Abstract
Diabetes promotes protein synthesis to induce kidney hypertrophy and increase renal matrix proteins. Increased capacity for mRNA translation by way of ribosomal biogenesis facilitates sustained stimulation of protein synthesis. We tested the hypothesis that high glucose induces ribosomal biogenesis as indicated by an increase in rRNA synthesis in the setting of augmented protein synthesis. High glucose (30 mM) increased global protein synthesis, expression of matrix proteins, laminin γ1 and fibronectin, and rDNA transcription in glomerular epithelial cells (GECs) compared with 5 mM glucose. High glucose induced Ser388 phosphorylation of upstream binding factor (UBF), an rDNA transcription factor, along with increased phosphorylation of Erk and p70S6 kinase. Inactivation of Erk and p70S6 kinase either by their respective chemical inhibitors or by expression of their inactive mutant constructs blocked high-glucose-induced UBF phosphorylation. High glucose reduced nuclear content of p19ARF and promoted dissolution of inactive UBF-p19ARF complex. High glucose also promoted association of UBF with RPA194, a subunit of RNA polymerase I. Inhibition of Erk, p70S6 kinase, and UBF1 by transfecting GECs with their respective inactive mutants abolished laminin γ1 synthesis, protein synthesis, and rDNA transcription. Renal cortex from type 1 diabetic rats and type 2 diabetic db/db mice showed increased phosphorylation of UBF, Erk, and p70S6 kinase coinciding with renal hypertrophy and onset of matrix accumulation. Our data suggest that augmented ribosome biogenesis occurs in an UBF-dependent manner during increased protein synthesis induced by high glucose in the GECs that correlates with UBF activation and renal hypertrophy in rodents with type 1 and type 2 diabetes.
Keywords: ribosomal deoxyribonucleic acid transcription, diabetic kidney disease, hypertrophy, mammalian target of rapamycin, ribonucleic acid, polymerase I
characteristic features of diabetic kidney disease include renal hypertrophy and accumulation of extracellular matrix (ECM) proteins. Both of these manifestations require augmented protein synthesis. It is generally recognized that mRNA translation is a key regulatory event in gene expression culminating in peptide synthesis (14). Ribosomes are the sites of peptide synthesis. The eukaryotic ribosome consists of a 60S subunit, which contains 28S, 5.8S, and 5S ribosomal RNAs (rRNAs) and 49 ribosomal proteins and a 40S subunit composed of the 18S rRNA and 33 ribosomal proteins (16). rRNA and proteins spontaneously fold into functional conformation based on their primary sequence (44). Inactivation of rRNA rather than the protein component of the ribosome inhibits protein synthesis, stressing the importance of rRNA in translation (32). rRNA is the most abundant form of RNA in the eukaryotic cell. Cell hypertrophy is known to be associated with increased 18S rRNA synthesis and augmented Ser388 phosphorylation of upstream binding factor 1 (UBF1), the nucleolar rDNA transcription factor that belongs to the high-mobility group family of proteins (27). Reversible phosphorylation of UBF at Ser388 plays a key role in modulating its DNA-binding activity and its interaction with other components of the transcriptional apparatus (39). However, the signal transduction pathways involved in this process are poorly understood.
Enhanced rate of protein synthesis is a result of increased translational rates by existing ribosomes (translational efficiency) and/or synthesis and recruitment of additional ribosomes (translational capacity) (42). Translational capacity is determined by the availability of components of the protein synthetic apparatus, including ribosomes, tRNAs, and translation factors. Accelerated synthesis and accumulation of ribosomes are fundamental for efficient cell growth and proliferation (27). Ribosomal biogenesis can be considered in two parts: generation of ribosomal proteins and rRNA. Much of the acute demand for ribosomal proteins arising from a growth stimulus is met by increased translation of encoding mRNAs (28). rRNA synthesis is stimulated through interaction of RNA polymerase I (RNA Pol I) with UBF (7, 34), selectivity factor 1, and factors containing the TATA-binding protein (8). Ribosomal gene transcription (rDNA transcription) by Pol I is a major rate-limiting step in ribosome biogenesis (13, 45).
Regulation of ribosomal generation in the kidney in diabetes, a setting in which protein synthesis is sustained for prolonged periods of time to induce parenchymal hypertrophy and deposition of matrix proteins, has not been studied. Glomerular epithelial cells (GECs) are important contributors to ECM production and accumulation in the glomerulus leading to matrix expansion and thickening of the glomerular basement membrane, a cardinal manifestation of diabetic kidney disease. We hypothesized that high glucose induces ribosomal biogenesis as indicated by the increase in rRNA synthesis in the setting of augmented protein synthesis in the GECs.
MATERIALS AND METHODS
Cell culture.
Rat GECs, kindly provided by Dr. Jeffrey Kreisberg (Department of Surgery, University of Texas Health Science Center at San Antonio), were cultured as described previously (15). GECs display several of the characteristics of visceral GECs in vivo, including expression of Heymann nephritis antigen (38), CD2AP, laminin, fibronectin, and basement membrane heparan sulfate proteoglycan (15) and sensitivity to puromycin aminonucleoside (17). GECs were grown in DMEM containing 7% FBS, 5 mM glucose, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. GECs were incubated with normal glucose (5 mM glucose), high glucose (30 mM glucose), or mannitol (5 mM glucose + 25 mM mannitol) as osmotic control for the indicated time points.
Extraction of nuclear and cytoplasmic fractions.
Nuclear extracts from cells and tissues were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific-Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. Purity of the nuclear fraction was ascertained by immunoblotting with lamin b antibody and that of cytoplasmic fraction with GAPDH antibody (Abcam, Cambridge, MA).
Immunoblotting.
Previously described methods were employed (23–25). Equal amounts of protein from cell lysate or nuclear extract were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with primary antibody for 3 h. Primary antibodies were from Cell Signaling (Beverly, MA) if not otherwise mentioned. Laminin γ1, UBF, phospho-UBF, and RPA194 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), and fibronectin antibody was from Sigma (St. Louis, MO). After being washed, the membrane was incubated with peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). Proteins were visualized by chemiluminescence using the enhanced chemiluminence reagent (Thermo Scientific-Pierce Biotechnology). Images of the bands were scanned by reflectance scanning densitometry, and the intensity of the bands was quantified using Scion Image software (23, 25).
Immunoprecipitation and immunoblotting.
We have previously described the methods employed (23–25). Nuclear extracts (300 μg protein) from cells treated with or without high glucose for the indicated time points were incubated with RPA194 antibody or UBF antibody at 4°C for 3 h. Washed protein A/G Agarose beads (20 μl) were added, and the samples were rotated for a further 1 h. The beads were washed three times with RIPA buffer containing 0.5% Nonidet P-40 followed by washing with 1× PBS one time. The samples were boiled, separated on SDS-PAGE, transferred, and Western blotted with anti-UBF or anti-p19ARF.
Transfections.
GECs were transiently transfected with empty vector plasmids or plasmids containing dominant-negative construct for Erk2 MAP kinase (kindly provided by Dr. T. W. Sturgill, University of Virginia) and inactive (pRc/CMV-FLAG-UBF1/S388G) or constitutively active (pRc/CMV-FLAG-UBF1/S388D) constructs of UBF1 (kindly provided by Dr. Renate Voit, German Cancer Research Center, Heidelberg, Germany), mammalian target of rapamycin (mTOR)-S2481A (kindly provided by Dr. S. L. Schreiber, Harvard University, Boston, MA), and p70S6 kinase-K100R (kindly provided by Dr. John Blenis via Addgene, Cambridge, MA). Transfection was performed using Fugene HD reagent (Roche, Pleasanton, CA) according to the manufacturer's protocol.
Protein synthesis measurement.
Serum-starved cells incubated with 30 mM glucose were labeled with 10 μCi/ml of [35S]methionine (Perkin-Elmer, Waltham, MA) for the terminal 2 h of incubation (22). Before serum starvation, cells were tranfected with indicated plasmid constructs or treated with respective inhibitors before adding glucose. Cells were washed in PBS and lysed in radioimmunoprecipitation assay buffer. Cell protein content was measured with Bio-Rad reagent (Bio-Rad, Hercules, CA). An equal amount of protein (30 μg) was spotted onto the 3-mm filter paper (Whatman). Filters were washed three times by boiling for 1 min in 10% TCA containing 0.1 g/l methionine. The filter papers were air-dried, and radioactivity was determined using a liquid scintillation counter.
Luciferase reporter assays.
Cells were plated in a 6- or 12-well plate and allowed to grow for 24 h. Ribosomal DNA promoter-IRES-luciferase reporter plasmid (kindly provided by Dr. Samson T. Jacob, Ohio State University, Columbus, OH) (6) and inactive plasmid constructs of Erk, UBF1, or p70S6 kinase were cotransfected using Fugene HD (Roche) transfection reagent according to the manufacturer's protocol. All transfections containing equal amounts of DNA (1 μg/ml media), were allowed to grow for 24 h, changed to serum-free media overnight, and treated as described above. Cell lysates were prepared using passive lysis buffer and assayed for luciferase activity using a luciferase assay kit (Promega, Madison, WI) according to the manufacturer's protocol (3). Luciferase activity was expressed as arbitrary units per milligram protein, and changes were shown in terms of percent of control.
Animal study.
Animal protocols were approved by the Institutional Animal Care and Use Committee. The C57BL6/KsJ lepr−/− db/db mice, a model of type 2 diabetes, and its lean littermates (db/m) (Jackson Labs, Bar Harbor ME) were maintained on regular laboratory chow. Blood glucose concentration was monitored for emergence of diabetes. In the present study, lean littermate control and diabetic mice were studied in the early phase, after 2 wk of onset of hyperglycemia. The db/db mice develop renal hypertrophy at 2 wk following onset of diabetes (9, 36, 37). Mice were killed at the end of the experimental period, and renal cortex was dissected out and processed for further analysis. To induce type 1 diabetes, male Sprague-Dawley rats weighing 200–220 g (Harlan, Indianapolis, IN) received a single injection of 60 mg/kg streptozotocin in citrate buffer (pH 4.5) in the tail vein (21). The rats became diabetic the next day as indicated by elevation in blood glucose concentration, which was measured by a glucometer (Ascensia Elite XL, Mishawaka, IN). On day 4 of hyperglycemia, rats were killed, and the renal cortex was dissected out for further analysis. Body weight and blood glucose concentration were measured daily in both rodents during the course of the study.
Immunofluorescent staining of kidney tissues.
Snap-frozen renal cortex from db/db type 2 diabetic mice along with their respective control db/m mice tissues were used for immunofluorescent studies using phospho-UBF and synaptopodin antibodies. Immunofluorescence histochemistry was performed as previously described (4). Six-micrometer-thick frozen kidney sections were cut using a cryostat and allowed to air-dry for 45 min. The sections were fixed in ice-cold acetone for 5 min, air-dried, and then rehydrated in PBS and 1% BSA. To identify podocytes, tissues were stained with a goat anti-synaptopodin antibody (Santa Cruz Biotechnology) followed by FITC-labeled donkey anti-goat IgG (Chemicon). After being washed, the sections were stained with a rabbit antibody directed against phospho-UBF (Santa Cruz Biotechnology) followed by Cy3-labeled donkey anti-rabbit IgG (Chemicon, Temecula, CA). After being mounted under glass cover slips, the sections were viewed, and digital images of random glomeruli representing each fluorochrome were taken using an AX70 Research microscope and a DP70 digital camera (Olympus, Melville, NY).
Statistical analysis.
All values are expressed as means ± SE obtained from at least three independent experiments. Statistical analysis was performed using one-way ANOVA for comparison between multiple groups and post hoc analysis using Student-Newman-Keuls multiple-comparison tests using GraphPad Prism 4 software. Statistical comparisons between two groups were performed by the Student's t-test. Statistical significance was assigned to values of P < 0.05.
RESULTS
High glucose increases protein synthesis and expression of laminin γ1 and fibronectin.
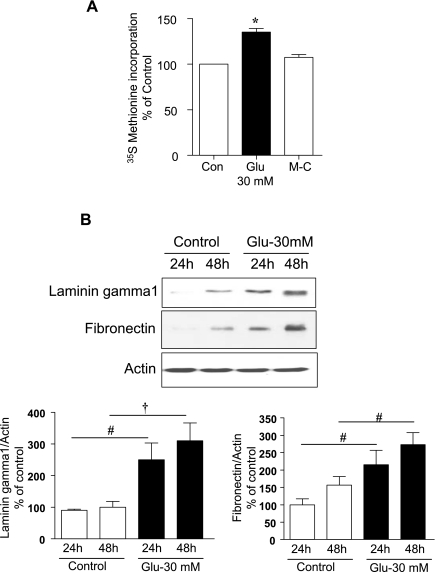
High glucose increased protein synthesis significantly by 40% following incubation for 3 days (P < 0.001, Fig. 1A). However, equimolar mannitol did not affect protein synthesis (Fig. 1A), suggesting that the high glucose effect was not by an osmotic mechanism. To determine the effect of high glucose on ECM protein synthesis, serum-starved GECs were incubated with or without 30 mM glucose for the time shown. Immunoblotting with specific antibodies showed a significant increase in contents of laminin γ1 (P < 0.05, 24 h and P < 0.01, 48 h) and fibronectin (P < 0.05, 24 and 48 h) matrix proteins in high-glucose-treated cells compared with control cells incubated in 5 mM glucose (Fig. 1B). The increase in ECM proteins was seen at 24 h and lasted for up to 48 h. There was no change in matrix protein synthesis following incubation with 5 mM glucose + 25 mM mannitol medium [Supplemental Fig. S1 (Supplemental data for this article may be found on the American Journal of Physiology: Renal Physiology website)].
Fig. 1.
A: high glucose stimulates protein synthesis in glomerular epithelial cells (GECs). Quiescent GEC were incubated with 5 mM glucose (control, Con), high glucose (30 mM, Glu), or 5 mM glucose + 25 mM mannitol (M-C) for 72 h. Serum-free media with 30 mM glucose was replaced after 48 h. During the last 2 h of the 72-h incubation [35S]methionine was added. Incorporation of radiolabel in TCA-precipitable protein was taken as a measure of de novo protein synthesis and expressed as a percentage of control. Composite data from 4 experiments, with triplicates for each condition for each experiment, are shown. *P < 0.001 high glucose vs. control, by ANOVA. B: high glucose stimulates synthesis of extracellular matrix proteins. Equal amounts of cell lysates following treatment under conditions shown were immunoblotted with specific antibodies. High glucose promoted an increase in synthesis of laminin γ1 and fibronectin starting at 24 h and lasting up to 48 h. Top, immunoblotting for actin to assess loading. A representative blot is shown. Histograms show quantitation of results from 3–4 independent experiments. #P < 0.05 and †P < 0.01 vs. control by ANOVA.
High glucose promotes phosphorylation of UBF, Erk1/2 MAP kinase, and p70S6 kinase.
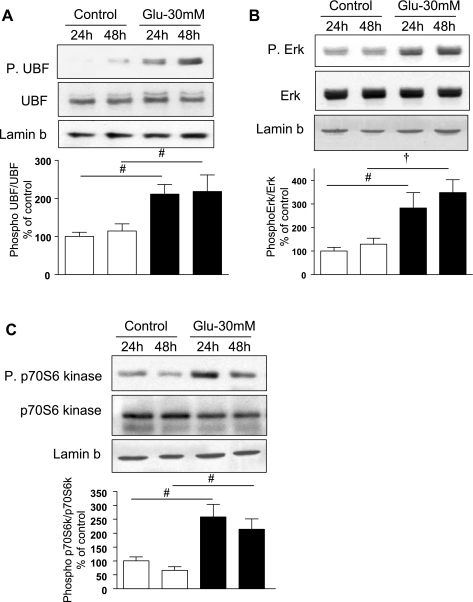
UBF is one of the key components of the RNA Pol I transcription initiation complex. Phosphorylation of UBF is required for its association with Pol I to form the rDNA transcription preinitiation complex in the nuclear compartment (43). Analysis of nuclear extracts from high-glucose-treated GECs showed increased phosphorylation of UBF at Ser388 at 24 h, and this effect persisted for 48 h (P < 0.05, Fig. 2A). We investigated the identity of kinases involved in UBF phosphorylation. Previous studies have implicated Erk1/2 MAP kinase (39) and/or p70S6 kinase (10) in UBF phosphorylation. Immunoblotting showed that high glucose increased phosphorylation of Erk (Fig. 2B) in the nuclear fractions at 24 h (P < 0.05) and 48 h (P < 0.01). High glucose also augmented Thr389 phosphorylation of nuclear p70S6 kinase at the same time (P < 0.05, Fig. 2C). Because Thr389 site on p70S6 kinase is phosphorylated and activated by mTOR, these data suggested that the Erk and mTOR/p70S6 kinase axis may be involved in high glucose regulation of UBF phosphorylation. These data also showed that high glucose promotes nuclear localization of phosphorylated Erk and p70S6 kinase in the GEC. It has been previously reported that a significant part of cytoplasmic Erk moves to the nucleus following stimulation, which may facilitate its access to nuclear substrates and allow for nuclear protein-protein interaction (46). Thus our data suggest that nuclear localization of phosphorylated, active Erk and p70S6 kinase in high-glucose-treated cells would help the kinases reach their potential substrate, UBF.
Fig. 2.
High glucose stimulates phosphorylation (P) of upstream binding factor 1 (UBF1), Erk1/2 MAP kinase, and p70S6 kinase in the nuclear compartment. Equal amounts of protein from nuclear extracts from cells treated with or without high glucose for 24 and 48 h were immunoblotted with antibodies that specifically detect phosphorylated forms of UBF1, Erk, and p70S6 kinase. Phosphorylation of UBF1 (A), Erk (B), and p70S6 kinase (C) was increased upon incubation with high glucose. Immunoblotting with antibodies against UBF, Erk, and p70S6 kinase is shown to evaluate changes in the respective proteins. Immunoblotting with antibody against lamin b, a nuclear marker, was performed to assess loading and establish purity of the nuclear preparation (top). Representative blots for each kinase from 3–5 experiments are shown, and composite data are given in histograms. #P < 0.05 and †P < 0.01 vs. control by ANOVA.
High glucose induction of UBF phosphorylation and laminin synthesis requires Erk and p70S6 kinase activation.
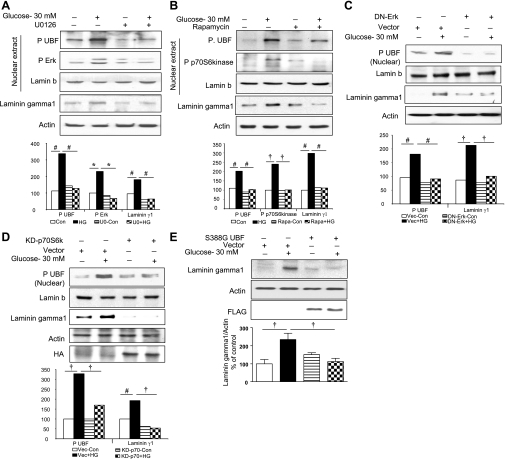
We investigated if Erk and mTOR/p70S6 kinase activation is required for UBF phosphorylation at Ser388. GECs were preincubated for 1 h with or without U-0126, a MEK inhibitor, or, rapamycin, an mTOR complex 1 (mTORC1) inhibitor, which leads to inhibition of mTOR-mediated phosphorylation of p70S6 kinase; cells were then incubated with high glucose for 24 h. Immunoblotting of nuclear lysates showed significant abrogation of high-glucose-induced UBF phosphorylation with either inhibitor (P < 0.05, Fig. 3, A and B). This was associated with inhibition of the high-glucose-induced increase in laminin γ1 synthesis in the cytosolic compartment (P < 0.05, Fig. 3, A and B) by both Erk and p70S6 kinase inhibitors. These data are consistent with the requirement for Erk and p70S6 kinase activation for high-glucose-stimulated UBF phosphorylation in the nuclear compartment. Requirement for activation of Erk and p70S6 kinase for high-glucose induction of UBF phosphorylation and laminin γ1 synthesis was further confirmed by expression of dominant-negative Erk or kinase-dead p70S6 kinase. Inactive mutant expression abrogated induction of UBF phosphroylation and laminin γ1 synthesis by high glucose (Fig. 3, C and D).
Fig. 3.
A–D: high-glucose (HG)-induced UBF1 phosphorylation and synthesis of laminin γ1 protein are dependent on Erk and p70S6 kinase phosphorylation. Serum-starved GEC were incubated with high glucose for 24 h with or without preincubation with 5 μM U-0126 (U0), an inhibitor of MEK, the upstream kinase of Erk (A), or 22 nM rapamycin (Rapa), an inhibitor of mammalian target of rapamycin complex 1 [mTORC1]-dependent phosphorylation of p70S6 kinase (B). C and D: GECs transfected with inactive dominant-negative (DN) mutant of Erk and kinase-dead p70S6 kinase mutant, respectively, followed by treatment with 30 mM glucose for 24 h. Equal amounts of nuclear extracts were resolved on SDS-PAGE and immunoblotted with phosphospecific antibodies for UBF1, Erk, and p70S6 kinase. Total cell lysates were probed for laminin γ1 content by immunoblotting. Lamin b and actin immunoblot analysis of the same samples was performed to assess loading in nuclear extracts and total lysates, respectively. Immunoblotting for hemagglutinin (HA) (D, top) was performed to assess transfection efficiency. Representative blots from 3 independent experiments are shown, and histograms represent means ± SE from 3 experiments. Statistically significant differences are indicated by #P < 0.05, †P < 0.01, and *P < 0.001 by ANOVA. E: laminin γ1 synthesis induced by high glucose requires UBF1 phosphorylation. GEC were transfected with 1 μg of a transcriptionally inactive UBF1 mutant (S388G)/well or an empty vector (Vec) without the construct as control and allowed to grow for 24 h. Cells were treated with or without 30 mM glucose for 24 h, and laminin γ1 protein was detected as described in Fig. 1B. Immunoblotting for actin (middle) and FLAG (bottom) was performed to assess loading and transfection efficiency, respectively. A representative blot from 4 experiments is shown along with quantitative data from all experiments (†P < 0.01 by ANOVA).
To determine if activation of UBF is required for induction of laminin γ1 synthesis by high glucose, we transiently transfected GECs with a transcriptionally inactive phosphorylation mutant of UBF (UBF-S388G). High glucose stimulated laminin γ1 synthesis in cells that were transfected with an empty vector (P < 0.01 compared with control, Fig. 3E) but not in cells expressing the inactive mutant of UBF (P < 0.01 compared with vector + glucose, Fig. 3E). Because laminin γ1 increment induced by high glucose was abrogated by inhibition of nuclear Erk and p70S6 kinase and by the expression of inactive UBF mutant, these data suggest that UBF-mediated rDNA transcription contributes to high-glucose-induced laminin γ1 synthesis in the GEC, implying the need for an increase in ribosomal biogenesis.
High-glucose regulation of p19ARF-UBF complex and association of UBF with the RPA194 subunit of Pol I.
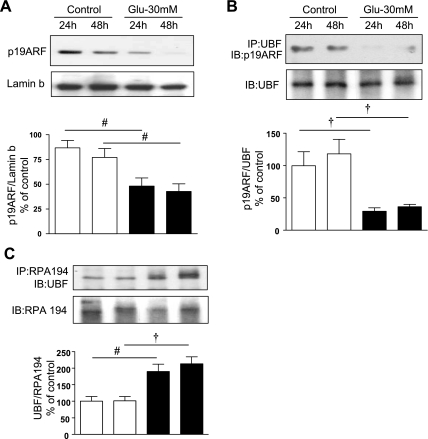
p19ARF keeps UBF in a complex making it unavailable for phophorylation, thus inhibiting rRNA generation (1, 40). Immunoblotting of nuclear extracts from GECs treated with high glucose showed a decrease in the content of p19ARF protein in nuclear preparations compared with control cells incubated with 5 mM glucose (Fig. 4A); the reduction was seen at 24 h (P < 0.05) and lasted for up to 48 h (P < 0.05). Concomitant with the reduced amount of p19ARF, immunoprecipitation of high-glucose-treated nuclear extracts with UBF antibody and immunoblotting with p19ARF antibody showed a >70% decrease in association of UBF with p19ARF at both 24 and 48 h (P < 0.01, Fig. 4B). These changes were in concordance with the temporal profile of increase in UBF phosphorylation (Fig. 2A).
Fig. 4.
A: high glucose induces loss of p19ARF in nuclear compartment. GEC were treated as described in Fig 2. Immunoblotting (IB) with antibody against p19ARF showed that high glucose reduced the nuclear content of p19ARF, a UBF1-associated protein, at 24 and 48 h. B and C: high glucose dissociates UBF-p19ARF complex and promotes association of UBF with RPA194 of Pol I. Equal amounts of protein (200 μg) from nuclear extracts immunoprecipitated (IP) with UBF1 antibody (B) or RPA194 antibody (C) were separated by SDS-PAGE and immunoblotted with an anti-p19ARF antibody or with an anti-UBF1 antibody, respectively. Immunoblotting with UBF1 and RPA194 antibodies was performed to assess loading (B and C, top). Note that high glucose augmented UBF-p19ARF dissociation and UBF-RPA194 association in the nucleus. Histograms represent means ± SE from 4 independent experiments. Significant differences between control and high-glucose-treated cells are indicated by #P < 0.05 and †P < 0.01 by ANOVA.
Previous studies have shown that UBF interacts with RNA Pol I subunits to facilitate formation of the transcription preinitiation complex (43). We examined if high glucose induced association of activated UBF with RPA194, a subunit of RNA Pol I, at the time when it stimulated general protein synthesis and augmented synthesis of matrix proteins. Immunoprecipitation of nuclear extracts with RPA194 antibody and immunoblotting with antibody against UBF showed that high glucose increased association of UBF with RNA Pol I by nearly twofold, starting at 24 h (P < 0.05) and persisting for at least 48 h (P < 0.01) (Fig. 4C). These data demonstrate that high glucose promotes formation of the rDNA transcription initiation complex containing RNA Pol I and UBF.
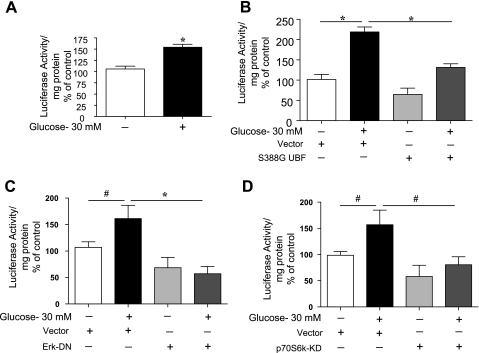
High glucose induces rDNA transcription that requires activation of UBF, Erk, and mTOR/p70S6 kinase.
Transcription of rDNA was studied employing GEC transfected with a reporter plasmid containing rDNA promoter driving IRES-luciferase fusion gene. Incubation of GECs with high glucose significantly augmented luciferase activity (Fig. 5A, P < 0.001), demonstrating an increase in rDNA transcription. Because UBF phosphorylation is required for laminin γ1 synthesis, suggested the need for ribosomal genesis to augment synthesis of laminin, we examined the effect of cotransfection of UBF S388G mutant with rDNA-IRES-Luc in GECs treated with high glucose. As shown in Fig. 5B, high-glucose-induced rDNA transcription (P < 0.001) was abrogated following coexpression of inactive UBF construct (P < 0.001). Although expression of UBF mutant decreased basal rDNA transcription, it did not achieve statistical significance. To determine if UBF-mediated rDNA transcription is dependent on Erk and p70S6 kinase activation, GECs were cotransfected with empty vector, dominant-negative Erk mutant, or kinase-dead p70S6 kinase construct and rDNA transcription reporter construct. High-glucose-induced rDNA transcription was abrogated in cells expressing inactive mutants of Erk (P < 0.001, Fig. 5C) and p70S6 kinase (P < 0.05, Fig. 5D). We observed similar changes when the cells were pretreated with rapamycin and U-0126, chemical inhibitors of mTOR/p70S6 kinase and Erk, respectively (Supplemental Fig. S2). Because p70S6 kinase is directly activated by mTORC1, data with rapamycin are consistent with the requirement for activation of p70S6 kinase for induction of rDNA transcription by high glucose. Taken together, these studies demonstrate that high-glucose-induced rDNA transcription is dependent on phosphorylation of Ser388 in UBF and on activation of mTORC1 and Erk.
Fig. 5.
A and B: high glucose augments rDNA transcription, which requires activation of UBF. GECs were transfected with rDNA-IRES-Luc reporter construct alone (A) or cotransfected with UBF S388G inactive mutant or an empty plasmid vector (B) for 24 h; they were then incubated with or without high glucose for an additional 24 h. The cells were harvested at the end of experiment, and lysates were subjected to luciferase activity assay as described in materials and methods. rDNA-IRES-Luc activity measured as arbitrary units/mg protein was expressed as %control. Composite data from 4–6 experiments done in triplicates are shown in histograms. *P < 0.001, by ANOVA (B). Student's t-test was employed to assess changes between 2 groups (A). C and D: Erk and mTORC1 activation is required for high-glucose-stimulated rDNA transcription in GEC cells. GECs were cotransfected with rDNA-IRES-Luc reporter construct and empty vector or inactive mutants of Erk (C) or p70S6 kinase (D) followed by treatment with or without high glucose. Luciferase activity assay was performed as described in A. Composite data from 4 experiments are shown in histograms. *P < 0.001 and #P < 0.05, by ANOVA.
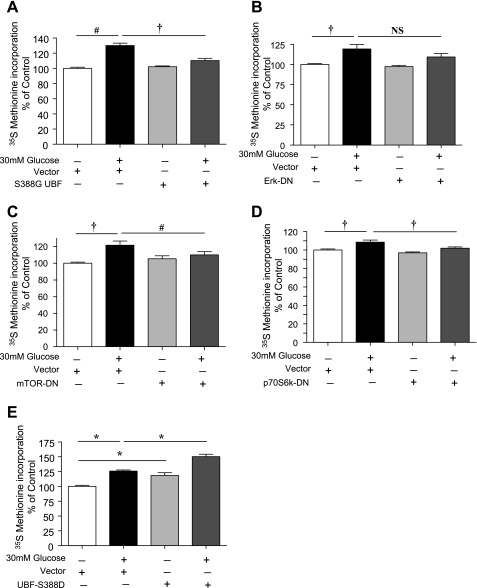
High-glucose-induced protein synthesis in GEC is dependent on activation of UBF, p70S6 kinase, mTOR, and Erk.
To further explore the role of signaling events stimulated by high glucose on protein synthesis, GECs were transfected with either empty vector plasmids or with respective inactive constructs for UBF, Erk, mTOR, and p70S6 kinase. High glucose stimulated protein synthesis in cells transfected with vector alone; expression of the inactive constructs abrogated the response to high glucose (Fig. 6, A–D). We examined the converse effect of constitutively active UBF on protein synthesis. Expression of S388D active mutant of UBF1 alone mimicked the effect of high glucose on protein synthesis (P < 0.001, Fig. 6E). Furthermore, UBF1 S388D produced an additive effect on protein synthesis in cells treated with high glucose (P < 0.001, Fig. 6E).
Fig. 6.
UBF, Erk, mTOR, and p70S6 kinase regulate high-glucose-induced protein synthesis. Dominant-negative constructs of UBF (A), Erk (B), mTOR (C), and p70S6 kinase (D) were employed to study their effect on high-glucose-stimulated protein synthesis in GECs. E: effect of constitutively active UBF1 (S388D) on high-glucose-induced protein synthesis. Following transfection, cells were allowed to grow for 24 h, changed to serum-free media, and treated with or without 30 mM glucose. [35S]methionine incorporation was measured as described in Fig. 1A. Means of 3 experiments with triplicates at each point in each experiment ± SE are shown in the histogram. Statistically significant changes are shown as *P < 0.001, †P < 0.01, #P < 0.05, and not significant (NS).
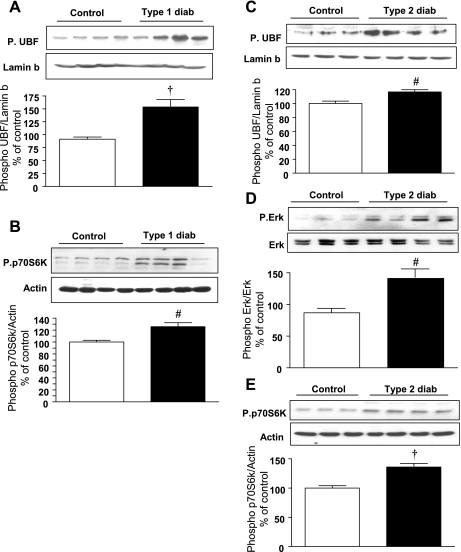
UBF phosphorylation is increased in hypertrophic kidneys from diabetic rodents.
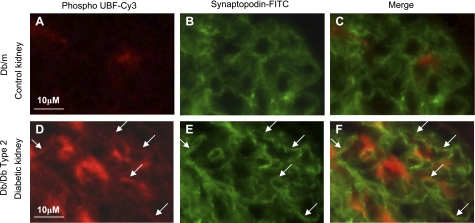
We investigated the status of UBF phosphorylation in nuclear extract prepared from kidney tissues of type 1 diabetic rats on day 4 after onset of hyperglycemia and type 2 diabetic db/db mice at 2 wk of hyperglycemia. At this time point, renal hypertrophy is seen in rodents with type 1 or type 2 diabetes (21, 36) and increase in renal cortical laminin expression in renal cortex of mice with type 2 diabetes (36). Clinical data, including blood glucose levels on these rodents, have been previously reported (21, 36). Kidney cortical nuclear extracts from mice with either type 1 or type 2 diabetes showed an increase in Ser388 phosphorylation of UBF (Fig. 7, A and C) compared with their respective control mice kidneys. Immunofluorescent staining of kidneys from control and diabetic mice was done using phospho-UBF (Ser388) antibody. UBF phosphorylation was increased in GECs and mesangium in mice with type 2 diabetes (Fig. 8, A vs. D) compared with nondiabetic control mice. Double immunostaining with anti-synaptopodin and anti-phospho UBF showed overlap, demonstrating localization of UBF phosphorylation to the podocyte. This was in agreement with the in vitro data on GECs incubated with high glucose.
Fig. 7.
Phosphorylation of UBF1, Erk, and p70S6 kinase is increased in the renal cortex of rodents with type 1 and type 2 diabetes. Rats were administered with streptozotocin to induce type 1 diabetes. On day 4 of hyperglycemia, they were killed. C57BL6/KsJ db/db mice with type 2 diabetes were killed at 2 wk of hyperglycemia. Equal amounts of renal cortical nuclear extracts from control and diabetic rodents were separated by SDS-PAGE and immunoblotted with antibodies against phospho-UBF1, phospho-Erk, and phospho-p70S6 kinase (A-E). Immunoblotting with antibodies against lamin b, actin, and Erk was done to show purity of the nuclear preparation and assess loading. Each lane in the blot represents material from an individual animal, and composite data from control and diabetic rodents are shown in a histogram. Statistical significance between the two groups is shown as #P < 0.05 and †P < 0.01, by Student's t-test, diabetes vs. control.
Fig. 8.
Immunofluorescent staining using Cy-3-conjugated phospho-Ser388-UBF and FITC-conjugated synaptopodin in kidney tissues from control and diabetic mice. Compared with control kidneys, sections from type 2 diabetic mice (A vs. D) showed increased phosphorylation of UBF in glomeruli. Double staining with synaptopodin as a marker for GECs showed overlapping of phospho-UBF with synaptopodin (F) staining, suggesting phosphorylation of UBF in the diabetic kidney podocytes. Three to six individual mouse tissues were examined in each group. Pol I, polymerase I.
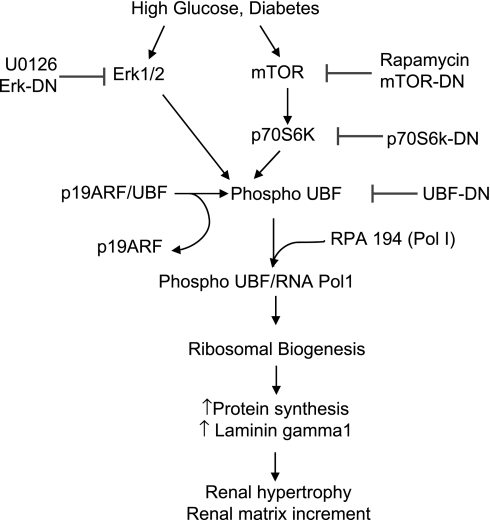
Phosphorylation of UBF was associated with an increase in phosphorylation of Erk in the renal cortex of mice with type 2 diabetes (Fig. 7D) and p70S6 kinase in rodents with either type 1 or type 2 diabetes (Fig. 7, B and E, respectively). We have previously reported an increase in Erk activity in the renal cortex of rats with type 1 diabetes at this time (21). Collectively, these results demonstrate that hyperglycemia leads to UBF phosphorylation, suggesting it leads to rDNA transcription, which would be required for induction of both renal hypertrophy and the increase in laminin synthesis in diabetic animals. Furthermore, these changes correlate with activation of Erk and p70S6 kinase in the kidney in diabetes. Figure 9 depicts the scheme proposed for the regulation of global and matrix protein synthesis at the level of rDNA transcription by high glucose in glomerular epithelial cells.
Fig. 9.
Signaling pathways involved in high-glucose regulation of UBF phosphorylation and general protein synthesis and synthesis of laminin γ1 in the GECs.
DISCUSSION
Our data show that high glucose augments rDNA transcription by 24 h preceding the increase in de novo protein synthesis in GECs. High glucose stimulates rDNA transcription and matrix protein expression via phosphorylation and activation of UBF, a nucleolar factor that regulates rDNA transcription in an Erk- and p70S6 kinase-dependent manner. Nuclear content of p19ARF, which complexes with UBF and keeps it inactive, is decreased by high glucose. High glucose promotes dissociation of UBF from p19ARF and facilitates increased association of UBF with RPA194, a subunit of RNA Pol I, leading to stimulation of rDNA transcription. Furthermore, phosphorylation of UBF on Ser388 and activation of Erk and mTORC1/p70S6 kinase are required for rDNA transcription, stimulation of general protein synthesis, and enhancement of laminin γ1 synthesis induced by high glucose. Thus high glucose promotes ribosomal biogenesis facilitating an increase in general protein synthesis and synthesis of matrix protein, laminin γ1 in the GEC. Augmented UBF phosphorylation and its localization to the podocyte, and activation of kinases involved in UBF phosphorylation, Erk, and p70S6 kinase, were also found in the renal cortex of rodents with type 1 and type 2 diabetes, suggesting induction of ribosomal biogenesis in vivo in the kidney in diabetes.
Stimulation of protein synthesis can be considered to involve two distinct events: an increase in efficiency of translation and an increase in capacity for translation. While the former is extensively studied in the form of regulation of eukaryotic initiation and elongation factors, capacity for translation, which involves ribosomal biogenesis, is not well understood. When the resting cell is stimulated, it can recruit an existing population of ribosomes to increase protein synthesis. Thus it is estimated that, in norepinephrine-induced cardiomyocyte hypertrophy, ∼90% of the pool of existing ribosomes is engaged in protein synthesis (29). However, for a sustained increase in protein synthesis, de novo ribosome biogenesis is required. There are several mechanisms by which production of ribosome can be stimulated and are probably distinct for its two components, i.e., rRNA and ribosomal proteins. For instance, increased expression of UBF has been correlated with increased rDNA transcription (rRNA production) and hypertrophic growth in cardiomyocytes (2, 11). However, high glucose did not change the content of UBF or RNA Pol I proteins in the GEC (Fig. 2A). Data from this study show that high glucose regulates rDNA transcription via UBF activation by phosphorylation on Ser388. This is supported by inhibition of high-glucose induction of both laminin γ1 synthesis and rDNA transcription by expression of inactive mutant of UBF that cannot be phosphorylated on Ser388 (S388G). Increased ribosomal biogenesis should assist induction of general protein synthesis by high glucose in the GEC. This is supported by our data showing that expression of constitutively active UBF was sufficient by itself to stimulate basal protein synthesis and further augmented high-glucose-induced protein synthesis (Fig. 6E). Furthermore, expression of S388G mutant abolished high-glucose induction of protein synthesis (Fig. 6A), suggesting that protein synthesis induced by high glucose requires an increase in ribosomal production in GEC. Augmented protein synthesis induced by high glucose results in GEC hypertrophy (21). GEC hypertrophy is a major manifestation of diabetic renal injury (19). It has been correlated with podocyte apoptosis in both diabetes (41) and in the model of subtotal nephrectomy (20). High-glucose stimulation of the laminin γ1 chain by the GECs contributes to matrix accumulation and thickening of the glomerular basement membrane, a characteristic feature of diabetic kidney disease (18).
Having established that Ser388 phosphorylation-mediated activation of UBF is a necessary condition for rDNA transcription in high-glucose-treated GEC, we next examined upstream kinases that may control UBF phosphorylation. Activation of Erk and p70S6 kinase was seen with rDNA transcription and UBF phosphorylation. In addition to indicating activation of p70S6 kinase, Thr389 phosphorylation of that kinase also provided evidence of mTORC1 activation (12). Corroborating the in vitro data in high-glucose-treated GEC, kidney cortex from type 1 and type 2 diabetic mice also showed increased Ser388 phosphorylation of UBF and its upstream regulators Erk and p70S6 kinase. Gallagher and Cobb (5) have demonstrated that UBF is phosphorylated by Erk1/2 MAP kinases. Erk activity is required not only for induction of transcription of rDNA but also for transcription of ribosomal protein genes, and the mechanism involves UBF phosphorylation (35). In addition to Erk, mTOR via p70S6 kinase controls several steps of ribosome biogenesis, including gene expression of rRNA and ribosomal proteins and processing of the 45S rRNA precursor (10, 26). Inhibition of high-glucose induction of UBF phosphorylation and laminin γ1 synthesis by rapamycin and kinase-dead mutant expression demonstrates requirement of mTORC1 and p70S6 kinase for these events. This is consistent with our previous reports that high glucose promotes the phosphatidylinositol 3-kinase (PI 3-kinase)-protein kinase B-mTOR axis in both the GECs and the renal proximal tubular epithelial cells (21, 23, 25). In our study, inhibition of either Erk or p70S6 kinase blocked UBF phosphorylation, rDNA transcription, general protein synthesis, and laminin γ1 synthesis induced by high glucose. These data suggest the possibility that Erk and mTORC1 may share a common signaling pathway. In renal proximal tubular epithelial cells, high-glucose stimulation of Erk is dependent on PI 3-kinase activation (25). A similar regulation of Erk by PI 3-kinase may also operate in the GEC exposed to high glucose. Signaling reactions involved in high-glucose induction of ribosomal production in the GECs are summarized in Fig. 9.
It is important to note that high glucose regulates several aspects of mRNA translation. High glucose promotes phosphorylation of 4E-binding protein 1 and eukaryotic initiation factor 4E in renal proximal tubular epithelial cells; both of these events have been implicated in augmenting the efficiency of translation (14, 23, 25). By stimulating rDNA transcription, high glucose augments the capacity for translation. Thus coordination between these aspects facilitates hypertrophy of renal cells and increase in specific proteins such as laminin. Ouellette et al. (33) have shown that rDNA transcription increased two- to threefold following unilateral nephrectomy-induced compensatory hypertrophy. RNA Pol I activity is increased during hypertrophy in neonatal cardiomyocytes (11, 30) and skeletal muscle (31). Thus augmented ribosomal biogenesis accompanies renal and cardiac growth in both physiological and pathological settings.
In conclusion, we report, for the first time, that high-glucose-induced Pol I- and UBF-mediated rDNA transcription to synthesize rRNA enhances protein synthesis and laminin γ1 synthesis in the GECs. This contributes to renal hypertrophy and matrix protein accumulation in rodents with type 1 or type 2 diabetes. Our data delineate the signaling mechanism by which high glucose induced rDNA transcription to augment translational capacity, culminating in increased protein synthesis and matrix protein increment in GECs. These data contribute to our understanding of the mechanisms underlying kidney hypertrophy and matrix expansion in diabetic kidney disease.
GRANTS
This study was supported by the National Institutes of Health Grants DK-077295 and RC2AG-036613 to B.S. Kasinath, DK-050190 to G. G. Choudhury, and DK-080106 to J. L. Barnes; a National Institute of Diabetes and Digestive and Kidney Diseases O'Brien Kidney Center Grant (B. S. Kasinath); the Veterans Affairs (VA) Medical Research Service (B. S. Kasinath, G. G. Choudhury, and J. L. Barnes); and Juvenile Diabetes Research Foundation Grants 3-2007-245 to M. M. Mariappan/B. S. Kasinath and 1-2008-185 to G. G. Choudhury. G. G. Choudhury is a recipient of VA Research Career Scientist Award.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
REFERENCES
- 1. Ayrault O, Andrique L, Fauvin D, Eymin B, Gazzeri S, Seite P. Human tumor suppressor p14ARF negatively regulates rRNA transcription and inhibits UBF1 transcription factor phosphorylation. Oncogene 25: 7577–7586, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Brandenburger Y, Jenkins A, Autelitano DJ, Hannan RD. Increased expression of UBF is a critical determinant for rRNA synthesis and hypertrophic growth of cardiac myocytes. Faseb J 15: 2051–2053, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Choudhury GG. A linear signal transduction pathway involving phosphatidylinositol 3-kinase, protein kinase Cepsilon, and MAPK in mesangial cells regulates interferon-gamma-induced STAT1alpha transcriptional activation. J Biol Chem 279: 27399–27409, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Faulkner JL, Szcykalski LM, Springer F, Barnes JL. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol 167: 1193–1205, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallagher ED, Cobb MH. ERKs weigh in on ribosome mass. Mol Cell 8: 932–933, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Ghoshal K, Majumder S, Datta J, Motiwala T, Bai S, Sharma SM, Frankel W, Jacob ST. Role of human ribosomal RNA (rRNA) promoter methylation and of methyl-CpG-binding protein MBD2 in the suppression of rRNA gene expression. J Biol Chem 279: 6783–6793, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grummt I. Life on a planet of its own: regulation of RNA polymerase I transcription in the nucleolus. Genes Dev 17: 1691–1702, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Grummt I. Regulation of mammalian ribosomal gene transcription by RNA polymerase I. Prog Nucleic Acid Res Mol Biol 62: 109–154, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Ha TS, Barnes JL, Stewart JL, Ko CW, Miner JH, Abrahamson DR, Sanes JR, Kasinath BS. Regulation of renal laminin in mice with type II diabetes. J Am Soc Nephrol 10: 1931–1939, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB, Hannan RD. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol 23: 8862–8877, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hannan RD, Stefanovsky V, Taylor L, Moss T, Rothblum LI. Overexpression of the transcription factor UBF1 is sufficient to increase ribosomal DNA transcription in neonatal cardiomyocytes: implications for cardiac hypertrophy. Proc Natl Acad Sci USA 93: 8750–8755, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569–580, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Jorgensen P, Nishikawa JL, Breitkreutz BJ, Tyers M. Systematic identification of pathways that couple cell growth and division in yeast. Science 297: 395–400, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Kasinath BS, Feliers D, Sataranatarajan K, Ghosh Choudhury G, Lee MJ, Mariappan MM. Regulation of mRNA translation in renal physiology and disease. Am J Physiol Renal Physiol 297: F1153–F1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kasinath BS, Grellier P, Choudhury GG, Abboud SL. Regulation of basement membrane heparan sulfate proteoglycan, perlecan, gene expression in glomerular epithelial cells by high glucose medium. J Cell Physiol 167: 131–136, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Kasinath BS, Mariappan MM, Sataranatarajan K, Lee MJ, Feliers D. mRNA translation: unexplored territory in renal science. J Am Soc Nephrol 17: 3281–3292, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kasinath BS, Singh AK, Kanwar YS, Lewis EJ. Effect of puromycin aminonucleoside on HSPG core protein content of glomerular epithelial cells. Am J Physiol Renal Fluid Electrolyte Physiol 255: F590–F596, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Kasinath BS, aK, YS Glomerular basement membrane-biology and physiology. In: Molecular and Cellular Aspects of Basement Membranes, edited by Rohrbach D, Timpl R. San Diego, CA: Academic, 1993, p. 89–106 [Google Scholar]
- 19. Kim NH, Rincon-Choles H, Bhandari B, Choudhury GG, Abboud HE, Gorin Y. Redox dependence of glomerular epithelial cell hypertrophy in response to glucose. Am J Physiol Renal Physiol 290: F741–F751, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286: F526–F533, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292: F617–F627, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Lee MJ, Feliers D, Sataranatarajan K, Mariappan MM, Li M, Barnes JL, Choudhury GG, Kasinath BS. Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell Signal 22: 65–70, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes 56: 476–485, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Mariappan MM, Senthil D, Natarajan KS, Choudhury GG, Kasinath BS. Phospholipase Cgamma-Erk Axis in vascular endothelial growth factor-induced eukaryotic initiation factor 4E phosphorylation and protein synthesis in renal epithelial cells. J Biol Chem 280: 28402–28411, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Mariappan MM, Shetty M, Sataranatarajan K, Choudhury GG, Kasinath BS. Glycogen synthase kinase 3beta is a novel regulator of high glucose- and high insulin-induced extracellular matrix protein synthesis in renal proximal tubular epithelial cells. J Biol Chem 283: 30566–30575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25: 6384–6391, 2006 [DOI] [PubMed] [Google Scholar]
- 27. McDermott PJ, Carl LL, Conner KJ, Allo SN. Transcriptional regulation of ribosomal RNA synthesis during growth of cardiac myocytes in culture. J Biol Chem 266: 4409–4416, 1991 [PubMed] [Google Scholar]
- 28. Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem 267: 6321–6330, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Morgan HECB, Russo L. Protein synthesis and degradation. In: The Heart and Cardiovascular System, edited by Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE. New York, NY: Academic, 1992, p. 1505–1524 [Google Scholar]
- 30. Moss T, Stefanovsky VY. Promotion and regulation of ribosomal transcription in eukaryotes by RNA polymerase I. Prog Nucleic Acid Res Mol Biol 50: 25–66, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol 289: C1457–C1465, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Noller HF, Hoffarth V, Zimniak L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science 256: 1416–1419, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Ouellette AJ, Moonka R, Zelenetz AD, Malt RA. Regulation of ribosome synthesis during compensatory renal hypertrophy in mice. Am J Physiol Cell Physiol 253: C506–C513, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Paule MR, White RJ. Survey and summary: transcription by RNA polymerases I and III. Nucleic Acids Res 28: 1283–1298, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Sataranatarajan K, Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, Kasinath BS. Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol 171: 1733–1742, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senthil D, Choudhury GG, McLaurin C, Kasinath BS. Vascular endothelial growth factor induces protein synthesis in renal epithelial cells: a potential role in diabetic nephropathy. Kidney Int 64: 468–479, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Singh AK, Kasinath BS. Metabolic fate of monovalent and multivalent antibodies of Heymann nephritis following formation of surface immune complexes on glomerular epithelial cells. Clin Exp Immunol 94: 403–411, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stefanovsky VY, Pelletier G, Hannan R, Gagnon-Kugler T, Rothblum LI, Moss T. An immediate response of ribosomal transcription to growth factor stimulation in mammals is mediated by ERK phosphorylation of UBF. Mol Cell 8: 1063–1073, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Sugimoto M, Kuo ML, Roussel MF, Sherr CJ. Nucleolar Arf tumor suppressor inhibits ribosomal RNA processing. Mol Cell 11: 415–424, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 42. Thomas G. An encore for ribosome biogenesis in the control of cell proliferation. Nat Cell Biol 2: E71–E72, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Voit R, Grummt I. Phosphorylation of UBF at serine 388 is required for interaction with RNA polymerase I and activation of rDNA transcription. Proc Natl Acad Sci USA 98: 13631–13636, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Williamson JR. The ribosome at atomic resolution. Cell 139: 1041–1043, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Wu A, Tu X, Prisco M, Baserga R. Regulation of upstream binding factor 1 activity by insulin-like growth factor I receptor signaling. J Biol Chem 280: 2863–2872, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Yao Z, Seger R. The ERK signaling cascade–views from different subcellular compartments. Biofactors 35: 407–416, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.