Abstract
The adaptor protein APPL1 mediates the stimulatory effect of adiponectin on p38 mitogen-activated protein kinase (MAPK) signaling, yet the underlying mechanism remains unclear. Here we show that, in C2C12 cells, overexpression or suppression of APPL1 enhanced or suppressed, respectively, adiponectin-stimulated p38 MAPK upstream kinase cascade, consisting of transforming growth factor-β-activated kinase 1 (TAK1) and mitogen-activated protein kinase kinase 3 (MKK3). In vitro affinity binding and coimmunoprecipitation experiments revealed that TAK1 and MKK3 bind to different regions of APPL1, suggesting that APPL1 functions as a scaffolding protein to facilitate adiponectin-stimulated p38 MAPK activation. Interestingly, suppressing APPL1 had no effect on TNFα-stimulated p38 MAPK phosphorylation in C2C12 myotubes, indicating that the stimulatory effect of APPL1 on p38 MAPK activation is selective. Taken together, our study demonstrated that the TAK1-MKK3 cascade mediates adiponectin signaling and uncovers a scaffolding role of APPL1 in regulating the TAK1-MKK3-p38 MAPK pathway, specifically in response to adiponectin stimulation.
Keywords: APPL1, transforming growth factor-β-activated kinase 1, mitogen-activated protein kinase kinase 3, p38 mitogen-activated protein kinase
adiponectin is an adipose-derived hormone that plays an important role in the regulation of energy homeostasis (2, 10, 28). The binding of adiponectin to its membrane receptors, such as AdipoR1 and AdipoR2, leads to the activation of two major signal pathways in muscle cells, the AMP-activated protein kinase (AMPK) and the p38 mitogen-activated protein kinase (MAPK) pathways (16, 27). Activation of these pathways has been shown to be essential for adiponectin-induced glucose uptake and fatty acid oxidation (16, 27, 28).
APPL1 (adaptor protein containing PH domain, PTB domain and leucine zipper motif-1) is an adaptor protein containing multiple protein-protein interaction domains and was originally reported as an associating protein that interacts with the catalytic subunit of phosphatidylinositol 3-kinase (p110) and Akt (17). Our laboratory has recently shown that APPL1 mediates adiponectin signaling to activate both the AMPK and the p38 MAPK signaling pathways (7, 16, 29). However, despite the finding that APPL1 mediates adiponectin-stimulated AMPK activation by promoting the cytosolic translocation of AMPK upstream kinase LKB1 (29), the molecular mechanism underlying APPL1-regulated p38 MAPK activation remains elusive.
The p38 MAPK is a major kinase in the MAPK family and plays essential roles in regulating cell proliferation, inflammation, and immune responses (19). Recent studies suggest that p38 MAPK acts as an essential mediator in regulating adiponectin-induced glucose uptake and fatty acid oxidation in C2C12 myotubes (16, 27). However, the molecular mechanism underlying adiponectin-stimulated p38 MAPK activation remains largely unknown. A kinase cascade consisting of transforming growth factor-β-activated kinase 1 (TAK1), mitogen-activated protein kinase kinase (MKK) 3, and MKK6 has been reported to activate p38 MAPK in response to extracellular stimuli, including growth factors and inflammatory cytokines (18, 19). It was unclear whether adiponectin activates p38 MAPK via a similar or a different mechanism.
In the present study, we showed that the TAK1-MKK3 kinase pathway mediates adiponectin-stimulated p38 MAPK activation in C2C12 myotubes. In addition, we demonstrated that APPL1 serves as a docking platform for scaffolding the TAK1-MKK3-p38 MAPK cascade in response to adiponectin stimulation. Altering the cellular expression level of APPL1 had no effect on TNFα-induced activation of the TAK1-MKK3-p38 MAPK kinase pathway, suggesting a selective role of this adaptor protein in regulating adiponectin signaling. Taken together, our study reveals that the APPL1-TAK1-MKK3 cascade mediates the adiponectin signaling to stimulate p38 MAPK activity in muscle cells.
MATERIAL AND METHODS
Plasmids, adiponectin, and antibodies.
The cDNAs encoding full-length and various truncation mutants of human APPL1 were generated by PCR and subcloned into the mammalian expression vector pcDNA3.1/Myc-His(+)A (Invitrogen) or the bacterial expression vector pGEX-4T1 (Amersham Pharmacia Biotechnology). The recombinant globular adiponectin was produced, as described previously (16). The short hairpin RNAs for TAK1 (V2M_193230), MKK3 (V2M_218674), MKK6 (V2M_188205), and AMPKα2 (RMM1766_96744125), as well as control vector pSM2c (RHS1704), were purchased from OpenBiosystem (Huntsville, AL). TNFα (catalog no. T7539) was purchased from Sigma-Aldrich (St. Louis, MO). The antibody to APPL1 and AdipoR1 was generated as described previously (16). All other antibodies were obtained from Cell Signaling Technologies (Danvers, MA).
Cell culture.
C2C12 myoblasts [from the American Type Culture Collection (ATCC), Manassas, VA] were grown in DMEM (ATCC) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Differentiation of C2C12 myoblasts into myotubes was induced by growing the cells in low-serum differentiation medium (99% DMEM, 0.1% fetal bovine serum, 1% penicillin-streptomycin, and 100 nM insulin). The medium was changed daily, and multinucleated myotubes were normally observed in 3–5 days. APPL1 scrambled and RNA interference (RNAi) stable cell lines were generated as described previously (16). Control and AMPKα2 RNAi stable cell lines were generated by transfecting the cells with pSM2C or pTRIPZ/AMPKα2 RNAi construct and selected by puromycin, following the manufacturer's protocol (OpenBiosystem, Huntsville, AL).
Immunoprecipitation and Western blot analysis.
GST in vitro affinity binding assays, coimmunoprecipitation, and Western blot experiments were carried out as described previously (16).
RESULTS
Adiponectin activates p38 MAPK through the TAK1-MKK3 kinase cascade.
Three kinases, TAK1, MKK3, and MKK6, are known as major upstream kinases, mediating p38 MAPK activation induced by extracellular stimuli, including stress and cytokines (5, 11, 19, 22). To determine whether these kinases are involved in adiponectin-stimulated p38 MAPK activation, we suppressed their expression levels by RNAi in C2C12 myocytes (Fig. 1A). The adiponectin-induced activations of p38 MAPK were notably reduced in cells in which the expression levels of TAK1 or MKK3 are suppressed, but not in the cells in which MKK6 is suppressed (Fig. 1A), demonstrating that the TAK1-MKK3, but not TAK1-MKK6, cascade is essential for mediating the stimulatory effect of adiponectin on p38 MAPK activation.
Fig. 1.
Transforming growth factor-β-activated kinase 1 (TAK1) and mitogen-activated protein kinase kinase 3 (MKK3) are essential in mediating adiponectin (Ad)-induced p38 mitogen-activated protein kinase (MAPK) activation. A: suppression of TAK1 or MKK3, but not MKK6, impaired Ad-induced p38 MAPK activation. C2C12 myocytes with TAK1, MKK3, or MKK6 suppressed by transfecting short hairpin RNA were serum starved for 6 h and treated with 1 μg/ml Ad for 10 min. The phosphorylated (P) (Thr180/Tyr182) and the protein levels of p38 MAPK, as well as TAK1, MKK3, MKK6, and tubulin, were detected by Western blot analysis with specific antibodies, as indicated. Graphic presentation indicated the effect of suppressing TAK1, MKK3, or MKK6 on the Ad-stimulated p38 MAPK activation shown in Western blot analysis. Values are means ± SE from three independent experiments. **P < 0.01. B: suppression of AMP-activated protein kinase (AMPK) expression does not affect Ad-stimulated p38 MAPK activation. The control or AMPKα2 RNA interference (RNAi) C2C12 myotubes were serum starved overnight and treated with 1 μg/ml Ad for 10 min. The P-p38 MAPK (Thr180/Tyr182) and the protein levels of p38 MAPK, as well as AMPKα2, were detected by Western blot analysis with specific antibodies, as indicated. Graphic presentation indicated the effect of suppressing AMPK on the Ad-stimulated p38 MAPK activation shown in Western blot analysis. Values are means ± SE from three independent experiments. **P < 0.01. C: Ad sequentially stimulates TAK1, MKK3, and p38 MAPK activities. C2C12 myotubes were serum starved overnight and treated with 1 μg/ml Ad for different times, as indicated. P-TAK1 (Thr184/187), P-p38 MAPK (Thr180/Tyr182), and their protein levels were detected by Western blot analysis with specific antibodies, as indicated. MKK3 was immunoprecipitated (IP) with an antibody specific to MKK3, and P-MKK3 was detected by Western blot with antibody to P-MKK3/6 (Ser189/207). Graphic presentation indicated the effect of Ad on activation of TAK1, MKK3, and p38 MAPK shown in Western blot analysis. Values are means ± SE from three independent experiments. The t-test was performed with the activity at each time point compared with the basal level. *P < 0.05 and **P < 0.01. Mock, mock control; Ctrl, control; ns, nonsignificant.
AMPK is not involved in adiponectin-stimulated p38 MAPK activation.
The AMPK has been suggested to be upstream of p38 MAPK in the ischemic heart (12). Since adiponectin activates both AMPK and p38 MAPK (16), we investigated whether AMPK is involved in the adiponectin-stimulated p38 MAPK activation. To this end, we generated C2C12 stable cell lines in which AMPKα2 was suppressed by RNAi, which impaired the intact activity of AMPK (12, 13, 21, 23, 24). As shown in Fig. 1B, adiponectin-stimulated p38 MAPK activation was not affected in the AMPKα2-suppressed cells. This observation is consistent with a report that AMPK and p38 MAPK pathways are dissociated in skeletal muscle (9).
Adiponectin sequentially activates TAK1, MKK3, and p38 MAPK.
We next examined whether adiponectin is able to activate TAK1 and MKK3. Time course studies showed that adiponectin stimulated TAK1 and MKK3 phosphorylation in a time-dependent manner, and the phosphorylation events occurred sequentially, i.e., TAK1, the kinase at the top of the cascade, was phosphorylated first, whereas p38 MAPK, the most downstream component of the cascade, was phosphorylated last (Fig. 1C, panels 1, 3, and 5). These results provided further evidence that TAK1 and MKK3 are the kinases involved in adiponectin-stimulated p38 MAPK activation.
APPL1 is essential for adiponectin-stimulated TAK1-MKK3-p38 MAPK activation.
APPL1 has been shown as a key mediator conveying adiponectin signaling toward p38 MAPK (16). To determine whether APPL1 is required for adiponectin-stimulated TAK1-MKK3 activation, we examined the effect of APPL1 protein expression on TAK1 and MKK3 phosphorylation in C2C12 myocytes. Overexpression of APPL1 significantly enhanced basal TAK1, MKK3, and p38 MAPK phosphorylation, but had little effect on adiponectin-stimulated phosphorylation due to high basal phosphorylation of these kinases (Fig. 2A, panels 1, 3, and 5, lane 5 vs. lane 4). The effect of APPL1 overexpression on the basal level of p38 MAPK (Fig. 2A, panel 5, lane 4) is consistent with what our laboratory reported previously (16). In addition, we also found that overexpression of APPL1 led to significant enhancement of basal activities of the kinases upstream of p38 MAPK (Fig. 2A, panels 1 and 3, lane 4). A possible explanation could be that a limited amount of endogenous APPL1 interacts with the adiponectin receptors, and the majority of the APPL1 is sequestrated away from the receptors by APPL2 under basal conditions (25). Overexpression of APPL1 disturbs the balance between APPL1 and APPL2 in cells, leading to excess amount of free APPL1 binding with the adiponectin receptors and subsequently activating the TAK1-MKK3-p38 MAPK pathway downstream of the adiponectin receptors. Conversely, suppressing APPL1 expression by RNAi significantly impaired adiponectin-stimulated phosphorylation of TAK1, MKK3, and p38 MAPK (Fig. 2B). However, suppression of APPL1 expression is not sufficient to completely inhibit adiponectin-stimulated TAK1-MKK3-p38 MAPK activation (Fig. 2B), suggesting that an APPL1-independent pathway may also contribute to adiponectin-stimulated p38 MAPK activation.
Fig. 2.
The role of APPL1 in mediating Ad-stimulated activation of TAK1-MKK3-p38 MAPK pathway. A: overexpression of APPL1 enhanced Ad-stimulated TAK1-MKK3-p38 MAPK cascade activation. C2C12 myocytes overexpressing pcDNA3.1/Myc-His(+)A vector (as a mock control) or pcDNA3.1/Myc-His(+)A/APPL1 were serum starved for 6 h and treated with 1 μg/ml Ad for different times, as indicated. P-TAK1 (Thr184/187) and P-p38 MAPK (Thr180/Tyr182) and their protein levels were detected by Western blot with specific antibodies, as indicated. MKK3 was IP with an antibody specific to MKK3, and P-MKK3 was detected by Western blot with antibody to P-MKK3/6 (Ser189/207). Graphic presentation indicated the effect of overexpression of APPL1 on Ad-stimulated activation of TAK1, MKK3, and p38 MAPK shown in the Western blot. Values are means ± SE from three independent experiments. The t-test was performed by comparing the activities between mock and overexpression group at each time point. *P < 0.05 and **P < 0.01. B: suppression of APPL1 expression impaired Ad-induced TAK1-MKK3-p38 MAPK cascade activation. The scrambled control or APPL1 RNAi C2C12 myotubes were serum starved overnight and treated with 1 μg/ml Ad for different times, as indicated. P-TAK1 (Thr184/187), P-p38 MAPK (Thr180/Tyr182), and their protein levels were detected by Western blot with specific antibodies, as indicated. MKK3 was IP with an antibody specific to MKK3, and P-MKK3 was detected by Western blot with antibody to P-MKK3/6 (Ser189/207). Graphic presentation indicated the effect of suppressing APPL1 expression on Ad-stimulated activation of TAK1, MKK3, and p38 MAPK shown in the Western blot. The t-test was performed by comparing the activities between the scramble and APPL1-RNAi group at each time point. Values are means ± SE from three independent experiments. *P < 0.05. au, Arbitrary units.
APPL1 plays a selective role in adiponectin and TNFα-stimulated p38 MAPK activation.
TNFα has been shown to stimulate p38 MAPK activation in C2C12 cells (Ref. 6 and Fig. 3A, lanes 2–4). However, suppressing the expression levels of APPL1 had little effect on TNFα-induced TAK1, MKK3, or p38 MAPK activation in C2C12 myotubes (Fig. 3A, lanes 6–8 vs. lanes 2–4), indicating that adiponectin and TNFα activate p38 MAPK through distinct mechanisms, and that the effect of APPL1 on p38 MAPK cascade is highly selective to adiponectin stimulation.
Fig. 3.
APPL1 is not involved in mediating TNFα-induced TAK1-MKK3-p38 MAPK activation. A: the scrambled control or APPL1 RNAi C2C12 myotubes were serum starved overnight and treated with TNFα (1 nM) for different times, as indicated. P-TAK1 (Thr184/187), P-p38 MAPK (Thr180/Tyr182), and their protein levels were detected by Western blot with specific antibodies, as indicated. MKK3 was IP with an antibody specific to MKK3, and P-MKK3 was detected by Western blot analysis with antibody to P-MKK3/6 (Ser189/207). Graphic presentation indicated the effect of suppressing APPL1 expression on TNFα-stimulated activation of TAK1, MKK3, and p38 MAPK shown in the Western blot. Values are means ± SE from three independent experiments. The t-test was performed by comparing the activities between scramble and RNAi groups at each time point. B: the effects of Ad and TNFα on the activation of α- and β-isoforms of p38 MAPK in C2C12 myotubes. C2C12 myotubes were serum starved overnight and treated with either Ad (1 μg/ml, 10 min) or TNFα (1 nM, 10 min). The α- and β-isoforms of p38 MAPK were IP, and P-p38 MAPK (Thr180/Tyr182) was detected by Western blot analysis with antibodies, as indicated. Graphic presentation indicated the effect of Ad or TNFα on activation of α- and β-isoforms of p38 MAPK shown in the Western blot. Values are means ± SE from three independent experiments. *P < 0.05. C: suppression of APPL1 impaired the effect of Ad on activation of the α- and β-isoform of p38 MAPK. The scrambled control or APPL1 RNAi C2C12 myotubes were serum starved overnight and treated with 1 μg/ml Ad for 10 min. The α- and β-isoforms of p38 MAPK were IP, and the P-p38 MAPK (Thr180/Tyr182) was detected by Western blot analysis with specific antibodies, as indicated. Graphic presentation indicates the effect of suppression of APPL1 on Ad-stimulated activation of the α- and β-isoforms of p38 MAPK shown in the Western blot. Values are means ± SE from three independent experiments. *P < 0.05. D: suppression of APPL1 had no effect of TNFα on activation of the β-isoform of p38 MAPK. The scrambled control or APPL1 RNAi C2C12 myotubes were serum starved overnight and treated with TNFα (1 nM) for 10 min. The β-isoform of p38 MAPK was IP, and the P-p38 MAPK (Thr180/Tyr182) was detected by Western blot analysis with specific antibodies, as indicated. Graphic presentation indicated the effect of suppression of APPL1 on TNFα-stimulated activation of β-isoform of p38 MAPK shown in the Western blot. Values are means ± SE from three independent experiments. *P < 0.05.
There are two major isoforms, α and β, of p38 MAPK in skeletal muscle cells (19), and the β-isoform of the kinase is selectively stimulated by TNFα (6). To determine whether the stimulatory effect of adiponectin is also selective, we examined the phosphorylation of the α- and β-isoforms of p38 MAPK immunoprecipitated from C2C12 cells treated with adiponectin or TNFα. Consistent with previous report (6), TNFα specifically activated the β-isoform, but not the α-isoform, of p38 MAPK (Fig. 3B, lane 3, panels 1 vs. 3). On the other hand, adiponectin treatment stimulated the phosphorylation of both isoforms (Fig. 3B, lane 2, panels 1 vs. 3). These data suggest that the α-isoform selectively mediates adiponectin, but not TNFα signaling, in skeletal muscle cells.
We next investigated the effects of APPL1 on adiponectin- or TNFα-stimulated activation of the α- and/or β-isoforms of p38 MAPK. As shown in Fig. 3C, suppression of APPL1 expression led to a significant inhibition of adiponectin-stimulated activation of both isoforms (panels 1 and 3, lane 4 vs. lane 2). In contrast, suppression of APPL1 expression had no effect on TNFα-induced activation of the β-isoform (Fig. 3D, panel 1, lane 4 vs. lane 2). Together, these data suggested that APPL1 selectively mediates adiponectin, but not TNFα signal, to activate both α- and β-isoforms of p38 MAPK.
APPL1 promotes p38 MAPK activation by scaffolding TAK1, MKK3, and p38 MAPK.
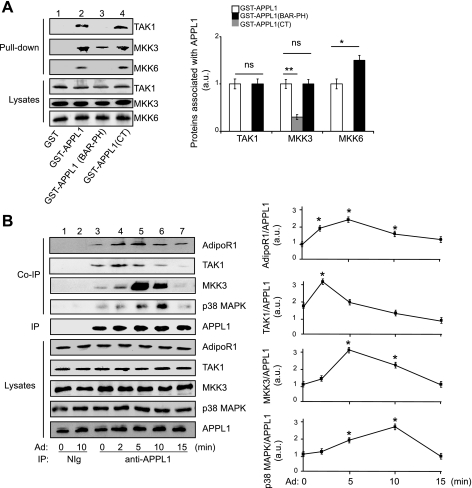
APPL1 consists of multiple protein-protein interaction domains, suggesting that this protein may function as an adaptor or a scaffold protein in a variety of pathways (7). Consistent with this hypothesis, GST-APPL1 fusion protein, but not GST itself, interacted with endogenous TAK1, MKK3, and MKK6 (Fig. 4A, panels 1–3, lane 2 vs. lane 1). In addition, GST-APPL1(BAR-PH), a mutated form of APPL1 with a truncation in the COOH-terminal region (16), interacted with MKK3, but not TAK1 and MKK6 (Fig. 4A, panels 1–3, lane 3), suggesting that TAK1 and MKK6 bind to the regions on APPL1 different from MKK3. The BAR-PH truncation of APPL1, which is unable to bind to the adiponectin receptors, has been shown to act as a dominant negative mutant that inhibits adiponectin-stimulated p38 MAPK activation (16). The deficiency of APPL1(BAR-PH) mutant in the interaction with TAK1 could also contribute to its inhibitory role in adiponectin-induced p38 MAPK activation.
Fig. 4.
APPL1 acts as a scaffold protein by interacting with TAK1-MKK3-p38 MAPK. A: APPL1 interacts with TAK1, MKK3, and MKK6 in vitro. GST or GST-APPL1 (full-length, BAR-PH or CT truncations) fusion protein was incubated with cell lysates of C2C12 myotubes. Endogenous TAK1, MKK3, and MKK6 associated with recombinant GST-APPL1 (full-length or truncations) and their protein levels in the lysates were detected by Western blot analysis with specific antibodies, as indicated. Graphic presentation indicated the binding affinity between APPL1 fusion proteins with TAK1, MKK3, and MKK6 shown in the Western blot. Values are means ± SE from three independent experiments. *P < 0.05 and **P < 0.01. B: the effect of Ad on the interactions of APPL1 with Ad receptor 1 (AdipoR1), TAK1, MKK3, and p38 MAPK in cells. C2C12 myotubes were serum starved overnight and treated with 1 μg/ml Ad for the indicated time. Endogenous APPL1 was IP with the antibody specific to APPL1. Coimmunoprecipitated (Co-IP) AdipoR1, TAK1, MKK3, and p38 MAPK, as well as their protein levels in the cell lysates, were detected by Western blot analysis with specific antibodies, as indicated. Graphic presentation indicated the effect of Ad on the interaction of APPL1 with AdipoR1, TAK1, MKK3, and p38 MAPK shown in the Western blot. The t-test was performed by comparing the affinity with the basal conditions. Values are means ± SE from three independent experiments. *P < 0.05.
By coimmunoprecipitation experiments, we found that endogenous APPL1 interacts with TAK1 and weakly associates with AdipoR1, MKK3, and p38 MAPK in C2C12 myotubes under the basal condition (Fig. 4B, panels 1–4, lane 3). Adiponectin treatment enhanced the interaction between APPL1 and TAK1 (Fig. 4B, panel 2, lane 4 vs. lane 3) and induced a binding of the APPL1-TAK1 complex with AdipoR1 (Fig. 4B, panel 1, lanes 4 and 5 vs. lane 3), which, subsequently, resulted in a sequential recruitment of MKK3 and p38 MAPK onto AdipoR1-APPL1-TAK1 complex (Fig. 4B, panels 3 and 4, lanes 5 and 6 vs. lane 3). Interestingly, the time course of the interaction between APPL1 with TAK1, MKK3, and p38 MAPK is correlated with adiponectin-stimulated activation of these kinases (Figs. 4B vs. 1C), suggesting the presence of a dynamic mechanism by which adiponectin activates p38 MAPK via promoting the interaction of APPL1 with all signal components in the p38 MAPK pathway.
It was noted that, while endogenous p38 MAPK is coimmunoprecipitated with APPL1 under the basal condition (Fig. 4B, panel 4, lane 3), we were unable to detect the interaction between p38 MAPK and APPL1 by in vitro binding assays (data not shown), implying that postmodification of APPL1 could play an essential role in the interaction of APPL1 with p38 MAPK or p38 MAPK binds with APPL1 indirectly in cells. Since the APPL1(BAR-PH) truncation mutant binds to MKK3 but not TAK1 (Fig. 4A, panel 1 vs. panel 2, lane 3), we tested whether altering the expression levels of this mutant activates MKK3. However, we found that overexpression of the APPL1(BAR-PH) truncation mutant was unable to activate MKK3 (data not shown), suggesting that an optimized binding and/or topological orientation of all components in TAK1-MKK3-p38 MAPK cascade is necessary for adiponectin-induced activation of MKK3. This observation is also consistent with our previous finding that overexpression of the APPL1(BAR-PH) mutant does not activate p38 MAPK activation in C2C12 myocytes (16).
DISCUSSION
Previous studies showed that adiponectin activates p38 MAPK through AdipoR1- and APPL1-dependent mechanism (16, 27). However, the underlying mechanism remains unknown. In this study, we demonstrated that the TAK1-MKK3 pathway mediates adiponectin-stimulated p38 MAPK activation, and that APPL1 functions as a scaffolding protein to form a complex with TAK1, MKK3, and p38 MAPK in muscle cells, leading to selective and effective activation of this cascade in response to adiponectin (Fig. 5).
Fig. 5.
A model of APPL1-regulated TAK1-MKK3-p38 MAPK pathway in response to Ad stimulation. C', indicates the COOH terminus of AdipoR1. Under the basal condition, APPL1 binds with inactive TAK1 and weakly associates with MKK3 and p38 MAPK. On Ad stimulation, APPL1 interacts with AdipoR1, leading to activation of TAK1 and, subsequently, recruitment of MKK3 and p38 MAPK to form a complex consisting of AdipoR1, APPL1, TAK1, MKK3, and p38 MAPK. Once MKK3 is activated, TAK1 dissociates from APPL1, and the activity of TAK1 is rapidly downregulated. This process occurs concurrently with the dissociation of APPL1 complex from AdipoR1 and, in turn, stimulates the downstream events regulated by TAK1-MKK3-p38 MAPK pathway.
Our study has demonstrated that APPL1 scaffolds TAK1 and MKK3 via different regions (Fig. 4A). While several MKKs have been shown to activate p38 MAPK in response to TNFα and UV stimulation (18, 19), we found that adiponectin-stimulated p38 MAPK activation selectively requires MKK3 in muscle cells (Figs. 1A). Interestingly, we found that MKK6 can also interact with APPL1 in vitro (Fig. 4A, panel 3), although it is not involved in adiponectin-stimulated p38 MAPK activation (Fig. 1A). This result suggests that MKK6 may be involved in another signaling event that is also mediated by APPL1. Recently, the TAK1-MKK6-p38MAPK axis was reported to be essential for the differentiation of C2C12 myocytes induced by low serum (3). Interestingly, the APPL1-mediated p38 MAPK activation is also involved in C2C12 myocyte differentiation (1). Therefore, it is possible that the interaction between APPL1 and MKK6 plays a role in myogenesis. Further investigations will be needed to test this possibility.
In the present study, we have found that adiponectin stimulation leads to a multiprotein complex formation (APPL1, AdipoR1, TAK1, MKK3, and p38 MAPK) (Fig. 4B), resulting in chronological activation of the TAK1-MKK3-p38 MAPK kinase cascade (Figs. 1C and 5). Interestingly, once activated, the components in this cascade dissociate from APPL1 (Figs. 4B and 5), followed by dephosphorylation of these kinases (Figs. 1C and 5). A possible explanation for these findings is that the interaction with APPL1 ensures timely activation of this cascade and prevents dephosphorylation of these kinases from the action of a protein phosphatase(s). Thus APPL1 acts as a docking platform to dynamically and efficiently regulate the TAK1-MKK3-p38 MAPK kinase cascade in response to adiponectin stimulation (Fig. 5).
The data from the affinity-binding assay suggest that p38 MAPK was unable to bind with GST-fused APPL1 under in vitro conditions (data not shown), although endogenous p38 MAPK was coimmunoprecipitated with APPL1 under basal conditions (Fig. 4B). One possible explanation is that posttranslational modification on APPL1 may contribute to the binding with p38 MAPK in cells. Alternatively, p38 MAPK may bind to the NH2-terminus of APPL1, and the GST protein fused to the NH2-terminus of APPL1 may interrupt this binding. The other possibility is that MKK3 acts as a “carrier” to bring p38 MAPK onto the APPL1-MKK3 complex in response to adiponectin stimulation. Together, our study indicates that APPL1 protein is essential for controlling adiponectin-induced TAK1-MKK3-p38 MAPK cascade activation, which is a highly dynamic process in cells.
It has been reported that AMPK functions as an upstream kinase of p38 MAPK in regulating glucose uptake stimulated by stretch and AICAR, however, it is still controversial whether AMPK-stimulated p38 MAPK activation is a common mechanism in skeletal muscle (4, 9, 12, 26). Suppression of AMPKα2 expression significantly affected AMPK activity and impaired the ischemia-induced p38 MAPK activation in ischemic heart (12), suggesting a role of AMPKα2 in the activation of p38 MAPK. To test whether a similar mechanism is involved in adiponectin-induced p38 MAPK activation, we generated a stable C2C12 cell line in which the expression levels of the AMPKα2 subunit are remarkably suppressed by RNAi (13). As shown in Fig. 1B, suppressing the expression of the α2-subunit of AMPK, a subunit essential for intact AMPK activity (12, 13, 21, 23, 24), had no significant effect on the stimulatory role of adiponectin in p38 MAPK activation in C2C12 cells (Fig. 1B), suggesting that AMPK is dispensable for adiponectin-stimulated p38 MAPK activation in C2C12 myotubes. Thus the involvement of AMPK in p38 MAPK activation may be a tissue- and pathway-specific event. Combined with the evidence that TAK1-MKK3 pathway transmits adiponectin signaling to p38 MAPK (Fig. 1A), our data demonstrate that the TAK1-MKK3 axis, but not AMPK, functions as a major regulator, mediating the effect of adiponectin toward p38 MAPK.
The p38 MAPK is a vital enzyme in maintaining metabolic homeostasis (14), and its activation is essential for exercise-, insulin-, and adiponectin-induced glucose and lipid utilization (8, 14–16, 27). However, aberrant activation of p38 MAPK could contribute to chronic inflammation-induced insulin resistance (6). Since both adiponectin and TNFα can activate p38 MAPK (6, 16), while adiponectin acts as an anti-inflammatory factor by inhibiting the expression and activity of TNFα in vivo (20), it implies that the activity of p38 MAPK is differently regulated by adiponectin and TNFα in cells. Consistent with this, our data showed that adiponectin activates both of the α-and the β-isoforms of p38 MAPK, whereas TNFα only stimulates the β-isoform of this kinase in muscle cells (Fig. 3B). In addition, we found that APPL1 specifically mediates adiponectin- but not TNFα-induced p38 MAPK activation (Fig. 3).
In summary, we have demonstrated TAK1-MKK3 as the upstream kinases for mediating adiponectin signal to stimulate p38 MAPK activity in muscle cells. In addition, we have shown that APPL1 acts as a scaffolding protein to orchestrate the activities of the TAK1-MKK3-p38 MAPK. Furthermore, we have demonstrated that the scaffolding action of APPL1 on TAK1-MKK3-p38 MAPK cascade is selective to adiponectin, but not TNFα. Identification of TAK1 and MKK3 as new signal components in adiponectin pathway and elucidation of the mechanism by which APPL1 scaffolds TAK1-MKK3-p38 MAPK pathway should provide important information for understanding the molecular mechanism of adiponectin action and identification of novel targets of insulin resistance and associated metabolic diseases.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grants (RO1 DK69930 to L. Q. Dong and R01 DK76902 to F. Liu) and a Pre-doctoral Fellowship from the American Heart Association (10PRE3180019 to X. Xin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Derong Hu for excellent technical assistance.
REFERENCES
- 1. Bae GU, Lee JR, Kim BG, Han JW, Leem YE, Lee HJ, Ho SM, Hahn MJ, Kang JS. Cdo interacts with APPL1 and activates AKT in myoblast differentiation. Mol Biol Cell 21: 2399–2411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beltowski J, Jamroz-Wiśniewska A, Widomska S. Adiponectin and its role in cardiovascular diseases. Cardiovasc Hematol Disord Drug Targets 8: 7–46, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bhatnagar S, Kumar A, Makonchuk DY, Li H, Kumar A. Transforming growth factor-beta-activated kinase 1 is an essential regulator of myogenic differentiation. J Biol Chem 285: 6401–6411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chambers MA, Moylan JS, Smith JD, Goodyear LJ, Reid MB. Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J Physiol 587: 3363–3373, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choo MK, Sakurai H, Koizumi K, Saiki I. TAK1-mediated stress signaling pathways are essential for TNF-alpha-promoted pulmonary metastasis of murine colon cancer cells. Int J Cancer 118: 2758–2764, 2006 [DOI] [PubMed] [Google Scholar]
- 6. de Alvaro C, Teruel T, Hernandez R, Lorenzo M. Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J Biol Chem 279: 17070–17078, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab 296: E22–E36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol 106: 929–934, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Ho RC, Fujii N, Witters LA, Hirshman MF, Goodyear LJ. Dissociation of AMP-activated protein kinase and p38 mitogen-activated protein kinase signalling in skeletall muscle. Biochem Biophys Res Commun 362: 354–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiemer AK, Weber NC, Fürst R, Bildner N, Kulhanek-Heinze S, Vollmar A. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res 90: 874–881, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, Young LH. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res 97: 872–879, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem 285: 36387–36394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Z, Cao W. p38 Mitogen-activated protein kinase: a critical node linking insulin resistance and cardiovascular diseases in type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets 9: 38–46, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Long Y, Widegren U, Zierath J. Exercise-induced mitogen-activated protein kinase signalling in skeletal muscle. Proc Nutr Soc 63: 277–232, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8: 516–523, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, Testa JR. Identification of a chromosome 3p14.3–211 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18: 4891–4898, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem 271: 13675–13679, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal 12: 1–13, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta 380: 24–30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J 334: 177–187, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takekawa M, Tatebayashi K, Saito H. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol Cell 18: 295–306, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Viollet B, Andreelli F, Jørgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans 31: 216–219, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Viollet B, Andreelli F, Jørgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 111: 91–98, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang C, Xin X, Xiang R, Ramos FJ, Liu M, Lee HJ, Chen H, Mao X, Kikani CK, Liu F, Dong LQ. Yin-yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem 284: 31608–31615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xi X, Han J, Zhang JZ. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J Biol Chem 276: 41029–41034, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno N, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, Liu F, Dong LQ. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem 284: 22426–22435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]