Abstract
Plasma volume (PV) expansion is required for optimal pregnancy outcomes; however, the mechanisms responsible for sodium and water retention in pregnancy remain undefined. This study was designed to test the “arterial underfill hypothesis” of pregnancy which proposes that an enlarged vascular compartment (due to systemic vasodilation and shunting of blood to the placenta) results in renal sodium and water retention and PV expansion. We produced chronic vasodilation by 14 days administration of nifedipine (NIF; 10 mg·kg−1·day−1) or sodium nitrite (NaNO2; 70 mg·kg−1·day−1) to normal, nonpregnant female Sprague-Dawley rats. Mean arterial pressure, monitored by telemetry, was reduced by both NIF and NaNO2 but was unchanged in control rats. At day 14, vasodilator treatment lowered hematocrit and increased PV (determined by Evans blue dye dilution). Plasma osmolarity (Posm), sodium (PNa), and total protein concentrations all fell. These responses resemble the responses to normal pregnancy with hemodilution, marked PV expansion, and decreased Posm and PNa. Our previous work indicates a role of increased inner medullary phosphodiesterase-5 (PDE5) in the sodium retention of pregnancy. Here, we found that inner medullary PDE5A mRNA and protein expression were increased by both NIF and NaNO2 treatment vs. control; however, neither renal cortical nor aortic PDE5 expression was changed by vasodilator treatment. We suggest that a primary, persistent vasodilation drives increased inner medullary PDE5 expression which facilitates continual renal Na retention causing “refilling” of the vasculature and volume expansion.
Keywords: nifedipine, sodium nitrite, phosphodiesterase-5, sodium balance
normal pregnant women undergo a progressive plasma volume expansion which begins in the first trimester, peaks near gestational week 32, and remains elevated until term (5, 12, 25). The demands of fetal development require this increased circulating volume, and suboptimal plasma volume expansion is associated with complications of pregnancy and “small for gestational age” (SGA) babies (6). Plasma volume expansion also occurs during pregnancy in the rat and is the result of net renal sodium and fluid retention (2). In the rat, metabolic cage studies demonstrated a positive sodium balance predominately during late pregnancy (7). Furthermore, inhibition of volume expansion by restricting sodium intake in the rat compromises pregnancy close to term (24).
The mechanism of sodium and water retention in pregnancy remains a mystery. One hypothesis to explain this phenomenon suggests that pregnancy is an “underfill” state (30). During pregnancy, there is an early systemic vasodilation that is combined with later placental arteriovenous shunting resulting in an enlarged vascular compartment. According to the “arterial underfill” hypothesis, the renal sodium retention of pregnancy represents an effort to “refill” the vasodilated vasculature. Therefore, this study was designed to determine whether chronic vasodilation in normal virgin female rats elicits plasma volume expansion and hemodilution similar to the changes that occur during normal pregnancy.
We previously reported that the activity and abundance of the cGMP-degrading enzyme phosphodiesterase-5 (PDE5) are selectively increased in the inner medullary collecting duct (IMCD) in normal pregnancy (23). In IMCD cells isolated from pregnant rats, there is blunting of the cGMP response to both atrial natriuretic peptide (ANP) and a nitric oxide (NO) donor (sodium nitroprusside), and this is normalized by inhibition of PDE5 (23, 29). We suggest that this increased inner medullary PDE5 inhibits all cGMP-mediated natriuretic responses, contributing to the volume expansion of normal pregnancy. In this study, we therefore also determined the effect of chronic vasodilation on the expression of PDE5 in the kidney and aorta of female rats to determine whether the underfill signal is responsible for the increase in inner medullary PDE5 abundance.
METHODS
All experiments were performed using female Sprague-Dawley rats (3–5 mo old; body wt: 245 ± 8 g; n = 8; Harlan Laboratories, Dublin, OH) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the University of Florida Institutional Animal Care and Use Committee. Rats were anesthetized with isoflurane (IsoFlo; Abbott Laboratories, North Chicago, IL), and telemetry transmitters (Data Sciences, St. Paul, MN) were implanted in the left femoral artery with the transmitter housed in the abdomen and attached to the abdominal wall according to the manufacturer's specifications. Rats were allowed to recover from surgery and returned to individual housing for 7 to 10 days before initiation of data acquisition. Arterial pressure waveforms were recorded continuously for 5 min every 30 min as described (28).
After baseline measurements of blood pressure (BP) were obtained, chronic vasodilation was produced by treating the rats for 14 days with either nifedipine (NIF; calcium channel blocker, 10 mg·kg−1·day−1 via diet; Sigma) (20) or sodium nitrite (NaNO2; 70 mg·kg−1·day−1 via drinking water; Sigma) (34). All rats received a gel diet containing all required nutrients and was made by dissolving 400 g of normal rodent chow (Harlan) and 9.75 g of agar (Becton Dickenson) in 450 ml of water. All rats received water ad libitum in addition to the water in the gel diet.
On day 14, each rat was weighed, and an acute experiment was performed to determine plasma volume (PV). Rats were anesthetized with isoflurane and placed on a heated table to maintain body temperature at 37 ± 1°C. The right femoral artery and vein were cannulated with PE-50 tubing, and an arterial blood sample was collected for determination of hematocrit, plasma sodium and protein concentrations, plasma osmolarity, and a “blank” for Evans blue measurement. Then, exactly 250 μl of Evans blue solution (0.3 mg/ml; Sigma) were injected into the venous line. An additional 200 μl of isotonic saline were also injected to ensure that all Evans blue was delivered to the rat. After 5 and 10 min, 300-μl blood samples were collected for Evans blue measurement. At the end of the experiment, the aorta and kidneys were removed, and the kidneys were separated into cortex and outer and inner medulla. Tissues were snap-frozen in liquid nitrogen and stored at −80°C.
Plasma osmolarity was measured using a VAPRO Vapor Pressure Osmometer (Wescor). The concentration of Evans blue in the plasma was measured on a Tecan Safire optical system (Tecan) at 620 nm, and the PV was calculated from the quantity of dye injected: concentration of dye in plasma. Sodium and potassium concentrations were measured on a flame photometer using cesium as the internal standard (1:100 dilution of sample in 1.5 mmol/l CsCl solution; Instrumentation Laboratory). Plasma renin activity (PRA) was measured using a commercially available RIA kit (DiaSorin, Stillwater, MN).
RT-PCR reaction.
All reagents, enzymes, and isolation kits were purchased from Qiagen Sciences. Total RNA extraction and first-strand cDNA synthesis were performed as previously described (11). PCR was performed in PCR buffer, 2 mM MgCl2, 2 mM dNTPs, 1.5 U Ampli Taq DNA polymerase, and 0.5 μM different PDE5A primers: forward (F): 5′-cgc cgc tgt cgc tgg gtc tgg ag-3′; reverse (R): 5′-ggc ggc cct tgt gtc tgg tga tgg-3′ (product length: 327 bp); PDE5A1: F: 5′-acg atc act ggg act tta cct tct c-3′; R: 5′-gag tttga gca ctg gtc ccc ttt-3′ (346 bp); PDE5A2: F: 5′-atg ttg ccc ttt gga gac aaa ac-3′; R: 5′-gag tttga gca ctg gtc ccc ttt-3′ (332 bp); PDE5A3: F: 5′-tgt ggc ctc tga tac ctt cc-3′; R: 5′-tgt ggc ctc tga tac ctt cc-3′ (101 bp); PDE5A4: F: 5′-atg gcc aag caa atg gtc ac-3′; R: 5′-gag cag caa aaca tgc tgc act ga-3′ (321 bp). The amplification profile consisted of denaturation at 94°C for 15 s, annealing 58°C for 15 s, and extension 72°C for 30 s for 33 amplification cycles. PCR products were analyzed on 2.5% agarose gel stained with ethidium bromide. The complementary DNA samples were also used to generate glyceraldehyde-3-phosphate dehydrogenase (GAPDH) PCR products which were used as internal control. GAPDH forward and reverse primers were as follows: F: 5′-ggt gaa ggt cgg agt caa cg-3′; R: 5′-ctc atc gcg ctt gcc agt g-3′ (product length: 496 bp). The amplification profile was the same as by PDE5A with a modification of annealing temperature to 55°C.
Western blot analysis.
Protein abundances were detected using Western blotting as previously described (38). Briefly, samples (200 μg of kidney cortex, 100 μg of inner medulla or outer medulla, and 140 μg of aorta) were loaded on 7.5% polyacrylamide gels and separated by electrophoresis. Membranes were incubated overnight with a specific antibody for PDE5A (H-120, 1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and then incubated with a corresponding anti-rabbit IgG secondary antibody (1:8,000 dilution; Santa Cruz Biotechnology). Bands of interest were visualized using enhanced chemiluminescence reagent and quantified by densitometry (VersaDoc imaging system and Quantity One Analysis software; Bio-Rad) as integrated optical density (IOD) after subtraction of background. The IOD was factored for Ponceau red staining (Sigma) to correct for any variations in total protein loading and for an internal positive control (rat lung). The protein abundance was represented as PDE5 IOD/Ponceau red/ positive control.
Statistical analysis.
The data were analyzed on STATISTICA.6 software (StatSoft). Data are presented as means ± SE. For multiple comparisons, ANOVA was used with Dunn's multiple comparison test. Mean arterial pressure data were compared using two-way repeated-measures ANOVA with Dunn's posttest. Criterion for significance was P < 0.05 in all experiments.
RESULTS
BP.
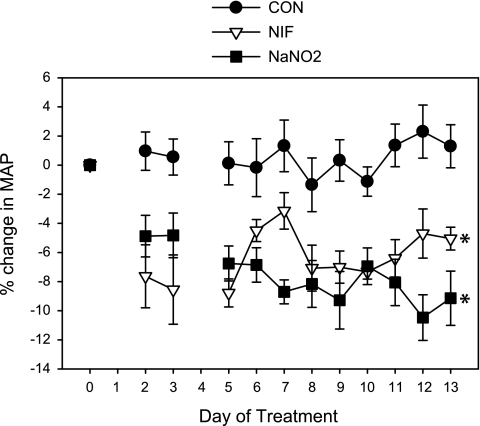
Mean arterial pressure (MAP) as measured by telemetric recording in conscious, freely moving rats was reduced by both NIF and NaNO2 (Fig. 1). Control (CON) rats maintained a constant MAP over the 2-wk treatment period. NIF and NaNO2 resulted in an early drop in MAP vs. CON by day 2 that was maintained through day 13 of treatment. The average falls in MAP over the 2-wk period were 6.7 ± 1.5% in NIF and 7.6 ± 1.8% in NaNO2-treated animals vs. baseline MAP levels.
Fig. 1.
Percent change in mean arterial pressure (MAP) as measured by telemetry in control (CON), nifedipine (NIF)-, and sodium nitrite (NaNO2)-treated female Sprague-Dawley rats (n = 7) during the 14-day treatment period. *P < 0.05 vs. CON from day 2 through day 13.
Plasma measurements.
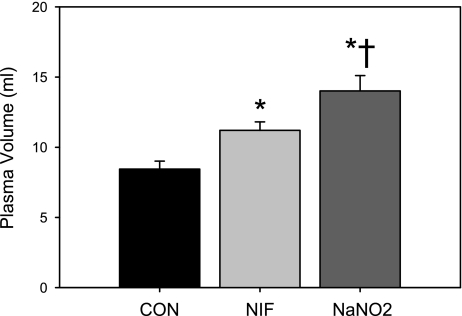
PV was increased by NIF treatment, and NaNO2 resulted in an even greater increase in PV (Fig. 2). As shown in Table 1, both vasodilator treatments lowered hematocrit (Hct), plasma protein and sodium concentration, and osmolarity. The falls in Hct and plasma sodium with NaNO2 treatment were greater than the effect observed with NIF treatment. Plasma potassium was not changed by either treatment. In a subset of CON and NaNO2-treated rats, PRA was not different after 14 days of treatment (CON = 6.1 ± 1.3, NaNO2 = 4.7 ± 0.4 ng·ml−1·h−1, P = 0.34, n = 6).
Fig. 2.
Plasma volume measured by Evans blue dilution in CON, NIF-, and NaNO2-treated female Sprague-Dawley rats after 14-day treatment (n = 6). *P < 0.05 vs. CON. †P < 0.05 vs. NIF.
Table 1.
Hematocrit and plasma protein, osmolarity, sodium, and potassium in female rats treated with vehicle, nifedipine, or sodium nitrite for 14 days
| Control | Nifedipine | Sodium Nitrite | |
|---|---|---|---|
| Hematocrit, % | 44 ± 1 | 40 ± 1* | 38 ± 1*† |
| Plasma protein, g/l | 6.6 ± 0.1 | 6.1 ± 0.02* | 6.1 ± 0.1* |
| Plasma osmolarity, mmol/l | 304 ± 2 | 297 ± 1* | 296 ± 1* |
| Plasma sodium, mmol/l | 154 ± 1 | 149 ± 1* | 143 ± 2*† |
| Plasma potassium, mmol/l | 3.9 ± 0.3 | 4.2 ± 0.2 | 3.9 ± 0.3 |
Values are means ± SE, n = 8.
P < 0.05 vs. control.
P < 0.05 vs. nifedipine.
PDE5A mRNA and protein expression.
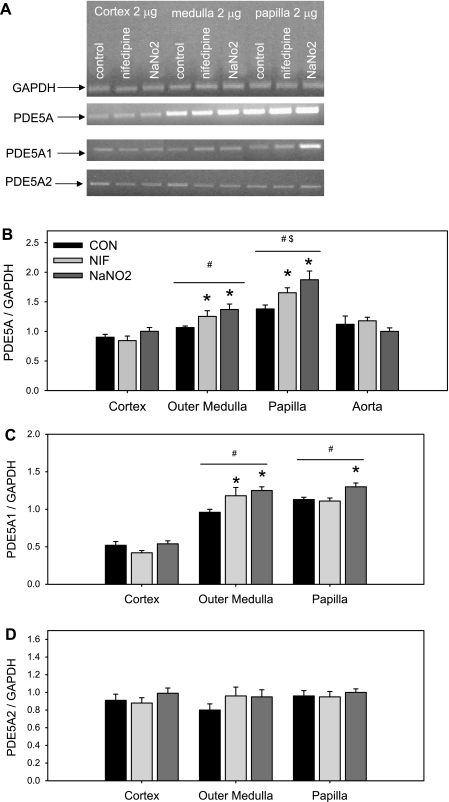
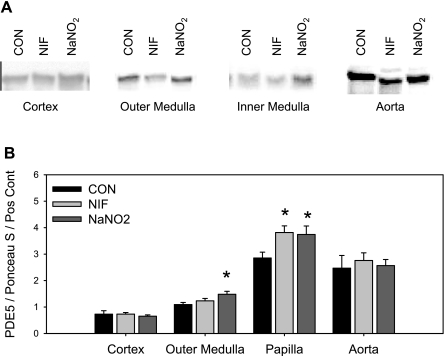
Both NIF and NaNO2 increased total PDE5A mRNA expression in the renal outer and inner medulla (Fig. 3A). No change in PDE5A mRNA expression was observed in the renal cortex or in the aorta. Furthermore, we found that PDE5A mRNA expression was less abundant in renal cortex than in the medulla and higher in the inner medulla than in the outer medulla, independent of the treatments (Fig. 3B). Using primers specific for the PDE5A1 isoform, we found a similar pattern (Fig. 3C), with the exception that NIF did not increase PDE5A1 expression in the inner medulla. PDEA2 mRNA levels did not differ among groups, and expression levels were similar in the three kidney regions (Fig. 3D). We did not detect the transcript for either PDE5A3 or PDE5A4 isoforms in any of the three regions of the kidney. Protein abundance of PDE5A followed the expression of PDE5A mRNA levels in that PDE5A protein levels were increased by both NIF and NaNO2 in the renal inner medulla (Fig. 4). NaNO2, but not NIF, treatment also increased renal outer medullary PDE5A abundance. Neither renal cortical nor aortic PDE5A protein abundance was changed by vasodilation.
Fig. 3.
Representative gels (A) and phosphodiesterase-5 (PDE5A; B), PDE5A1 (C), and PDE5A2 (D) mRNA expression in kidney cortex, outer medulla, and inner medulla of CON, NIF-, and NaNO2-treated female Sprague-Dawley rats after 14-day treatment (n = 7). *P < 0.05 vs. CON. #P < 0.05 vs. respective cortex. $P < 0.05 vs. respective outer medulla. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Fig. 4.
PDE5A protein level in kidney cortex, outer and inner medulla, and aorta of CON, NIF-, and NaNO2-treated female Sprague-Dawley rats after 14-day treatment (n = 7). Pos Cont, positive control (rat lung homogenate). *P < 0.05 vs. CON.
DISCUSSION
The main findings of this study are that chronic vasodilation in normal female rats, achieved with two distinct vasodilators, results in PV expansion, hemodilution, and falls in plasma osmolarity and sodium concentration. These changes closely resemble the responses in normal pregnancy. We also found that these changes are accompanied by an increase in renal medullary PDE5 mRNA and protein expression, similar to the changes in PDE5 protein abundance we previously observed in normal pregnant rats (23). Therefore, these data suggest that a primary underfill signal causes an increase in renal inner medullary PDE5, leading to loss of tubular natriuretic responses. It is remarkable that the kidney adapts during chronic vasodilation to diminish natriuretic signaling, thereby protecting the animal from hypotension. This facilitates continued renal sodium retention and volume expansion to “refill” the vasculature and may reflect the mechanism of PV expansion during normal pregnancy.
During normal pregnancy in both women and rats, there is a significant PV expansion due to net renal sodium retention (2, 6, 12, 25), and failure of volume expansion is associated with pregnancy complications and SGA babies (6, 12). In rats, sodium restriction during the last week of pregnancy results in reduced maternal PV and growth-restricted pups (24, 27). While we know that PV expansion is required for optimal pregnancy outcomes, the mechanisms responsible for these changes in maternal volume homeostasis during pregnancy remain unknown.
One potential explanation for the renal sodium retention and PV expansion of pregnancy was suggested by Schrier (30). He posits that the renal sodium retention of normal pregnancy results from a primary “underfill” signal, driven first by peripheral vasodilation and later by opening of the uteroplacental “shunt”. It is notable that despite the marked increase in PV during normal pregnancy, BP decreases significantly during pregnancy in women and late in gestation in rats due to a major reduction of peripheral vascular resistance (2, 5, 13, 33). In fact, a fall in peripheral resistance and PV expansion is seen as early as 6 wk after conception in normal women (5). Unfortunately, there have been no studies to date which clarify the sequence of these events earlier in pregnancy. The present study gives some insight; however, since we find that a primary, persistent widespread peripheral vasodilation causes a PV expansion. The specific vasodilatory signal is apparently unimportant since we observe PV expansion secondary to both calcium channel blockade and NO-dependent vasodilation. In addition to cumulative PV expansion due to renal sodium retention during pregnancy, there is also an excess of water retention, leading to falls in plasma osmolarity and complex resetting of both osmotic and nonosmotic AVP release (15, 32). We also observed falls in plasma osmolarity in the present study, suggesting that both the sodium and water retention of normal pregnancy are driven by primary, persistent vasodilation. In this study, we did not observe an increase in PRA after 2-wk treatment with NaNO2, possibly because the vasculature was somewhat “refilled” after 14 days of chronic vasodilation, and therefore the initial signaling mechanisms could no longer be detected. However, our findings do not exclude a continuing role of the renin-angiotensin-aldosterone system (RAAS) in the sodium retention secondary to vasodilation. It is possible that chronic vasodilation induces changes in systemic or local angiotensin concentrations independent of changes in PRA, and there could also be changes in the sensitivity of the angiotensin II receptors in the kidney. The sodium transporters that are targets of the RAAS may also undergo adaptive changes to facilitate continued sodium retention, although these possibilities remain to be explored.
The cause of the systemic vasodilation of pregnancy remains obscure and endothelial factors, angiotensin 1–7, VEGF, and relaxin have all been implicated (19, 35). However, the renal vasodilation in the pregnant rat is certainly due to increased renal NO production (3, 4, 8–10). This makes the renal sodium retention of pregnancy particularly difficult to explain, given the potent natriuretic action of NO in nonpregnant animals (1, 26).
Our previous studies in normal pregnancy suggest a mechanism that allows the kidney cortex to vasodilate to NO while becoming unresponsive to the tubular actions in medulla. Specifically, we observed an increase in inner medullary PDE5 protein abundance and activity in normal pregnancy with no change in the renal cortex (23). Since NO (and ANP) signal through cGMP, this allows a cGMP-dependent renal vasodilation to persist while specifically inhibiting the cGMP-dependent natriuresis. This is consistent with our previous observations that the pregnant rat is refractory to the natriuretic actions of ANP (17) and exhibits a blunted acute pressure natriuresis (which is mediated by NO) (16, 18). We also reported that intrarenal PDE5 inhibition will restore the natriuretic effect of ANP and NO in pregnant rats (14, 29), further supporting a role for increased medullary PDE5 in the sodium retention of pregnancy. Overall, this selective blunting of the normal cGMP-dependent natriuretic influences, together with an underfill-activated renin, angiotensin, aldosterone system (30, 32) will allow the sodium-retaining influences to predominate and cumulative PV expansion to occur. In this study, we found that chronic treatment with either NIF or NaNO2 increased PDE5 expression. Because these two agents have distinct mechanisms of action, it is likely that the change in PDE5 expression is secondary to the chronic vasodilation rather than a specific effect of either agent. Future studies are needed to investigate the pathways involved in this upregulation of PDE5A1 and how this is related to the initial underfill signal that occurs as a result of the systemic vasodilation during both pregnancy and other states of arterial underfill.
In addition to increasing our understanding of the sodium and water retention of pregnancy, this work also has important implications in edematous disorders such as cirrhosis, heart failure, and nephrotic syndrome. These patients are also thought to have a decreased effective blood volume, and therefore the pathological sodium and water retention in these conditions could also be secondary to arterial underfill (31). Animal models of cirrhosis and nephrotic syndrome also exhibit an increase in inner medullary PDE5, similar to what is seen in normal pregnancy (21, 22, 36, 37). Therefore, we propose that in both healthy pregnancy and in disease states, systemic arterial vasodilation stimulates inner medullary PDE5 expression and promotes sodium and water retention to allow PV expansion and “refilling” of the arterial vasculature.
GRANTS
This work was supported by the University of Florida Multidisciplinary Training Program in Hypertension (T32 HL083810) to J. M. Sasser and National Institutes of Health Grants R01-HD-041571 and R01-DK-56843 to C. Baylis. A. Fekete is a recipient of Bolyai-Kelly and Magyary scholarships.
DISCLOSURES
Portions of this work have appeared in abstract form (FASEB J and J Am Soc Nephrol 2009).
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank B. Cunningham for expert technical assistance.
REFERENCES
- 1. Alberola A, Pinilla JM, Quesada T, Romero JC, Salom MG, Salazar FJ. Role of nitric oxide in mediating renal response to volume expansion. Hypertension 19: 780–784, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Baylis C. Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin Obstet Gynaecol 8: 235–264, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Baylis C, Engels K. Adverse interactions between pregnancy and a new model of systemic hypertension produced by chronic blockade of EDRF in the rat. Clin Exp Hypertens B11: 117–129, 1992 [Google Scholar]
- 4. Cadnapaphornchai MA, Ohara M, Morris KG, Jr, Knotek M, Rogachev B, Ladtkow T, Carter EP, Schrier RW. Chronic NOS inhibition reverses systemic vasodilation and glomerular hyperfiltration in pregnancy. Am J Physiol Renal Physiol 280: F592–F598, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54: 2056–2063, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Chesley LC, Lindheimer MD. Renal hemodynamics and intravascular volume in normal and hypertensive pregnancy. In: Hypertension: Hypertension in Pregnancy, edited by Rubin PC. Amsterdam: Elsevier, 1988, p. 38 [Google Scholar]
- 7. Churchill SE, Bengele HH, Alexander EA. Sodium balance during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 239: R143–R148, 1980 [DOI] [PubMed] [Google Scholar]
- 8. Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Chavez JE, Mosher MD. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J 7: 566–571, 1993 [PubMed] [Google Scholar]
- 9. Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest 96: 482–490, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deng A, Engels K, Baylis C. Increased nitric oxide production plays a critical role in the maternal blood pressure and glomerular hemodynamic adaptations to pregnancy in the rat. Kidney Int 50: 1132–1138, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Fekete A, Vannay A, Vér A, Vásárhelyi B, Müller V, Ouyang N, Reusz G, Tulassay T, Szabó AJ. Sex differences in the alterations of Na+, K+-ATPase following ischaemia-reperfusion injury in the rat kidney. J Physiol 555: 471–480, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallery EDM, Hunyor SN, Gyory AZ. Plasma volume concentration: a significant factor in both pregnancy-associated hypertension (preeclampsia) and chronic hypertension in pregnancy. Q J Med 48: 593–602, 1979 [PubMed] [Google Scholar]
- 13. Gilson GJ, Mosher MD, Conrad KP. Systemic hemodynamics and oxygen transport during pregnancy in chronically instrumented, conscious rats. Am J Physiol Heart Circ Physiol 263: H1911–H1918, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Knight S, Snellen H, Humphreys M, Baylis C. Increased renal phosphodiesterase-5 activity mediates the blunted natriuretic response to ANP in the pregnant rat. Am J Physiol Renal Physiol 292: F655–F659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindheimer MD, Barron WM, Davison JM. Osmoregulation of thirst and vasopressin release in pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 257: F159–F169, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol Renal Fluid Electrolyte Physiol 264: F79–F87, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Masilamani S, Castro L, Baylis C. Pregnant rats are refractory to the natriuretic actions of atrial natriuretic peptide. Am J Physiol Regul Integr Comp Physiol 267: R1611–R1616, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Masilamani S, Hobbs GR, Baylis C. The acute pressure natriuresis response is blunted and the blood pressure response reset in the normal pregnant rat. Am J Obstet Gynecol 179: 486–491, 1998 [DOI] [PubMed] [Google Scholar]
- 19. McGuane JT, Debrah JE, Debrah DO, Rubin JP, Segal M, Shroff SG, Conrad KP. Role of relaxin in maternal systemic and renal vascular adaptations during gestation. Ann NY Acad Sci 1160: 304–312, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Morgan PE, Aiello EA, ChiappedeCingolani GE, Mattiazzi AR, Cingolani HE. Chronic administration of nifedipine induces upregulation of functional calcium channels in rat myocardium. J Mol Cell Cardiol 31: 1873–1883, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Ni XP, Cheng Y, Cao L, Gardner DG, Humphreys MH. Mechanisms contributing to renal resistance to atrial natriuretic peptide in rats with common bile duct ligation. J Am Soc Nephrol 7: 2110–2118, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Ni XP, Safai M, Gardner DG, Humphreys MH. Increased cGMP phosphodiesterase activity mediates renal resistance to ANP in rats with bile duct ligation. Kidney Int 59: 1264–1273, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Ni XP, Safai M, Rishi R, Baylis C, Humphreys MH. Increased activity of cGMP-specific phosphodiesterase (PDE5) contributes to resistance to atrial natriuretic peptide natriuresis in the pregnant rat. J Am Soc Nephrol 15: 1254–1260, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pike RL. Sodium requirement of the rat during pregnancy. In: Hypertension in Pregnancy, edited by Linheimer MD, Katz AI, Zuspan FP. New York: Wiley and Sons, 1976, p. 207–215 [PubMed] [Google Scholar]
- 25. Pirani BB, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. J Obstet Gynaecol Br Commonw 80: 884–887, 1973 [DOI] [PubMed] [Google Scholar]
- 26. Romero JC, Lahera V, Salom MG, Biondi ML. Role of the endothelium-dependent relaxing factor nitric oxide on renal function. J Am Soc Nephrol 2: 1371–1387, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Roy-Clavel E, Picard S, St-Louis J, Brochu M. Induction of intrauterine growth restriction with low-sodium diet fed to pregnant rats. Am J Obstet Gynecol 180: 608–613, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Sasser JM, Baylis C. Effects of sildenafil on maternal hemodynamics and fetal growth in normal rat pregnancy. Am J Physiol Regul Integr Comp Physiol 298: R433–R438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sasser JM, Ni XP, Humphreys MH, Baylis C. Increased renal phosphodiesterase-5 activity mediates the blunted natriuretic response to a nitric oxide donor in the pregnant rat. Am J Physiol Renal Physiol 299: F810–F814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schrier RW. Body fluid volume regulation in health and disease: a unifying hypothesis. Ann Intern Med 113: 155–159, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Schrier RW. Decreased effective blood volume in edematous disorders: what does this mean? J Am Soc Nephrol 18: 2028–2031, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Schrier RW, Ohara M. Dilemmas in human and rat pregnancy: proposed mechanisms relating to arterial vasodilation. J Neuroendocrinol 22: 400–406, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Slangen BF, Out IC, Verkeste CM, Peeters LL. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am J Physiol Heart Circ Physiol 270: H1779–H1784, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Tsuchiya K, Kanematsu Y, Yoshizumi M, Ohnishi H, Kirima K, Izawa Y, Shikishima M, Ishida T, Kondo S, Kagami S, Takiguchi Y, Tamaki T. Nitrite is an alternative source of NO in vivo. Am J Physiol Heart Circ Physiol 288: H2163–H2170, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol 7: 79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valentin JP, Qiu C, Muldowney WP, Ying WZ, Gardner DG, Humphreys MH. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest 90: 1302–1312, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valentin JP, Ying WZ, Sechi LA, Ling KT, Qiu C, Couser WG, Humphreys MH. Phosphodiesterase inhibitors correct resistance to natriuretic peptides in rats with Heymann nephritis. J Am Soc Nephrol 7: 582–593, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol Renal Physiol 280: F996–F1000, 2001 [DOI] [PubMed] [Google Scholar]