Abstract
Peritubular fibroblasts in the kidney are the major erythropoietin-producing cells and also contribute to renal repair following acute kidney injury (AKI). Although few fibroblasts were observed in the interstitium adjacent to damaged tubular epithelium in the early phase of AKI, the underlying mechanism by which their numbers were reduced remains unknown. In this study, we tested the hypothesis that damaged renal epithelial cells directly induce renal interstitial fibroblast death by releasing intracellular ATP and activating purinergic signaling. Exposure of a cultured rat renal interstitial fibroblast cell line (NRK-49F) to necrotic renal proximal tubular cells (RPTC) lysate or supernatant induced NRK-49F cell death by apoptosis and necrosis. Depletion of ATP with apyrase or inhibition of the P2X purinergic receptor with pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid blocked the deleterious effect of necrotic RPTC supernatant. The P2X7 receptor, an ATP-sensitive purinergic receptor, was not detected in cultured NRK-49F cells but was inducible by necrotic RPTC supernatant. Treatment with A438079, a highly selective P2X7 receptor inhibitor, or knockdown of the P2X7 receptor with small interference RNA diminished renal fibroblast death induced by necrotic RPTC supernatant. Conversely, overexpression of the P2X7 receptor potentiated this response. Collectively, these findings provide strong evidence that damaged renal epithelial cells can directly induce the death of renal interstitial fibroblasts by ATP activation of the P2X7 receptor.
Keywords: renal proximal tubular cells, acute kidney injury
fibroblasts in the kidney, situated in the peritubular compartment, play important roles in mediating intercellular communication with neighboring cells and extracellular matrix (ECM) as well as in maintaining renal tissue architecture (23). The cortical fibroblast is also the principle cell type that produces erythropoietin (EPO), a substance that stimulates the division and differentiation of erythroid precursors in the bone marrow (12, 23). Thus preservation of renal fibroblasts is critical for renal repair as well as stability of red blood cells after acute kidney injury (AKI).
Previous studies have indicated a reduced number of peritubular EPO-producing fibroblasts in the injured kidney as the result of a variety of insults (i.e., ischemia, ureteric obstruction, and focal needle stick injury) in rats, mostly in the area adjacent to the damaged tubular epithelium (26). This suggests that peritubular fibroblasts may die upon exposure to some toxic factor or factors released by injured tubular cells. Although the nature of this factor (s) is currently unknown, ATP may be a candidate since it can be released from renal epithelial cells and play a role in regulating the function of adjacent cells under both physiological and pathological conditions. For example, ATP produced primarily by renal tubular epithelial cells can reach the vascular smooth muscle cells and influence renal microvascular function (20). In the case of AKI, renal tubular cells die by apoptosis and necrosis, which are accompanied by the damage of tubular basement membrane (TBM) (27). A large amount of ATP from necrotic tubular cells might be released quickly into the interstitium at the injury sites and act directly on the adjacent interstitial fibroblasts, influencing their fate. This hypothesis has not yet been tested.
Most cellular effects of ATP are mediated through its interaction with specific membrane-bound purinergic, or P2, receptors, which are classified into two subfamilies: the G protein-coupled P2Y receptors and P2X receptors. The P2X super-family consists of seven members (P2X1–7) in which the P2X7 receptor is unique and has low affinity for ATP. P2X7 requires 10–100 times higher concentrations of ATP (100 μmol/l) for activation compared with other purinergic receptors (30). Sustained activation of the P2X7 receptor triggers formation of a large nonselective pore for molecules up to 900 kDa and causes necrotic cell death (37). Stimulation of the P2X7 receptor also leads to apoptosis in macrophages (25), dendritic cells (7), and mesangial cells (16, 34).
The P2X7 receptor is normally expressed in the cells of the immune system, and there is very little expression in normal kidney tissue (17). Under pathological conditions, its expression is upregulated at sites of tissue damage and inflammation in the kidney. For example, a high level of the P2X7 receptor was detected in the glomeruli of diabetic and hypertensive rats (43) and in several cell types such as podocytes in response to stresses (18, 43) and in cultured mesangial cells upon exposure to TNF-α (16). Currently, the functional role of P2X7 in renal interstitial fibroblasts after renal tubular damage is not understood. It is also unclear whether ATP could act as a mediator for renal tubular cells to communicate with renal fibroblasts in the nearby interstitium, leading to their death.
In this study, we examined the effect of damaged renal epithelial cells on the viability of renal interstitial fibroblasts in a coculture system and investigated the molecular mechanisms involved. Our data showed that ATP released from necrotic renal proximal tubule cells (RPTC) induces death of renal interstitial fibroblasts through its action on the P2X7 receptor.
MATERIALS AND METHODS
Chemicals and antibodies.
A-438079 and MRS-2500 were purchased from Tocris Bioscience (Ellisville, MO). Benzyloxycarbonyl- Val-Ala-Asp-(OMe)fluoro-methylketone (ZVAD-fmk) was obtained from Enzo Life Science (Plymouth Meeting, PA). The small interference RNA (siRNA) specific for P2X7 was purchased from Invitrogen (Carlsbad, CA). Antibodies to P2X7 and P2Y1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and Abcam (Cambridge, MA), respectively. All other antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA). ATP, suramin, and pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPAD), apyrase (grade VII), and all other chemicals were purchased from Sigma (St Louis, MO).
Cell culture.
Rat renal interstitial fibroblasts (NRK-49F) and immortalized mouse RPTC were used in this study. Both of them were cultured in DMEM/Nutrient-F12 (DMEM/F12, Sigma-Aldrich, St. Louis, MO) containing 5% FBS, penicillin, and streptomycin in an atmosphere of 5% CO2-95% air at 37°C. NRK-49F were confluent when used for various treatments. When necessary, various inhibitors were directly added to the culture and then incubated for desired time as indicated in the figure legends.
Preparation of cell lysate.
RPTC and NRK-49F were harvested and washed twice with sterile PBS, and then reconstituted at different cell numbers (2 × 104, 2 × 105, and 2 × 106 /ml) in complete culture media. These cells were immediately used for cell lysate preparation by repetitive (5 cycles) freezing at −80°C and thawing at 37°C. Then, the cell lysate was centrifuged at 15,000 rpm for 20 min to obtain a supernatant. The pellet was washed twice and dispersed in a volume of fresh medium equal to the volume of the supernatant. For the experiments, volume (330 μl/ml) of medium was removed and 330 μl of whole cell lysate or supernatant was added to the culture. When we replaced 330 μl/ml of medium with lysate or supernatant from 2 × 106 cells/ml, the final concentration of cell lysate was equal to the lysate from 6.6 × 105 cells/ml.
Measurement of ATP.
The ATP level in RPTC and NRK-49F was measured by using an Enzylight ATP assay kit (Bioassay Systems). This method is based on bioluminescence by the reaction of ATP with d-luciferin in the presence of luciferase. In brief, 90 μl of assay reagent (mixture of 95 μl of assay buffer, 1 μl of substrate, and 1 μl of enzyme) was added to 100 μl cell lysate and incubated in room temperature for 10 min. Then, luminescence of samples was read in a Spectramax M5 plate reader with integration time of 5 s.
Transfection of siRNA into cells.
siRNA oligonucleotides targeted specifically to rat P2X7 were used in this experiment. siRNA (750 pmol) was transfected into NRK-49F (1 × 106 cells) using a Nucleofector Kit V and the Amaxa Nucleofector device according to the manufacturer's instructions (Gaithersburg, MD). In parallel, 750 pmol of scrambled siRNA was used to control for off-target changes in NRK-49F. After transfection, cells were cultured in DMEM/F-12 for 24 h and used for the experiments.
Determination of cell viability by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After treatment, MTT was added (final concentration, 0.5 mg/ml) and incubated for 1 h. Tetrazolium was released by the addition of DMSO, and the optical density was determined with a spectrophotometer (570-nm reader, Molecular Devices, Sunnyvale, CA).
Immunoblot analysis.
After various treatments, cells were washed once with ice-cold PBS and harvested in a cell lysis buffer. Proteins (20 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% skim milk overnight at 4°C, membranes were incubated with a primary antibody for 1 h at room temperature and then incubated with an appropriate horseradish peroxidase-conjugated secondary antibody for an additional 1 h. Bound antibodies were visualized by chemiluminescence detection.
Statistical analysis.
Data are presented as means ± SD and were subjected to one-way analysis of variance. Multiple means were compared using Tukey's test, and differences between two groups were determined by Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Necrotic RPTC induce death of renal interstitial fibroblasts.
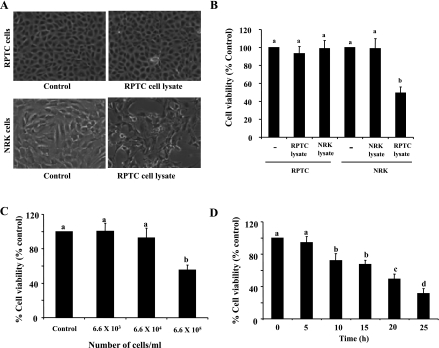
Previous pathological studies have shown a reduced number of EPO-expressing renal interstitial fibroblasts in the area adjacent to the damaged renal tubular epithelium following acute injury (26), suggesting that injured tubular cells may affect the viability of renal interstitial fibroblasts. However, it is uncertain whether a cross talk exists in these two cell types. To address this issue, we treated renal NRK-49F, a normal rat interstitial fibroblast line, with necrotic RPTC lysates. Necrotic RPTC were prepared by repetitive freezing and thawing through five cycles, at the end of which cells lost normal morphology and became debris and generated a major poly(ADP-ribose) polymerase (PARP) fragment at ∼55 kDa, a signature of necrosis (5, 15, 35) (Supplemental Fig. 1; supplementary material for this article is available online at the journal web site). Exposure of confluent NRK-49F to the necrotic RPTC (6.6 × 105) led to NRK-49F round-up, with some of the cells detached from the dishes (Fig. 1). Cell viability was reduced by ∼50% as measured by the MTT assay (Fig. 1, A and B). As a control, we also treated RPTC with necrotic RPTC lysates (Fig. 1A), RPTC with necrotic NRK-49F lysates, or NRK-49F with necrotic NRK-49F lysates (Fig. 1B). Under all those conditions, there were no alterations in cell morphology and viability. Therefore, necrotic RPTC uniquely cause death of renal interstitial fibroblasts but not themselves. Furthermore, necrotic renal fibroblasts do not have any deleterious effect on either cell type.
Fig. 1.
Effect of renal proximal tubule cells (RPTC) and normal renal kidney (NRK) cell lysate on cell viability. Confluent NRK-49F (NRK) and RPTC were treated with control culture medium (control) or NRK-49F and RPTC cell lysate from 6.6 × 105 cells for 20 h (A and B). After 20 h, cells were photographed (A), and cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (B). NRK-49F cells were treated with RPTC lysates from the indicated number of cells for 20 h (C) or treated with RPTC lysate from 6.6 ×105 cells for the indicated time (D), and cell viability was assessed by the MTT assay. Values are means ± SD of 3 independent experiments conducted in triplicate and expressed as the percentage of control (B–D). Bars with different letters (a–d) are significantly different from one another (P < 0.05).
Necrotic RPTC-induced death of NRK-49F occurred in a dose- and time-dependent manner. Although incubation of NRK-49F with the cell lysate from 6.6 × 105 necrotic RPTC resulted in reduced NRK-49F viability up to 50%, the cell lysate from 6.6 × 103 or 6.6 × 104 necrotic RPTC did not alter the viability of NRK-49F (Fig. 1C). The time course study showed that NRK-49F cell survival began to decline at 10 h after exposure to necrotic RPTC (6.6 × 105) and then was further reduced over time. At 20 and 25 h, cell viability declined by ∼55 and 40%, respectively (Fig. 1D). Based on these results, 6.6 × 105 cells/ml necrotic RPTC and 20 h incubation time were used for all of other experiments in this study.
Necrotic RPTC induce both necrosis and apoptosis of cultured renal interstitial fibroblasts.
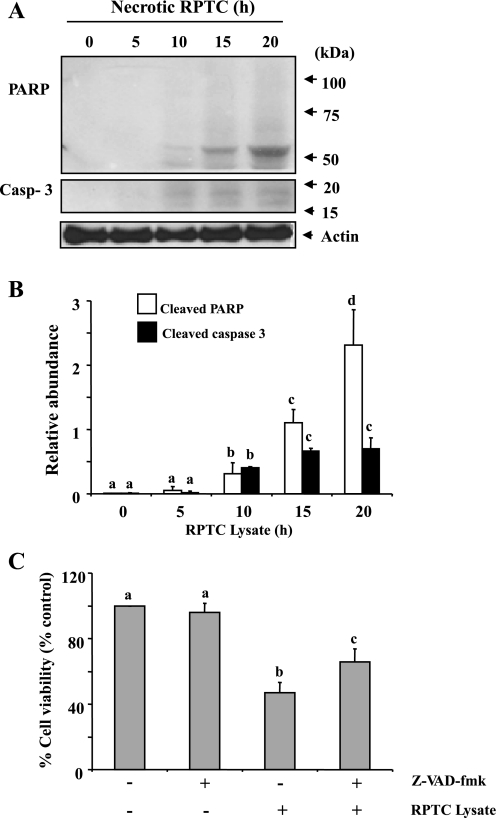
To determine the mode of cell death of renal interstitial fibroblasts induced by necrotic RPTC, necrotic RPTC-treated NRK-49F were subjected to immunoblot analysis for detection of active forms of PARP and caspase-3. It has been reported that cleavage of PARP-1 to a 55-kDa fragment (15) and cleavage of caspase-3 to small fragments of 17 and 19 kDa (13) are hallmarks of necrotic and apoptotic cell death, respectively. Thus specific antibodies against cleaved PARP-1 and caspase-3 were used to detect these cleaved fragments. As shown in Fig. 2, A and B, treatment of NRK-49F with necrotic RPTC resulted in the formation of a PARP-1 fragment at 55 kDa and caspase-3 fragments at 17 and 19 kDa in NRK-49F. Those fragments appeared in NRK-49F at 10 h after treatment with necrotic RPTC lysates and further increased at 15 and 20 h. These data suggest that renal interstitial fibroblasts undergo both necrosis and apoptosis in response to the supernatant from necrotic RPTC.
Fig. 2.
RPTC lysate induces fibroblast cell death in a caspase-3-dependent and -independent manner. Confluent NRK-49F cells were treated with RPTC lysate (prepared from 6.6 × 105 cells) for the indicated time period (A and B) or pretreated with Z-VAD-fmk (50 μM) for 1 h followed by exposed to RPTC lysate for 20 h (C). Cell lysate were subjected to immunoblot analysis for cleaved poly(ADP-ribose) polymerase (PARP), cleaved caspase 3, and actin (A). The levels of the indicated proteins were quantified by densitometry and normalized with actin (B). Cell viability was assessed by the MTT assay (C). Values are means ± SD of 3 independent experiments and expressed as the percentage of control. Bars with different letters (a–d) are significantly different from one another (P < 0.05).
As caspases are the major mediator of apoptosis, we further assessed the effect of Z-VAD-fmk, a pan-caspase inhibitor, on the death of NRK-49F following necrotic RPTC exposure. Treatment with 50 μM Z-VAD-fmk, a dose that was reported to completely block caspase-3 activity (8), led to an increased viability of NRK-49F by ∼20% compared to RPTC lysate alone treated cells (Fig. 2C). These data support the above observation that apoptotic cell death only in part contributes to death of renal interstitial fibroblasts following exposure to necrotic RPTC lysates.
A cell supernatant but not cell debris of necrotic RPTC induces death of renal interstitial fibroblasts.
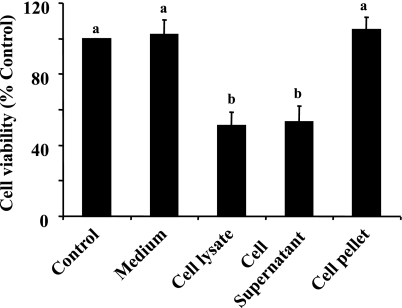
We hypothesized that a soluble factor (s) in necrotic RPTC is responsible for induction of renal fibroblast death. To test this hypothesis, we separated the supernatant and debris of RPTC by ultracentrifugation and then examined their effects on the viability of NRK-49F. Addition of the supernatant to the cultured fibroblast reduced cell viability to the level similar to that in cells treated with the whole necrotic RPTC lysates. In contrast, fresh suspension of necrotic RPTC pellets did not alter cell viability and morphology in NRK-49F (Fig. 3 and data not shown). As a control, the frozen and thawed medium was also added to the cultured NRK-49F, and it did not affect cell viability. These results indicate that the factor that causes the death of renal interstitial fibroblasts is soluble and present in the supernatant of necrotic RPTC.
Fig. 3.
Effects of cell supernatant and pellet on renal fibroblast viability. Confluent NRK-49F cells were treated with RPTC supernatant and cell debris pellet prepared from RPTC lysate (as described in materials and methods), and cell viability was assessed by MTT assay. Values are means ± SD of 3 independent experiments and expressed as the percentage of control. Bars with different letters (a and b) are significantly different from one another (P < 0.05).
Identification of ATP as a prodeath factor in necrotic RPTC.
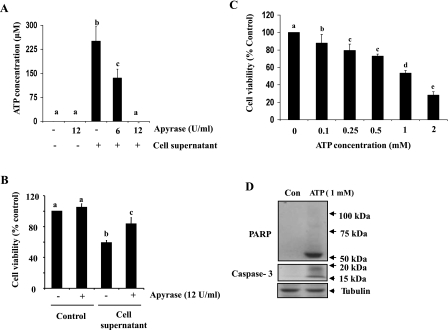
It is well known that ATP can be rapidly released into the extracellular milieu from damaged and necrotic cells (9). To determine whether ATP present in necrotic RPTC is the primary prodeath factor, we examined the effect of apyrase, an enzyme that catalyzes the hydrolysis of ATP degradation, on the viability of NRK-49F. Figure 4A showed that treatment with apyrase at 12 U/ml resulted in a complete degradation of ATP released from 2 × 106 necrotic cells/ml. In parallel, this dose of apyrase also reduced supernatant-induced renal fibroblast cell death (Fig. 4B). In addition, addition of exogenous ATP to cultured NRK-49F reduced cell viability and induced cleavage of PARP-1 to a fragment of 55 kDa and caspase-3 to fragments of 17 and 19 kDa, similar to the pattern observed after treatment with necrotic RPTC (Fig. 4, C and D). As such, these data suggest that ATP released from necrotic RPTC is the primary factor that contributes to renal fibroblast death.
Fig. 4.
ATP levels in cell supernatant and effect of apyrase on necrotic RPTC supernatant-induced renal fibroblast death. The supernatant from necrotic RPTC (2 × 105) were incubated in the presence or absence of apyrase (6 and/or 12 U/ml) in culture medium (1 ml) without cells for 6 h (A) or with NRK-49F for 20 h (B). The concentration of ATP was measured as described in materials and methods (A), or cell viability was assessed by the MTT assay (B). NRK-49F cells were treated with ATP for 20 h at the concentrations as indicated, and cell viability was assessed by the MTT assay (C), or 1 mM ATP and cells were harvested for immunoblot analysis of active PARP-1 and caspase-3 (D). Values are means ± SD of 3 independent experiments and expressed as the percentage of control. Bars with different letters (a–c) are significantly different from one another (P < 0.05).
Figure 1C shows that cell lysates prepared from RPTC, but not renal fibroblasts, induced renal fibroblast death even when the same number of cells were used. A possible explanation is that RPTC contain more ATP than renal fibroblasts. We measured the ATP concentration in the cell lysate prepared from RPTC and renal fibroblasts. A higher level of ATP (11.74 ± 0.66 nM/mg protein) was detected in RPTC compared with that in renal fibroblasts (6.79 ± 0.33 nM/mg protein) (Supplemental Fig. 2). These findings further support the importance of ATP in deleterious renal epithelial-fibroblast cross talk.
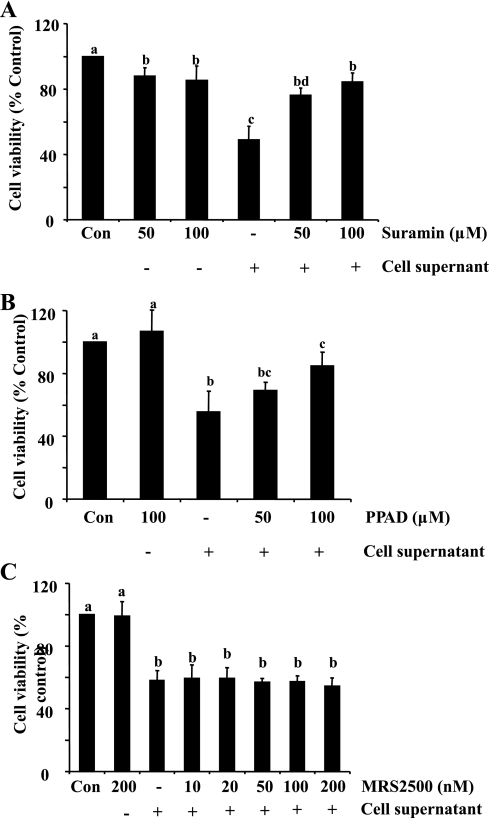
Effect of P2 receptor antagonists on necrotic RPTC-induced death of renal interstitial fibroblasts.
It has been documented that ATP-induced biological effects occur through purinergic P2 receptors, which are classified into P2Y and P2X receptors. To determine whether P2 receptors mediate death of renal interstitial fibroblasts following exposure to necrotic RPTC and if so, to identify which class is responsible, we examined the effect of suramin, PPADS, and MRS-2500 on the viability of NRK-49F. Suramin is a general P2 inhibitor (14); PPADS is a selective inhibitor of P2X receptors (14); and MRS-2500 is a selective inhibitor of P2Y receptors (19). As shown in Fig. 5, A and B, treatment with suramin and PPAD significantly reduced necrotic RPTC supernatant-induced death of NRK-49F at 50 and 100 μM, respectively. However, treatment with MRS-2500 did not improve cell survival of NRK-49F in the range of doses from 10 to 200 nM that was reported to effectively inhibit P2Y receptors (19) (Fig. 5C). On this basis, we suggest that P2X, but not P2Y receptors mediate necrotic RPTC-induced death of renal interstitial fibroblasts.
Fig. 5.
Effect of P2 receptor antagonists on cell supernatant-induced cell death. NRK-49F were treated with different concentrations of suramin (50 and 100 μM), PPAD (50 and 100 μM), or MRS-2500 (10–200 nM) for 1 h and then treated with cell supernatant for 20 h (A–C). Cell viability was determined by MTT assay. Values are means ± SD of 3 independent experiments conducted in triplicate and expressed as the percentage of control. Bars with different letters (a–d) are significantly different from one another (P < 0.05).
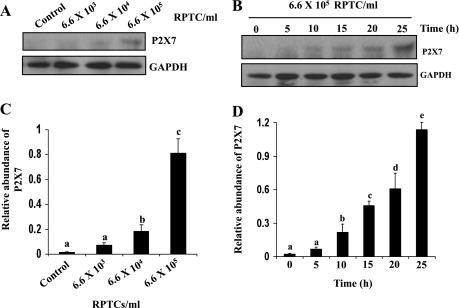
Necrotic RPTC induce expression of P2X7 in renal interstitial fibroblasts.
Of the P2X receptor family members, the P2X7 receptor has been reported to be involved in cell death in other cell types, including mesangial cells (40, 41). To determine whether P2X7 receptors mediate renal fibroblast death induced by RPTC lysates, we first examined its expression in NRK-49F exposed to the necrotic RPTC supernatant. As shown in Fig. 6A, P2X7 protein was not detectable in normally cultured NRK-49F, but induced to be expressed after treatment with the supernatant from 6.6 × 105/ml necrotic RPTC (Fig. 6, A and C). P2X7 expression in NRK-49F also occurred in a time-dependent manner. A significant increase in P2X7 expression was observed at 10 h and a maximum at 25 h following exposure to the necrotic RPTC supernatant (Fig. 6, B and D). The dose- and time-dependent induction of P2X7 expression is consistent with timing and dosing by which necrotic RPTC induced death of NRK-49F (see Fig. 1).
Fig. 6.
Induction of P2X7 expression in NRK-49F cells by cell supernatant. NRK-49F cells were treated with cell lysate prepared from different density of cells for 20 h (A) or treated with RPTC lysate from 6.6 × 105 for the indicated time (B). Cells were harvested and subjected to immunoblot analysis for the level of P2X7 and actin. The level of P2X7 was quantified by densitometry and normalized with actin (C and D). Representative immunoblots are from 3 or more experiments. Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.05).
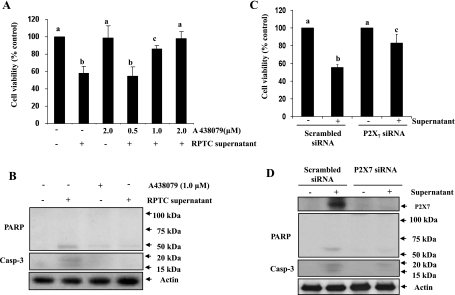
Blockade of the P2X7 receptor inhibits necrotic RPTC- induced cell death in renal interstitial fibroblasts.
To determine the role of P2X7 in cell death induced by necrotic RPTC, two approaches were used. First, we examined the effect of A438079, a highly selective antagonist for P2X7 (29), on the survival of NRK-49F. Figure 7A showed that A438079 dose dependently inhibited necrotic RPTC supernatant-induced cell death. At 2 μM, A438079 completely blocked cell death. A438079 also blocked cleavage of PARP and caspase-3 induced by the necrotic RPTC supernatant (Fig. 7C). Next, we examined the effect of knockdown of P2X7 on the viability of NRK-49F using siRNA specific for P2X7. Necrotic RPTC supernatant-induced cell death was significantly inhibited in NRK-49F transfected with the P2X7siRNA whereas transfection with an equal amount of scrambled siRNA did not affect cell survival (Fig. 7B). P2X7 siRNA also significantly reduced RPTC supernatant-induced expression of P2X7 and formation of cleaved PARP and caspase-3 in NRK-49F (Fig. 7D). P2X7 was completely knocked out by its siRNA as evaluated by immunoblot analysis (Fig. 7D). Collectively, these data indicate that P2X7 mediates death of renal fibroblasts.
Fig. 7.
Effect of P2X7 inhibition on cell supernatant-induced cell death in NRK-49F cells. NRK-49F cells were incubated with different concentration (0.5–2 μM) of A438079 for 1 h and then treated with cell supernatant for 20 h, and cell viability was assessed the MTT assay (A). Cells were treated with supernatant for 20 h in the presence or absence of 1 μM of A438079 and then harvested for immunoblot analysis for P2X7, active PARP, cleaved caspase-3, and actin (B). NRK-49F cells were transfected with siRNA targeting P2X7 or scrambled siRNA and allowed to grow for 24 h and then treated with supernatant for 20 h. Cell viability was assessed by the MTT assay (C), and cell lysates were subjected to immunoblot analysis for P2X7, cleaved PARP, cleaved caspase-3, and actin (D). Values are means ± SD of 3 independent experiments. Bars with different letters (a–c) are significantly different from one another (P < 0.05).
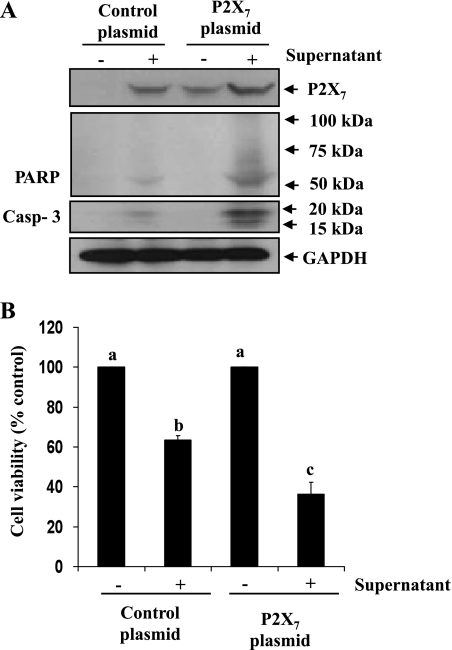
Overexpression of the P2X7 receptor enhances necrotic RPTC-induced renal interstitial fibroblast cell death.
To confirm the role of the P2X7 receptor in mediating the death of renal fibroblasts, we further examined the effect of overexpression of P2X7 on necrotic RPTC-induced renal fibroblast death. As in renal fibroblasts without vector transfection, P2X7 was not detectable in renal fibroblasts transfected with empty vector, but inducible by the necrotic RPTC supernatant (Fig. 8A, lanes 1 and 2). P2X7 expression was detected in renal fibroblasts transfected with P2X7 plasmid and increased after stimulation with the necrotic RPTC supernatant (Fig. 8A, lanes 3 and 4). Necrotic RPTC-induced activation of PARP-1 and caspase-3 was also significantly enhanced in fibroblasts overexpressing P2X7 relative to those overexpressing the empty vector (Fig. 8A, lanes 2 and 4). We also examined the effect of P2X7 overexpression on the viability of renal fibroblasts. As shown in Fig. 8B, the cell viability in fibroblasts transfected with P2X7 was dramatically decreased in response to the necrotic RPTC supernatant compared with that in necrotic RPTC-treated renal fibroblasts transfected with empty vector (Fig. 8B). Therefore, these studies further confirm the role of the P2X7 receptor in mediating necrotic RPTC supernatant-induced death of renal fibroblasts.
Fig. 8.
Effect of overexpression of P2X7 on necrotic RPTC-induced renal interstitial fibroblast death. NRK-49F were transiently transfected with the empty vector or plasmid encoding wild-types of P2X7. After 24 h, cells were treated with necrotic RPTC supernatant for 20 h and then harvested for immunoblot analysis of P2X7, PARP, cleaved caspase-3, and GAPDH (A), or cell viability was assessed by the MTT assay (B). Values are means ± SD of 3 independent experiments. Bars with different letters (a–c) are significantly different from one another (P < 0.05).
DISCUSSION
Pathological studies have shown that RPTC are frankly damaged in AKI induced by a variety of stimuli, including ischemia-reperfusion and nephrotoxins (3, 31). Necrotic death of RPTC resulted from severe kidney injury leads to the leak of cellular contents to the interstitium with back-leaked filtrate through the denuded TBM. Since renal interstitial fibroblasts are directly connected to the TBM (1), TBM damage may results in direct exposure of these cells to the high concentration of cellular contents/factors released from necrotic RPTC at the injury sites, leading to a change in their fate. To test this hypothesis, we examined the effect of necrotic RPTC on the viability of NRK-49F, normal rat kidney interstitial fibroblasts, in a coculture system. Our results demonstrated that direct exposure of NRK-49F to necrotic RPTC lysates reduces their survival, suggesting a deleterious cross talk existing between necrotic epithelial cells and renal interstitial fibroblasts.
Given the fact that most cells contain ATP in the millimolar range and dying cells can release high concentrations of ATP into the pericellular space, resulting from cell death (11), we assumed that ATP might act as a prodeath factor contributing to the reduced viability of renal interstitial fibroblasts. In support of this speculation, our data showed that degradation of ATP with apyrase largely inhibited renal fibroblast death at the dose that completely destroyed ATP present in the RPTC supernatant. There are three possibilities for incomplete blockade of death of NRK-49F by apyrase. First, other nucleotides such as ADP and AMP generated from degradation of ATP may reduce the protective effect of apyrase since these nucleotides can also induce activation of P2X7, a death-mediating purinergic receptor, although weakly (6). Second, cells with P2X7 activation may become a self-activating source of ATP due to prolonged stimulation and cytolysis, thereby releasing ATP from the fibroblast itself, which may reduce the rate of ATP breakdown. Finally, in addition to ATP, other toxic factors may also be released by necrotic RPTC and contribute to induction of renal fibroblast death. In this regard, it has been reported that some cytokines such as TNF-α and Fas synthesized in RPTC (2, 10, 24) can induce death of numerous cell types, including fibroblasts (4). Additional studies are needed to examine these possibilities.
Interestingly, we found that the cell lysate prepared from the same number of necrotic renal interstitial fibroblasts does not cause death of themselves. Considering the importance of ATP as a death inducer in necrotic RPTC, ATP must also be released from damaged renal fibroblasts. A possible explanation is that renal interstitial fibroblasts may contain a lower level of ATP. Indeed, the ATP level was lower in NRK-49F than that in RPTC (11.74 ± 0.66 vs. 6.79 ± 0.33 nM/mg protein). These results are consistent with a previous report indicating that half the amount of ATP is present in NIH-3T3 fibroblasts compared with that in tracheal epithelial cells (39). Currently, it is unclear that this is the sole factor responsible for the distinct abilities of cell lysates from necrotic RPTC and renal fibroblasts in triggering the death of renal fibroblasts or that other factors and mechanisms also contribute to this process. We will address this issue in our future studies.
Our data indicated that the P2X7 receptor mediates necrotic RPTC-induced death of renal interstitial fibroblasts. First, treatment with either suramin, a general P2 receptor antagonist (14), or A438079, a highly selective inhibitor of the P2X7 receptor, abolished necrotic RPTC-induced death of renal fibroblasts. Second, knockdown of P2X7 with siRNA improved survival of renal fibroblasts under this condition. Third and finally, overexpression of the P2X7 receptor enhanced necrotic RPTC supernatant-induced death of NRK-49F. Although P2Y1 is also expressed in renal fibroblasts in the normal kidney, pretreatment with MRS-2500, a selective P2Y1 inhibitor (19), did not interfere with the death of renal fibroblasts in response to necrotic RPTC, suggesting that this receptor does not mediate renal fibroblast death.
It is generally accepted that P2X7 is constitutively expressed on the majority of cells of the immune system, but not on most nonimmune cell types (30) under physiological conditions. Indeed, we did not detect P2X7 receptor protein in normally cultured NRK-49F. However, exposure of NRK-49F to necrotic RPTC supernatant resulted in the expression of this receptor, which occurs in a time- and dose-dependent manner, suggesting that necrotic RPTC contains the factor(s) that is able to stimulate expression of P2X7. Although it was previously reported that exogenous ATP can stimulate expression of P2X7 in macrophages (42), it is unlikely that ATP mediates P2X7 expression in renal fibroblasts exposed to the necrotic RPTC supernatant because ATP depletion with apyrase did not affect P2X7 expression (data not shown). In addition to ATP, other soluble factors such as IL-1β and TNF-α have also been reported to be able to upregulate expression of P2X7 in a variety of cell types (16, 28) and RPTC also express these cytokines (21, 33). Thus it is possible that these cytokines are responsible for induction of P2X77 receptor expression in renal fibroblasts.
The functional role of renal interstitial fibroblasts after AKI remains incompletely understood. It is generally believed that they take part in promoting renal tubular regeneration by synthesis of extracellular matrix components and repair of damaged TBM structure during the early recovery process. In addition, renal interstitial fibroblasts have an ability to synthesize EPO. EPO not only promotes production of erythrocytes but also protects renal tubular cells from further renal injury and accelerates their proliferation and repair (22, 36, 38). Given that necrotic RPTC can stimulate expression of P2X7 receptors and that P2X7 mediates the death of renal fibroblasts, inhibition of the P2X7 receptor may have therapeutic benefits in AKI. In this context, it has been reported that systemic administration of brilliant blue G, a selective P2X7 receptor antagonist, improved motor recovery after spinal cord injury (32). Recently, we also showed that administration of suramin, a general inhibitor of purinergic receptors, improves renal functional recovery and promotes renal tubular cell proliferation (44). Additional animal studies are required to assess the effect of specific inhibition of the P2X7 receptor on renal repair and regeneration.
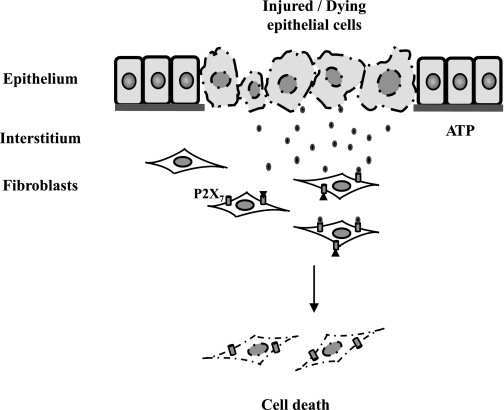
In summary, our data demonstrate for the first time that necrotic renal epithelial cells can directly induce death of renal fibroblasts with ATP as a death factor and the P2X7 receptor as a critical mediator. These findings provide an explanation, at least in part, for the reduced number of peritubular EPO-producing fibroblasts in the injured kidney after a variety of acute insults. Following acute injury, ATP from necrotic tubular cells might be released quickly into the interstitium, where it acts directly on the adjacent interstitial fibroblasts to induce their death by activating P2X7 signaling (Fig. 9). Since renal fibroblasts contribute to renal repair and EPO production, inhibition of P2X7 may promote renal structural and functional recovery after AKI. Therefore, the P2X7 receptor may be a potential target for therapeutic intervention in AKI.
Fig. 9.
Mechanism of necrotic RPTC-induced death of renal interstitial fibroblasts. Following severe acute injury, RPTC die by necrosis, releasing ATP, which acts on P2X7 expressed on renal fibroblasts and subsequently induces fibroblast cell death.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-071997.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Bachmann S, Le Hir M, Eckardt KU. Co-localization of erythropoietin mRNA, and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem, 41: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Baud L, Ardaillou R. Tumor necrosis factor in renal injury. Miner Electrolyte Metab 21: 336–341, 1995 [PubMed] [Google Scholar]
- 3. Bonegio R, Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens 11: 301–308, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Cao H, Mattison J, Zhao Y, Joki N, Grasso M, Chang NS. Regulation of tumor necrosis factor-and Fas-mediated apoptotic cell death by a novel cDNA TR2L. Biochem Biophys Res Commun 227: 266–272, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Casiano CA, Ochs Rl, Tan EM. Distinct cleavage products of nuclear proteins in apoptosis, and necrosis revealed by autoantibody probes. Cell Death Differ 5: 183–190, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Seguela P. ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci 22: 3061–3069, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coutinho-Silva R, Persechini PM, Bisaggio RD, Perfettini JL, Neto AC, Kanellopoulos JM, Motta-Ly I, Dautry-Varsat A, Ojcius DM. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol Cell Physiol 276: C1139–C1147, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302: 8–17, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Di Virgilio F. ATP as a death factor. Biofactors 8: 301–303, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Du C, Jiang J, Guan Q, Yin Z, Masterson M, Parbtani A, Zhong R, Jevnikar AM. Renal tubular epithelial cell self-injury through Fas/Fas ligand interaction promotes renal allograft injury. Am J Transplant 4: 1583–1594, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Duan S, Neary JT. P2X7 receptors: properties and relevance to CNS function. Glia 54: 738–46, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Eckardt KU. Erythropoietin production in liver and kidneys. Curr Opin Nephrol Hypertens 5: 28–34, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Estrov Z, Thall PF, Talpaz M, Estey EH, Kantarjian HM, Andreeff M, Harris D, Van Q, Walterscheid M, Kornblau SM. Caspase 2 and caspase 3 protein levels as predictors of survival in acute myelogenous leukemia. Blood 92: 3090–3097, 1998 [PubMed] [Google Scholar]
- 14. Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors). Mol Pharmacol 48: 178–183, 1995 [PubMed] [Google Scholar]
- 15. Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (P.A.R.P.-1): implication of lysosomal proteases. Cell Death Differ 8: 588–594, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Harada H, Chan CM, Loesch A, Unwin R, Burnstock G. Induction of proliferation, and apoptotic cell death via P.2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int 57: 949–958, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Hillman KA, Burnstock G, Unwin RJ. The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron Exp Nephrol 101: e24–e30, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hillman KA, Woolf AS, Johnson TM, Wade A, Unwin RJ, Winyard PJ. The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem Biophys Res Commun 322: 434–439, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Jacobson KA, Costanzi S, Joshi BV, Besada P, Shin DH, Ko H, Ivanov AA, Mamedova L. Agonists and antagonists for P2 receptors. Novartis Found Symp 276: 58–68, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jankowski V, Karadogan S, Vanholder R, Nofer JR, Herget-Rosenthal S, van der Giet M, Tolle M, Tran TN, Zidek W, Jankowski J. Paracrine stimulation of vascular smooth muscle proliferation by diadenosine polyphosphates released from proximal tubule epithelial cells. Kidney Int 71: 994–1000, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Jenkins DA, Wojtacha DR, Swan P, Fleming S, Cumming AD. Intrarenal localization of interleukin-1 beta mRNA in crescentic glomerulonephritis. Nephrol Dial Transplant 9: 1228–1233, 1994 [PubMed] [Google Scholar]
- 22. Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury, and failure. Kidney Int 69: 1806–1813, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Kaissling B, Le Hir M. The renal cortical interstitium: morphological, and functional aspects. Histochem Cell Biol 130: 247–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koide N, Narita K, Kato Y, Sugiyama T, Chakravortty D, Morikawa A, Yoshida T, Yokochi T. Expression of Fas, and Fas ligand on mouse renal tubular epithelial cells in the generalized Shwartzman reaction and its relationship to apoptosis. Infect Immun 67: 4112–4118, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J Biol Chem 277: 3210–3218, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Maxwell PH, Ferguson DJ, Nicholls LG, Johnson MH, Ratcliffe PJ. The interstitial response to renal injury: fibroblast-like cells show phenotypic changes, and have reduced potential for erythropoietin gene expression. Kidney Int 52: 715–724, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Mene P, Polci R, Festuccia F. Mechanisms of repair after kidney injury. J Nephrol 16: 186–195, 2003 [PubMed] [Google Scholar]
- 28. Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL1beta transiently enhances P2X7 receptor expression, and function in human astrocytes. Glia 49: 245–258, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donnelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR, Carroll WA. Structure-activity relationship studies on a series of novel substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem 49: 3659–3666, 2006 [DOI] [PubMed] [Google Scholar]
- 30. North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608–F627, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA 106: 12489–12493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramesh G, Brian Reeves W. Cisplatin increases TNFalpha mRNA stability in kidney proximal tubule cells. Ren Fail 28: 583–592, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brune B, Sterzel RB. Extracellular ATP causes apoptosis, and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol Renal Physiol 275: F962–F971, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Shah GM, Shah RG, Poirier GG. Different cleavage pattern for poly(ADP-ribose) polymerase during necrosis, and apoptosis in HL-60 cells. Biochem Biophys Res Commun 229: 838–844, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM. Erythropoietin protects the kidney against the injury, and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol 15: 2115–2124, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24: 337–345, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Spandou E, Tsouchnikas I, Karkavelas G, Dounousi E, Simeonidou C, Guiba-Tziampiri O, Tsakiris D. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant 21: 330–336, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Takahashi T, Matsushita K, Welsh MJ, Stokes JB. Effect of cAMP on intracellular, and extracellular ATP content of Cl−-secreting epithelia and 3T3 fibroblasts. J Biol Chem 269: 17853–17857, 1994 [PubMed] [Google Scholar]
- 40. Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, Pickering MC, Whitehouse DL, Cook HT, Burnstock G, Pusey CD, Unwin RJ, Tam FW. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 1275–1281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner CM, Elliott JI, Tam FW. P2 receptors in renal pathophysiology. Purinergic Signal 5: 513–520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, Licata G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem 9: 1240–1260, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Vonend O, Turner CM, Chan CM, Loesch A, Dell'Anna GC, Srai KS, Burnstock G, Unwin RJ. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int 66: 157–166, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Zhuang S, Lu B, Daubert RA, Chavin KD, Wang L, Schnellmann RG. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int 75: 304–311, 2009 [DOI] [PubMed] [Google Scholar]