Abstract
Renal tubular cell apoptosis is a critical detrimental event that leads to chronic kidney injury in association with renal fibrosis. The present study was designed to investigate the role of galectin-3 (Gal-3), an important regulator of multiple apoptotic pathways, in chronic kidney disease induced by unilateral ureteral obstruction (UUO). After UUO, Gal-3 expression significantly increased compared with basal levels reaching a peak increase of 95-fold by day 7. Upregulated Gal-3 is predominantly tubular at early time points after UUO but shifts to interstitial cells as the injury progresses. On day 14, there was a significant increase in TdT-mediated dUTP nick end labeling-positive cells (129%) and cytochrome c release (29%), and a decrease in BrdU-positive cells (62%) in Gal-3-deficient compared with wild-type mice. The degree of renal damage was more extensive in Gal-3-deficient mice at days 14 and 21, 35 and 21% increase in total collagen, respectively. Despite more severe fibrosis, myofibroblasts were significantly decreased by 58% on day 14 in the Gal-3-deficient compared with wild-type mice. There was also a corresponding 80% decrease in extracellular matrix synthesis in Gal-3-deficient compared with wild-type mice. Endo180 is a recently recognized receptor for intracellular collagen degradation that is expressed by interstitial cells during renal fibrogenesis. Endo180 expression was significantly decreased by greater than 50% in Gal-3-deficient compared with wild-type mice. Taken together, these results suggested that Gal-3 not only protects renal tubules from chronic injury by limiting apoptosis but that it may lead to enhanced matrix remodeling and fibrosis attenuation.
Keywords: chronic kidney disease, nephron loss, matrix degradation, tubular apoptosis, defective tissue remodeling
chronic kidney disease (CKD) represents a significant global health problem with few therapeutic options currently known to slow its progression. The prevalence of moderate to advanced stages of CKD has increased by an alarming 42% over the past decade (48). Progressive renal disease is the consequence of a relentless expansion of interstitial extracellular matrix which leads to nephron loss. Two critical pathways following renal injury have a significant impact on fibrosis severity; tubular apoptosis and defective tissue remodeling characterized by the imbalance between matrix synthesis and matrix degradation.
Renal tubular cell apoptosis and subsequent tubular atrophy are an important cause of progressive loss of kidney functional decline (11). Apoptosis is the end result of a complex regulatory system balancing survival factors and cell activation/injury signals. Apoptosis occurs principally through two separate yet interlinked signaling mechanisms: the extrinsic pathway, activated by proapoptotic receptor signals at the cell surface, and the intrinsic pathway, activated by mitochondrial signals from within the cell.
Tubular cell injury occurs in parallel with interstitial matrix expansion. During the final phases of classical wound repair, extracellular matrix synthesis and degradation reach equilibrium and normal tissue architecture is restored. By contrast, in progressive renal fibrosis, this final phase is altered and the normal architecture is irreversibly damaged. Several studies demonstrated that inhibiting matrix synthesis by targeting myofibroblasts reduced fibrosis severity (25, 36, 53), but no studies to date have elucidated the key matrix degradation pathways in progressive kidney disease.
Galectin-3 (Gal-3) is a 32- to 35-kDa multifunctional lectin protein expressed by epithelial cells, endothelial cells, and macrophages that regulates numerous biological processes through interactions between its carbohydrate recognition domain and via carbohydrate-independent mechanisms. Although it is predominantly located in the cytoplasm, Gal-3 can be secreted extracellularly and it can also shuttle into the nucleus. Intracellular Gal-3 is an important protein for cell survival due to its ability to block the intrinsic apoptotic pathway, while in the nucleus Gal-3 promotes cell proliferation–both via carbohydrate-independent mechanisms (10, 18, 26). Extracellular Gal-3 modulates important interactions between epithelial cells and extracellular matrix through its carbohydrate domain and it plays an important role during embryonic development of collecting ducts (5, 34). Several studies also suggest that Gal-3 is important in alternative macrophage activation (28), macrophage phagocytosis (42), and clearance of advanced glycation end-products (40). Due to its functional diversity, the role of Gal-3 in progressive renal disease remains controversial and is likely context dependent (17, 21, 22). The present study investigates the primary functions of Gal-3 in an experimental model of progressive renal fibrosis by comparing Gal-3-deficient and wild-type mice. Our findings suggest that Gal-3 attenuates renal fibrosis by limiting renal tubular apoptosis and modulating extracellular matrix remodeling.
METHODS
Experimental Design
Breeding pairs of Gal-3-deficient (Gal-3−/−) mice on a C57BL/6 background obtained from Dr. Fu-Tong Liu's colony were bred in our animal facility in Seattle. In brief, Gal-3-deficient mice were generated in Dr. Liu's laboratory using blastocysts from C57BL/6J mice and sent to us after nine generations (19). Wild-type C57BL/6 male mice, 8–10 wk of age, were purchased from Jackson Laboratory (Maine, CT). Unilateral ureteral obstruction (UUO) surgery was performed on Gal-3−/− and wild-type male mice, 8–10 wk of age (n = 6–10 each), and they were killed at 3, 7, 14, or 21 days after surgery. For mice in the UUO group, the left ureter was exposed through a midabdominal incision and ligated using 4–0 silk. All surgeries were performed under general anesthesia with isoflurane. All procedures were performed in accordance with the guidelines established by National Research Council Guide for the Care and Use of Laboratory Animals and approval of our Institute Animal Care and Use Committee (IACUC). Contralateral and UUO kidneys were harvested and processed for RNA and protein extraction and histological studies as previously described (32, 37, 38). Frozen tissue samples were stored at −80°C.
Genotyping
Genotyping was performed by PCR using genomic DNA isolated from tails. PCR primer sequences were obtained from Dr. Liu and genotyping was performed as described previously (19). Primers for the wild-type Gal-3 allele are 5′-GTAGGTGAGAGTCACAAGCTGGAGGCC; 3′-CACTCTCAAAGGGGAAGGCTGACTGTC (band size ∼450 bp). The primers for the Gal-3-deficient allele include the 5′-GGCTGACCGCTTCCTCGTGCTTTACGG; and the 3′ wild-type Gal-3 primer (band size ∼300 bp).
Collagen Content
Hydroxyproline content of kidney tissue (μg of hydroxyproline per mg of wet wt kidney section) was measured by acid hydrolysis of the tissue section using procedures established in our laboratory (32, 37, 38).
Histological Examination
Immunohistochemical staining was performed on sections of paraffin-embedded tissue or cryosections of snap-frozen tissue using procedures established in our laboratory with VECTASTAIN Elite ABC Kits (Vector Laboratories, Burlingame, CA) and AEC Substrate Chromogen K3464 (Dako, Carpinteria, CA). Sections were blocked with avidin/biotin blocking kit (Vector Laboratories). Confocal microscopy was performed on 5-μm cryosections fixed with 4% paraformaldehyde and imaged with the Zeiss LSM 5 Pascal confocal microscope with LSM software (Thornwood, NY). Confocal z-stack images were analyzed with Imaris 7.0 software (Bitplane, St. Paul, MN). In some cases, tyramide signal amplification was utilized (TSA kit #3–488 tyramide and TSA kit #4–546 tyramide; Invitrogen, Carlsbad, CA). Nuclei were stained with TO-PRO-3 iodide. Primary antibodies used were reactive with Gal-3 (mouse anti-mouse monoclonal-FITC; Cedarlane Laboratories, Burlington, NC), F4/80 (rat anti-mouse F4/80 monoclonal; AbD Serotec, Raleigh, NC), α-smooth muscle actin (α-SMA; mouse anti-mouse monoclonal α-SMA-Cy3; Sigma, St. Louis, MO), Endo180 (sheep anti-mouse Mrc2; R&D Systems, Minneapolis, MN), E cadherin (goat anti-mouse E cadherin; R&D Systems), and IκB-α and phosphorylated IκB-α (rabbit anti-human IκBα polyclonal and rabbit anti-human phospho IκB-α polyclonal; Cell Signaling Technology, Danvers, MA). Interstitial myofibroblasts were quantified by staining using peroxidase-conjugated murine anti-human α-SMA 1A4 monoclonal antibody (Dako) as described previously (32, 35, 49). TdT-mediated dUTP nick end labeling (TUNEL) and Picrosirius red staining was performed as previously described (32, 49). Secondary antibodies were shown to be nonreactive with tissue sections stained without the primary antibody. Semiquantitative computer-assisted image analysis of tubulointerstitial proteins was performed on six randomly selected ×400 magnified images of slides from individual animals with Image-Pro Plus software (Mediatech). Glomerular areas and space not occupied by tissue were subtracted in the analysis. Interstitial macrophage density was expressed as percent F4/80-positive interstitial area on fluorescent-stained cryosections. The percent macrophage density was determined from results of six randomly selected ×400 magnified images of slides from individual animals with assistance from Image-Pro Plus software (Mediatech). The investigator was blinded to the experimental groups at the time of analysis. Analysis of fluorescent dual stained kidney sections by confocal microscopy was performed using the histogram function in the LSM software with thresholds set by staining specificity.
Western Blotting
Protein was isolated from homogenized frozen kidney and Western blotting was performed as previously described (37). The primary antibodies are described above. Bands were normalized using beta actin (anti-mouse beta actin; Sigma). The secondary antibodies were anti-rabbit, anti-sheep, anti-goat, and anti-mouse IR700Dye and IR800Dye (Rockland Immunochemicals, Gilbertsville, PA). Protein bands were visualized and quantified using the Odyssey (Li-Cor Biosciences, Lincoln, NE).
Semiquantitative Real-Time qPCR
Total RNA from frozen kidney tissue homogenate was obtained using the Maxwell 16 instrument (Promega, Madison, WI). RNA samples were loaded on a Agilent RNA 6000 Nano Chip and analyzed in the Agilent 2100 Bioanalyzer (Agilent Technologies, Foster City, CA) for RNA concentration and quality; samples with RNA integrity numbers greater than 8.0 were utilized for cDNA synthesis. First-strand cDNA was prepared from 1 μg of total RNA using the Bio-Rad iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA). Semiquantitative real-time qPCR was performed according to the IQ SYBR Green Supermix kit (Bio-Rad Laboratories) using Gal-3 primers (forward 5′-CCCTTTGAGAGTGGCAAACCA; reverse 5′-GTAGGTGAGCATCGTTGACCG), fibronectin (forward 5′-GACCAGCCCAGGGAGTCATC; reverse 3′- CTCTGTAGCGTCCGTCACACG), pocollagen I (Col 1A: forward 5′- AGAAGTCTCAAGATGGTGGCCG; reverse 3′-GGTCACGAACCACGTTAGCATC), procollagen III (Col 3A: forward 5′-CAGCTATGGCCCTCCTGATCTT; reverse 3′-GTAATGTTCTGGGAGGCCCG), Endo180 (forward 5′- GTCTGCAAGCTCCCTAGAGTGG; reverse 3′-GTAGAGGATGAGGGCTGC), primers for the housekeeping genes 18S (forward 5′-GGTGAAATTCTTGGACCGGC; reverse 5′-GACTTTGGTTTCCCTTAAGC), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward 5′-TCACCACCATGGAGAAGGC; reverse 5′-GCTAAGCAGTTGGTGGTGCA; Invitrogen). Real-time qPCR reactions containing 1.5 μl of cDNA, 0.2 μM primers, and the 2× SYBR Green Supermix were run in the Bio-Rad iQ thermal cycler with programs specific for the respective gene. Reactions were run in triplicate and genes of interest were normalized to both 18S and GAPDH housekeeping genes. Absence of primer-dimers was confirmed for primer specificity. Single amplicons were confirmed by gel electrophoresis. Data analysis was performed using the Pfaffl algorithm with the REST analysis software version 1.9.9 (Corbett Research) and the REST-MCS (multiple condition solver, version 2). Boxplot with whiskers were generated from REST software; boxes represent interquartile ranges, dotted line represents median gene expression, and whiskers represent minimum and maximum observations.
Statistical Analysis
All data are presented as means ± SE. A nested ANOVA was utilized for all semiquantitative computer-assisted image analysis. For image analysis data, the arithmetic mean of six randomly selected images of slides for each animal was used to calculate the reported mean of the group and SE. All other results were analyzed by unpaired Student's t-test. Nonparametric data were analyzed using the Mann-Whitney U-test. F statistic values are reported for all ANOVAs and z values are reported for Mann-Whitney tests. A P value <0.05 was considered statistically significant.
RESULTS
Gal-3 Is Protective During Chronic Kidney Injury
Gal-3 expression increases during UUO.
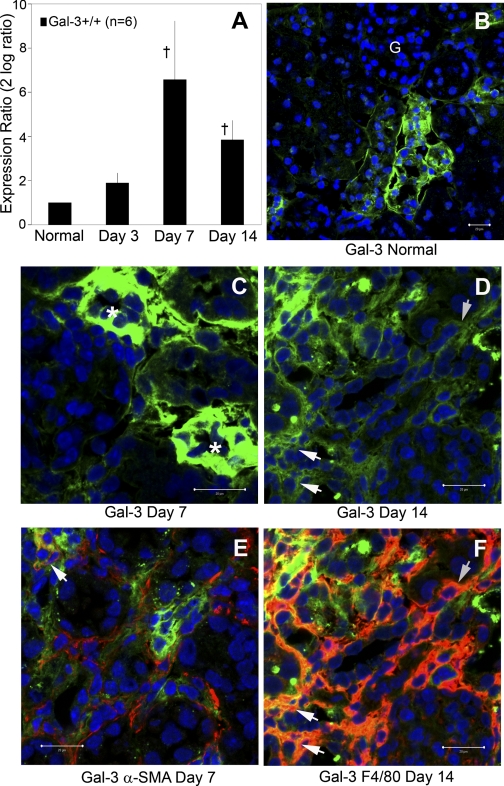
In normal kidneys, basal Gal-3 expression level is low. In response to chronic injury induced by obstruction, Gal-3 steady-state mRNA levels dramatically increased with a peak relative expression (95-fold increase, 2 log = 6.6) observed 7 days after UUO in wild-type mice measured by semiquantitative real-time qPCR (normalized to 18S, UUO relative to contralateral kidney, n = 6/group, P = 0.001; Fig. 1A). By immunolocalization, Gal-3 expression was upregulated predominantly in renal tubular epithelial cells at early time points (days 3 and 7). As the kidney injury progressed over time, there was a shift in Gal-3 expression from tubular cells to interstitial cells (Fig. 1, B, C, D). By dual-staining confocal microscopy, macrophages were identified as the primary interstitial cells expressing Gal-3 during later time points, while only a small proportion of myofibroblasts were Gal-3-positive (Fig. 1, E and F).
Fig. 1.
Galectin-3 (Gal-3) expression is increased after unilateral ureteral obstruction (UUO) in tubular and interstitial cells. A: graph summarizes analysis of relative Gal-3 mRNA expression normalized to 18S, UUO relative to contralateral, by semiquantitative real-time qPCR (†P < 0.01; n = 6) in wild-type mice. Representative confocal photomicrographs of Gal-3 (green) localization demonstrated low basal expression in tubular cells of normal kidneys (B) that is upregulated in tubular cells (*) at early time points (C) and transitioned to interstitial cells (arrows) by day 14 (D). A small subpopulation of α-smooth muscle actin (α-SMA+; red) myofibroblasts expressed Gal-3 (yellow merged, indicated by arrow; E) while the majority of the Gal-3 interstitial cells are F4/80+ (red) macrophages (F; arrows indicate an aggregated subpopulation of dual stained Gal-3+F4/80+ cells). Bar = 20 μm. Magnification ×400. G, glomeruli.
Fibrosis severity worse in gal-3-deficient mice.
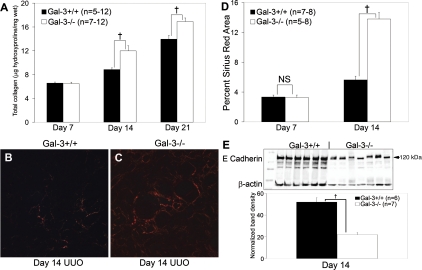
To investigate whether Gal-3 modulated fibrosis severity after UUO, total collagen levels were measured as hydroxyproline content per wet weight from obstructed kidneys. Fibrosis severity was significantly increased by 35 and 21% in Gal-3-deficient compared with wild-type mice at 14 and 21 days after UUO, respectively (n = 5–12/group, P < 0.01; Fig. 2A). Picrosirius red staining provided histological confirmation that there was significantly more interstitial collagen deposited at day 14 in Gal-3-deficient compared with wild-type mice (n = 7–8/group, F = 19.6, P = 0.0007; Fig. 2, B-D). There was no difference in baseline levels of interstitial collagen in normal kidneys of Gal-3-deficient mice compared with wild-type mice measured by Picrosirius red staining (wild-type vs. Gal-3-deficient, n = 5/group, contralateral: 0.3 ± 0.04 vs. 0.4 ± 0.04%, P = 0.2; Supplemental Fig. 1; the online version of this article contains supplemental data). A limitation of the UUO model is the inability to measure the functional consequences of the structural damage due to contralateral kidney compensation. E-cadherin levels have been used as a surrogate measure of the number of intact tubules (20, 32, 38). Using this parameter, renal tubular integrity was significantly diminished by 58% in normalized E-cadherin protein levels at day 14 in obstructed kidneys of Gal-3-deficient compared with wild-type mice (n = 6–7/group, normalized to β-actin, P = 0.00008; Fig. 2E). Collectively, these results indicate that Gal-3 expression attenuated fibrosis severity and preserved renal tubules during more advanced stages (days 14 and 21) of chronic kidney injury.
Fig. 2.
A: total kidney collagen content measured by the hydroxyproline assay is significantly increased in obstructed kidneys from Gal-3-deficient mice compared with wild-type mice. B and C: representative polarized light microscopy photomicrographs (×200) illustrate increased collagen deposition by Picrosirius red staining at day 14 in Gal-3-deficient mice. D: graph summarizes the results of polarized Picrosirius red quantification by computer-assisted image analysis, expressed as the percent positive area. E: renal E-cadherin Western blot illustrates significantly lower levels in Gal-3-deficient mice at day 14 after UUO reflecting the loss of intact tubules. The graph below summarizes the results of single-band density measurements, expressed as β-actin-normalized E-cadherin band levels. Filled bars represent wild-type mice and open bars represent Gal-3-deficient mice. Results are expressed as means ± SE. †P < 0.01, wild-type vs. Gal-3-deficient.
Gal-3 Attenuates Apoptosis in Damaged Renal Tubular Cells
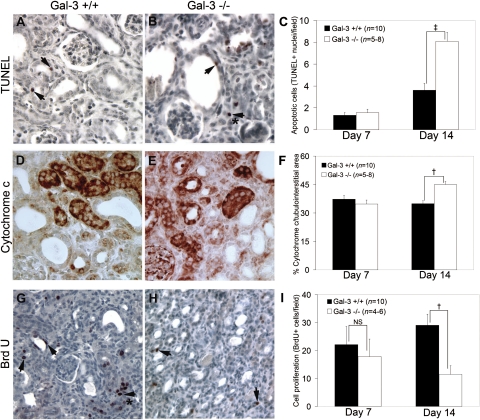
Intracellular Gal-3 is primarily localized to the cytoplasm where it functions as an anti-apoptotic factor by inhibiting caspase activation similar to Bcl2 in the intrinsic pathway (47). However, extracellular Gal-3 can form oligomers and bind to cell surface receptors in a carbohydrate-dependent manner and promote apoptosis (16, 46, 54). Since Gal-3 expression was initially prominent in tubular epithelial cells and tubular E-cadherin levels were significantly lower in Gal-3-deficient mice, its effects on apoptosis were investigated. The number of TUNEL-positive cells was significantly increased by 123% at day 14 after UUO in Gal-3-deficient compared with wild-type mice (n = 8–10/group, z = 3.1, P = 0.002; Fig. 3, A–C). Cytochrome c release from mitochondria represents an important step in activating the intrinsic pathway of apoptosis (24, 43). Semiquantitative analysis of kidney sections stained for cytochrome c showed significantly more positive tubules (29%) at day 14 after UUO in Gal-3-deficient compared with wild-type mice (n = 8/group, F = 6.6, P = 0.02; Fig. 3, D and E). In addition to the increase in apoptotic cells, there was a significant decrease in cell proliferation with a 62% decrease in the number of BrdU-positive tubulointerstitial cells at day 14 in Gal-3-deficient compared with wild-type mice (n = 6–10/group, z = −2.4, P = 0.02; Fig. 3, F-H). By immunolocalization, the majority of BrdU-positive cells are tubular cells (see arrows Fig. 3G). These results suggested that during chronic kidney injury induced by obstruction, intracellular Gal-3 limited caspase activation and subsequent apoptosis in tubular epithelial cells and promoted recovery through tubular cell proliferation.
Fig. 3.
A and B: representative photomicrographs (×400) and the graph (C) demonstrate increased TdT-mediated dUTP nick end labeling (TUNEL+) cells in Gal-3-deficient mice on UUO day 14. The arrows highlight TUNEL+ tubular cells and the (*) interstitial cells. D and E: representative photomicrographs illustrate more cytochrome c-positive tubules in Gal-3-deficient mice and the graph (F) summarizes total cytochrome c levels on UUO day 14. G and H: representative photomicrographs illustrate more proliferating BrdU+ tubular and (*) interstitial cells in wild-type mice and the graph (I) summarizes the differences in proliferating BrdU+ cells on UUO day 14. All results are expressed as means ± SE. NS, not significant. †P < 0.05, ‡P < 0.01, wild-type vs. Gal-3-deficient.
Gal-3 Does Not Affect Indicators of Inflammation
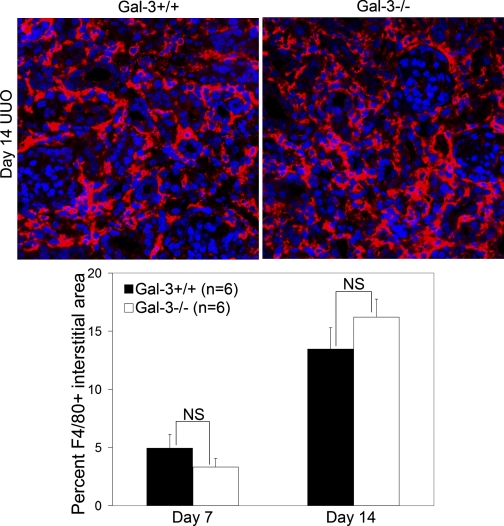
Gal-3 has been reported to be an important factor in alternative macrophage activation (28) and interstitial macrophage infiltration is a requisite step in kidney fibrosis. Therefore, since Gal-3 affected later time points in collagen accumulation, interstitial macrophage infiltration was examined at days 7 and 14 after UUO. Significant differences were not observed in F4/80+ interstitial area at either time points examined (wild-type vs. Gal-3-deficient, n = 6/group, P = 0.3; Fig. 4). By dual fluorescent confocal microscopy, only a small subpopulation of the interstitial F4/80+ macrophages expressed Gal-3; these numbers did not change as fibrosis advanced (day 7 vs. day 14, n = 6/group: 11 ± 3.7 vs. 14 ± 2.7%, P = 0.7). To consider the possibility that proinflammatory signaling might differ between Gal-3-deficient and wild-type macrophages despite similar recruitment levels, phosphorylated IκB-α (pIκB-α)-to-total IκB-α ratios were measured by Western blotting. There was no difference in NF-κB activation between Gal-3-deficient and wild-type mice at day 3 or day 7 after UUO (pIκB-α total IκB-α wild-type vs. Gal-3-deficient, n = 5–6/group: day 3: 0.8 ± 0.02 vs. 0.8 ± 0.04, P = 0.7; day 7: 0.7 ± 0.002 vs. 0.7 ± 0.009, P = 0.6).
Fig. 4.
Gal-3 deficiency did not alter macrophage infiltration after ureteral obstruction. Representative confocal photomicrographs (×400) are shown at top and the graph at the bottom summarizes the quantitative results of F4/80-positive (red) interstitial staining. Results are expressed as means ± SE. NS, wild-type vs. Gal-3-deficient.
Gal-3 Modulates Matrix Turnover
Paradoxical decreased interstitial myofibroblasts despite increased fibrosis.
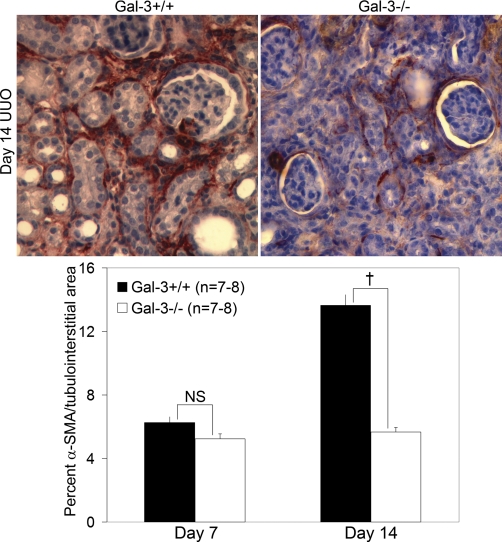
Since fibrosis severity was significantly increased in Gal-3-deficient mice, studies were performed to examine its potential effect on interstitial myofibroblast accumulation. Despite more severe fibrosis, the number of interstitial α-SMA-positive interstitial cells was significantly decreased by 58% in Gal-3-deficient compared with wild-type mice at day 14 after UUO (n = 7–8/group, F = 49.6, P = 0.00001; Fig. 5). Fibronectin and interstitial collagens are primary components of the extracellular matrix in kidney fibrosis. Since α-SMA-positive myofibroblasts are the primary source of these extracellular matrix proteins during kidney injury, matrix gene transcription was examined by real-time semiquantitative qPCR. Consistent with the decrease in α-SMA-positive myofibroblasts, there was a significant decrease by more than 80% in steady-state mRNA levels of fibronectin and procollagen I in Gal-3-deficient compared with wild-type mice by real-time semiquantitative qPCR at day 14 after UUO (normalized to 18S and GAPDH, n = 6/group, P < 0.01; Table 1). These findings suggested that myofibroblast accumulation and matrix synthesis rates cannot explain the worse fibrosis that developed in Gal-3-deficient mice.
Fig. 5.
Interstitial α-SMA+ myofibroblast numbers were significantly lower in Gal-3-deficient mice compared with wild-type mice at day 14 after UUO despite an increase in fibrosis severity. Representative α-SMA-stained immunohistochemical photomicrographs (×400) are shown at top and the graph at the bottom summarizes the quantitative results of α-SMA interstitial staining. Results are expressed as means ± SE. †P < 0.01, wild-type vs. Gal-3-deficient.
Table 1.
Relative expression of extracellular matrix genes in Gal-3-deficient mice
| Day 7 UUO | P | Day 14 UUO | P | |
|---|---|---|---|---|
| Fibronectin | 1.2 (0.4–7.8) | 0.77 | 0.2 (0.07–0.7) | 0.003* |
| Collagen I | 0.4 (0.1–1.2) | 0.11 | 0.2 (0.05–0.6) | 0.005* |
| Collagen III | 0.9 (0.5–1.4) | 0.61 | 0.8 (0.4–2.0) | 0.46 |
Semiquantitative real-time qPCR of galectin-3 (Gal-3)-deficient compared with wild-type mice. Data were analyzed with REST software using both GAPDH and 18S as reference housekeeping genes. Each sample was performed in triplicate (n = 6/group; 95%tile confidence interval in parentheses).
UUO, unilateral ureteral obstruction.
P < 0.01.
Alterations in matrix remodeling.
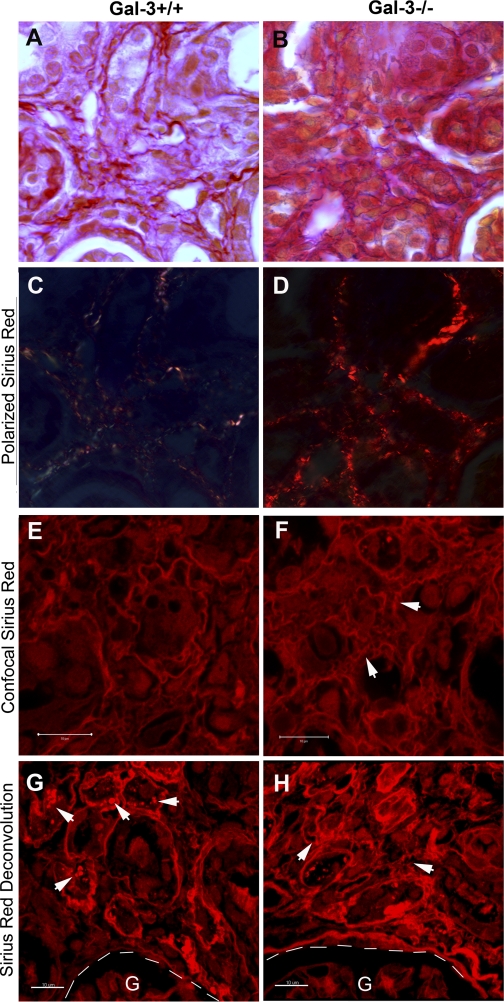
Polarized light microscopy of tissues stained with Picrosirius red can be used qualitatively to evaluate matrix maturity: the closely packed thick fibrils of type I-like collagen stain an intense red-orange, as seen in advanced scar, while faint yellow-green staining of smaller, thinner collagen fibers typifies immature scar and in remodeled fetal wounds (2, 8, 33). Polarized microscopy of day 14 UUO tissue demonstrated a more intense red-orange birefringence pattern with broader expansion of the interstitial space in Gal-3-deficient mice compared with the thinner, helical, faint yellow-green pattern seen in wild-type mice (Fig. 6, A–D). This difference in Picrosirius red staining pattern could also be appreciated by confocal microscopy and confirmed that the collagen fibers within the interstitial space were shorter and more disorganized (see arrows in Fig. 6F) at day 14 after UUO in Gal-3-deficient compared with wild-type mice (Fig. 6, E and F). Confocal z-stack Picrosirius red images of wild-type and Gal-3-deficient mice at day 21 after UUO were analyzed with the Imaris 7.0 software. Sirius red-positive vesicle-type structures were observed in wild-type mice (arrows in Fig. 6G) and suggested phagocytic uptake of extracellular matrix during advanced stages of fibrosis. Three-dimensional deconvolution images of Gal-3-deficient obstructed kidneys at day 21 further confirmed a disorganized interstitial matrix pattern (arrows in Fig. 6H). Dual fluorescent confocal staining with collagen I and F4/80 demonstrated that macrophages are intimately integrated within the histoarchitecture of the interstitial collagen I matrix, suggesting that differences in macrophage phagocytic function may account for the disorganized matrix pattern that was observed in the Gal-3-deficient mice (Fig. 7A).
Fig. 6.
Gal-3 expression alters matrix remodeling. A and B: representative Picrosirius red photomicrographs (×1,000) and corresponding polarized images (C, D) demonstrate increased intensity of red-orange collagen matrix in Gal-3-deficient mice on UUO day 14. E and F: representative confocal photomicrographs of Picrosirius red staining illustrates disordered interstitial matrix patterns (arrows) in Gal-3-deficient mice at day 14. G and H: representative 3-dimensional confocal image using Imaris 7.0 software illustrates vesicles-like structures containing Picrosirius red-positive matrix (arrows, G) in wild-type mice and confirmed disorganized interstitial matrix patterns in Gal-3-deficient mice (arrows, H). G, glomerulus. Magnifications are ×1,000 (A–D). Bar = 10 μm (E–H).
Fig. 7.
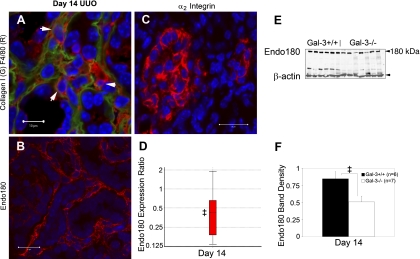
Attenuated expression of Endo180 in Gal-3-deficient mice. A: dual stained confocal microscopy of collagen I (green) and F4/80 (red). Arrows highlight F4/80+ macrophages integrated within collagen matrix. B: photomicrograph illustrates the diffuse interstitial infiltrate of Endo180+ cells (red) at day 14 after UUO. C: photomicrograph illustrates interstitial expression of α2 integrin, a potential binding partner for Gal-3. D: boxplot summarizes analysis of relative Endo180 mRNA expression normalized to both GAPDH and 18S, Gal-3-deficient to wild-type, measured by semiquantitative real-time qPCR. E: Endo180 Western blot of total kidney protein shows decreased expression levels and graph (F) summarizes results of normalized Endo180 to β-actin levels at day 14. Results are expressed as means ± SE. ‡P < 0.05, wild-type vs. Gal-3-deficient. Bar = 10 μm (A); bar = 20 μm (B, C).
Lower Endo180 levels associated with altered matrix turnover.
Endo180, also known as urokinase receptor-associated protein, UPARAP, and mannose receptor 2, Mrc2, has recently been identified as a transmembrane endocytic receptor for several collagens and it appears to function as an intracellular pathway for collagen degradation (7). Endo180 is expressed by interstitial cells during renal fibrogenesis (Fig. 7B). It is known to be expressed by both macrophages and fibroblasts. There was a 58% decrease in steady-state Endo180 mRNA levels at day 14 after UUO in Gal-3-deficient compared with wild-type mice as measured by semiquantitative real-time qPCR (normalized to 18S and GAPDH, n = 6/group, Gal-3-deficient relative to wild-type: day 7: expression ratio 0.98, 95% CI 0.77–1.27, P = 0.9; day 14: expression ratio 0.42, 95% CI 0.15–1.84, P = 0.03; Fig. 7D). In the Gal-3-deficient mice, Endo180 protein expression also decreased by Western blot at day 14 after UUO compared with wild-type mice (n = 6–7/group, P = 0.03; Fig. 7, E and F). α2β1 Integrin is both a potential binding partner for Gal-3 and another known receptor for collagen (15). During UUO, interstitial cells were shown to express α2 integrin at day 14 (Fig. 7C), raising the possibility of a link with Gal-3 and Endo180.
Matrix metalloproteinases, such as MMP9, MMP12, and MMP13, can also degrade interstitial collagen matrices. There was no difference in steady-state mRNA levels of MMP9, MMP12, and MMP13 between Gal-3-deficient mice and wild-types at day 7 and day 14 as measured by quantitative real-time qPCR (normalized to 18S and GAPDH, Gal-3-deficient relative to wild-type, n = 5/group; day 14 UUO, MMP9: 0.9, 95% CI 0.4–2.2, P = 0.8; MMP12: 1.0, 95% CI 0.4–2.8, P = 1.0; MMP13: 1.6, 95% CI 0.4–15.4, P = 0.6).
DISCUSSION
The results of the present study demonstrated that Gal-3 protects the kidney from progressive damage due to chronic injury by modulating two important pathways: tubular apoptosis and extracellular matrix remodeling. Renal tubular epithelial cell apoptosis and subsequent tubular atrophy are an important cause of nephron loss that causes progressive functional deterioration. Whether a cell is committed to a fate of apoptosis or recovery following injury or severe stress is determined by the balance between proapoptotic factors, such as BH3-only proteins, and anti-apoptotic factors, such as Bcl-2 (4). BH3-only proteins can lead to the permeabilization of the outer mitochondrial membrane with release of cytochrome c and other proteins into the cytoplasm where they promote caspase-mediated apoptosis (43). Intracellular Gal-3 is a well-established anti-apoptotic factor that can block both the intrinsic and extrinsic pathways (26, 31, 51). Basal Gal-3 expression levels in normal kidneys are low. With the onset of damage initiated by ureteral obstruction, tubular Gal-3 expression is dramatically upregulated, within the first week. Gal-3 is not a member of the Bcl-2 family but shares significant structural similarities with Bcl-2: both proteins are rich in proline, glycine, and alanine amino acid residues within their NH2-terminal domains and contain the Asp-Trp-Gly-Arg (NWGR) sequence in the COOH terminus which is critical for anti-apoptotic function (1, 50, 51). In the present study, there were significantly increased cytosolic levels of cytochrome c and greater numbers of tubular apoptotic cells in Gal-3-deficient compared with wild-type mice after UUO consistent with an anti-apoptotic role during chronic kidney injury. In addition, Gal-3 may enhance tubular cell proliferation which is an important component of the adaptive response during obstructive kidney injury. Nuclear Gal-3 is known to be an important regulator of cell proliferation via its ability to regulate the cell cycle and to serve as a pre-mRNA splicing factor (9, 23). Significantly fewer proliferating tubular cells were detected in Gal-3-deficient compared with wild-type mice after UUO, suggesting that Gal-3 facilitates tubular cell regeneration during chronic injury. Taken together, these data establish that intracellular Gal-3 expression is upregulated in tubular cells during chronic kidney injury where it appears to function as an important survival factor by blocking apoptosis and promoting tubular cell proliferation.
Our data also suggest that a second major pathway is modulated by Gal-3 during chronic kidney injury that has important effects on extracellular matrix remodeling. In contrast to the study by Henderson and colleagues (17) that focused on the initial phase of UUO-induced kidney injury and reported less fibrosis at 7 days, the present study demonstrated that fibrosis severity was increased at day 14 and persisted through day 21 in Gal-3-deficient compared with wild-type mice. In the present study, fibrosis severity was measured both biochemically (hydroxyproline content) and histologically (Picrosirius red) and extended to structural consequences on renal tubular integrity (E-cadherin Western). Consistent with the findings of Henderson and colleagues, we confirmed significantly fewer α-SMA-positive interstitial myofibroblasts which likely accounted for lower rates of extracellular matrix gene transcription in Gal-3-deficient compared with wild-type mice. These findings suggested the possibility that the anti-fibrotic effects of Gal-3 might be due to enhanced extracellular matrix turnover.
The histoarchitecture of the interstitial matrix in the Gal-3-deficient mice was more disorganized and characterized by thicker, more intensely red-orange fibrils compared with wild-type mice. Wound remodeling studies suggest that this pattern indicates the predominance of collagen I-type fibrils (8). A recent study by Oliveria and colleagues (39) demonstrated that collagen fibers surrounding acute and chronic granulomas in Gal-3-deficient mice were loosely packed and oriented in a disorganized pattern in a schistosomiasis model of liver fibrosis. Extracellular matrix remodeling is a critical process that involves three components: synthesis, deposition, and degradation. Despite intense interest in the contribution of matrix degradation to renal fibrogenesis, the specific pathways that are involved remain elusive. It had been assumed that matrix remodeling is an extracellular process mediated by MMPs, but this hypothesis has yet to be definitively established. Previous studies on certain serine protease and gelatinase systems in chronic kidney injury suggest that these proteases do not mediate significant matrix degradation but likely have a more prominent role in fibrogenic signaling that may actually enhance fibrosis (6, 12, 13, 32, 52). Endo180 has recently been identified as an endocytic receptor for collagen I and IV and is likely a key receptor for matrix degradation during wound remodeling (7, 29). Studies in our lab showed that Endo180 protein levels increased steadily after the onset of obstruction and that renal fibrosis is significantly worse in Endo180-deficient mice (27). In the present study, Endo180 mRNA and protein levels were significantly lower in Gal-3-deficient compared with wild-type mice. Since Gal-3 is not a transmembrane receptor, it is not yet clear how Gal-3 might regulate Endo180 expression.
Gal-3 may also play a more direct role in intracellular matrix turnover through extracellular interactions with one of its known binding partners, the α2β1 integrin. Gal-3 binds α2β1 integrin through its carbohydrate recognition domain (54) and a recent study reported that Gal-3 was an important regulator of α2β1 integrin-mediated adhesion to collagen I and collagen IV (15). In addition to promoting adhesion, functional β1 integrins also stabilize fibronectin matrix fibrils and promote endocytosis of matrix fibronectin (45). Shi and colleagues (44) recently demonstrated that endocytosis of collagen I from extracellular matrix is dependent on both β1 integrin and Endo180 and the authors further speculated that Endo180 may interact with β1 integrin to modulate collagen I endocytosis. However, the specific mechanisms of this interaction remain unknown. We speculate that extracellular Gal-3 may form oligomers to direct β1 integrin/Endo180-mediated collagen endocytosis and is the subject of future investigations.
Both macrophages and fibroblasts can express Endo180 and are capable of performing Endo180-mediated collagen endocytosis (29, 30); whether this process resides within myofibroblasts and/or macrophages during chronic kidney injury remains to be determined. In the present study, macrophages were identified as the primary Gal-3-positive interstitial cell population, representing ∼10 to 14% of all F4/80-positive macrophages. The importance of macrophage functional heterogeneity is increasingly recognized, although the specific phenotype and function of fibrosis-associated macrophages are not yet clear (41). Although classically associated with matrix production, fibroblasts are also known to be functionally heterogeneous and may contribute to intracellular matrix degradation (3, 14). In the present study, a small subpopulation of interstitial α-SMA-positive myofibroblasts was shown to express Gal-3. Future studies are needed to determine whether Gal-3 expression identifies subpopulations of macrophages and/or fibroblasts that phenotypically define cells that promote matrix degradation.
In summary, the present study demonstrated that Gal-3 not only protects renal tubules from chronic injury by limiting apoptosis but that it may be an important factor in matrix remodeling and fibrosis attenuation. Based on our findings and previously published studies (15, 26, 34, 54), they further suggest that intracellular Gal-3 through carbohydrate-independent mechanism may preserve renal tubules while extracellular Gal-3 through its carbohydrate domain may direct Endo180-mediated collagen degradation.
GRANTS
The authors gratefully acknowledge research grant support from the National Institutes of Health [DK-54500 and DK-0044757 (to A. A. Eddy)], Child Health Research Center Scholar Award, K12 HD0-43376 (to D. M. Okamura), K08-DK-073497 (to D. M. Okamura), and a National Kidney Foundation Young Investigator Grant (to D. M. Okamura).
DISCLOSURES
Part of this work was presented as an abstract at the annual meeting of the American Society of Nephrology in October 2007 and October 2009.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. J. Garrigues for technical expertise with the Zeiss LSM5 confocal microscope and LSM analysis software.
REFERENCES
- 1. Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res 57: 5272–5276, 1997 [PubMed] [Google Scholar]
- 2. Andrade GB, Montes GS, Conceicao GM, Saldiva PH. Use of the Picrosirius-polarization method to age fibrotic lesions in the hepatic granulomas produced in experimental murine schistosomiasis. Ann Trop Med Parasitol 93: 265–272, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Arora PD, Manolson MF, Downey GP, Sodek J, McCulloch CA. A novel model system for characterization of phagosomal maturation, acidification, and intracellular collagen degradation in fibroblasts. J Biol Chem 275: 35432–35441, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest 118: 1979–1990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bullock SL, Johnson TM, Bao Q, Hughes RC, Winyard PJ, Woolf AS. Galectin-3 modulates ureteric bud branching in organ culture of the developing mouse kidney. J Am Soc Nephrol 12: 515–523, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J 20: 1898–1900, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Curino AC, Engelholm LH, Yamada SS, Holmbeck K, Lund LR, Molinolo AA, Behrendt N, Nielsen BS, Bugge TH. Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J Cell Biol 169: 977–985, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. Collagen in the scarless fetal skin wound: detection with picrosirius polarization. Wound Repair Regen 13: 198–204, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci USA 92: 1213–1217, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davidson PJ, Davis MJ, Patterson RJ, Ripoche MA, Poirier F, Wang JL. Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology 12: 329–337, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Eddy AA. Serine proteases, inhibitors and receptors in renal fibrosis. Thromb Haemost 101: 656–664, 2009 [PMC free article] [PubMed] [Google Scholar]
- 13. Eddy AA, Kim H, Lopez-Guisa J, Oda T, Soloway PD. Interstitial fibrosis in mice with overload proteinuria: deficiency of TIMP-1 is not protective. Kidney Int 58: 618–628, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Everts V, van der Zee E, Creemers L, Beertsen W. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem J 28: 229–245, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Friedrichs J, Manninen A, Muller DJ, Helenius J. Galectin-3 regulates integrin alpha2beta1-mediated adhesion to collagen-I and -IV. J Biol Chem 283: 32264–32272, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res 63: 8302–8311, 2003 [PubMed] [Google Scholar]
- 17. Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 172: 288–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsu DK, Liu FT. Regulation of cellular homeostasis by galectins. Glycoconj J 19: 507–515, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol 156: 1073–1083, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huguenin M, Muller EJ, Trachsel-Rosmann S, Oneda B, Ambort D, Sterchi EE, Lottaz D. The metalloprotease meprinbeta processes E-cadherin and weakens intercellular adhesion. PLoS One 3: e2153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iacobini C, Menini S, Oddi G, Ricci C, Amadio L, Pricci F, Olivieri A, Sorcini M, Di Mario U, Pesce C, Pugliese G. Galectin-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury: evidence for a protective role of galectin-3 as an AGE receptor. FASEB J 18: 1773–1775, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Iacobini C, Oddi G, Menini S, Amadio L, Ricci C, Di Pippo C, Sorcini M, Pricci F, Pugliese F, Pugliese G. Development of age-dependent glomerular lesions in galectin-3/AGE receptor-3 knockout mice. Am J Physiol Renal Physiol 289: F611–F621, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res 59: 4148–4154, 1999 [PubMed] [Google Scholar]
- 24. Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ, Fritz LC. Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-xL has activity independent of cytochrome c release. J Biol Chem 272: 30299–30305, 1997. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol 20: 1907–1918, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta 1572: 263–273, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Lopez-Guisa JMBT, Isacke CM, Collins SJ, Cai S, Eddy AE. Endo180/uPAR-associated protein is an important regular of renal fibrogenesis. In: 42nd Annual Meeting American Society of Nephrology. San Diego, CA: 2009 [Google Scholar]
- 28. MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol 180: 2650–2658, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjoller L, Gardsvoll H, Hoyer-Hansen G, Holmbeck K, Bugge TH, Behrendt N. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem 282: 27037–27045, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Martinez-Pomares L, Wienke D, Stillion R, McKenzie EJ, Arnold JN, Harris J, McGreal E, Sim RB, Isacke CM, Gordon S. Carbohydrate-independent recognition of collagens by the macrophage mannose receptor. Eur J Immunol 36: 1074–1082, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Matarrese P, Tinari N, Semeraro ML, Natoli C, Iacobelli S, Malorni W. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett 473: 311–315, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67: 2221–2238, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Montes GS, Junqueira LC. The use of the Picrosirius polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz 86, Suppl 3: 1–11, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J 19: 527–535, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, Eddy AA. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60: 587–596, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16: 133–143, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Okamura DM, Lopez-Guisa JM, Koelsch K, Collins S, Eddy AA. Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol 293: F575–F585, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Okamura DM, Pennathur S, Pasichnyk K, Lopez-Guisa JM, Collins S, Febbraio M, Heinecke J, Eddy AA. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J Am Soc Nephrol 20: 495–505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oliveira FL, Frazao P, Chammas R, Hsu DK, Liu FT, Borojevic R, Takiya CM, El-Cheikh MC. Kinetics of mobilization and differentiation of lymphohematopoietic cells during experimental murine schistosomiasis in galectin-3 −/− mice. J Leukoc Biol 82: 300–310, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Pricci F, Leto G, Amadio L, Iacobini C, Romeo G, Cordone S, Gradini R, Barsotti P, Liu FT, Di Mario U, Pugliese G. Role of galectin-3 as a receptor for advanced glycosylation end products. Kidney Int Suppl 77: S31–S39, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest 112: 389–397, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanz AB, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol 19: 1634–1642, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Shi F, Harman J, Fujiwara K, Sottile J. Collagen I matrix turnover is regulated by fibronectin polymerization. Am J Physiol Cell Physiol 298: C1265–C1275, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shi F, Sottile J. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci 121: 2360–2371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 176: 778–789, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, Bresalier RS, Raz A. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol 24: 4395–4406, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. US Renal Data System 2008 Annual Data Report Bethesda: National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 49. Yamaguchi I, Lopez-Guisa JM, Cai X, Collins SJ, Okamura DM, Eddy AA. Endogenous urokinase lacks antifibrotic activity during progressive renal injury. Am J Physiol Renal Physiol 293: F12–F19, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA 93: 6737–6742, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang RY, Liu FT. Galectins in cell growth and apoptosis. Cell Mol Life Sci 60: 267–276, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang G, Kernan KA, Collins SJ, Cai X, Lopez-Guisa JM, Degen JL, Shvil Y, Eddy AA. Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: role of plasmin-activated signals. J Am Soc Nephrol 18: 846–859, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Zhang G, Kim H, Cai X, Lopez-Guisa JM, Alpers CE, Liu Y, Carmeliet P, Eddy AA. Urokinase receptor deficiency accelerates renal fibrosis in obstructive nephropathy. J Am Soc Nephrol 14: 1254–1271, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Zhuo Y, Chammas R, Bellis SL. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem 283: 22177–22185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.