Abstract
Resveratrol, a polyphenol found in many plants, has antioxidant and anti-inflammatory actions. It also improves endothelial function and may be cardioprotective. Tumor necrosis factor-α (TNFα) causes oxidative stress and microvascular endothelial dysfunction. Whether resveratrol affects microvascular function in vivo and, if so, whether inflammatory cytokines antagonize its microvascular action are not clear. In cultured bovine aortic endothelial cells (BAECs), reserveratrol (100 nM) increased the phosphorylation of protein kinase B (Akt), endothelial nitric oxide (NO) synthase (eNOS), and ERK1/2 within 15 min by more than twofold, and this effect lasted for at least 2 h. Treatment of BAECs with TNFα (10 ng/ml) significantly increased the NADPH oxidase activity and the production of hydrogen peroxide and superoxide. Pretreatment of cells with resveratrol (100 nM) prevented each of these. Injection (ip) of resveratrol in rats potently increased muscle microvascular blood volume (MBV; P = 0.007) and flow (MBF; P < 0.02) within 30 min, and this was sustained for at least 2 h. The phosphorylation of Akt in liver or muscle was unchanged. Superimposed systemic infusion of l-NAME (NOS inhibitor) completely abolished resveratrol-induced increases in MBV and MBF. Similarly, systemic infusion of TNFα prevented resveratrol-induced muscle microvascular recruitment. In conclusion, resveratrol activates eNOS and increases muscle microvascular recruitment via an NO-dependent mechanism. Despite the potent antioxidant effect of resveratrol, TNFα at concentrations that block insulin-mediated muscle microvascular recruitment completely neutralized resveratrol's microvascular action. Thus, chronic inflammation, as seen in type 2 diabetes, may limit resveratrol's vasodilatory actions on muscle microvasculature.
Keywords: tumor necrosis factor-α, muscle, microvascular recruitment, inflammation, oxidative stress
the major function of the muscle microcirculation is to regulate tissue perfusion to ensure adequate delivery of nutrients, oxygen, and hormones despite changing needs based on activity and feeding patterns. To do this, the endothelium provides an exchange surface area between the plasma compartment and tissue interstitium that can change based upon tissue needs (2). Microvascular perfusion is determined by relaxing precapillary terminal arterioles, which expands exchange surface area (capillary recruitment). A number of physiological and pathological factors can regulate this process (2). Among them, insulin, exercise, and mixed meals have each been shown to increase muscle microvascular perfusion, whereas tumor necrosis factor-α (TNFα) and elevated free fatty acid concentrations inhibit recruitment (2, 8).
Oxidative stress plays a pivotal role in the pathogenesis of endothelial dysfunction and vascular complications in patients with type 2 diabetes (T2DM), consistent with T2DM being a chronic inflammatory disorder (29, 32). Patients and animal models of T2DM have elevated tissue and plasma levels of various inflammatory cytokines that are capable of inducing oxidative stress. Excess production of reactive oxygen species (ROS) reduces nitric oxide (NO) bioavailability and increases the production of cytotoxic peroxynitrite (42). TNFα is a widely studied inflammatory cytokine that has clearly been linked to insulin resistance and endothelial dysfunction. It stimulates superoxide production via activation of NADPH oxidase and induces insulin resistance via the p38 MAPK-dependent pathway in cultured endothelial cells (14, 21, 30, 44). Perfusion of dissected resistance arterioles with TNFα blocks insulin-mediated vasodilation, whereas systemic infusion of TNFα completely abrogates insulin-mediated muscle capillary recruitment in rats (13, 43, 46).
Resveratrol (3,5,4′-trihydroxystilbene) is a naturally occurring polyphenolic phytoalexin found in many plants and has been shown to prolong lifespan in lower organisms. Foods and drinks rich in resveratrol, such as the Mediterranean diets and French wine, are associated with a reduced risk of cardiovascular mortality in humans, and evidence has confirmed that resveratrol has antiapoptosis, anti-inflammatory, anti-aging, and anticancer as well as cardiovascular protective effects (3). Although the underlying mechanisms remain unclear, resveratrol's antioxidant and anti-inflammatory actions may have played an important role in these processes.
Resveratrol is also vasoactive and has been shown to cause vasodilation and to improve aortic endothelial function in diabetic mice (44). In db/db mice, resveratrol restored endothelium-dependent vasorelaxation to acetylcholine, attenuated TNFα expression, inhibited NADPH oxidase, and preserved endothelial NO synthase (eNOS) phosphorylation (44). Whether resveratrol acts more distantly to recruit muscle microvasculature and enhance tissue perfusion and, if so, whether inflammatory cytokines affect this action were examined in the current study. Our results suggest that resveratrol potently induced muscle microvascular recruitment; however, this action was blocked by TNFα despite the potent antioxidant effect of resveratrol.
MATERIALS AND METHODS
Culture of bovine arterial endothelial cells.
Bovine arterial endothelial cells (BAECs) in primary culture were purchased from Cambrex BioSciences (Walkersville, MD) and cultured in endothelial basic medium supplemented with 5% fetal bovine serum, bovine brain extract, human epidermal growth factor (10 ng/ml), gentamicin sulfate (50 μg/ml), and hydrocortisone (1 μg/ml). Cells (passages 3–8) were grown to confluence and then used for experiments after serum starvation for 14 h. Cells were incubated with resveratrol (100 nM; Sigma-Aldrich, St. Louis, MO) in the absence or presence of TNFα (10 ng/ml; R & D Systems, Minneapolis, MN) for various amounts of time and then used for either Western blotting or measurement of hydrogen peroxide, superoxide anion, or NADPH oxidase activity.
Western blotting.
Cells were lysed in ice-cold lysis buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM EGTA, 1 mM sodium orthovanadate, 1 mM NaF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 mM phenylmethylsulfonyl fluoride) and used for Western blotting, as described previously (4, 21, 22). The membranes were probed with antibodies against phospho-eNOS (Ser1177), phospho-ERK1/2 (Thr202/Tyr204; Cell Signaling Technology, Danvers, MA), or phospho-Akt1 (Ser473; Upstate Biotechnology) overnight at 4°C. After the membranes were incubated with respective secondary antibody conjugated with horseradish peroxidase (Amersham, Buckinghamshire, UK), the blots were developed using enhanced chemiluminescence (Amersham Life Science, Piscataway, NJ), and the autoradiographic films were scanned and quantified using ImageQuant TL (Bio-Rad).
Measurement of hydrogen peroxide production.
Endothelial production of hydrogen peroxide (H2O2) was measured using the fluorescent probe 5(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester (CM-H2DCFDA; Molecular Probes, Eugene, OR). In brief, BAECs (1 × 105/0.2 ml culture medium) were plated in 96-well microplates, grown to confluence, and incubated with CM-H2DCFDA (10 μM) at 37°C for 30 min. The fluorescence intensity was measured using a Wallac 1420 Victor2 multilabel counter (excitation 485 nm, emission 535 nm; Wallac, Turku, Finland). Cells treated with hydrogen peroxide (1 mM; Sigma) for 30 min at 37°C were used as positive control.
Determination of superoxide production and NADPH oxidase activity.
Superoxide production was assessed with the lucigenin chemiluminescence method, using superoxide anion assay kit (Sigma) based on the manufacturer's instructions. After cells were homogenized in ice-cold lysis buffer, proteins (100 μg) were added to 96-well luminometer plates containing 5 μM lucigenin (Sigma). Luminescence of the samples was measured using a Wallac 1420 Victor2 multilabel counter, and its intensity (relative light unit) was normalized to sample protein concentration.
The endothelial NADPH oxidase activity was determined by assessing NADPH-dependent superoxide generation. Superoxide generation was measured with or without NADPH (100 μM; Sigma), and the reaction was started by the addition of NADPH to each well. The specificity of the superoxide assay was assessed by inhibition of the reaction with apocynin (100 μM, 1 h; Sigma).
Animal preparations and experimental protocols.
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing ∼300 g were housed at 22 ± 2°C, kept on a 12:12-h light-dark cycle, and fed a standard laboratory chow and water ad libitum prior to the study. After an overnight fast, rats were anesthetized with pentobarbital sodium (50 mg/kg ip; Abbott Laboratories, North Chicago, IL), placed in a supine position, and intubated with a PE-240 tubing to maintain airway patency. The carotid artery and external jugular vein were then cannulated with polyethylene PE50 through a midline neck incision. Rats were observed for 30 min to ensure hemodynamic and anesthesia stability and then studied under one of the following four groups: group 1 (control) received an ip injection of vehicle (50% ethanol at 1 ml/kg); group 2 (resveratrol) received an ip injection of resveratrol (25 mg/kg dissolved in 50% ethanol); group 3 received an ip injection of resveratrol 30 min after systemic infusion of NO synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 50 μg·kg−1·min−1) was begun [l-NAME infusion persisted throughout the study, and at the dose selected it increased mean arterial blood pressure (MAP) by ∼20–30 mmHg and blocked insulin- and losartan-induced increases in muscle microvascular blood volume (MBV) (5, 36)]; and group 4 received an ip injection of resveratrol 60 min after a systemic infusion of TNFα (0.5 μg·kg−1·h−1) was begun, which persisted throughout the study. TNFα, at the dose selected, had no significant effect on either MAP or muscle capillary recruitment but blocked insulin-mediated capillary recruitment in skeletal muscle (43, 46). Muscle microvascular parameters were measured using contrast-enhanced ultrasound (CEU) technique at baseline and 30, 60, 90, and 120 min after vehicle or resveratrol injection.
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996). The study protocol was approved by the Animal Care and Use Committee of the University of Virginia.
Measurement of muscle microvascular parameters.
Microvascular parameters, including MBV and microvascular flow velocity (MFV), were measured using CEU with a HDI-5000 ultrasound system and a L7-4 linear array transducer (Philips Medical Systems, Andover, MA), and microvascular blood flow (MBF) was calculated as described previously (5, 17, 37). In brief, CEU was performed on the proximal adductor muscles (adductor magnus and semimembranosus) of the right hindlimb at a transmission frequency of 3.3 MHz. The contrast agent (Definity; Lantheus Medical Imaging), microbubbles composed of a lipid shell filled with a perfluorocarbon gas, was infused intravenously at a rate of 16 μl/min. Once the systemic microbubble concentrations reached steady state (after 5–8 min of continuous infusion), images were digitally acquired at pulsing intervals (PIs) from 0.5 to 20 s, with a mechanical index of 0.8 (5, 36). Depth, focus, and gains (overall gain, time-gain compensation, and lateral-gain compensation) were optimized at the beginning of each study and held constant throughout. All muscle CEU images were analyzed using the QLAB software (Philips Medical Systems, Andover, MA), and background-subtracted video intensity in the region of interest was determined as described previously (24, 25, 40, 41). The PI (time) vs. video-intensity curve was generated and fitted to an exponential function: y = A (1 − e−βt), where y is video intensity at a PI t, A is the plateau video intensity representing MBV, and β is the rate constant, which reflects the rate of rise of video intensity (i.e., MFV). MBF is the product of MBV and MFV.
Throughout the study, MAP and heart rate were monitored via a sensor connected to the carotid arterial catheter (AD Instruments, Colorado Springs, CO). Pentobarbital sodium was infused at a variable rate to maintain steady levels of anesthesia and blood pressure throughout the study. Euthermia was maintained with a heating pad. Arterial blood glucose concentrations were determined using an Accu-Chek Advantage blood glucometer (Roche).
Statistical analysis.
All data are presented as means ± SE. Statistical analyses were performed using Sigma Stat 3.1.1 software (Systat Software), using one-way ANOVA with post hoc Dunn's analysis, or unpaired Student's t-test as appropriate. A P value of <0.05 was considered statistically significant.
RESULTS
Resveratrol stimulates phosphorylation of endothelial Akt, eNOS, and ERK1/2 in vitro.
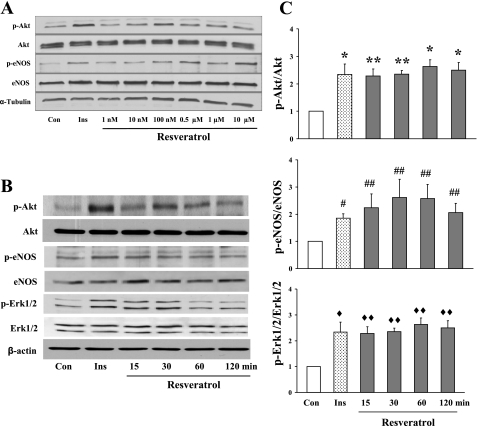
To examine the effects of resveratrol on the phosphorylation of Akt, eNOS, and ERK1/2 in endothelial cells, BAECs were cultured to confluence and then treated with 100 nM resveratrol for various times after an overnight fast. We first carried out a dose response study and found that resveratrol at 100 nM potently increased the phosphorylation of Akt and eNOS by 30 min of incubation (Fig. 1A). Using this concentration, we examined the time course of resveratrol on endothelial Akt, eNOS, and ERK1/2 phosphorylation. As shown in Fig. 1, B and C, resveratrol treatment acutely increased the phosphorylation of Akt, eNOS, and ERK1/2 within 15 min by more than twofold, and this effect lasted for ≥2 h.
Fig. 1.
Effects of resveratrol on the phosphorylation of Akt, endothelial nitric oxide synthase (eNOS), and ERK1/2 in cultured endothelial cells. A: representative gel of dose response study. B: representative gel of time course study. C: quantification of protein phosphorylation. Con, control; Ins, insulin vs. Con. *P < 0.01; **P < 0.04; #P < 0.004; ##P < 0.001; ♦P < 0.004; ♦♦P < 0.03.
Resveratrol abolishes TNFα-induced ROS production and NADPH oxidase activity in endothelial cells.
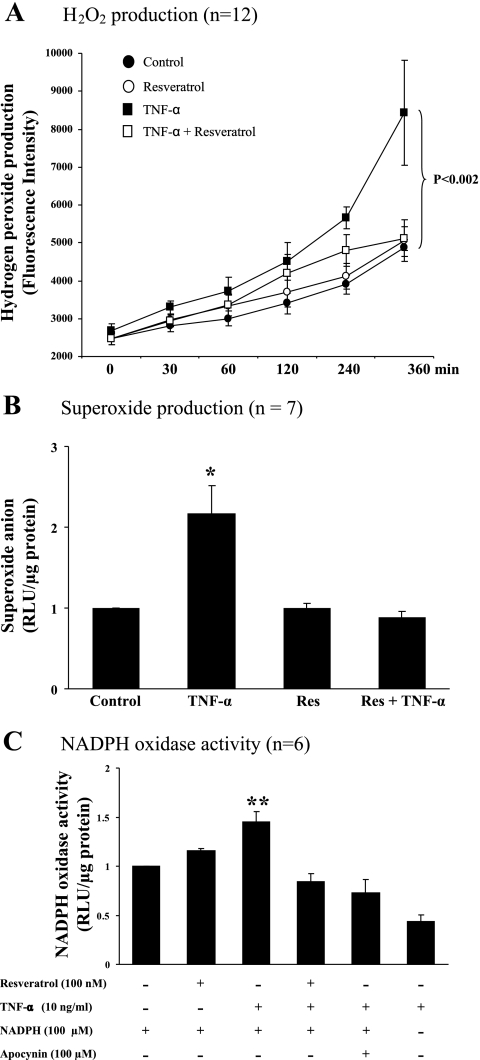
Resveratrol is a potent antioxidant and has been shown to prevent TNFα-induced ROS production in endothelial cells. To ascertain that resveratrol does decrease TNFα-induced ROS and NADPH oxidase activity, we incubated BAECs with TNFα in the presence or absence of resveratrol. As shown in Fig. 2, TNFα potently increased the endothelial production of H2O2 (P < 0.002) and superoxide anion (P < 0.02) and increased the NADPH oxidase activity (P < 0.004). However, treatment with resveratrol at the same time completely restored the levels of H2O2 and superoxide anion and the activity of NADPH oxidase back to the control levels.
Fig. 2.
Effects of resveratrol on TNFα-induced oxidative stress in cultured endothelial cells. *P < 0.02 and **P < 0.004 vs. control. Res, resveratrol; RLU, relative light units.
Resveratrol increases muscle microvascular perfusion in vivo via NO-dependent pathway.
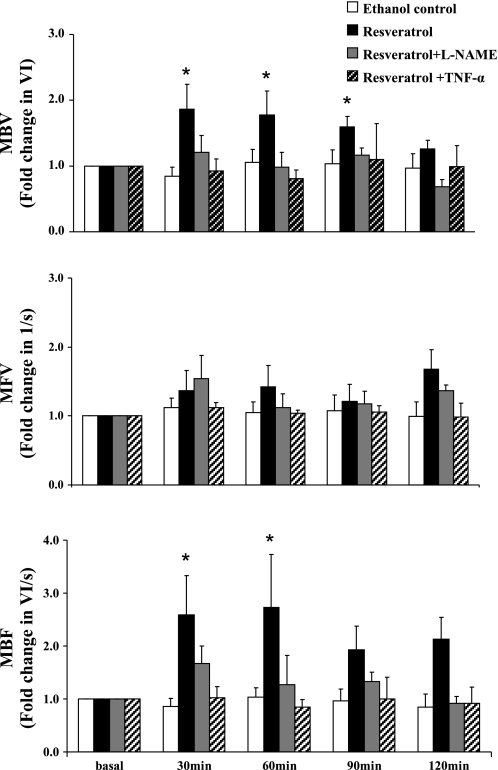
To assess the physiological significance of the resveratrol-induced eNOS phosphorylation, we next carried out in vivo studies examining the effects of resveratrol on muscle microvascular perfusion, since previous studies have shown repeatedly that activation of eNOS by insulin potently increases muscle microvascular perfusion by increasing MBV without affecting MFV (9, 10, 25, 36–38). As shown in Fig. 3, ip resveratrol potently increased muscle MBV at 30 min, and this effect lasted for ∼120 min (P = 0.007, ANOVA). Although MFV trended up after resveratrol injection, the increase was not statistically significant (P = 0.233). As a result, MBF, which is derived from the product of MBV and MFV, increased significantly after resveratrol injection (P < 0.02). Resveratrol clearly increased MBF without inducing significant changes in either MAP or blood glucose concentrations (Table 1).
Fig. 3.
Effects of resveratrol on muscle microvascular parameters in vivo. Resveratrol significantly increased microvascular blood volume (MBV; P = 0.007, ANOVA) and microvascular blood flow (MBF; P < 0.02, ANOVA) vs. basal. *P < 0.05. MFV, microvascular flow velocity; l-NAME, Nω-nitro-l-arginine methyl ester.
Table 1.
Changes in MAP and blood glucose during the in vivo studies
| Basal | 30 min | 60 min | 90 min | 120 min | |
|---|---|---|---|---|---|
| MAP, mmHg | |||||
| Control | 107 ± 5 | 99 ± 4 | 100 ± 3 | 100 ± 3 | 100 ± 4 |
| Resveratrol | 111 ± 2 | 104 ± 4 | 106 ± 4 | 104 ± 4 | 101 ± 4 |
| Resveratrol + l-NAME | 113 ± 4 | 125 ± 4* | 130 ± 4* | 133 ± 4* | 130 ± 2* |
| Resveratrol + TNF-α | 114 ± 4 | 109 ± 4 | 104 ± 4 | 106 ± 3 | 109 ± 3 |
| Blood glucose, mg/dl | |||||
| Control | 98 ± 6 | 93 ± 8 | 96 ± 5 | 101 ± 6 | 107 ± 7 |
| Resveratrol | 101 ± 1 | 91 ± 4 | 101 ± 3 | 96 ± 4 | 102 ± 4 |
| Resveratrol + l-NAME | 90 ± 3 | 86 ± 2 | 88 ± 3 | 92 ± 3 | 98 ± 4 |
| Resveratrol + TNFα | 107 ± 2 | 109 ± 5 | 107 ± 5 | 111 ± 5 | 113 ± 4 |
Values are means ± SE. MAP, mean arterial blood pressure; l-NAME, Nω-nitro-l-arginine methyl ester.
P < 0.001.
Because a previous study has confirmed that resveratrol enhances NO release and improves endothelial function in diabetic mice (44), and increased NO availability by either insulin infusion (36, 37, 45) or losartan injection (5) potently increases MBV, we further examined the role of NO in resveratrol-induced increase of muscle MBV. As shown in Fig. 3, continuous infusion of l-NAME, a selective NOS inhibitor, completely abolished resveratrol-induced increases in MBV and MBF (P = 0.122 and 0.073, respectively; Fig. 3). As expected, continuous infusion of l-NAME at this dose raised the MAP by ∼20 mmHg (P < 0.001; Table 1) without affecting blood glucose levels.
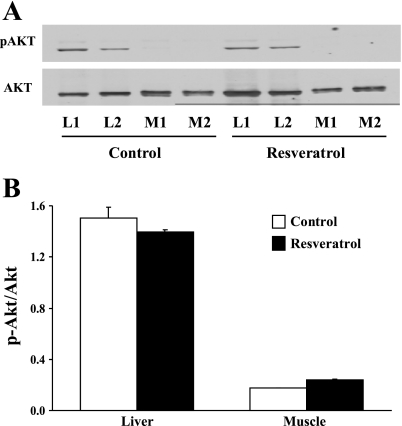
Resveratrol does not increase the phosphorylation of Akt in either liver or muscle in vivo.
Because we have shown that resveratrol increases the phosphorylation of Akt in cultured endothelial cells, we further examined whether resveratrol also stimulated the phosphorylation of Akt in liver and muscle. We selected these tissues because liver has a high blood flow and discontinuous endothelium, allowing easy access to the hepatocyte, whereas muscle has tight endothelial junctions. As shown in Fig. 4, resveratrol did not increase the phosphorylation of Akt in either tissue, suggesting that the resveratrol effect is relatively endothelium specific in our study setting.
Fig. 4.
Effects of resveratrol on Akt phosphorylation in liver and muscle in vivo. A: representative gels. L1 and L2, liver samples 1 and 2, respectively; M1 and M2, muscle samples 1 and 2, respectively. B: quantification of Akt phosphorylation.
TNFα abolishes resveratrol-induced increases in muscle microvascular perfusion.
Chronic inflammation contributes to endothelial dysfunction. Although resveratrol restores vascular function in diabetic mice, the improvement is abolished by incubating the vessel with TNFα in vitro (44). To further examine whether TNFα impairs the vasodilatory effect of resveratrol on muscle microcirculation, we systemically infused TNFα at a concentration that has been shown to abolish insulin-mediated capillary recruitment in rats 60 min prior to resveratrol ip injection. TNFα infusion had no significant effect on MAP or blood glucose concentrations (Table 1) but completely reversed resveratrol-induced increases in skeletal muscle MBV and MBF (P = 0.659 and 0.834, respectively; Fig. 3).
DISCUSSION
The present study demonstrated for the first time that resveratrol exerts potent vasodilatory action in the skeletal muscle microcirculation via a NO-dependent mechanism. Despite the potent antioxidant effect of resveratrol, TNFα infusion completely neutralized resveratrol's microvascular vasodilatory actions. Because it is in the microcirculation where substrate extraction takes place and a relatively small increase (decrease) in the microvascular surface area could markedly increase (decrease) substrate extraction by muscle, antioxidants like resveratrol may play an important role in regulating substrate exchange in the muscle by increasing endothelial surface area.
Resveratrol has been shown to have multiple potential health benefits, including cardiovascular protection (3). Although the underlying mechanisms remain to be elucidated, evidence suggests that this might be related to resveratrol's antioxidant and anti-inflammatory properties and its ability to improve endothelial function (3). Resveratrol can relax isolated murine aorta or porcine mesenteric and uterine arteries (6, 27). It appears that both endothelium-dependent (NO-mediated; Refs. 28 and 33) and endothelium-independent (Ca++-activated K+ channels; Ref. 23) pathways are involved in resveratrol-induced vascular relaxation. In the present study, we observed that l-NAME completely abolished resveratrol-mediated muscle microvascular recruitment in vivo. This strongly suggests that resveratrol increases muscle microvascular perfusion via the endothelium-dependent, NO-mediated process. The observation that resveratrol increased eNOS phosphorylation within 15 min and recruited microvasculature within 30 min suggests that resveratrol can acutely activate eNOS to increase NO production. This is consistent with a previous report showing that resveratrol induced endothelial NO production after only a 2-min incubation (39). In addition, longer treatment with resveratrol upregulated eNOS mRNA and protein expression, increased eNOS promoter activity, and stabilized eNOS mRNA in cultured human umbilical vein endothelial cells (39). Resveratrol appears to affect eNOS via SIRT1 to cause upregulation and deacetylation (26) and perhaps also via ERα to induce eNOS phosphorylation, which is mediated by ERK1/2 (19, 20).
The effect of resveratrol on the muscle microcirculation resembles that of physiological concentrations of insulin. We and others have demonstrated repeatedly that insulin at physiological concentrations potently recruits microvasculature in muscle in both humans and experimental animals (9, 11, 24, 25, 36–38, 45). This is mediated via the Akt-eNOS signaling pathway. Although we did not measure insulin concentrations in the present study, it is unlikely that resveratrol exerted its actions via insulin secretion since plasma glucose concentrations remained steady during the study, and there was no evidence of increased Akt phosphorylation in biopsies of either skeletal muscle or liver following resveratrol treatment (Fig. 4). Furthermore, in vitro studies have shown that resveratrol actually inhibits insulin release, likely by blocking the voltage-gated Ca++ channels (18, 34).
That resveratrol increased muscle microvascular blood flow by more than twofold without significantly changing plasma glucose concentrations is not surprising, since the basal use of glucose by muscle is low and the increased glucose use associated with the muscle microvascular recruitment is likely less than the hepatic glucose production in the postabsorptive state. Similarly, we have demonstrated previously that increasing muscle microvascular recruitment and muscle glucose use by threefold after losartan administration does not alter plasma glucose concentrations (5).
Endothelial dysfunction is an early event in the development of atherosclerosis and is closely associated with insulin resistance, obesity, and diabetes. Many factors are associated with endothelial dysfunction, including chronic inflammation. Resveratrol treatment has been shown to improve endothelial function in hypertensive rats (31), in rats a fed high-fat diet (1), and in diabetic mice (44). This is not surprising since resveratrol has been shown to have potent antioxidant and anti-inflammatory properties, likely via its effect on the NADPH oxidase (7, 33, 44). Consistent with previous reports, we have shown in the present study that resveratrol prevented TNFα-induced increases in NADPH oxidase activity and ROS production (Fig. 2).
In the present study, we observed that TNFα completely neutralized the vasodilatory actions of resveratrol on muscle microvasculature (Fig. 3). TNFα at the dose infused increased plasma TNFα concentrations to ∼350 pg/ml (46) and did not affect basal hemodynamic parameters, glucose metabolism, or muscle microvascular perfusion (43). However, this dose was clearly enough to completely block muscle microvascular recruitment induced by physiological hyperinsulinemia. Alhough this concentration is higher than ∼90 pg/ml observed in obese, insulin-resistant rodents (16), it is likely comparable with tissue concentrations, since most TNFα is released from various tissues. Indeed, in fructose-fed rats, each gram of skeletal muscle contains ∼500–600 pg of TNFα (35). Further studies are warranted to elucidate the mechanisms underlying the inhibitory effect of TNFα on resveratrol-mediated muscle microvascular recruitment. Compared with the in vitro studies the TNFα concentration was 30-fold less, and resveratrol concentrations were fourfold higher. As such, it is reasonable to assume that resveratrol prevented TNFα-induced ROS production in vivo as well. However, it is likely that TNFα still stimulates some superoxide production, which then interacts with NO to form peroxynitrite, resulting in decreased NO bioavailability (42). It is also possible that TNFα may act via ROS-independent mechanisms. We have demonstrated previously that TNFα potently activates p38 MAPK, which subsequently causes serine phosphorylation of insulin receptor substrate-1 and impairs insulin-stimulated activation of Akt and eNOS in cultured endothelial cells (21). Whether TNFα attenuates resveratrol's effect on muscle microvasculature via a similar mechanism warrants further study. Our results suggest that chronic inflammation, as seen in patients with diabetes, may limit resveratrol's vasodilatory effect in the muscle microcirculation. Interestingly, in arteries isolated from humans with coronary heart disease, NO-dependent vasorelaxation in response to resveratrol is also lost (12).
At the dose (25 mg/kg ip) selected, plasma resveratrol concentrations reach ∼100 ng/ml (∼0.4 μM) at 120 min (47), which is similar to those achieved with intravenous injection (15). This was significantly lower than previous studies demonstrating potential health benefits of resveratrol (1–100 μM) (3). At this concentration, we were able to show that resveratrol potently recruited muscle microvasculature in vivo and abolished high concentrations of TNFα-induced increases in NADPH oxidase activity and ROS production in vitro. Whether resveratrol at lower concentrations has similar effects warrants further studies.
In conclusion, resveratrol acutely activates eNOS and recruits muscle microvasculature via an NO-dependent mechanism. Despite the potent antioxidant effect of resveratrol, TNFα at concentrations that block insulin-mediated muscle microvascular recruitment completely neutralized resveratrol's microvascular action. Thus, our results suggest that chronic inflammation, as seen in type 2 diabetes, may limit resveratrol's vasodilatory actions on muscle microvasculature.
GRANTS
This work was supported by American Diabetes Association Grants 7-07-CR-34 and 7-09-NOVO-11 (Z. Liu) and National Institutes of Health Grants R01-HL-094722 (Z. Liu), R01-DK-057878 (E. J. Barrett), and R01-073759 (E. J. Barrett).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Aubin MC, Lajoie C, Clément R, Gosselin H, Calderone A, Perrault LP. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J Pharmacol Exp Ther 325: 961–968, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Chai W, Liu Z. p38 mitogen-activated protein kinase mediates palmitate-induced apoptosis but not inhibitor of nuclear factor-kappaB degradation in human coronary artery endothelial cells. Endocrinology 148: 1622–1628, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, Liu Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 55: 523–530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol 27: 363–366, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol 102: 1520–1527, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 295: E732–E750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clerk LH, Rattigan S, Clark MG. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51: 1138–1145, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev 20: 3–12, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Cruz MN, Luksha L, Logman H, Poston L, Agewall S, Kublickiene K. Acute responses to phytoestrogens in small arteries from men with coronary heart disease. Am J Physiol Heart Circ Physiol 290: H1969–H1975, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Eringa EC, Stehouwer CD, Walburg K, Clark AD, van Nieuw Amerongen GP, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-alpha dependence on c-Jun N-terminal kinase. Arterioscler Thromb Vasc Biol 26: 274–280, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 15. He H, Chen X, Wang G, Wang J, Davey AK. High-performance liquid chromatography spectrometric analysis of trans-resveratrol in rat plasma. J Chromatogr B Analyt Technol Biomed Life Sci 832: 177–180, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 56: 2194–2200, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Jakab M, Lach S, Bacová Z, Langelüddecke C, Strbák V, Schmidt S, Iglseder E, Paulmichl M, Geibel J, Ritter M. Resveratrol inhibits electrical activity and insulin release from insulinoma cells by block of voltage-gated Ca+ channels and swelling-dependent Cl− currents. Cell Physiol Biochem 22: 567–578, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors α and β in endothelial cells. J Biol Chem 280: 7460–7468, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J 22: 2185–2197, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Li G, Barrett EJ, Barrett MO, Cao W, Liu Z. Tumor necrosis factor-α induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology 148: 3356–3363, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146: 4690–4696, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Li HF, Chen SA, Wu SN. Evidence for the stimulatory effect of resveratrol on Ca2+-activated K+ current in vascular endothelial cells. Cardiovasc Res 45: 1035–1045, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 293: E1250–E1255, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 94: 3543–3549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naderali EK, Doyle PJ, Williams G. Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female guinea-pigs. Clin Sci 98: 537–543, 2000 [PubMed] [Google Scholar]
- 28. Orallo F, Alvarez E, Camina M, Leiro JM, Gomez E, Fernandez P. The possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol Pharmacol 61: 294–302, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Perseghin G, Petersen K, Shulman GI. Cellular mechanism of insulin resistance: potential links with inflammation. Int J Obes Relat Metab Disord 27, Suppl 3: S6–S11, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-α induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99: 69–77, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Rush JW, Quadrilatero J, Levy AS, Ford RJ. Chronic resveratrol enhances endothelium-dependent relaxation but does not alter eNOS levels in aorta of spontaneously hypertensive rats. Exp Biol Med (Maywood) 232: 814–822, 2007 [PubMed] [Google Scholar]
- 32. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silvia B, Livia B, Alessandro V. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev 22: 169–188, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Szkudelski T. Resveratrol-induced inhibition of insulin secretion from rat pancreatic islets: evidence for pivotal role of metabolic disturbances. Am J Physiol Endocrinol Metab 293: E901–E907, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Togashi N, Ura N, Higashiura K, Murakami H, Shimamoto K. The contribution of skeletal muscle tumor necrosis factor-α to insulin resistance and hypertension in fructose-fed rats. J Hypertens 18: 1605–1610, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285: E123–E129, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53: 1418–1423, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 51: 42–48, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106: 1652–1658, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97: 473–483, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Wei K, Ragosta M, Thorpe J, Coggins M, Moos S, Kaul S. Noninvasive quantification of coronary blood flow reserve in humans using myocardial contrast echocardiography. Circulation 103: 2560–2565, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 120: 1266–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Youd JM, Rattigan S, Clark MG. Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-α. Diabetes 49: 1904–1909, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function—role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29: 1164–1171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53: 447–453, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Zhang L, Wheatley CM, Richards SM, Barrett EJ, Clark MG, Rattigan S. TNF-α acutely inhibits vascular effects of physiological but not high insulin or contraction. Am J Physiol Endocrinol Metab 285: E654–E660, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Zhu Y, Huang T, Cregor M, Long H, Kissinger CB, Kissinger PT. Liquid chromatography with multichannel electrochemical detection for the determination of trans-resveratrol in rat blood utilizing an automated blood sampling device. J Chromatogr B Biomed Sci Appl 740: 129–133, 2000 [DOI] [PubMed] [Google Scholar]