Abstract
“Humanized” mice are a promising translational model for studying human hematopoiesis and immunity. Their utility has been enhanced by the development of new stocks of immunodeficient hosts, most notably mouse strains such as NOD-scid IL2rγ null mice that lack the IL-2 receptor common gamma chain. These stocks of mice lack adaptive immune function, display multiple defects in innate immunity, and support heightened levels of human hematolymphoid engraftment. Humanized mice can support studies in many areas of immunology, including autoimmunity, transplantation, infectious diseases, and cancer. These models are particularly valuable in experimentation where there is no appropriate small animal model of the human disease, as in the case of certain viral infections. This unit details the creation of humanized mice by engraftment of immunodeficient mice with hematopoietic stem cells or peripheral blood mononuclear cells, provides methods for evaluating engraftment, and discusses considerations for choosing the appropriate model system to meet specific goals.
Keywords: humanize mice, NOD-SCID IL2rγ null, hu-SRC-SCID, hu-PBL-SCID, stem cell transplantation
INTRODUCTION
“Humanized” mouse models of immunity refer to normal, immunocompetent mice expressing human genes via transgenesis (e.g., HLA or human immunoglobulin transgenic mice) or to immunodeficient mice engrafted with human hematopoietic and lymphoid cells or tissues. The protocols presented in this unit describe approaches for generating the latter type of humanized mice. Two basic protocols describe generating humanized mice: Basic Protocol 1 deals with hematopoietic stem cell (HSC) engraftment (human SCID repopulating cell; hu-SRC) and Basic Protocol 2 addresses engraftment with human peripheral blood mononuclear cells (PBMC) (human peripheral blood leukocyte; hu-PBL). Additionally, Alternate Protocols 1 and 2 respectively describe procedures for creating hu-SRC and hu-PBL severe combined immunodeficient (SCID) mice that differ from Basic Protocols 1 and 2 in timing and/or route of engraftment with human cells. Finally the Support Protocol outlines the steps needed to verify engraftment levels in humanized mice. These methods are complimentary, each with its individual strengths and limitations, and can be used to address different experimental questions. Creation of another type of humanized mouse, the SCID-hu mouse, a model where human fetal thymus and liver tissues are transplanted into the renal subcapsular space of immunodeficient mice, is detailed in UNIT 4.8.
The main advantage of the HSC engraftment model (hu-SRC-SCID) is that the human T and B cells develop from human stem cells engrafted in the mouse, undergo negative selection during differentiation into T and B cells, and are therefore tolerant of the mouse host. This model allows for investigation of hematopoietic lineage development and mechanisms of immune system development and the generation of primary immune responses by a naïve immune system.
The PBMC model (hu-PBL-SCID) utilizes leukocytes isolated from peripheral whole blood or spleen and allows for rapid analysis of human immune function because the transferred lymphocytes are functionally mature. This model is best suited for studies of immune function from patients with immunologic disorders, analyses of antigen recall responses, investigations of allograft rejection, and other short-term (~4-week) experiments. The sources and availability of HSC and PBMC, methods of engraftment, and evaluation of reconstitution are considered in this unit.
NOTE: Human tissues, mice engrafted with human tissues, as well as the mouse bedding and caging from humanized mice, should be considered potential biological hazards and handled with proper personal protective equipment at animal biosafety level 2 (ABSL2), in accordance with governmental and institutional biosafety guidelines.
NOTE: Immunodeficient mice should be housed in a specific pathogen free (SPF) environment, using sterile techniques and microisolator caging. See UNIT 1.2 for details on care of immunodeficient mice.
STRATEGIC PLANNING
There are several factors to address before commencing with the generation of humanized mice. Outlined below are several key areas to consider. Additional details regarding vendors for immunodeficient mice and mechanisms for obtaining human tissues are provided in the Internet Resources section at the end of this chapter.
Immunodeficient Mouse Strains
Several reports have now been published using BALB/c and NOD strain immunodeficient mice that lack the IL-2r common γ chain. While there is no data in the literature directly comparing these strains (NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ, NOD.Cg-PrkdcscidIl2rgtm1Sug, C.129(Cg)-Rag2tm1FwaIl2rgtm1Sug, and (H2d)-Rag2tm1Fwa Il2rgtm1Krf), published reports appear to indicate similar levels of engraftment and immune function after reconstitution with HSC (Gimeno et al., 2004; Traggiai et al., 2004; Ishikawa et al., 2005; Shultz et al., 2005). In addition, (C57BL/6J × C57BL/10SgSnAi)-[KO]γc-[KO]Rag2 are also commercially available, but there have been no published reports of their successful use as recipients of human HSC or PBMC.
As of this writing, for immunodeficient IL2rγ null mice that have been used as recipients of human cells and tissues, only NOD/Lt-scid IL2rγ null mice are widely available from a mouse repository. For investigators desiring to use BALB/c-Rag2 null IL2rγ null mice, there are three approaches for obtaining this strain: (1) Breeding stock can be requested from Dr. M. Manz (see Table 15.21.1). (2) Investigators can generate this strain by crossing the commercially available C.129S6(B6)-Rag2tm1Fwe with C.129S4-Il2rgtm1Wjl/J (BALB/c-IL2rγ null cryopreserved embryos. (3) Another approach would be using the C.129S7(B6)-Rag1tm1Mom/J strain in place of the BALB/c-Rag2 null strain (see Internet Resources). Many previous strains of immunodeficient hosts, particularly those based on the NOD/Lt-scid or NOD/Lt-Rag1 null strains, are available from The Jackson Laboratory, and these mice can also be readily obtained. See Table 15.21.1 for more information.
Table 15.21.1.
Immunodeficient Strains of Mice for Human Engraftment Studies
| Strain | Alternate designation | Sourcea | Reference |
|---|---|---|---|
| NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ | NOD/Lt-scid IL2rγ null | The Jackson Laboratory (005557) | Shultz et al., 2005 |
| NOD.Cg-PrkdcscidIl2rgtm1Sug | NOD/Shi-scid IL2rγ null | Dr. M. Ito, Central Institute for Experimental Animals, Kawasaki, Japan | Ito et al., 2002 |
| C.129(Cg)-Rag2tm1FwaIl2rgtm1Sug | BALB/c-Rag2nullIL2rγ null | Dr. M. Manz, Institute for Research in Biomedicine, Bellinzona, Switzerland | Traggiai et al., 2004 |
| C.129(Cg)-Rag2tm1FwaIl2rgtm1Sug progeny from C.129S6(B6)-Rag2tm1Fwe mated with C.129S4-Il2rgtm1Wjl/J | BALB/c-Rag2null IL2rγnull progeny from BALB/c-Rag2null mated with BALB/c-IL2rγ null | Mating stocks: Taconic Farms (000601), The Jackson Laboratory (cryopreserved embryos, 003169) | |
| C.129S7(B6)-Rag1tm1Mom/J | BALB/c-IL2rγ null | The Jackson Laboratory (003145) | |
| (H2d)-Rag2tm1Fwa Il2rgtm1Krf | H2d Rag2null Il2rγ null | Dr. B. Biom, Academic Medical Center, University of Amsterdam, The Netherlands | Gimeno et al., 2004 |
| (C57BL/6J × C57BL/10SgSnAi)-[KO]γc-[KO]Rag2 | C57BL/6 Rag2null IL2rγnull | Taconic Farms (004111-MT-M) | |
| Additional immunodeficient strains based on the NOD/Lt-PrkdcSCID or NOD/Lt-Rag1 null strains | The Jackson Laboratory | Shultz et al., 2007 |
See Internet Resources for commercial suppliers.
HSC and PBMC Sources
Hematopoietic stem cells for use in generating humanized mice can be obtained from several different tissues, including umbilical cord blood (UCB; Ito et al., 2002; Traggiai et al., 2004; Ishikawa et al., 2005), bone marrow (Holyoake et al., 1999) G-CSF-mobilized peripheral blood (Shultz et al., 2005), and fetal liver (Holyoake et al., 1999). Human HSC isolated from UCB and mobilized stem cells have been used to generate a human immune system in BALB/c-Rag2 null IL2rγ null, NOD/Lt-scid IL2rγ null, and NOD/Shi-scid IL2r null mice. While a direct comparison of these stem cell sources has not yet been reported, all appear capable of generating a human immune system following human HSC engraftment.
Obtaining human HSC for research purposes requires appropriate Institutional Review Board (IRB) approval. UCB is an abundant tissue source for HSC and is easiest to obtain for most investigators, as most umbilical cords are considered by IRBs as discarded tissue that does not require informed consent. Chapter 22 provides methods of tissue sample collection and HSC enrichment and isolation. Bone marrow aspirates and G-CSF mobilized peripheral blood samples are most often obtained from volunteers following informed consent, and they require controlled harvesting procedures necessitating coordination with a clinical trials unit or hematology department at a medical center. Finally, human tissue banks such as the NDRI (see Internet Resources) provide a nonprofit fee-for-service resource for obtaining human tissues and HSC.
Obtaining PBMC samples for engraftment into immunodeficient mice is relatively straightforward. With appropriate IRB approval, volunteers (following informed consent) can be enrolled to donate peripheral whole blood, which can be collected via standard venipuncture techniques by a trained phlebotomist (see APPENDIX 3F and UNIT 7.1). Multiple individuals can be enrolled, minimizing the need to perform repeated draws on the same donor. Approximately 150 ml of whole blood collected in a single draw will provide enough PBMC to engraft into 10 to 15 immunodeficient NOD/Lt-scid IL2rγ null mice using a dose of 20 × 106 cells/mouse. Each recipient should receive PBMC from an individual donor.
HEMATOPOIETIC STEM CELL ENGRAFTMENT OF ADULT IMMUNODEFICIENT MICE
This protocol describes the generation of HSC-engrafted immunodeficient mice using a simple method: the intravenous injection of T cell–depleted UCB containing at least 3 × 104 CD34+ hematopoietic stem cells into irradiated adult immunodeficient mice. The mouse strain used for all basic protocols in this unit is the NOD/Lt-scid IL2rγ null strain. HSC engraftment of newborn mice is described in Alternate Protocol 1.
Materials
NOD/Lt-scid IL2rγ null mice: males or females, 5 to 12 weeks old (official strain designation, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; The Jackson Laboratory, 005557; see Internet Resources), housed in microisolator cages
Phosphate-buffered saline (PBS; APPENDIX 2A)
137Cs gamma irradiator and autoclaved, filtered, ventilated device for housing mice while in irradiator chamber (design varies, depending on model of irradiator used)
Laminar flow biocontainment hood
1-cc tuberculin syringes with 25-G × ⅝-in. needle
Additional reagents and equipment for preparing CD3 T cell–depleted HSC (Chapter 22) and restraining and injecting mice (UNITS 1.3 and 1.6)
-
Place 5- to 12-week-old NOD-scid IL2rγ null mice into an autoclaved, filtered, ventilated housing device for containment during irradiation.
Mouse containment devices will often vary based on model/manufacturer of the irradiator and may need to be specifically manufactured by an institutional machine shop.
The transfer of mice from the microisolator cage to the filtered sterile container should be done in a laminar flow biocontainment hood to minimize exposure of the immunodeficient mice to environmental infectious agents.
-
Irradiate mice with 240 cGy whole body gamma irradiation (most commonly performed by exposure to a 137Cs radioactive source).
Depending on the age and attenuation of the 137Cs source, rates of irradiation can vary from 50 to 250 cGy/min, but the rate of delivery of irradiation does not appear to alter the ability of human HSC to engraft.
Return the mice to their microisolator cages, again using a biocontainment hood for transfer and wait for 4 but not more than 24 hr before injecting the human HSC.
-
Prepare CD3+ T cell–depleted UBC (see Chapter 22), enumerate, suspend in PBS at 3 × 104 CD34+ cells/0.5 ml, and maintain at 4°C. Load a 1-cc tuberculin syringe with 25-G × ⅝-in. with 0.5 ml HSC for injection.
The 3 × 104 CD34+ cells translate to an inoculum that will contain 3–5 × 106 total nucleated cells in 0.5 ml.
Fresly prepared or previously frozen preparations may be used.
Inject 0.5 ml T cell–depleted HSC containing 3 × 104 CD34+ cells into the lateral tail vein of irradiated mice (see UNIT 1.6).
-
Allow human HSC to engraft in mice for a minimum of 10 to 12 weeks.
Numbers of human T and B cells will increase over an additional 4 to 8 weeks.
-
Perform flow cytometric analysis on peripheral blood of recipient mice (Support Protocol) for evaluation of engraftment levels 10 to 12 weeks after human HSC injection.
Mice can be monitored for engraftment of human HSC over the 10- to 12-week period by flow cytometric analysis for detection of human CD45+ cells in the mouse peripheral blood (see Chapter 5 and Support Protocol). Human CD45+ cells can routinely be detected in the peripheral blood as early as 4 weeks after human HSC injection.
Use mice with appropriate levels of engraftment in experiments. Appropriate levels of engraftment will depend on the specific experimental design and the needs of the individual investigator. See Commentary.
PERIPHERAL BLOOD MONONUCLEAR CELL ENGRAFTMENT OF ADULT IMMUNODEFICIENT MICE
This protocol describes engraftment of NOD/Lt-scid IL2rγ null mice with PBMC via the intravenous route. Previous generations of immunodeficient host strains did not support human PBMC engraftment following intravenous injection but were readily engrafted following intraperitoneal injection of human PBMC. The ability of NOD/Lt-scid IL2rγ null mice to support PBMC engraftment via intravenous injection allows the injection of PBMC directly into the host’s circulation as opposed to requiring migration of the engrafted PBMC from the peritoneal cavity to the circulation. Alternate Protocol 2 describes an additional method for delivering PBMC directly into the spleen to achieve PBMC engraftment in the circulation of NOD/Lt-scid IL2rγ null and other immunodeficient strains of mice.
Materials
Ficoll-isolated human PBMC: 20 × 106 cells per recipient mouse; freshly prepared from whole blood or single cell suspensions of splenocytes (see APPENDIX 3F and UNIT 7.1)
Phosphate-buffered saline (PBS; APPENDIX 2A), sterile
NOD/Lt-scid IL2rγ null mice: males or females, 5 to 12 weeks old (official strain designation, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; The Jackson Laboratory, 005557; see Internet Resources), housed in microisolater cages
1-cc tuberculin syringes with 25-G × ⅝-in. needles
Additional reagents and equipment for isolating and enumerating PBMC (UNIT 7.1) and restraining (UNIT 1.3) and injecting (UNIT 1.6) mice
Enumerate PBMC and suspend the cells at 20 × 106/0.5 ml in sterile PBS.
Load 1-cc tuberculin syringes with 25-G × ⅝-in. needles with 0.5 ml cell suspension.
-
Inject 0.5 ml PBMC (i.e., 20 × 106 cells) into the lateral tail vein of recipient mice.
PBMC-engrafted mice can be used immediately in experiments.
Most experiments should reach an end point within 4 weeks after engraftment or at the onset of xenogeneic graft-versus-host disease (GVHD) symptoms that can be monitored by weight loss.
Weight loss of > 15% of original starting weight is a sign of GVHD development and necessitates euthanasia of the PBMC-engrafted mice.
HEMATOPOIETIC STEM CELL ENGRAFTMENT OF NEWBORN IMMUNODEFICIENT MICE
HSC engraftment of newborn mice is commonly performed and requires only modest increases in time, resources, and technical proficiency. The protocol can use one of a number of different engraftment routes that include intrahepatic, intravenous via the facial vein, intravenous via the intracardiac route, and intraperitoneal injections. This protocol will describe two of the most technically simple newborn engraftment routes that lead to robust human HSC engraftment: intrahepatic and intracardiac injection.
Materials
NOD/Lt-scid IL2rγ null breeder pairs: as many as are required to produce sufficient newborn mice for experimental needs (official strain designation, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; The Jackson Laboratory, 005557; see Internet Resources)
Phosphate-buffered saline (PBS), sterile
Topical petrolatum-based nasal decongestant (e.g., Vicks Vaporub)
6-well tissue culture plate
100-mm2 petri dishes (~1 dish per litter to be injected)
137Cs gamma irradiator and autoclaved, filtered, ventilated device for housing mice while in irradiator chamber (design varies, depending on model of irradiator used)
Ice bucket with ice
Disposable weigh boats or sterile gauze
1-cc tuberculin syringes (BD)
27-G × ½-in. winged infusion set with 8-in. tubing (Terumo, SV*27EL) Heating pad or warming lamp
Additional reagents and equipment for preparing CD34+ T cell–depleted umbilical cord blood (UCB) hematopoietic stem cells (see Chapter 22)
-
Monitor breeder pairs for the birth of new litters.
Engraftment procedures should be performed on newborn pups 24 to 48 hr post-natal.
The presence of milk in the stomach, seen as a white spot in the abdomen (see Fig. 15.21.2B), indicates that the pups have nursed and are old enough to inject.
Timed matings can be set up and provide a more predictable window in which to expect litters (see Commentary).
-
Prepare T cell–depleted UCB stem cells (see Chapter 22), enumerate, and suspend in PBS at 6 × 105 CD34+ cells/ml.
Freshly prepared or previously frozen preparations may be used.
-
Dispense the cell suspension (3 × 104 CD34+ cells) into the well(s) of a 6-well tissue culture plate.
If the total volume is <3 ml, only one well need be used.
Other vessels for holding the cells may be used, but tissue culture plates are convenient because the needle can easily reach the bottom of the wells.
-
Place 24- to 48-hr post-natal pups from a single litter into a 100-mm2 petri dish along with a small amount of bedding material from the breeder cage (see Fig. 15.21.1).
This is done to help minimize foreign odors being imparted onto the pups and to improve the chance that the parents will accept their pups back into the cage after the procedure.
Additionally, all investigators handling pups should rub clean bedding between their hands to further mask any foreign odors on recipient pups.
Irradiate pups with 100 cGy whole body irradiation (WBI) by exposure to a 137Cs source.
-
Place irradiated pups on a weigh boat or on a gauze pad on ice for 5 to 10 min, until anesthetized.
Be sure that the pups are not placed directly onto the ice. Sufficient anesthesia is achieved when gross movement ceases.
-
Attach a 27-G × ½-in. winged infusion kit to the end of a 1-cc tuberculin syringe. Load the syringe with the UCB cell suspension.
Approximately 300 μl of cell suspension is required to load the tubing. Therefore, leaving the air space in the syringe barrel is acceptable so as to avoid wasting large quantities of cells.
Dispensing volumes of 50 μl can be accomplished reasonable accurately with a 1-cc syringe; however, accuracy is compromised with volumes of <50 μl.
Figure 15.21.2.
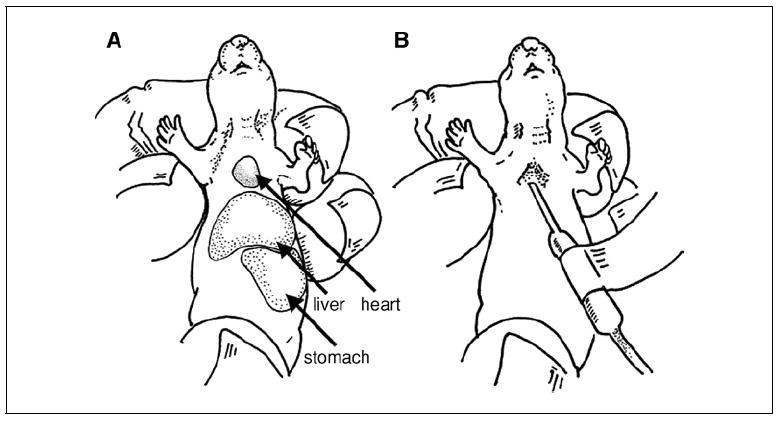
(A) Close up of a newborn NOD/Lt-scid IL2rγnull pup, showing restraint technique for intracardiac injection. The stomach, filled with milk, is clearly visible as a white area. (B) Demonstration of needle placement for intracardiac injection into a newborn NOD/Lt-scid IL2rγnull pup. The appearance of a “flash” of blood in the IV tubing provides confirmation of proper placement for delivery of HSC into the circulation.
Figure 15.21.1.
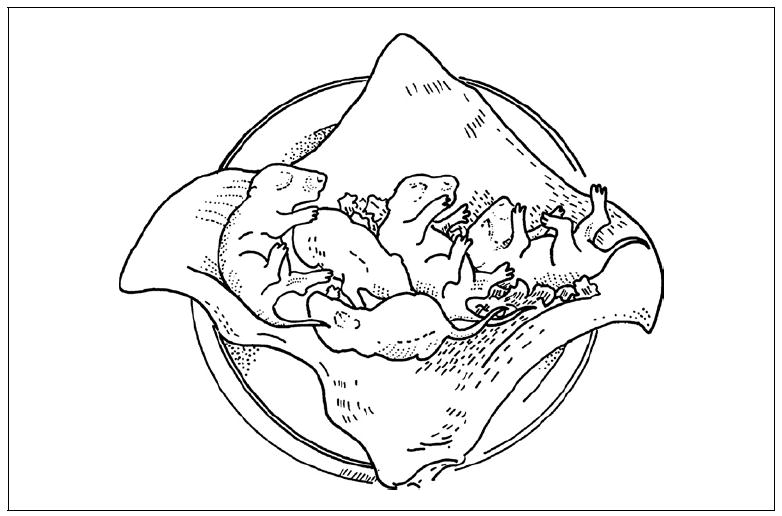
Newborn NOD/Lt-scid IL2rγnull pups placed on a gauze pad in a petri dish for irradiation and subsequent injection.
-
8a
For engraftment via intracardiac injection: Upon sufficient anesthesia, immediately inoculate each pup with 3 × 104 CD34+ cells in a 50-μl volume. (see Fig. 15.21.2). Visualize the apex of the heart and insert the needle slightly superior to the apex, in a cephalad/superior direction.
It is most efficient for one investigator to hold the pup and a second to administer the injections.
If performing the injections without a second investigator to restrain the pups, a restraint similar to that described in UNIT 1.10 for newborn thymectomy can be employed.
A “flash” of blood should appear where the catheter tubing meets the base of the needle (see Fig. 15.21.2 B). If the flash does not spontaneously appear, pulling back slightly on the syringe plunger may be necessary. The appearance of the backflow of blood into the catheter provides confirmation that the needle has been inserted into the proper location for injection.
Slight hydrodynamic resistance will also be observed when the needle is correctly placed for intracardiac injection. Flow of cell volume from the tubing into the recipient will progress somewhat slowly. Take care not to remove the needle before the full 50μl volume has been delivered.
-
8b
For engraftment via intrahepatic injection: Restrain pups in same manner as for intracardiac injection. Deliver a 50-μl volume of cells directly into the liver.
A shallow needle angle should be used when injecting to avoid completely piercing the liver (see Fig. 15.21.3).
In contrast to intra-cardiac injections where a flash of blood can be seen, there is no immediate confirmation that the injection is reaching the intended site.
-
9
Place the pups on a warming pad or under an incandescent lamp for 1 to 2 min
-
10
Immediately before returning the pups to their parents, apply a small amount of petrolatum-based topical decongestant to the snout of both parents.
Vicks Vaporub is strongly aromatic and needs to be used only sparingly.
This is a further cautionary step to dampen the sense of smell of the parents to cover scents that have transferred to the pups during handling. This will facilitate the acceptance of the pups by the parents. See ILAR (2003).
-
11
Wean pups normally between 21 and 25 days of age and verify engraftment in peripheral blood by flow cytometry (Support Protocol) at 12 weeks of age.
Mice can be monitored for engraftment of human HSC over the 10 to 12 week period by flow cytometric analysis for detection of human CD45+ cells in the mouse peripheral blood (see Chapter 5 and Support Protocol). Human CD45+ cells can routinely be detected in the peripheral blood as early as 4 weeks after human HSC injection.
The weight of the weaned pups will be less than normally observed due to effects of the irradiation on the weight gain of the newborn pups.
Figure 15.21.3.
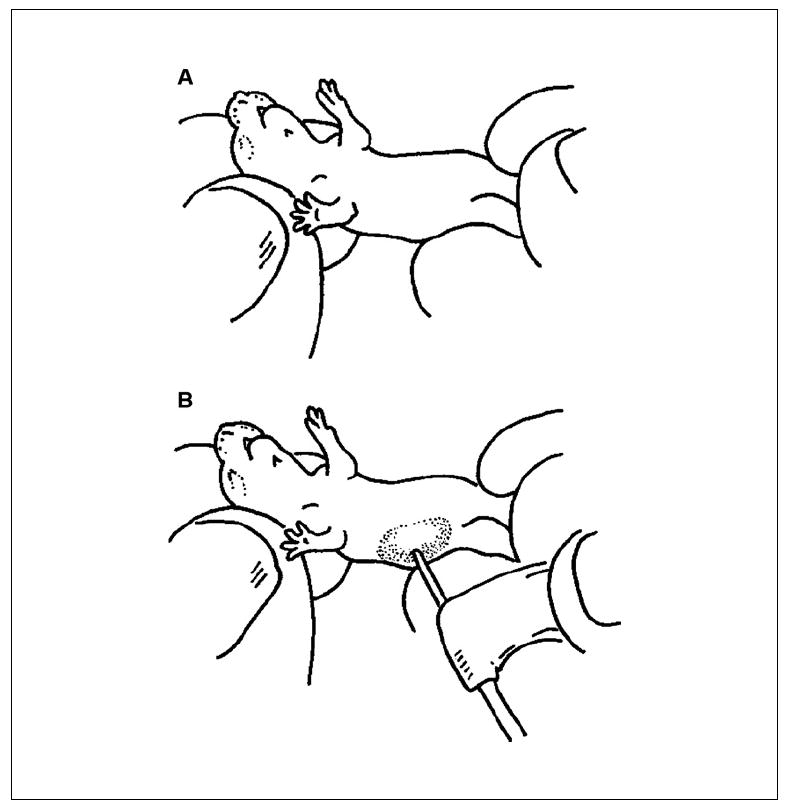
(A) Close up of a newborn NOD/Lt-scid IL2rγnull pup, showing restraint technique for intrahepatic injection. (B) Demonstration of needle placement for intrahepatic injection into a newborn NOD/Lt-scid IL2rγnull pup.
PERIPHERAL BLOOD MONONUCLEAR CELL ENGRAFTMENT VIA INTRASPLENIC INJECTION
NOD-scid/Lt IL2rγ null mice support equivalent levels of PBMC engraftment via intravenous, intraperitoneal, or intrasplenic routes, so investigators can choose the most appropriate route of injection for their studies. The procedures for delivering PBMC via the intrasplenic route is provided in this protocol.
Materials
NOD/Lt-scid IL2rγ null mice: males or females, 5 to 12 weeks old (official strain designation, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; The Jackson Laboratory, 005557; see Internet Resources)
20/4 mg/ml ketamine/xylazine in PBS (e.g., ketaset/zylaject, Webster Veterinary Supply)
Sterile petrolatum ophthalmic ointment (e.g., Purelube veterinary ointment, Webster Veterinary Supply)
Ficoll-isolated human PBMC, 20 × 106 cells per recipient mouse: freshly prepared from whole blood (see APPENDIX 3F and UNIT 7.1)
Phosphate-buffered saline (PBS; APPENDIX 2A), sterile
0.01 mg/ml buprenorphine (see recipe)
Electric clippers
70% (v/v) ethanol
Betadine
Sterile surgical instruments: iris scissors, forceps, needle holder
1-cc syringes with 27-G × ½-in. needle
Sterile cotton swabs
3-0 coated vicryl suture
Wound clips
Sterile gauze
Warming pad or warming lamp
-
Anesthetize mice by intraperitoneal injection of 20/4 mg/ml ketamine/xylazine cocktail at a dosage of 100 mg/kg ketamine and 20 mg/kg xylazine (see UNIT 1.4) and administer a dose of buprenorphine (0.05 mg/kg) via subcutaneous injection. During anesthesia, smear sterile petrolatum ophthalmic ointment onto the eyes to prevent drying during anesthesia.
Buprenorphine is given as an analgesic and additional doses should be given every 6-8 hr during the first 48 hr following the surgery. This also provides an opportunity for the investigator to assess the post-operative condition of the mice.
Place the anesthetized recipient in right lateral recumbency, so that the left lateral side is exposed.
Shave the hair over the left lateral side using veterinary electric clippers and wipe the area with 70% ethanol and sterile gauze; scrub area twice with Betadine.
Make a 1-in. vertical incision through the skin, above the spleen to expose the peritoneal musculature.
Make a second incision of the peritoneal musculature under the opening in the skin.
-
Using forceps, gently exteriorize the spleen through the two incisions.
For additional information on the procedure to exteriorize the spleen, see UNIT 1.10.
-
Use forceps to stabilize the spleen and inject 20 × 106 human PBMCs in a volume of 50 μl directly into the spleen.
The spleens of immunodeficient mice are considerably smaller than immunocompetent mice. To minimize the possibility of completely piercing the spleen, use a shallow angle of insertion with the needle and do not insert the full length of the needle into the spleen.
The hydrodynamic pressure of injecting the cell volume should produce a “ballooning” of the splenic capsule, providing positive feedback of a successful injection.
If desired, other cells or tissues can be delivered simultaneously (e.g., islet transplants; Banuelos et al., 2004).
-
With the needle still inserted into the spleen, place a cotton swab at the injection site. Hold the swab at the injection site while removing the needle and for an additional 5 sec after removing the needle.
This is done to minimize the chance of backflow of cells after the needle is withdrawn. Minimal backflow should occur.
Carefully replace the spleen inside the peritoneal wall and suture the peritoneal cavity using 3-0 coated vicryl in an interrupted stitch pattern. Close outer incision with three wound clips.
-
Wrap the mouse loosely in sterile gauze and place on a warming pad or under a warming lamp until the mouse is fully recovered from anesthesia. Once ambulatory, return the mice to their cage.
Monitor mice for signs of distress and treat humanely in accordance with institutional animal care protocols.
Wound clips should be removed after 7 to 10 days.
FLOW CYTOMETRIC ANALYSIS OF HUMANIZED MICE
Flow cytometry is a convenient method for evaluating human cell engraftment in humanized mice. The unique aspects of the method for achieving interpretable results in chimeric humanized mice are highlighted in this protocol. All anti-human antibodies are potentially useful for evaluating human leukocyte engraftment in humanized mice, with the only limitation being the ability of the cell type bearing a particular antigen to engraft and develop in immunodeficient mice. All antibodies should be first titered on a 1:5 mixture of human PBMC and splenocytes from a nonengrafted NOD/Lt-scid IL2rγ null mouse to ensure specificity and non-cross-reactivity with mouse cells. The protocol below outlines a four-color analysis of whole blood, but staining other lymphoid/hematopoietic tissues and scale-up to six parameter or greater analysis can easily be accomplished. Antibodies specific for murine CD45 are used for the single color controls to ensure a strong signal when calibrating the flow cytometer. The approach for flow cytometric analysis of human cell engraftment is described in detail for assessment of chimerism in the blood. Similar methodology can be used to determine human cell engraftment in the spleen, bone marrow, thymus, and lymph nodes of the engrafted mice.
Materials
- Commonly used antibodies (additional formats available), labeled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinine chlorophyll-a protein (PerCP), allophycocyanin (APC):
- Mouse pan-CD45, clone 30-F11, BD Biosciences catalog numbers: 553079 (FITC), 553081 (PE), 557235 (PerCP), 559864 (APC); isotype control, clone A95-1 (rat IgG2b, k), BD Biosciences 552991 (PerCP)
- Human CD45, clone HI30, BD Biosciences 555485 (APC); isotype control, clone MOPC-21 (mouse IgG1, k), BD Biosciences 555751 (APC)
- Human CD3, clone UCHT1, BD Biosciences 555333 (PE); isotype control, clone MOPC-21 (mouse IgG1, k), BD Biosciences 555749 (PE)
- Human CD20, clone 2H7, BD Biosciences 555622 (FITC); isotype control, clone 27-35 (mouse IgG2b, k), BD Biosciences 555742 (FITC)
- Anti-Fcγ RI (Fc Block), clone 2.4G2, BD Biosciences (purified)
FACS buffer (see UNIT 5.3)
BD FACS lysing solution (BD), prepared according to manufacturer’s instructions
12 × 75–mm round bottom tubes for flow cytometry
Flow cytometer capable of supporting four-color cytometry (e.g., FACScalibur, BD; see UNIT 5.4)
Additional reagents and equipment for collecting blood from mice (UNIT 1.7)
Prepare cells for flow cytometry
-
Collect ~200 μl whole blood from all mice to be tested (see UNIT 1.7), including a whole blood sample from a nonengrafted NOD/Lt-scid IL2rγ null mouse as a negative control. Keep all blood ice-cold.
All handling of blood samples during staining for flow cytometry should be carried out according to guidelines in UNIT 5.3 and 5.4.
Dispense a 100-μl aliquot of each sample into separate “experimental” 12 × 75–mm tubes, reserving the remaining for a “pooled” sample.
Combine remaining ~100-μl samples into a single “pooled” sample (e.g., for tubes 1 to 8 in Table 15.21.2).
-
Dispense ~50 μl of this sample into tubes 2 to 8. Dispense the remaining pooled sample (up to 250 μl) into tube 1 (for single color and unstained compensation controls).
At the completion of the staining, 50 μl of unstained samples will be added to tubes 2 to 5. This is done to result in a ~1:1 mix of unstained cells and fluorophore-positive cells in the single color controls, as anti-mCD45 MAb is a pan-leukocyte marker that stains virtually every murine nucleated hematolymphoid cell. The 1/1 mixture of unstained/fluorophore-positive cells aids in accurately determining the settings of the cytometer.
-
Stain with antibodies, lyse red blood cells, and fix samples using standard flow cytometric technique (see UNITS 5.3 and 5.4), including an FcR blocking step.
Lysis of red blood cells is typically done after staining using BD FACS lysing solution or ACK lysis buffer (see UNIT 3.1).
If analysis of human platelets and erythrocytes is desired, the red blood cell lysis step should be omitted.
After lysis and fixing, add 50 μl of unstained sample to tubes 2 to 5, reserving at least 50 μl of the unstained cells for tube 1.
Collect data on all viable cells using a flow cytometer capable of supporting four-color cytometry (see UNIT 5.4). Collect at least 50,000 total events for each sample.
Table 15.21.2.
Example of a Flow Cytometry Antibody Panel for Monitoring Human Engraftment in Immunodeficient Mice
| Sample | Samplea | FITC | PE | PerCP | APC |
|---|---|---|---|---|---|
| 1 | Pooled | — | — | — | — |
| 2 | Pooled | mCD45 | — | — | — |
| 3 | Pooled | — | mCD45 | — | — |
| 4 | Pooled | — | — | mCD45 | — |
| 5 | Pooled | — | — | — | mCD45 |
| 6 | Pooled | mIgG2b, k | hCD3 | mCD45 | hCD45 |
| 7 | Pooled | hCD20 | mIgG1, k | mCD45 | hCD45 |
| 8 | Pooled | hCD20 | hCD3 | mCD45 | mIgG2b, k |
| 9+ | Experimentals | hCD20 | hCD3 | mCD45 | hCD45 |
Pooled sample consists of a mixture of samples from all the individual mice to be tested and is used for the single color and compensation control samples. This has the added important benefit of mitigating the risk that the sample used to set up the cytometer is from a nonengrafted or poorly engrafted recipient. Experimentals refers to the staining that will be done on each individual engrafted mouse to be tested (i.e., one experimental tube per mouse).
Analyze samples
-
8
Open the file in FSC-H × SSC-H view. Draw a large gate including all viable cells and excluding only cell debris (see Fig. 15.21.4A).
As human cells engraft in multiple lineages (especially in HSC-engrafted mice), a comprehensive gate of all viable cells is required to get an accurate assessment of overall chimerism levels.
-
9
Plot this new gate in FL3-H (PerCP) × FL4-H (APC) view. This plot will allow for human cell chimerism to be visualized (see Fig. 15.21.4B). Draw a tight gate around cells that stain positive for human CD45 and negative for mouse CD45.
This step allows for human and mouse cells to be distinguished from each other. Gating on cells that are single positive for human CD45 ensures that only human cells are being examined in subsequent plots.
Human engraftment levels are defined as the proportion of total nucleated cells that stain single positive for human CD45.
-
10
Plot the human CD45+ gate in a new window, viewing FL1-H (FITC) × FL2-H (PE) to visualize lineage development.
Contribution of each of the lineages to overall human cell engraftment is determined using this view (see Fig. 15.21.4C).
Visualizing additional lineages can be accomplished by two means: (1) scaling up to five or more parameters in a single sample or (2) running additional aliquots of the same specimen with different markers in the FITC and PE channels.
The staining strategy based on first identifying human CD45+ cells will not permit identification of human-derived nucleated erythroblasts and red blood cells that are CD45−. These cells can be identified using anti-human Glycophorin A antibody. In addition, different light-scatter gating strategies are required for detection of human-cell-derived platelets (Ishikawa et al., 2005).
Figure 15.21.4.
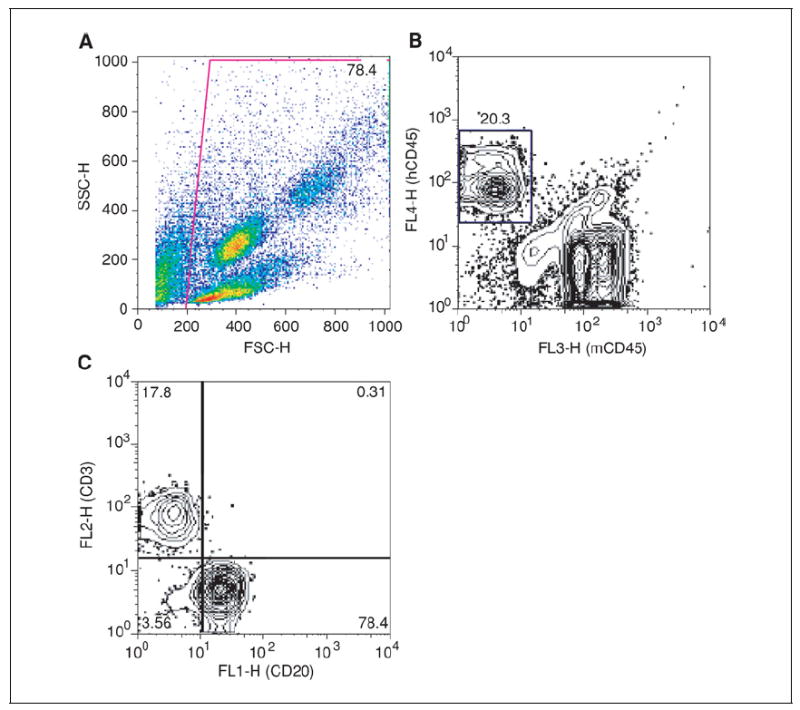
(A) Forward scatter by side scatter plot of all collected events from the peripheral blood of a NOD/Lt-scid IL2rγnull mouse 12 weeks after HSC engraftment, showing gating strategy. (B) Contour plot of gated events from Panel A, showing human hematopoietic chimerism by plotting mCD45 (x axis) by hCD45 (y axis). (C) Contour plot gated on human CD45+ cells from Panel B plotted to show B cells (CD20, x axis) and T cells (CD3, y axis).
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see APPENDIX 5.
Buprenorphine (0.01 mg/ml)
Add 200 μl of 0.3 mg/ml buprenorphine (Buprenex, J.A. Webster) to 5.8 ml sterile saline (APPENDIX 2A) in a sterile vial. Store at room temperature up to expiration date indicated on original packaging.
Buprenorphine is a controlled substance requiring DEA licensing and must be kept under lock and key.
COMMENTARY
Background Information
The ability to engraft mice with human hematopoietic tissues was first made possible by the discovery of the scid mutation in the CB17 strain mice over 20 years ago (Bosma et al., 1983). These mice were found to be B and T cell deficient as a result of a mutation in the gene encoding the DNA repair enzyme protein kinase, DNA-activated, catalytic polypeptide (Prkdcscid, hereafter referred to as scid). However, CB17-scid mice supported only low levels of human hematopoietic cell engraftment due to a robust innate immune system (Shultz et al., 2007). The intervening twenty-plus years of research in humanized mice has focused on improving human hematopoietic engraftment by decreasing levels of innate immunity, particularly in immunodeficient NOD strains (Shultz et al., 2007). Creation of scid, Rag1 null, or Rag2null mice that also lack the IL-2rγ subunit (γc, CD132) are the new “gold standard” as immunodeficient hosts for human cell and tissue engraftment. This is because they support higher levels and greater multilineage development of human hematopoietic cells than any of the previous generations of immunodeficient hosts (Ito et al., 2002; Traggiai et al., 2004; Ishikawa et al., 2005; Shultz et al., 2005; Watanabe et al., 2006). The different strains of immunodeficient mice that have been cited in the published literature and are available for “humanized” mouse studies are be discussed below.
Humanized mice are of great interest to immunologists who are focused on translating basic research findings into clinical applications. The need for humanized mice evolved from several key needs: first, human and rodent immune systems are different, and results obtained in animal models have not always translated into human therapies; additionally, there are a number of human diseases that don’t have appropriate animal models, or the animal models that do exist have significant differences from the human counterpart. Examples of these situations include viral infections such as dengue and human immunodeficiency virus, which do not replicate in murine tissues, but have been reported to infect humanized mice (Hesselton et al., 1995; Bente et al., 2005; Watanabe et al., 2006). While many exciting preliminary reports have demonstrated the utility of humanized mice, it is important to remember that humanized mouse models are still under development, and efforts continue to improve the models to facilitate human cell engraftment and function.
NOD/Lt-scid IL2rγ null, NOD/Shi-scid IL2rγ null, and BALB/c-Rag2 null IL2rγ null mice represent a technological leap over previous generations of immunodeficient hosts as recipients of human hematolymphoid cells. Most importantly, these IL2r null stocks of immunodeficient mice support high levels of de novo human T cell development from stem cell precursors, previously a rare event in other immunodeficient strains of mice. Additionally, in earlier models of hu-PBMC-SCID mice, reliable engraftment required much higher cell doses, and engraftment via the intravenous route was not routinely achieved.
Critical Parameters and Troubleshooting
Humanized immunodeficient mice can be used to study a number of immunologic and hematologic processes. However, it is important to note that the protocols presented in this unit are specifically directed towards the engraftment of NOD/Lt-scid IL2rγ null mice with a human immune system for the generation of functional T and B cell responses. Other uses of this model, including engraftment with human stem cells of nonhematopoietic origin or human tumor cells, may require additional optimization.
Choice of model
The two basic humanized mouse models presented in this chapter offer the investigator a choice when developing an experimental model system. The decision of which model to use must be based on the particular experimental objective and the hypothesis to be tested. The hu-PBL-SCID model is well suited for examining the function of mature cells of the immune system; it has been employed to study islet and skin allograft rejection (Turgeon et al., 2003; Banuelos et al., 2004). The hu-PBL-SCID model system also has utility for the study of recall responses to vaccinations or infection (Lim et al., 2007) and for investigation of hematological malignancies (Fujii et al., 2007). The HSC-engrafted humanized mouse model (hu-SRC-SCID) is useful for studying questions related to immune system development or hematopoiesis, and virtually all areas of immune function (Shultz et al., 2007). In many cases, it may be advantageous for investigators to pursue testing their hypothesis in both model systems.
Host mouse strains
There are many publications reporting the study of immunodeficient mice engrafted with human cells and tissues. However, many different strains of mice have been used in these reports, and to reproduce these data, investigators must take notice of the precise model system used. Even though the immunodeficient IL2rγ null mouse models have gained in popularity, many studies continue to use other immunodeficient mouse hosts, including CB17-scid and NOD/Lt-scid mice. Furthermore, there are different genetic stocks of IL-2rγ null immunodeficient hosts, including those that differ in host background (NOD/Shi-scid versus NOD/Lt-scid versus BALB/c-Rag2 null) and the targeted mutation used to disrupt the IL-2rγ subunit, the fully null IL2rgtm1Wjl (Cao et al., 1995) and the truncated IL2rgtm1Sug (Ito et al., 2002). A caveat regarding the use of NOD/Lt-scid IL2rγ null mice is that the strain is still being characterized; adapting many protocols (e.g., treatment with anti-CD122 monoclonal antibody) devised using other host strains (e.g., NOD-scid) has not in all cases been thoroughly investigated. However, previous generations of immunodeficient hosts remain accessible, and an excellent overview of immunodeficient hosts available for humanized mouse studies can be obtained from the Jackson Laboratory Web site (see Internet Resources). On the other hand, given the high levels of human cell engraftment that are achieved in NOD-scid IL2rγ null mice, this strain is strongly recommended as the strain of choice for studying virtually all aspects of human immune function in humanized mice.
Age of host
When deciding between using newborn or adult HSC engraftment model systems, there are additional practical considerations. First, there is a cost advantage to newborn engraftment methods, because newborns stay with parents until weaning, thus reducing per diem housing fees. Second, an additional advantage of the newborn engraftment protocol is that a humanized immune system is developed at a younger absolute age (≥ 12 weeks of age versus 17 to 24 weeks of age in mice engrafted as adults).
The major drawback to the newborn engraftment protocol is the need for increased resource management. For example, the constant monitoring of breeder cages for new litters can be labor intensive, especially if multiple litters are needed for experiments within a narrow time frame. One potential solution to this problem is to set up timed matings, which has the benefit of allowing for more precise scheduling of experiments. The protocol for timed matings has been described by Nagy (2003).
Another drawback to using the newborn protocol is the timing of newborn litters with availability of the human HSC for injection. However, HSC from umbilical cord blood or bone marrow can be T cell depleted, frozen in aliquots in liquid nitrogen, and thawed immediately prior to injection. This approach overcomes the major logistical problem of coordinating the timing of obtaining and preparing HSC samples and having recipient mice ready for engraftment.
Human donor cells
In immunodeficient NOD-scid IL2rγ null mice, excellent engraftment with mature human T cells in the hu-PBL-SCID model has been observed (King et al., 2008). Because of this, in the HSC engraftment model, extensive T cell depletion of cord blood must be achieved to prevent the engraftment and expansion of mature human T cells that could lead to GVHD. This phenomenon is readily detectable by flow cytometry, where up to 90% of the CD45+ cells can be CD3+ mature T cells in mice engrafted with non/T cell–depleted cord blood HSC populations. Mice exhibiting a mature T cell outgrowth often appear ill, with hunched posture and weight loss characteristic of GVHD. To minimize GVHD, efficient T cell depletion of HSC should be verified by flow cytometry prior to injection of the HSC into mice.
In many cases, it is desirable to inject HSC populations that are highly enriched for CD34+ cells rather than injecting T cell-depleted HSC populations. For example, an experimental protocol may involve transduction of human CD34+ hematopoietic stem cells with adenovirus or lentivirus prior to engraftment in immunodeficient hosts. For these experiments, enrichment of the CD34+ cell population can be achieved using lineage depletion or positive selection methodology such as Miltenyi or Stem Cell Technologies cell separation methodology (see Internet Resources). These methodologies are widely used protocols for the positive selection and enrichment of human CD34+ cells. Enrichment of CD34+ cells would reduce the number of cells that will need to be transduced. This will reduce the amount of virus required for efficient transduction; high titer stocks of these viruses are often difficult to generate and are expensive. In these experiments, the absolute number of human CD34+ cells to be injected should be increased to 1 × 105/mouse to achieve optimal engraftment.
Irradiation
Irradiation doses will vary with the strain used and even the dose needed within the same strain may vary between investigators and facilities. A dose of 240 cGy represents a conservative dose for preconditioning adult NOD/Lt-scid IL2rγ null mice, as levels of whole body irradiation (WBI) up to 325 cGy have been reported in this strain (Shultz et al., 2005). Rag1 null or Rag2 null mice are more radioresistant than scid mice and require higher irradiation doses. Indeed, newborn BALB/c-Rag2 null IL2rγ null mice withstand 400 cGy of split dose WBI for HSC engraftment (Traggiai et al., 2004) while 100 cGy of WBI is sufficient for HSC engraftment in newborn NOD/Lt-scid IL2rγ null mice (Ishikawa et al., 2005).
Improving engraftment
Finally, the protocols in this chapter describe only the generation of humanized mice and their phenotypic characterization by flow cytometry. However, this only represents the jumping off point for humanized mouse studies. Many protocols for improving human PBMC and HSC engraftment have been reported using previous generations of humanized mice. These protocols include depletion of mouse macrophages or granulocytes prior to engraftment, treatment of engrafted mice with human cytokines, or co-engraftment with mesenchymal stem cells (Pearson et al., 2008). In addition, other routes of engraftment have been reported in previous models of humanized mice to enhance engraftment (Shultz et al., 2007). However, the use of these alternative engraftment routes have not yet been described in immunodeficient IL2rγ null mice. Each investigator must determine what scientific questions he or she wishes to address and then tailor the assays and functional readouts to specific needs. This aspect of humanized mouse studies represents the real challenge (but also potential benefit) of this system.
Anticipated Results
hu-PBL-SCID model
In PBMC-engrafted NOD/Lt-scid IL2rγ null mice, a single injection (intravenous, intraperitoneal, or intrasplenic) of 2 × 107 cells typically results in 15% to 45% human CD45+ cells in the peripheral blood within 4 weeks. This percentage of human CD45+ cells in the blood will correspond to approximately 50% human CD45+ or 2–3 × 107 total human CD45+ cells engrafted in the spleen at 4 weeks. Virtually all PBMC injected NOD/Lt-scid IL2rγ null mice engraft, unless the injection itself is a technical failure. The majority of the human CD45+ cells will be CD3+ T cells, with a memory/effector CD45RO phenotype and a CD4:CD8 ratio of ~1:1 at 4 weeks post-engraftment. At earlier time points, the CD4:CD8 ratio will be higher, ~2 to 3:1 but reaches a stable 1:1 ratio by 3 weeks post-engraftment. It is currently unknown if this represents an attrition of CD4+ T cells or the late preferential expansion of CD8+ T cells. B cells are detectable in the spleens of PBMC-engrafted mice, but only at low levels (0.5 to 1.0%). B cell engraftment is confirmed by the persistence of human Ig in the serum of engrafted mice. The serum Ig levels in PBMC-engrafted NOD/Lt-scid IL2rγ null mice remains high over the 4-week period of study (unpublished data).
PBMC-engrafted NOD/Lt-scid IL2rγ null mice will begin to exhibit symptoms of xeno-GVHD, such as weight loss and hunched posture sometime after 4 weeks following injection of human PBMC. The development of GVHD is a major limiting factor for long-term studies using the hu-PBL-SCID model. Engraftment of cell lineages in addition to B and T cells is poor and/or brief in PBMC-engrafted NOD/Lt-scid IL2rγ null mice. While some antigen presenting cells are a component of the initial PBMC inoculum, they are undetectable using flow cytometry by 4 weeks after PBMC injection.
Previous generations of hu-PBL-SCID mice were hindered by high levels of variability in engraftment from donor to donor. The NOD/Lt-scid IL2rγ null stock appears to have overcome this problem, with uniformly high engraftment in the spleen 4 weeks after IV injection of PBMC, regardless of the donor used. It is important to note that engraftment in the peripheral blood of NOD/Lt-scid IL2rγ null mice may show some donor-to-donor variability, but splenic human PBMC engraftment appears to be extremely reproducible between individual mice and between different donors. In summary, the NOD/Lt-scid IL2rγ null mouse is a much more robust host for human PBMC engraftment than previously available immunodeficient strains of mice.
hu-SRC-SCID model
The level of human hematopoietic development in HSC-engrafted NOD/Lt-scid IL2rγ null mice is dependent on several factors and will, in most cases, be more variable than PBMC engraftment. First, the CD34+ cell dose will have a large impact on total human CD45+ cell engraftment levels in the periphery of mice at 12 weeks after HSC injection. CD34+ cell inoculums as low as 3 × 104 per recipient give reliable, but highly variable, levels from less than 1% to greater than 30% human CD45+ in the peripheral blood at 12 weeks post-engraftment. Increasing the CD34+ dose will generally increase the total CD45 engraftment at the time of analysis. The 10- to 12-week time point is used because it is the earliest point at which reliable human T cell generation can be detected, but total CD45 levels and human B and T cell levels will continue to increase over the following weeks. Human CD45+ cells are detectable in the blood of HSC-engrafted mice by as early as 4 weeks post-engraftment, but at low levels, and CD3+ T cells are not present.
Head to head comparisons of NOD/Lt-scid IL2rγ null mice engrafted with the same HSC source as newborns and adults reveals that the total CD45+ cell levels are roughly equivalent. However, T cell development is superior in newborn engrafted mice as compared to comparably engrafted adult mice (T. Pearson, un-pub observ.). A comparison of NOD/Lt-scid IL2rγ null mice engrafted as newborns via the intracardiac or intrahepatic routes of injection revealed essentially similar levels of human CD45+ cell engraftment and T cell development (T. Pearson, unpub. observ.). Facial vein injection of newborns also can also be used. However, this method is technically more challenging.
Human CD45+ cell engraftment levels after HSC injection are also variable depending on the lymphoid tissue examined. Blood, while a convenient tissue for evaluating human cell engraftment, contains human cells at lower proportions than is observed in other hematopoietic lymphoid tissues. The highest engraftment of human cells is observed in the bone marrow, where human CD45+ cell levels of greater than 75% are commonly observed following an original injected cell dose of 3 × 104 CD34+ cells in a T cell–depleted UCB. Therefore, using human CD45+ levels in the blood as a tissue for evaluating engraftment in the immunodeficient mice will underestimate total engraftment levels and may yield some false negatives. Intermediate between blood and bone marrow is the spleen, with CD45+ cell engraftment levels typically ranging from 25% to 75% of nucleated splenocytes.
Multilineage hematopoietic development and stable engraftment is much more reliable in HSC-engrafted NOD/Lt-scid IL2rγ null, NOD/Shi-scid IL2rγ null and BALB/c-Rag2 null IL2rγ null mice than in any of the previous generations of immunodeficient hosts (Shultz et al., 2007). Importantly, human myeloid and plasmacytoid dendritic cells are generated, as well as T and B cell populations and other human leukocyte subsets. Table 15.21.3 provides a list of lineages that have been reported to be present in HSC-engrafted NOD/Lt-scid IL2rγ null mice. To date, no direct comparisons of HSC or PBMC engraftment between NOD/Lt-scid IL2rγ null and BALB/c-Rag2 null IL2rγ null mice have been reported. However, published studies suggest that all three strains are well suited as hosts for a human immune system generated by engraftment of HSC (Ito et al., 2002; Traggiai et al., 2004; Shultz et al., 2005; Ishikawa et al., 2006). Future studies should continue to reveal the optimal host and protocol for creating humanized mice.
Table 15.21.3.
Cell Surface Antigens Expressed on Human Cells in HSC-Engrafted Micea
| Cell surface antigen | Tissue | Lineage |
|---|---|---|
| CD3 | LN, THY, SPL, PBL | T cell |
| CD4 | LN, THY, SPL, PBL | T cell |
| CD8 | LN, THY, SPL, PBL | T cell |
| CD10 | BM, SPL, PBL | B cell |
| CD11c | BM, SPL | mDC |
| CD13 | PBL | Myelomonocytic |
| CD14 | BM, SPL, PBL | Myelomonocytic |
| CD19 | BM, SPL, PBL | B cell |
| CD20 | BM, SPL, PBL | B cell |
| CD27 | BM, SPL | B cell |
| CD33 | BM, SPL, PBL | Myelomonocytic |
| CD34 | BM | HSC |
| CD38 | BM | HSC |
| CD40 | BM | DC |
| CD45RA | LN, THY, SPL, PBL | Naïve lymphocytes |
| CD45RO | LN, THY, SPL, PBL | Activated lymphocytes |
| CD56 | PBL | NK cell |
| CD64 | PBL | Monocyte |
| CD71 | BM | All proliferating cells |
| CD80 | BM, SPL | Pan-APC |
| CD83 | BM | mDC |
| CD86 | BM, SPL | Pan-APC |
| CD123 | BM, SPL | pDC |
| CD133 | BM | HSC |
| CD138 | BM, SPL | B cell |
| CD235a | BM | Erythroid |
| BDCA2 | BM, SPL, PBL | pDC |
| BDCA4 | BM | pDC |
| CCR7 | LN | T cell |
| HLA-DR | BM, SPL | Pan-APC |
| TLR2/3 | BM, SPL | mDC |
| TLR4 | BM | mDC |
| TCRα/β | THY, SPL, PBL | T cell |
| TCRγ/δ | THY, SPL, PBL | T cell |
| IgA | BM, SPL | B cell |
| IgD | BM, PBL, SPL | B cell |
| IgG | PBL, SPL | B cell |
| IgM | BM, SPL | B cell |
List of cell surface antigens expressed on human cells that can be detected by flow cytometry in HSC-engrafted mice. Markers and/or tissues combinations not listed in this table are not necessarily indicative of the failure of the particular lineage to develop, as this panel of markers represents only those that have been currently tested and/or published in Traggiai et al. (2004), Ishikawa et al. (2005), and Shultz et al. (2005).
Abbreviations: BM, bone marrow; HSC, hematopoietic stem cell; LN, lymph node;mDC,myeloid dendritic cell; pan-APC, antigen-presenting cell; pDC, plasmacytoid dendritic cell; PBL, peripheral blood; SPL, spleen; THY, thymus.
Time Considerations
HSC or PBMC engraftment of adult mice
The time required to engraft NOD/Lt-scid IL2rγ null mice with human cells (either PBMC or HSC) depends on the method of engraftment used and the degree of input cell preparation needed.
HSC engraftment of adult NOD/Lt-scid IL2rγ null mice using a standard intravenous injection (Basic Protocol 1) requires a 4-hr resting period for the recipient mice after WBI, which is the most important timing consideration for this protocol. The need for this resting period has been associated with increased expression of stromal derived factor-1 (SDF-1), a chemoattractant for HSC that is produced by bone marrow stromal cells and is up-regulated within 4 hr after irradiation (Ponomaryov et al., 2000).
The time for irradiation will vary as a function of the rate of γ emission of the 137Cs source. However, exposure to 240 to 325 cGy will take less than 10 min for most irradiators and commonly used dose rates. If T cell depleted CD34+ stem cells have been previously prepared and frozen (either in-house or from an outside source such as NDRI), the thawing and dilution of the stem cells can easily be accomplished during the 4-hr period between irradiation of the mice and the time for injection of the human HSC.
If the T cell depletion of the HSC is being performed on the day of injection, the time required will vary according to the protocol being followed, but it should also be completed in about a half day (see Chapter 22 for additional time considerations regarding stem cell isolation).
Following stem cell preparation and the rest time following irradiation, the final time consideration is the time for performing intravenous injections, which will vary depending on individual investigator’s technical proficiency and the number of mice to be injected. In total, Basic Protocol 1 can be accomplished in ~6 hr.
For intravenous engraftment of adult NOD-scid IL2rγ null mice with PBMC (Basic Protocol 2), the major time considerations are the collection and preparation of the PBMC from whole blood, which can be accomplished in 2 to 3 hr, and the time to perform intravenous injections (as described above). Generally, 4 to 5 hr in total should be sufficient for Basic Protocol 2.
Engraftment of newborn mice
Engraftment of newborn NOD/Lt-scid IL2rγ null mice with HSC (by either the intracardiac or intrahepatic routes; Alternate Protocol 1) requires relatively less time to complete because the pups can be injected immediately after irradiation. Preconditioning of newborn BALB/c-Rag2 null IL2rγ null mice with 400 cGy in two split doses of irradiation separated by 3 to 4 hr, followed by a 4- to 12- hr wait before injection of the human HSC after completion of the last irradiation has been reported (Traggiai et al., 2004), suggesting that engraftment of newborn mice, similar to adult mice, may benefit from a 4-hr wait following irradiation before human HSC injection. However, initial experiments suggest that there are no differences in the engraftment levels of human HSC by injection of human HSC immediately after irradiation as compared to waiting for 4 hrs (T. Pearson, unpub. observ.). Immediate injection also eliminates the additional handling and separation of the newborns from their parents.
In addition, the injection techniques take less time to perform than a standard intravenous injection in adult mice. Therefore, the time needed to prepare the HSC for injection is a major time consideration, but can be minimized on the day of injection by having the cells previously prepared and frozen. If this is the case, Alternate Protocol 1 can be accomplished comfortably in a half day.
Intrasplenic engraftment with PBMC
Intrasplenic injections (Alternate Protocol 2) are more time consuming than the standard intravenous route, with surgical proficiency of the individual investigator being the major consideration. After injection with ketamine/ xylazine, it will take several minutes for the recipient to be deeply anesthetized before the surgical procedure can begin. With practice, it is possible for a single investigator to perform surgeries and injections on several groups of mice in a single day, with each recipient requiring ~10 min, once anesthetized. Efficiency can be maximized when three or four mice can be anesthetized simultaneously and the procedure performed on each recipient before regaining consciousness. Thus, it is reasonable to collect and prepare PBMC from whole blood and perform 12 to 15 intrasplenic injections in a single day.
Acknowledgments
We thank Drs. Thomas Chase and Bonnie Lyons for critical review of this unit. Supported by the Beta Cell Biology Consortium and Autoimmunity Prevention Centers from NIH, the Juvenile Diabetes Research Foundation, International, the American Diabetes Association, the Diabetes Endocrinology Center, the National Cancer Institute, National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute of NIH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Internet Resources
The Jackson Laboratory. This Web site provides detailed information on nomenclature, genotyping protocols and phenotypes for several of the immunodeficient strains described in this unit, and is the sole repository and provider of the NOD.Cg-Prkdcscid Il2rgtm1Wjll (NOD-scid IL2rγ null)stock.
http://jaxmice.jax.org/library/notes/501a.html
Choosing an Immunodeficient Mouse Model, Jax Notes, Spring 2006
Taconic Farm Web sites. A comprehensive overview of immunodeficient hosts for HSC engraftment, including salient features of each strain, availability, and references. Taconic Farms is another supplier of immunodeficient mice, including CB17-scid, NOD-scid, BALB/c-Rag2 null, and “C57BL/6-Rag2 null IL2rgnull (C57BL/6J × C57BL/10SgSnAi)-[KO]γc-[KO]Rag2”
National Disease Research Exchange (NDRI) is a nonprofit resource providing access (through an application process) to human biomaterials for investigators’ research studies.
http://www.miltenyibiotec.com/en/NN_625_Hematopoietic_stem_and_progenito_cells.aspx
Miltenyi Biotec is a commercial supplier of cell separation technologies to isolate/enrich for human hematopoietic stem cells.
https://www.stemcell.com/productcatalog/huprog.asp
Stem Cell Technologies is a commercial supplier of cell separation technologies to isolate/enrich for human hematopoietic stem cells.
Literature Cited
- Banuelos SJ, Shultz LD, Greiner DL, Burzenski LM, Gott B, Lyons BL, Rossini AA, Appel MC. Rejection of human islets and human HLA-A2.1 transgenic mouse islets by alloreactive human lymphocytes in immunodeficient NOD-scid and NOD-Rag1(null)Prf1(null) mice. Clin Immunol. 2004;112:273–283. doi: 10.1016/j.clim.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–13799. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, Paul WE, Katz SI, Love PE, Leonard WJ. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Fujii H, Trudeau JD, Teachey D, Fish JD, Grupp SA, Schultz KR, Reid GS. In vivo control of acute lymphoblastic leukemia by immunostimulatory CpG oligonucleotides. Blood. 2007;109:2008–2013. doi: 10.1182/blood-2006-02-002055. [DOI] [PubMed] [Google Scholar]
- Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL, Wijnands E, Blom B, Spits H. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: Functional inactivation of p53 in developing T cells. Blood. 2004;104:3886–3893. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- Hesselton RM, Greiner DL, Mordes JP, Rajan TV, Sullivan JL, Shultz LD. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. J Infect Dis. 1995;172:974–982. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- Holyoake TL, Nicolini FE, Eaves CJ. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp Hematol. 1999;27:1418–1427. doi: 10.1016/s0301-472x(99)00078-8. [DOI] [PubMed] [Google Scholar]
- ILAR. Institute for Laboratory Animal Research (U.S.) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research: Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press; Washington, D.C.: 2003. [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain (null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F, Shimazu H, Shultz LD, Fukata M, Nakamura R, Lyons B, Shimoda K, Shimoda S, Kanemaru T, Nakamura K, Ito H, Kaji Y, Perry AC, Harada M. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FAESB J. 2006;20:950–952. doi: 10.1096/fj.05-4863fje. [DOI] [PubMed] [Google Scholar]
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, Atkinson MA, Wasserfall C, Herold KC, Woodland RT, Schmidt MR, Woda BA, Thompson MJ, Rossini AA, Greiner DL. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Lim WH, Kireta S, Russ GR, Coates PT. Human plasmacytoid dendritic cells regulate immune responses to Epstein-Barr virus (EBV) infection and delay EBV-related mortality in humanized NOD-SCID mice. Blood. 2007;109:1043–1050. doi: 10.1182/blood-2005-12-024802. [DOI] [PubMed] [Google Scholar]
- Nagy A. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2003. [Google Scholar]
- Pearson T, Greiner DL, Shultz LD. Humanized SCID mouse models for biomedical research. In: Nomura T, Watanabe T, Habu S, editors. Current Topics in Microbiology and Immunology. Vol. 324. Springer; Berlin: 2008. pp. 25–52. [DOI] [PubMed] [Google Scholar]
- Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D, Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell–transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Turgeon NA, Banuelos SJ, Shultz LD, Lyons BL, Iwakoshi N, Greiner DL, Mordes JP, Rossini AA, Appel MC. Alloimmune injury and rejection of human skin grafts on human peripheral blood lymphocyte-reconstituted nonobese diabetic severe combined immunodeficient beta2-microglobulin-null mice. Exp Biol Med. 2003;228:1096–1104. doi: 10.1177/153537020322800918. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, Dewan MZ, Yu Z, Ito M, Morio T, Shimizu N, Honda M, Yamamoto N. Hematopoietic stem cell-engrafted NOD/SCID/IL2R {gamma} null mice develop human lymphoid system and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2006;109:212–218. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]


