Abstract
The role electrical charge plays in determining glomerular permeability to macromolecules remains unclear. If the glomerular basement membrane (GBM) has any significant role in permselectivity, physical principles would suggest a negatively charged GBM would reject similarly charged more than neutral species. However, recent in vivo studies with negative and neutral glomerular probes showed the opposite. Whether this observation is due to unique characteristics of the probes used or is a general physiological phenomenon remains to be seen. The goal of this study was to use the basement membrane deposited by Madin-Darby canine kidney epithelial cells as a simple model of a biologically derived, negatively charged filter to evaluate size- and charge-based sieving properties. Fluorescein isothiocyanate-labeled carboxymethylated Ficoll 400 (FITC-CM Ficoll 400) and amino-4-methyl-coumarin-labeled Ficoll 400 (AMC Ficoll 400) were used as negatively charged and neutral tracer molecules, respectively, during pressure-driven filtration. Streaming potential measurement indicated the presence of fixed, negative charge in the basal lamina. The sieving coefficient for neutral Ficoll 400 decreased by ∼0.0013 for each 1-Å increment in solute radius, compared with a decrease of 0.0023 per Å for the anionic Ficoll 400. In this system, molecular charge played a significant role in determining the sieving characteristics of the membrane, pointing to solute charge as a potential contributor to GBM permselectivity.
Keywords: extracellular matrix, glomerular permeability, Ficoll
the appearance of plasma proteins in the urine is pathological and leads to renal failure, a common public health problem that consumes resources out of proportion to its prevalence. The mechanism that controls glomerular filtration is not fully understood, hampering efforts to optimize drug therapy for proteinuric kidney disease. In particular, the role played by electrostatic hindrance in glomerular protein retention remains controversial.
Many constituents of the extracellular matrix are anionic at physiologic pH, and it is appealing to consider an electrostatic component in the capillary wall's impressive barrier to albumin. Multiple physical mechanisms might contribute to an electrostatic barrier, including a Donnan equilibrium between blood and extracellular matrix, direct effects on partitioning of solutes due to increased energy of interaction between the electrical double layers around solute and gel fiber, and, most intriguingly, the possibility that electrophoresis might drive anionic proteins opposite to the convective flow of glomerular filtration (29, 32, 39, 40). Seminal studies by Brenner and Deen (6, 30, 37, 38) using inert tracers suggested that glomerular transport of negatively charged solutes was much lower than that of comparably sized neutral solutes. Multiple investigators published studies comparing charged and neutral solutes consistent with a glomerular charge barrier. Other investigators challenged that conclusion after careful study of inert tracers or after enzymatic manipulation of the glomerular basement membrane (GBM) to remove anionic components of the matrix (2, 3, 25, 27, 32–34, 44).
Computational complexity renders quantitative modeling of electrostatic effects on filtration a highly challenging exercise. In general, partitioning effects (the concentration in a particular medium compared with the concentration in bulk solution) dominate transport as the partition coefficient appears as a multiplier in all terms of a generalized transport equation describing membrane flux (45). Thus, an examination of electrostatic effects on partitioning is likely to be informative about differences in filtration of charged and neutral solutes. Johnson and Deen (29) used the linearized Poisson-Boltzmannn equation to estimate the change in free energy associated with electrostatic interactions between a sphere and a cylinder, and they demonstrated an increase in partition coefficient for unlike charges and a decrease in partition coefficient for like charges, although the effect appeared small for solutions at physiologic tonicity. Ohlson et al. (32) derived a qualitatively similar result for albumin partitioning using a Donnan equilibrium approach, but concluded that albumin concentration in the GBM ought to be ∼10% of the plasma value based on electrostatics alone. Thus, experimental and analytical tools indicated that the extracellular matrix posed an electrostatic barrier to filtration.
Against this backdrop, two independent groups using carboxymethylated polysaccharides observed a paradoxical increase in transmission of anionic tracers compared with neutral tracers (2, 25). The basis for the apparently paradoxical effect was attributed either to an absence of electrostatic hindrance or to conformational differences between neutral and anionic Ficoll (2, 25). To probe this question more deeply, our group developed an anionic Ficoll that appeared to be conformationally similar to neutral Ficoll and was retarded by anionic membranes (24). Having completed some very preliminary studies on solute partitioning by extracellular matrices, we sought evidence to support either enhanced or retarded transmission of anionic polysaccharides through a model of the GBM.
It appears challenging to isolate GBM separately from other extracellular matrix components of the kidney. Extracellular matrix that has been solubilized from tumor matrix lacks collagen or contains collagen that is not crosslinked. However, polarized epithelial cells, such as Madin-Darby canine kidney (MDCK) cells, appear to deposit an organized basal lamina containing laminin, collagen, and heparan sulfate proteoglycans (9, 13, 16, 41–43). As such, cultured MDCK cells may offer an avenue to explore the role of basement membranes in size- and charge-dependent filtration. We now extended our prior models of filtration through extracellular matrix to include measurements of the effect of electrostatic charge on filtration.
It is also important to assess the electrical character of the membrane itself, as the presence of fixed anionic charges in the capillary wall is critical to models of glomerular permselectivity that include an electrostatic barrier to albumin. Various efforts to define the distribution of charges have generally used cationic probes to stain and localize anionic structures in the capillary wall. The applicability of these histological data to transport physiology is not always clear or quantitative. Instead, we chose to measure a functional assessment of electrostatic contributions to transport, called the streaming potential. The flow of an electrolyte through a charged porous medium gives rise to an electrical potential gradient; similarly, application of a voltage across a charged porous structure bathed in electrolyte causes flow of fluid, called electroosmotic flow (31). Briefly, bulk electrical neutrality demands that immobile ions in a gel or filter must be balanced by counterions in solution. Thus, when there is fluid flow, there must be net flow of charge, as the counterions are free to move and the fixed charges are not. The magnitude of the voltage is limited by electrical conduction through the electrolyte, which dissipates the charge gradient. The measured streaming potential varies directly with charge density in the medium, and inversely with tonicity of the electrolyte and inversely with pore size. Thus, streaming potential measurements provide insight into electrostatic contributions to hindered transport through a membrane. The potential measurement itself does not, however, describe size selectivity nor necessarily the magnitude of the electrostatic component to hindrance.
As an extracellular matrix model for the GBM incorporating crosslinked collagen, the basal lamina deposited by MDCK cells were prepared on porous substrates, decellularized, and mounted in ultrafiltration cells. Extracellular matrix charge was qualitatively estimated by measuring streaming potential through the decellularized basal laminae. Anionic and neutral Ficoll solutions were filtered through the extracellular matrix and sieving coefficients were measured.
METHODS
Ficoll preparation and characterization.
Fluorescently labeled neutral and anionic polysaccharide probes were prepared as described (24). Generally, the carboxymethylation reaction of polysaccharides is done using strong NaOH and monochloroacetic acid (MCA) in aqueous medium at elevated temperature. Briefly, 1 g Ficoll 400 (Cat. no. 46326 and 46327, respectively; Fluka, St. Louis, MO) was mixed with 26.5 ml deionized water and stirred for 30 min at 40°C. A 10-M NaOH solution (20 ml) was slowly added. After that, MCA (15%, 53.5 ml) was added dropwise into the mixture. Thus, the reaction mixture was initially 1 M in chloroacetate and 2 M in NaOH. The mixture was stirred at 40°C for 3 h. Then, the mixture was neutralized with 5 M HCl and dialyzed against distilled water for 4 days. The solid carboxymethylated products were recovered by freeze-drying.
Labeling of Ficoll with FITC (fluorescein isothiocyanate) was performed after the method of Ohlson et al. (33). Ficoll was coupled with 7-amino-4-methylcoumarin (AMC) by reductive amination in the presence of sodium cyanoborohydride. Briefly, Ficoll was dissolved in a solution of AMC in DMSO/glacial acetic acid/sodium cyanoborohydride. The solution was then incubated at 80°C for 2 h. After conjugation with AMC, labeled Ficoll were precipitated in ethanol, centrifuged, and dissolved in deionized water. Labeled Ficoll was separated from free AMC with a PD-10 column. Labeled samples were analyzed by size-exclusion chromatography as previously described, except that the excitation and emission wavelengths of the fluorescence detector were set to the appropriate wavelengths for each fluorophore (19, 22).
Basal lamina preparation and charge characterization.
MDCK epithelial cells were obtained from the American Type Culture Collection. Cells were grown in UltraMDCK medium (12–749Q; Lonza, Basel, Switzerland) with 10 ml/l antibiotic-antimycotic solution (15250; Invitrogen, Carlsbad, CA) at 37°C in a humidified atmosphere with 5% CO2. Culture medium was replaced three times per week.
Rat tail collagen (∼3.9 mg/ml in 0.02 N acetic acid) was obtained from BD Biosciences (354236; Bedford, MA). The collagen solution was brought to neutrality with sterile 1 M NaOH and mixed with phosphate-buffered saline to a final concentration of 3 mg/ml. Collagen solution (600 μl) was added to each 24-mm Transwell cell culture insert (3412; Corning, Lowell, MA) and gelled for 2 h at 37°C.
MDCK basal laminas were prepared using a similar method to that described previously (1, 42). MDCK cells were seeded on the rat tail collagen gels at 200,000 cells/insert. Cells were grown for 28–31 days with medium replacement three times weekly. MDCK cells were lysed with 10 mM Tris·HCl with 0.1% bovine serum albumin and 1 mM CaCl2 at pH 7.5. Cells were dissolved in 0.2% sodium deoxycholate (D6750; Sigma) in lysis buffer for 10 min at 37°C with fresh solution added after 5 min. Samples were washed with 0.5% IGEPAL CA-630 (I3021; Sigma) in lysis buffer for 2 min. Samples were carefully rinsed with PBS three times.
Streaming potential measurements.
Decellularized cell culture inserts were positioned on a rigid silicon frit (0.5-μm-pore size) and were mounted in a custom ultrafiltration cell equipped with silver-silver chloride electrodes. The filtration cell was flooded with 10 mM KCl in water buffered with 1 mM TRIS (pH 7.0). Transmembrane pressure was applied to the membrane and the voltage difference between the electrodes was measured by a digital multimeter (Keithley 2000, Keithley Instruments, Cleveland, OH). Voltage-pressure curves were mapped over a range of pressures (3.5–13.8 kPa). While the streaming potential was measured across multiple membranes in series (silicon microporous frit, polycarbonate, collagen, and MDCK cell basal lamina), the streaming potential (voltage) developed across each individual membrane is a direct function of the individual pressure drop across that membrane. Due to hydraulic permeability of the basal lamina being much lower than the other layers, the pressure gradient across the ultrafiltration cell is almost completely realized across the basal lamina. As such, the change in pressures, and thus change in transmembrane voltages, for the support layers of the filter is negligible. Therefore, the entire streaming potential can be attributed to the basal lamina even in the presence of the higher permeability membranes.
Filtration.
Filtration procedures for membranes and extracellular matrix have been described previously (18, 22). Briefly, after the extracellular matrix was decellularized, the Transwell membranes were cut from their supports and laid on a rigid 0.5-μm-pore size silicon frit. A second Transwell membrane was laid face-to-face on top of the first and the sandwich of extracellular matrix between two Transwell membranes was mounted in an ultrafiltration cell. A schematic of the sample configuration can be found in Fig. 1. A single solution of 50 μg/ml each of FITC-labeled 400 (Ficoll) and AMC-labeled carboxymethyl Ficoll 400 (CM-Ficoll) in Dulbecco's PBS (DPBS) with calcium chloride and magnesium chloride (D8662; Sigma) was introduced into the feed reservoir and continuously circulated through the feed side of the ultrafiltration cell by a peristaltic pump. The reservoirs and tubing were covered with aluminum foil to minimize photobleaching of the fluorophores. The feed side circuit was pressurized to two pounds per square inch (13.8 kPa, the same pressure at which the streaming potential was measured) with compressed air and ultrafiltrate samples were collected. The first sample collection was discarded as it may be diluted by the DPBS used to wet the membranes. Samples were collected in darkened microcentrifuge tubes and refrigerated. Sieving coefficients were calculated as the ratio of fluorescence in the ultrafiltrate divided by the fluorescence in the feed.
Fig. 1.
Schematic of Madin-Darby canine kidney (MDCK) basal lamina filtration experiment consisting of a rigid microporous frit and a stack of 2 polycarbonate Transwell membranes, rat tail collagen gels, and decellularized MDCK basal laminas.
Statistics.
Experiments were repeated in triplicate, except for chromatography of neutral Ficoll, which was performed in duplicate as results were identical to our previous extensive testing (20). Data were analyzed using linear mixed models incorporating molecular radius, charge, and radius-charge interaction. All analyses were run on SAS version 9.2 (SAS, Cary, NC).
RESULTS
Basement membrane characterization.
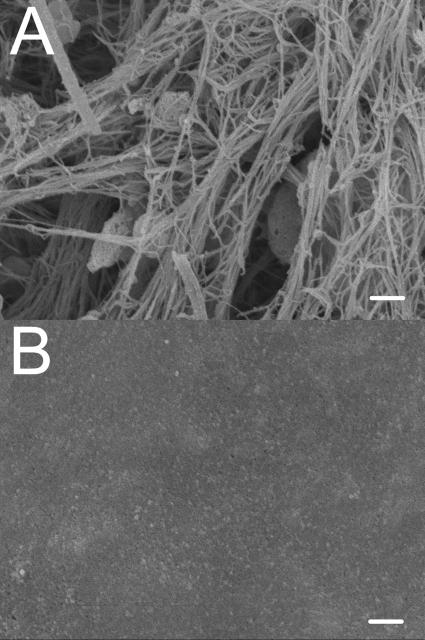
MDCK cells grew to confluence rapidly and were maintained in cell culture for 28–31 days, and then removed as described. The underlying matrix was exceptionally smooth when observed by scanning electron microscopy compared with collagen gels (Fig. 2, A and B). Streaming potentials in basement membranes derived from MDCK cells using 10 mM KCl at 37°C average −0.05 ± 0.02 mV/kPa, consistent with the presence of fixed negative charges in the extracellular matrix. The results from the measurements taken with PBS at 37°C appear to demonstrate a slight negative charge as well with an average streaming potential measurement of −0.01 ± 0.01 mV/kPa (t-test, P < 0.02 vs. a slope of 0).
Fig. 2.
A: rat tail collagen gel underlying MDCK basal lamina. B: basal lamina deposited by MDCK cells. The rat tail collagen is a loose, fibrous gel while the basal lamina is a dense, smooth layer. Scale bar in both micrographs is 2 μm.
Permselectivity of MDCK-generated basement membranes.
Basement membranes deposited by MDCK cells displayed size-dependent filtration of neutral Ficoll and charged Ficoll (Fig. 3). The rat tail collagen matrix also displayed faint trends towards size- and charge selectivity (Fig. 3). Linear mixed-model analysis of the sieving data indicated that charged and neutral populations were significantly different from each other (P < 0.0001) and there was a significant interaction between molecular radius and carboxymethylation (P < 0.0001).
Fig. 3.
Sieving of neutral and anionic Ficoll by MDCK cell basal lamina. y-Axis, sieving coefficient. x-Axis, Ficoll radius in Å. Both neutral Ficoll (gray lines) and anionic Ficoll (black lines) were retained by the basal lamina (solid lines, means ± SE), whereas the support structure of frit, polycarbonate membrane, and collagen gel were minimally selective (dashed lines, means ± SE). The anionic Ficoll was retained more than the neutral Ficoll (P < 0.0001).
DISCUSSION
It is generally accepted that each of the multiple layers of the glomerular capillary wall (GCW) contributes to the permeability barrier (26). The pathophysiology by which disease disrupts the barrier remains obscure, leaving mechanism-based treatments for proteinuric renal disease out of reach. The reason why the permeability barrier remains persistently enigmatic is in part attributable to the fragility of the normal glomerulus. The endothelial glycocalyx has been thought a significant contributor to permselectivity in vivo but remains frustratingly evanescent in culture or biopsy samples (28, 35, 36). The basement membrane, long held to be a critical component of the permeability barrier, is much thinner than the wavelength of visible light, frustrating efforts at optical microscopy approaches to measuring spatial solute distribution. In addition, the ultrastructural appearance of the GBM seems sensitive to fixation, and the GBM cannot be isolated from biopsy samples independent of mesangial matrix (4, 5, 7, 11, 12, 14, 15, 30, 37, 38). Structural rearrangement of the glomerular podocyte almost invariably accompanies significant proteinuria, yet study of this intriguing cell has been limited as it does not appear to assume the same phenotype in vitro as it does in vivo, frustrating efforts to isolate the contribution of the slit diaphragm apparatus to the permeability barrier. In the face of these challenges, we sought to develop analogies to components of the capillary wall and explore the physical principles underlying transport within these structures (8, 10, 17–19, 21–23). The analogy may not be perfect, but the insights gained may assist in interpreting in vivo data.
To this end, we explored solute partitioning into Matrigel and bovine lens capsule basement membranes, and now we report filtration by a basal lamina produced by renal epithelial cells. The MDCK cell-generated basal lamina we employed has been described and used as a tool to investigate basement membrane assembly. In our hands, the basal lamina displayed charge selectivity, albeit weakly. This is consistent with the presence of fixed anionic charges within the basement membrane and tends to corroborate the physical intuition that most proteins, including constituents of basement membranes, are negatively charged at physiologic pH, and a gel of such proteins would be expected to reject anionic solutes based on charge as well as size. The size selectivity of this extracellular matrix model of the GBM is orders of magnitude less stringent than observed for the living kidney, or for isolated GBM as reported by Edwards et al. (14). This suggests that the transport properties of different capillary beds are sensitive to extracellular matrix composition.
The apparent range of pore sizes in the basal lamina tested in these experiments is probably an order of magnitude larger than those hypothesized to form the permeability barrier in the GCW. The polydisperse Ficoll 400 we used contains solutes that are hundreds of Ångstroms in radius, so we were able to carry out the experiment at this length scale. What does not scale, however, is the size of the electrical double layer. That we observed a charge effect at all seems remarkable given that the double layer might only be 3–5% of the size of the solute. This makes a strong case for more detailed modeling of charge effects in the complex medium of biologic gels. The measurements reported here suggest that the effect of electrostatics in glomerular permeability has the potential to be significant. This is consonant with a wealth of data in the literature stretching back several decades suggesting a role for charge, as well as size, in glomerular filtration.
It remains a challenge to integrate these in vitro data with animal data published on neutral and CM-Ficoll which appears to show enhanced transport of anionic Ficoll compared with neutral. Those observations were regarded as anomalous by the authors. We would speculate that having prepared and characterized our own anionic samples, there may have been a physicochemical difference between CM-Ficoll examined by others and the CM-Ficoll we examined.
The physiological relevance of these in vitro data to understanding of renal physiology and kidney disease is less tenuous than it might appear. Vigorous debate regarding structure-function relationships in the capillary wall persists and clouds development of mechanism-specific therapies for proteinuric renal disease. The publications by Guimaraes and Asgeirsson (2, 25) challenged conventional physical intuition about glomerular physiology, and in their aftermath, it has been debated whether the test solute they employed was in some way producing incongruous data or whether there was a new and unexplained phenomenon in the glomerular capillary that demanded explanation. Our choice to synthesize and characterize our solutes ourselves allowed us to confirm that the anionic solutes behave as expected in a highly structured silicon membrane (24), and now are retarded by a complex organized extracellular matrix secreted by epithelial cells. These data are consistent with the work of Deen and Ohlson and others (11, 12, 32, 33) predicting that an anionic extracellular matrix will retard passage of anionic solutes compared with neutral ones. To our knowledge, we controlled other variables besides electrostatics (in particular, size and shape) that affect transport. Future work will lie in defining the charge densities in the extracellular matrix and the charge on the tracers themselves. These tools, in combination with molecular biologic approaches and novel imaging techniques, may allow investigators to probe dynamic interactions between layers of the filtration barrier in vivo.
GRANTS
This work was supported by a Carl W. Gottschalk Research Scholar Award from the American Society of Nephrology and National Institutes of Health Grant T32-DK-007470–25.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ajani G, Sato N, Mack JA, Maytin EV, Ajani G, Sato N, Mack JA, Maytin EV. Cellular responses to disruption of the permeability barrier in a three-dimensional organotypic epidermal model. Exp Cell Res 313: 3005–3015, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asgeirsson D, Venturoli D, Rippe B, Rippe C. Increased glomerular permeability to negatively charged Ficoll relative to neutral Ficoll in rats. Am J Physiol Renal Physiol 291: F1083–F1089, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bakoush O, Tencer J, Torffvit O, Tenstad O, Skogvall I, Rippe B. Increased glomerular albumin permeability in old spontaneously hypertensive rats. Nephrol Dial Transplant 19: 1724–1731, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Caulfield J, Farquhar M. Distribution of anionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci USA 73: 1646–1650, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caulfield JP, Farquhar MG. The permeability of glomerular capillaries to graded dextrans: identification of the basement membrane as the primary filtration barrier. J Cell Biol 63: 883–903, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang RL, Deen WM, Robertson CR, Brenner BM. Permselectivity of the glomerular capillary wall. III. Restricted transport of polyanions. Kidney Int 8: 212–218, 1975 [DOI] [PubMed] [Google Scholar]
- 7. Cochrane SM, Robinson GB. In vitro glycation of glomerular basement membrane alters its permeability: a possible mechanism in diabetic complications. FEBS Lett 375: 41–44, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Conlisk AT, Datta S, Fissell WH, Roy S. Biomolecular transport through hemofiltration membranes. Ann Biomed Eng 37: 722–736, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cook JR, Crute BE, Patrone LM, Gabriels J, Lane ME, Van Buskirk RG. Microporosity of the substratum regulates differentiation of MDCK cells in vitro. In Vitro Cell Dev Biol 25: 914–922, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Datta S, Conlisk AT, Kanani DM, Zydney AL, Fissell WH, Roy S. Characterizing the surface charge of synthetic nanomembranes by the streaming potential method. J Colloid and Interface Science 348: 85–95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deen WM. Cellular contributions to glomerular size selectivity. Kidney Int 69: 1295–1297, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 281: F579–F596, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Ecay TW, Valentich JD. Basal lamina formation by epithelial cell lines correlates with laminin A chain synthesis and secretion. Exp Cell Res 203: 32–38, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Edwards A, Daniels BS, Deen WM. Hindered transport of macromolecules in isolated glomeruli. II. Convection and pressure effects in basement membrane. Biophys J 72: 214–222, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edwards A, Deen WM, Daniels BS. Hindered transport of macromolecules in isolated glomeruli. I. Diffusion across intact and cell-free capillaries. Biophys J 72: 204–213, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erickson AC, Couchman JR. Basement membrane and interstitial proteoglycans produced by MDCK cells correspond to those expressed in the kidney cortex. Matrix Biol 19: 769–778, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Ferrell N, Desai RR, Fleischman AJ, Roy S, Humes HD, Fissell WH. A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnology and Bioengineering 107: 707–716, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fissell W, Hofmann C, Ferrell N, Schnell L, Dubnisheva A, Zydney A, Yurchenco P, Roy S. Solute partitioning and filtration by extracellular matrices. Am J Physiol Renal Physiol 297: F1092–F1100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fissell WH, Dubnisheva A, Eldridge AN, Fleischman AJ, Zydney AL, Roy S. High-performance silicon nanopore hemofiltration membranes. J Memb Sci 326: 58–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fissell WH, Hofmann CL, Smith R, Chen MH. Size and conformation of Ficoll as determined by size-exclusion chromatography followed by multi-angle light scattering. Am J Physiol Renal Physiol 298: F205–F208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fissell WH, Humes HD. New insights into mechanisms of glomerular permselectivity. Minerva Urol Nefrol 61: 397–410, 2009 [PubMed] [Google Scholar]
- 22. Fissell WH, Manley S, Dubnisheva A, Glass J, Magistrelli J, Eldridge AN, Fleischman AJ, Zydney AL, Roy S. Ficoll is not a rigid sphere. Am J Physiol Renal Physiol 293: F1209–F1213, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Fissell WH, Manley S, Westover A, Humes HD, Fleischman AJ, Roy S. Differentiated growth of human renal tubule cells on thin-film and nanostructured materials. ASAIO J 52: 221–227, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Groszek J, Li L, Ferrell N, Smith R, Zorman CA, Hofmann CL, Roy S, Fissell WH. Molecular conformation and filtration properties of anionic Ficoll. Am J Physiol Renal Physiol 299: F752–F757, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Guimaraes MAM, Nikolovski J, Pratt LM, Greive K, Comper WD. Anomalous fractional clearance of negatively charged Ficoll relative to uncharged Ficoll. Am J Physiol Renal Physiol 285: F1118–F1124, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 171: 139–152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeansson M, Haraldsson B, Jeansson M, Haraldsson B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol 290: F111–F116, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Johnson EM, Deen WM. Electrostatic effects on the equilibrium partitioning of spherical colloids in random fibrous media. J Colloid Interface Sci 178: 749–756, 1996 [Google Scholar]
- 30. Kanwar Y, Farquhar M. Anionic sites in the glomerular basement membrane: in vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol 81: 137–153, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirby BJ, Hasselbrink EF. Zeta potential of microfluidic substrates. 1. Theory, experimental techniques, and effects on separations. Electrophoresis 25: 187–202, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Ohlson M, Sorensson J, Haraldsson B. A gel-membrane model of glomerular charge and size selectivity in series. Am J Physiol Renal Physiol 280: F396–F405, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Ohlson M, Sorensson J, Haraldsson B. Glomerular size and charge selectivity in the rat as revealed by FITC-Ficoll and albumin. Am J Physiol Renal Physiol 279: F84–F91, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Osicka TM, Pratt LM, Comper WD. Glomerular capillary wall permeability to albumin and horseradish peroxidase. Nephrology 2: 199–212, 1996 [Google Scholar]
- 35. Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res 102: 770–776, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res 104: 1318–1325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ryan GB, Karnovsky MJ. Distribution of endogenous albumin in the rat glomerulus: role of hemodynamic factors in glomerular barrier function. Kidney Int 9: 36–45, 1976 [DOI] [PubMed] [Google Scholar]
- 38. Ryan GB, Karnovsky MJ. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int 8: 219–232, 1975 [DOI] [PubMed] [Google Scholar]
- 39. Somers D. Diagenic membrane barriers and the function of the slit diaphragm. J Am Soc Nephrol 16: 441A, 2005 [Google Scholar]
- 40. Somers D. On the nature of the glomerular barrier to albuminuria: electrokinetic glomerulus theory. J Am Soc Nephrol 16: 109a, 2005. 15563564 [Google Scholar]
- 41. Svennevig K, Prydz K, Kolset SO. Proteoglycans in polarized epithelial Madin-Darby canine kidney cells. Biochem J 311: 881–888, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tammi RH, Tammi MI, Hascall VC, Hogg M, Pasonen S, MacCallum DK. A preformed basal lamina alters the metabolism and distribution of hyaluronan in epidermal keratinocyte “organotypic” cultures grown on collagen matrices. Histochem Cell Biol 113: 265–277, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Tveit H, Dick G, Skibeli V, Prydz K, Tveit H, Dick G, Skibeli V, Prydz K. A proteoglycan undergoes different modifications en route to the apical and basolateral surfaces of Madin-Darby canine kidney cells. J Biol Chem 280: 29596–29603, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Wijnhoven TJ, Lensen JF, Wismans RG, Lamrani M, Monnens LA, Wevers RA, Rops AL, van der Vlag J, Berden JH, van den Heuvel LP, van Kuppevelt TH, Wijnhoven TJM, Lensen JFM, Wismans RGP, Lamrani M, Monnens LAH, Wevers RA, Rops ALWMM, van der Vlag J, Berden JHM, van den Heuvel LPWJ, van Kuppevelt TH. In vivo degradation of heparan sulfates in the glomerular basement membrane does not result in proteinuria. J Am Soc Nephrol 18: 823–832, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Zeman LJ, Zydney AL. Microfiltration and Ultrafiltration. New York: Marcel Dekker, 1996 [Google Scholar]