mouse monoclonal anti-FLAG (Sigma) and a rabbit polyclonal anti-caveolin-1 (Santa Cruz Biotechnology).
Real-time RT-PCR.
Total RNAs were extracted from the cultured bAECs with an RNeasy kit (Qiagen). A total of 2 μg of total cellular RNA from each sample was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). The cDNA products were then amplified using the iQ SYBR Green supermix and the iCycler apparatus (Bio-Rad). For the amplification of caveolin-1 gene products, the following primers were designed: forward 5′-AGCCCAACAACAAGGCTATG-3′ and reverse 5′-GATGCCATCGAAACTGTGTG-3′, and the caveolin-1 mRNA levels were normalized to the housekeeping gene (GAPDH) mRNAs (primers designed: forward 5′-gggtcatcatctctgcacct-3′, and reverse 5′-ggtcataagtccctccacga-3′) (Integrated DNA Technologies, Coralville, IA). Standard curves for each mRNA were generated by serial dilution of cDNA synthesized from the extracted total RNA and were included in each iCycler real-time PCR experiment. The specificity of the desired product was verified by the analysis of the melting curve.
Plasma membrane sheet preparation.
Plasma membrane sheets were prepared as previously described (50). Briefly, bAECs were cultured on a coverslip in the wells of six-well plates and treated as described above. The cells were washed once with ice-cold PBS followed by incubation on ice with 0.5 mg/ml of poly-l-lysine in PBS for 30 s. The cells were then washed three times with ice-cold hypotonic buffer (23 mM KCl, 10 mM HEPES, pH7.5, 2 mM MgCl2, 1 mM EGTA). The swollen cells were then sonicated for 3 s (550 Fisher sonic dismembrator) in ice-cold sonication buffer (70 mM KCl, 30 mM HEPES, pH7.5, 5 mM MgCl2, 3 mM EGTA, 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride) followed by two washes with the ice-cold sonication buffer and used for immunocytochemical staining (see below).
Cell viability tests.
To assess any contribution of nonspecific cell injury caused by 24 h of serum-free culture in the presence of either 20 ng/ml IL-6 or 20 ng/ml TNFα, we used LDH (lactate dehydrogenase) leakage, intracellular ATP concentration (Promega), and trypan blue staining (Mediatech) as measures of cell viability following the manufacturer's protocols.
Immunocytochemistry.
After fixation, cells were washed three times in TBS, permeabilized in TBS containing Triton X-100 (0.05%) and 1% horse serum for 30 min at room temperature, and incubated with two different primary antibodies against two different target proteins (double labeling) overnight at 4°C. Primary antibodies used were mouse monoclonal anti-caveolin-1 and rabbit polyclonal anti-fluorescein (FITC) (Molecular Probes), rabbit polyclonal anti-IRβ and rabbit polyclonal anti-IGF-IRβ (Santa Cruz Biotechnology). The cells were washed three times in PBS and then incubated with species-specific secondary antibodies conjugated with fluorochrome for 45 min at room temperature. The following secondary antibodies were used: donkey anti-rabbit IgG conjugated to Cy3 and donkey anti-mouse IgG conjugated to Cy2 (Jackson ImmunoResearch, West Grove, PA). The cells were washed three times in TBS and then coverslipped with the anti-fade mounting medium.
Imaging.
The double immunocytochemical labeling was examined simultaneously using a two-color Olympus BX50 WI confocal microscope equipped with Krypton and Argon laser as described previously (47, 49). An x-y-z axis scanning method was employed. The images were scanned through up to ×100 objectives, and stored in 24-bit TIFF format. To demonstrate that the specific immunoreactivity was located within cells, a series of optical sections at a step size of 0.6 μm was acquired from the top to the bottom of the cells along the z-axis and saved individually. Fluorescence intensity of individual cells reflecting FITC-insulin uptake was quantified using Image J software (National Institutes of Health) as described previously (49). Briefly, 45 cells (or as noted) were measured for each treatment group. All images from each experimental group were processed identically. During image acquisition, the individual microscopic field was selected to include at least 15 cells but was otherwise random. Individual cells were outlined by the polygonal tool, and the integrated fluorescence intensities were measured. To quantify fluorescence intensity of the staining from plasma membrane (PM) sheets, the images from randomly selected microscopic fields containing stained PM sheets were outlined, and the integrated fluorescence intensities were measured using the Image J software as described previously (50).
Statistical analysis.
Data are presented as mean ± SE. Statistical comparisons among different groups were made using one-way ANOVA with post hoc testing performed by the method of Student-Newman-Keuls. Statistical significance was defined as P ≤ 0.05.
RESULTS
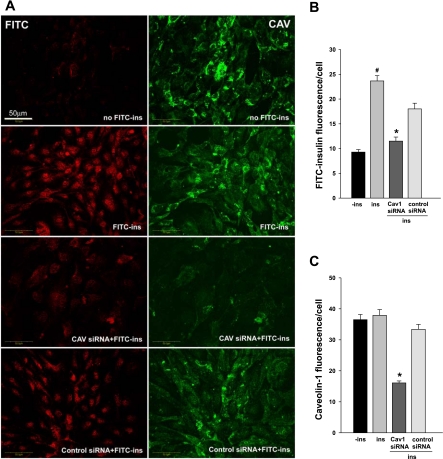
First, we used siRNA to decrease caveolin-1 mRNA and protein and assess its effect on FITC-insulin uptake by cultured bAECs. Supplemental Fig. 1 illustrates that, compared with the scrambled siRNA control, the caveolin-1 siRNA reduced caveolin-1 expression by ∼70% by 48 h after transfection as determined by either real-time RT-PCR (Supplemental Fig. 1C; supplementary materials are found in ther online version of this paper at the Journal website) or Western blot (Supplemental Fig. 1B) (P < 0.05, in each case). Figure 1 A, right, and C show that transfection with caveolin-1 siRNA reduced caveolin-1 staining (P < 0.05) compared with the scrambled siRNA control. Although delivering scrambled siRNA to bAECs appeared to have a small but significant (P < 0.05) negative effect on FITC-insulin uptake, knockdown of caveolin-1 significantly reduced FITC-insulin uptake compared with the scrambled controls (P < 0.05; Fig. 1A, left, and B). FITC-insulin uptake, assessed by confocal microscopy, does not reflect nonspecific surface binding phenomena (49) but movement to an intracellular locus. The observation that decreasing caveolin-1, an essential structural protein of caveolae (9), simultaneously inhibited insulin uptake is consistent with a role for caveolin-1/caveolae in insulin transport (12, 26).
Fig. 1.
Effect of siRNA-mediated knockdown of caveolin-1 (Cav1) expression on insulin uptake. Bovine aorta endothelial cells (bAECs) were transfected with either caveolin-1 siRNA or control siRNA. Forty-eight hours after transfection, cells were serum starved for 16 h followed by incubation with or without 50 nM FITC-insulin (FITC-Ins) for 30 min. Cells then were fixed and doubly stained using anti-FITC (red, revealed by Cy3, left) and anti-caveolin-1 (green, revealed by Cy2, right) primary antibodies. A: confocal images from single optical sections. Histograms indicate quantitation of FITC-insulin (B) and caveolin-1 (C) fluorescence intensity observed in 3 experiments [*P < 0.05 vs. Ins (FITC-insulin treated without transfection of siRNA) or control siRNA; #P < 0.05 vs. all groups]. −Ins, incubated in basal medium without FITC-insulin.
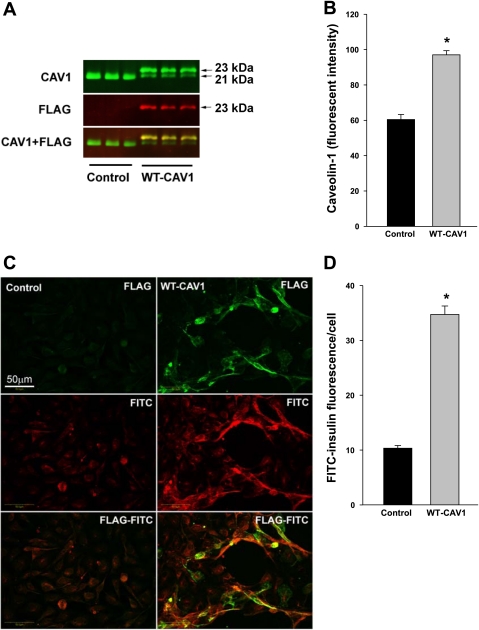
Next, we examined the effects of overexpression of caveolin-1 in cultured bAECs on FITC-insulin uptake. As shown in Fig. 2A, the transfection of FLAG-tagged caveolin-1 into the bAECs resulted in expression of FLAG-tagged as well as native caveolin-1 protein (Fig. 2, A and B). Although in the cells transfected with FLAG-tagged caveolin-1 the endogenous caveolin-1 protein expression appeared to decrease (the mechanism by which this occurred is not clear), the total caveolin-1 expression (endogenous + transfected) significantly increased by over 30% compared with the control (Fig. 2, A and B). Cells selected for their expressing abundant FLAG-caveolin-1 (as shown by fluorescence with anti-FLAG) had a significant 2.5-fold increased FITC-insulin uptake (Fig. 2C) compared with control cells (P < 0.05; Fig. 2D). To further examine the role of caveolin-1 in mediating insulin uptake, we incubated MAECs from caveolin-1 knockout mice with FITC-insulin. The caveolin-1−/− MAECs failed to take up FITC-insulin (supplemental Fig. 2, left) relative to background. However, transfecting these caveolin-1−/− MAECs with FLAG-tagged caveolin-1 DNA restored FITC-insulin uptake (supplemental Fig. 2, right), again indicating a requirement for caveolin-1 in mediating insulin uptake.
Fig. 2.
Overexpression of FLAG-tagged caveolin-1 in bAECs enhanced FITC-insulin uptake. Top: 48 h after bAECs ± transfection with FLAG-tagged WT caveolin-1 DNA, cells were processed for simultaneous detection of caveolin-1 and FLAG using an Odyssey LI-COR infrared imaging system. A: representative blots. B: quantification of changes (n = 3). Each bar represents a summation of endogenous caveolin-1 and FLAG-caveolin-1. Bottom: 48 h after bAECs ± transfection with FLAG-tagged caveolin-1 DNA, cells were serum starved for 6 h followed by treatment with 50 nM FITC-insulin for 30 min. Cells were doubly immunostained for FITC and FLAG. C: representative confocal images (single optical sections) stained using anti-FITC (red, revealed by Cy3) and anti-FLAG (green, revealed by Cy2) primary antibodies. D: average fluorescent intensity of individual cells measured by Image J software (n = 24; counting 8 cells randomly selected from either the control group or FLAG-tagged caveolin-1-transfected group in each of 3 independent experiments). In the FLAG-tagged caveolin-1-transfected group, only strongly FLAG-positive cells were selected for counting. CAV1, caveolin-1; WT-CAV1, FLAG-tagged WT caveolin-1.*P < 0.05 vs. control group.
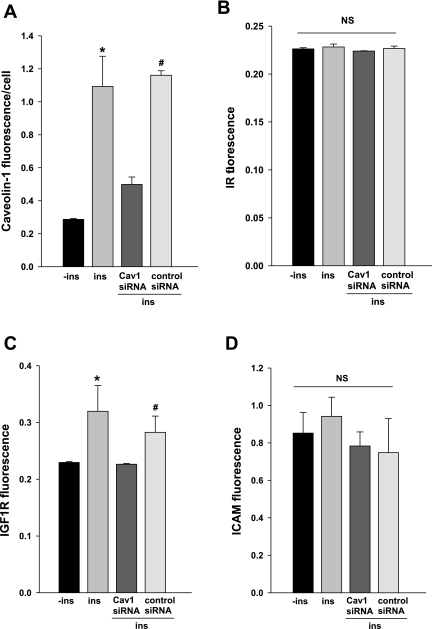
Since caveolin-1 not only plays a role in formation of transporting caveolae but is enriched with insulin receptors (11) and interacts with a variety of signaling molecules through its scaffolding domain (34), we examined whether decreasing caveolin-1 interfered with insulin signaling, thereby affecting insulin uptake. Figure 3A shows that insulin treatment for 30 min did not significantly change the caveolin-1, IR, or IGF-1R content in either nontransfected bAECs or the cells transfected with either caveolin-1 siRNA or control siRNA (P > 0.05 for each). Knockdown of caveolin-1 (Fig. 3B) significantly decreased cellular IR (P < 0.05; Fig. 3C) but not IGF-1R (Fig. 3D) protein (P > 0.05). We also observed that downregulating caveolin-1 decreased insulin-induced phosphorylation of Akt1 compared with either nontransfected or scrambled siRNA transfected group (P < 0.05 for each). Compared with the scrambled siRNA control group, insulin stimulation resulted in only a weak (P > 0.05) increase in phosphorylation of Akt1 in the caveolin-1 siRNA group (Fig. 3E). Curiously, we found that delivering either specific siRNA against caveolin-1 or scrambled siRNA significantly increased basal ERK1/2 phosphorylation, and adding insulin had no further effect (Supplemental Fig. 3). This suggests that the transfection process per se had a nonspecific stimulatory effect on ERK1/2 phosphorylation, and this might have impeded our detecting any further insulin-stimulated phosphorylation of this kinase. These data suggest that knockdown of caveolin-1 might, at least in part, impair intracellular insulin signaling.
Fig. 3.
Effects of knockdown of caveolin-1 on insulin receptor (IR) or IGF-I receptor (IGF-1R) protein level and insulin-stimulated Akt1 phosphorylation. bAECs ± transfection of either caveolin-1-siRNA or scrambled siRNA (control) duplex were serum starved for 6 h followed by ±insulin treatment for 30 min, and then cell lysates were separated on a 4–20% Tris·HCl Criterion precast gel. A: representative blots. B: quantification of caveolin-1. *P < 0.05 vs. controls in both no-siRNA and control siRNA groups, respectively. C: quantification of IR. *P < 0.05 vs. controls in both no-siRNA and control siRNA groups, respectively. D: quantification of IGF-1R. E: quantification of phospho-Akt1. *P < 0.05 vs. insulin-free control, both insulin free and insulin stimulated, in caveolin-1 siRNA group and insulin free in control siRNA group, but P > 0.05 vs. insulin-stimulated in control siRNA group, respectively; #P < 0.05 vs. insulin-free control and both insulin-free and insulin-stimulated in caveolin-1 siRNA group, respectively. Blots are representative of 4–6 independent experiments. NS, not significant statistically.
We also examined the effects of knockdown of caveolin-1 on the localization of both IR and IGF-1R at the PM by using PM sheet preparations (50). Figure 4 (also see Supplemental Fig. 4) shows that with 6-h serum starvation, knockdown of caveolin-1 significantly reduced the amount of caveolin-1 at the PM (50) compared with the scrambled siRNA control (Fig. 4A), but the localization of IR to the PM appeared not to be significantly affected by either insulin treatment or the downregulation of caveolin-1 (Fig. 4B). However, with this period of serum starvation (6 h), insulin treatment induced a significant increase of IGF-1R in the PM, and knockdown of caveolin-1 eliminated the insulin's effect (Fig. 4C). It has been reported that the IGF-1Rs are as much as 10-fold more abundant than IRs in vascular ECs (1). The low IR abundance expressed in ECs may have limited our ability to detect changes with this PM sheet preparation. These data, in aggregate, suggest that the inhibition of insulin uptake caused by downregulation of caveolin-1 expression may be mainly due to reduction of transporting cavaolae, but the reduction of both IR expression and insulin-induced Akt phosphorylation by knockdown of caveolin-1 might also contribute to the reduced FITC-insulin uptake.
Fig. 4.
Knockdown of caveolin-1 inhibited insulin-induced translocation of caveolin-1 and IGF-1R but did not significantly affect the amount of IR in the plasma membrane (PM). bAECs ± transfection of caveolin-1 siRNA (CAV) or scrambled siRNA duplex were serum starved for 6 h followed by insulin treatment for 30 min. PM sheets were prepared followed by double staining for CAV-1 and IR or IGF-1R or intercellular adhesion molecule-1(ICAM; used as a control). For illustrations see Supplemental Fig. 3. A–D: quantification of changes in fluorescence intensity of caveolin-1, IR, IGF-1R, and ICAM, respectively. *P < 0.05 vs. −ins (neither insulin added nor siRNA transfected) or caveolin-1 siRNA; #P < 0.05 vs. caveolin-1 siRNA, but P > 0.05 vs. Ins (insulin treated but not siRNA transfected).
We next examined the effects of 20 ng/ml IL-6 or 20 ng/ml TNFα for 24 h (37) on FITC-insulin uptake. Figure 5 shows that IL-6 treatment nearly completely abolished FITC-insulin uptake and TNFα treatment reduced FITC-insulin uptake by ∼70% (P < 0.05 for each). These effects of IL-6 or TNFα to inhibit insulin uptake did not appear to be secondary to loss of cell viability. Compared with control cells, there were no significant changes in LDH leakage (Supplemental Fig. 5A), intracellular ATP content (Supplemental Fig. 5B), or trypan blue staining (not shown) after treatment with either cytokines.
Fig. 5.
Exposure to either IL-6 or TNFα inhibited FITC-insulin uptake by cultured bAECs. Cultured bAECs were serum starved in the absence or presence of IL-6 or TNFα (20 ng/ml each) for 24 h before incubation with 50 nM FITC-insulin (INS) for 30 min. Cells were then processed for immunocytochemical staining. A: confocal images (single optical sections) stained using anti-FITC primary antibody (revealed by Cy3). B: average fluorescent intensity of individual cells measured by Image J software (counting 15 randomly selected cells in each of 3 independent experiments). *P < 0.05 vs. all other groups. −INS, incubated in basal medium only.
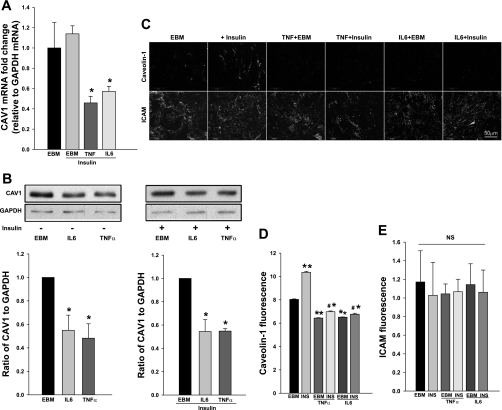
We next examined caveolin-1 expression by the bAECs treated with either IL-6 or TNFα. Figure 6 shows that, compared with the control, IL-6 and TNFα each inhibited both caveolin-1 mRNA (Fig. 6A) and its protein expression (Fig. 6B) by ∼50% as well as the caveolin-1 amount on the PM (Fig. 6, C–E) irrespective of whether insulin was present (P < 0.05 for each). The finding that both insulin uptake and caveolin-1 expression are impaired by IL-6 and TNFα suggests that these agents may inhibit insulin uptake, at least in part, by impairing caveolin-1 synthesis.
Fig. 6.
IL-6/TNFα inhibited caveolin-1 expression. bAECs ± IL-6 or TNFα (20 ng/ml each) were serum starved for 24 h then for an additional 30 min in absence or presence of insulin. Cells were then processed for real-time RT-PCR (A) or Western blotting (B) or preparation of PM sheets (PM) for immunocytochemical staining (C). D: quantification of changes in fluorescence intensity of caveolin-1 in the PM; E: quantification of changes in fluorescence intensity of ICAM (used as a control) in the PM. *P < 0.05 vs. endothelial basal medium (EBM) + insulin (A) or EBM (B), respectively; **P < 0.001 vs. EBM, #P < 0.001 vs. insulin only (INS) group, and *P < 0.05 vs. basal medium only (EBM); (D); NS, not statistically significant (E).
DISCUSSION
The findings presented here provide substantial evidence that caveolin-1 is required for normal insulin uptake by endothelial cells. Additionally, these data indicate that the proinflammatory cytokines IL-6 and TNFα may reduce insulin uptake, at least in part, by inhibiting caveolin-1 synthesis.
Caveolae are abundant in microvasculature, where (depending on the tissue) they can constitute ∼15% of the total endothelial cell volume (42). Several laboratories have reported that IRs colocalize with caveolae in the PM of adipocytes (14, 18). While this was not without some controversy, recent EM immunocytochemsitry has convincingly shown that IRs are present throughout in the PM but are particularly concentrated at the neck of caveolae in 3T3-L1 adipocytes (11). IGF-1Rs appear to have similar lipid raft/caveolae localization in the PM (16). We (47) have previously reported that antibodies against IR and caveolin-1 mutually coimmunoprecipitate one another from bAECs, and others have reported that IR binds to caveolin-1 scaffolding domain through its caveolin-1 binding domain (7, 27). IRs appear to mediate not only insulin uptake but also insulin TET when physiological insulin concentrations were used (2, 19). Lack of saturation of insulin TET in muscle observed in some in vivo studies appears to be due to the higher insulin doses used in these studies (4, 15, 45), which would allow additional binding and transport via IGF-1Rs and hybrid IR/IGF-1R, both of which are abundant on vascular ECs (21, 47). Using the same concentration of FITC-insulin (50 nM) as used in present study, we previously showed that both unlabeled insulin and IGF-I peptides compete with FITC-insulin for TET across bAECs grown on Transwell plates, indicating that both IR and IGF-1R can mediate insulin TET (47).
Direct microscopic studies have shown that insulin TET follows a transcellular pathway in vivo (47). Consistent with this, depletion of caveolae by use of filipin strongly inhibits vascular endothelial insulin uptake (47) and TET (39) without affecting TET of the polysaccharide inulin [which is similar to insulin in size but crosses the endothelium via a nonmediated diffusional path (39)]. Compartmentation of insulin to caveolae might allow efficient and rapid coupling of activated receptors to one or more effector systems (13, 38). Interestingly, albumin can bind to a surface receptor and initiate a process that enhances caveolin-1 oligomerization, caveolae formation, and albumin internalization within caveolae and its TET (25, 44).
That caveolin-1 is required for the formation of caveolae has been demonstrated by the observations that either targeted deletion of the gene for caveolin-1 (Cav-1−/−) (9) or knockdown of caveolin-1 expression by use of specific siRNA (26) results in the loss of caveolae. In contrast, expression of exogenous caveolin-1 in lymphocytes (which are normally devoid of caveolae) induces the de novo formation of caveolae, and the level of caveolin-1 expression is directly correlated to the number of caveolae (12). Another recently described protein (Cavin/PTRF) is also required for normal caveolae formation (22), but its abundance in vascular ECs appears to be very low, and we failed to detect it using Western blots (data not shown). In addition, its role in endocytosis of proteins has not, to our knowledge, been explored. Until recently, caveolae were thought to be sessile structures with few free caveolae detectable in the cytosol of ECs (35). However, a recent study using total internal reflectance microscopy has demonstrated the dynamic nature of caveolae trafficking in living cells, with about one-third of total cellular caveolae engaged in a rapid, local fission-fusion cycle with the PM (29).
The mechanism by which caveolin-1 mediates insulin uptake is not clear. Caveolae not only mediate endocytosis/transcytosis but also serve to regulate intracellular signaling by a variety of ligands via caveolin-1's scaffolding domain. We have previously shown that inhibition of insulin signaling at either PI 3-kinase or ERK1/2 significantly reduces EC FITC-insulin uptake (49). In the present study, we found that knockdown of caveolin-1 decreased the amount of cellular IR (but not IGF-1R) in bAECs and eliminated insulin-stimulated IGF-1R recruitment to the PM. In the present study, caveolin-1 knockdown did not significantly affect insulin-stimulated IR recruitment to the PM. Compared with IGF-1R, bAECs express many fewer IRs (1), and this low abundance may have possibly limited our ability to detect small changes at the PM. Our present finding is consistent with a role of IGF-1Rs (in addition to IRs) in mediating insulin uptake when supraphysiological insulin concentration is applied (50 nM) (47). It has been previously reported that in caveolin-1-null mice IR protein (not mRNA) expression was selectively inhibited in adipose tissue. This may have contributed to the insulin resistance seen in these mice (6). Here, we also found that knockdown of caveolin-1 appeared to downregulate cellular IR protein expression (Fig. 3C). Consistent with this observation, knockdown of caveolin-1 also appeared to blunt insulin-stimulated Akt1 phosphorylation. This is consistent with the previous finding in adipose tissue of caveolin-1 knockout mice (6). This is also consistent with our previous observation that insulin signaling plays an important role in mediating its own uptake by vascular ECs (49). On the other hand, insulin uptake by the cells transfected with the scrambled siRNA was slightly lower than in the control nontransfected cells. That might be attributed to a modest, nonspecific decrease of caveolin-1 expression. However, insulin uptake by cells transfected with scrambled siRNA was still significantly greater than by cells treated with caveolin-1 siRNA with a comparable insulin-stimulated Akt1 phosphorylation level. By contrast, transfection with caveolin-1 siRNA resulted in an ∼70% reduction of caveolin-1 expression and a comparable inhibition of FITC-insulin uptake. Knockdown of caveolin-1 also eliminated insulin-induced caveolin-1 recruitment to the PM. Thus, although the reduced insulin uptake by bAECs after knockdown of caveolin-1 may be mainly due to reduced numbers of transporting caveolae (28), the partially impaired intracellular insulin signaling might also contribute to this process. The increased insulin uptake following transfection with FLAG-caveolin-1 in both bAECs and caveolin-1−/− MAECs is consistent with this conclusion.
In the present study, we found that IL-6 (20 ng/ml) and TNFα (20 ng/ml) treatment for 24 h each reduced insulin uptake and inhibited expression of both caveolin-1 mRNA and protein. IL-6 and TNFα treatment also significantly reduced caveolin-1 amounts at the PM. In a previous study we did not observe significant changes of caveolin-1 protein expression in the bAECs treated with a lower dose of TNFα (5 ng/ml) for a shorter time (6 h) (49). However, that dose of TNFα inhibited insulin uptake, suggesting that other actions of TNFα also can adversely impact insulin uptake (20, 49). This is consistent with the observation here that TNFα and IL-6 inhibited insulin uptake to a greater extent (on a percentage basis) than the observed decline in caveolin-1 protein. Previous studies have found that treatment of adipocytes with TNFα results in elimination of IR from the caveolae fraction (17) and dissociation of IR from caveolin-1 (18). Very recently, we (48) found that pretreatment of cultured bAECs with either TNFα (20 ng/ml) or IL-6 (20 ng/ml) for 24 h significantly inhibited insulin-induced IR/IGF-1R translocation to the PM and their colocalization with caveolin-1 at the PM. In other studies TNFα and IL-6 (20 ng/ml each for 24 h) have been found to inhibit IRS-1 expression and insulin-stimulated IRS-1 tyrosine phosphorylation, although insulin-stimulated IR tyrosine phosphorylation appeared unaffected (37). In addition, IL-6 has recently been reported to impair insulin signaling in vascular ECs (52). These findings suggest that these cytokines might inhibit insulin uptake not only through inhibition of caveolin-1 protein synthesis but also through inhibition of insulin signaling (20, 49, 52).
Our current findings address only the first step by which insulin crosses the endothelium, i.e., endothelial uptake of insulin. Although it would seem desirable to examine the role of caveolin-1 in mediating insulin TET using either caveolin-1−/− knockout mice or caveolin-1−/− MAECs cultured on Transwell plates (47), lack of caveolin-1 has been shown to induce a paracellular leak of macromolecules attributed to the loss of the tonic caveolin-1 inhibition of endothelial NO syntrhase activity that restrains paracellular pathway permeability (40). Interestingly, although the caveolin-1-null mice on normal-chow diet showed an obvious insulin resistance during insulin tolerance testing, both their fasting and postprandial plasma insulin levels were surprisingly normal, consistent with a leaky endothelial barrier in these mice, minimizing hyperinsulinemia (6). The findings presented here provide evidence that the pathway by which insulin uptake by vascular endothelial cells requires caveolin-1 and that this process is inhibited by proinflammatory cytokines that induce insulin resistance.
GRANTS
This work was supported by research grants from the National Institute of Diabetes Diabetes and Digestive and Kidney Diseases (DK-057878 and DK-073059) to E. J. Barrett.
DISCLOSURES
No conflicts of interest are reported by the authors.
Supplementary Material
REFERENCES
- 1. Bar RS, Boes M, Dake BL, Booth BA, Henley SA, Sandra A. Insulin, insulin-like growth factors, and vascular endothelium. Am J Med 85: 59–70, 1988 [DOI] [PubMed] [Google Scholar]
- 2. Bar RS, Boes M, Sandra A. Vascular transport of insulin to rat cardiac muscle. Central role of the capillary endothelium. J Clin Invest 81: 1225–1233, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baron AD, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol Endocrinol Metab 266: E248–E253, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Brunner F, Wascher TC. Contribution of the endothelium to transcapillary insulin transport in rat isolated perfused hearts. Diabetes 47: 1127–1134, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Carey AL, Febbraio MA. Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia 47: 1135–1142, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 285: C222–C235, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem 272: 6525–6533, 1997 [DOI] [PubMed] [Google Scholar]
- 8. del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol 7: 901–908, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab 86: 1154–1159, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Foti M, Porcheron G, Fournier M, Maeder C, Carpentier JL. The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 104: 1242–1247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci USA 92: 8655–8659, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell 106: 403–411, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Stralfors P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J 13: 1961–1971, 1999 [PubMed] [Google Scholar]
- 15. Hamilton-Wessler M, Ader M, Dea MK, Moore D, Loftager M, Markussen J, Bergman RN. Mode of transcapillary transport of insulin and insulin analog NN304 in dog hindlimb: evidence for passive diffusion. Diabetes 51: 574–582, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K. Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. J Biol Chem 278: 11561–11569, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Kabayama K, Sato T, Kitamura F, Uemura S, Kang BW, Igarashi Y, Inokuchi J. TNFalpha-induced insulin resistance in adipocytes as a membrane microdomain disorder: involvement of ganglioside GM3. Glycobiology 15: 21–29, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci USA 104: 13678–13683, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science 227: 1583–1586, 1985 [DOI] [PubMed] [Google Scholar]
- 20. Li G, Barrett EJ, Barrett MO, Cao W, Liu Z. Tumor necrosis factor-alpha induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology 148: 3356–3363, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146: 4690–4696, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab 8: 310–317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miles PD, Levisetti M, Reichart D, Khoursheed M, Moossa AR, Olefsky JM. Kinetics of insulin action in vivo. Identification of rate-limiting steps. Diabetes 44: 947–953, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Miles PD, Li S, Hart M, Romeo O, Cheng J, Cohen A, Raafat K, Moossa AR, Olefsky JM. Mechanisms of insulin resistance in experimental hyperinsulinemic dogs. J Clin Invest 101: 202–211, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol 150: 1057–1070, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol 290: L405–L413, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Nystrom FH, Chen H, Cong LN, Li Y, Quon MJ. Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected Cos-7 cells and rat adipose cells. Mol Endocrinol 13: 2013–2024, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185–194, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature 436: 128–133, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol 57, Suppl 10: 43–51, 2006 [PubMed] [Google Scholar]
- 31. Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40: 1286–1292, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327–334, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 3: 46–56, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev 54: 431–467, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Rippe B, Rosengren BI, Carlsson O, Venturoli D. Transendothelial transport: the vesicle controversy. J Vasc Res 39: 375–390, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 68: 673–682, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 278: 45777–45784, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science 269: 1435–1439, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol 127: 1217–1232, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, l-name, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem 277: 40091–40098, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Siddiqui SS, Siddiqui ZK, Uddin S, Minshall RD, Malik AB. p38 MAPK activation coupled to endocytosis is a determinant of endothelial monolayer integrity. Am J Physiol Lung Cell Mol Physiol 292: L114–L124, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Simionescu M, Simionescu N, Palade GE. Morphometric data on the endothelium of blood capillaries. J Cell Biol 60: 128–152, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sjostrand M, Gudbjornsdottir S, Holmang A, Lonn L, Strindberg L, Lonnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes 51: 2742–2748, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Smart EJ, Ying YS, Anderson RG. Hormonal regulation of caveolae internalization. J Cell Biol 131: 929–938, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest 97: 1497–1503, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53: 1418–1423, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Wang H, Liu Z, Li G, Barrett EJ. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am J Physiol Endocrinol Metab 291: E323–E332, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Wang H, Wang AX, Barrett EJ. Insulin promotes and cytokines inhibit both trafficking of IR and IGF-1R to the plasma membrane and endothelial cell insulin transport. Diabetes 58: A333, 2009 [Google Scholar]
- 49. Wang H, Wang AX, Liu Z, Barrett EJ. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes 57: 540–547, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Wang H, Wang AX, Liu Z, Chai W, Barrett EJ. The trafficking/interaction of eNOS and caveolin-1 induced by insulin modulates endothelial nitric oxide production. Mol Endocrinol 23: 1613–1623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 84: 1620–1628, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuen DY, Dwyer RM, Matthews VB, Zhang L, Drew BG, Neill B, Kingwell BA, Clark MG, Rattigan S, Febbraio MA. Interleukin-6 attenuates insulin-mediated increases in endothelial cell signaling but augments skeletal muscle insulin action via differential effects on tumor necrosis factor-alpha expression. Diabetes 58: 1086–1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang L, Wheatley CM, Richards SM, Barrett EJ, Clark MG, Rattigan S. TNF-α acutely inhibits vascular effects of physiological but not high insulin or contraction. Am J Physiol Endocrinol Metab 285: E654–E660, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.