Abstract
Erythrocyte free hemoglobin (Hb) induces vasoconstriction due to nitric oxide (NO) scavenging, limiting the NO available for vascular smooth muscle. The central objective of this study was to restore NO bioavailability using long-lived circulating NO-releasing nanoparticles (NO-np) to reverse the vasoconstriction and hypertension induced by polymerized bovine Hb (PBH) NO scavenging. PBH (13 g/dl) was infused in a volume equal to 10% of the animal blood volume. Intravascular NO supplementation was provided with an infusion of NO-np (10 and 20 mg/kg body wt). This study was performed using the hamster window chamber model to concurrently access systemic and microvascular hemodynamics. Infusion of PBH increased blood pressure and induced vasoconstriction. Treatment with 10 and 20 mg/kg NO-np reduced the blood pressure and vasoconstriction induced by PBH. Moreover, the higher dose of NO-np decreased blood pressure and induced vasodilation compared with baseline, respectively. Treatment with NO-np to decrease PBH-induced vasoconstriction increased methemoglobin levels and plasma nitrite and nitrate. In conclusion, NO-np counteracted both systemic hypertension and decreased the vasoconstrictor effects of PBH infusion, improving systemic and microvascular function. Based on the observed physiological properties, NO-np has clear potential as a therapeutic agent to replenish NO in situations where NO production is impaired, insufficient, or consumed, thereby preventing vascular complications.
Keywords: transfusion medicine, endothelial dysfunction, hypertension, nanotechnology, blood substitutes, hemolysis, gas delivery
allogeneic red blood cell (RBC) transfusion is the gold standard of therapy after significant blood loss (17). Currently, the American Red Cross tests donated blood for hepatitis B and C viruses, human immunodeficiency virus, human T cell lymphotropic virus, syphilis, West Nile virus, and Chagas disease (40). These and emerging bloodborne infectious diseases will further increase the cost per unit of blood as well as increase limitations on legitmate and approved sources (9). Even more problematic is the blood safety in developing countries (29) as well as its availability in emergency situations or natural disasters. Beyond issues of availability, transfusion-related adverse events, both short and long term, are among some of the costliest contributors to healthcare expenditures (3). Taking into account the increased liability and regulatory hemovigilance, the costs of blood products will clearly trend exponentially upward. Therefore, the race to develop an efficacious and safe universal alternative to RBC transfusion has been fierce but unfortunately unsuccessful.
Hemoglobin (Hb), in the RBC, is the protein responsible for transporting and delivering oxygen and other gaseous Hb ligands to the tissues (30). Mimicking nature's approach, Hb-based oxygen carriers (HBOCs) have been synthesized and formulated to replace the RBC oxygen carrying capacity (1). An early HBOC example is stroma-free Hb, which was found to be associated with a variety of serious side effects including renal failure, intravascular coagulation, and anaphylactic reactions (2, 12). Removal of RBC stroma from the Hb solution reduced, but did not prevent, toxicity because the Hb tetramers easily dissociate into dimers and monomers, which then go on to impart renal injury (4). Polymerization of Hb with dysfunctional cross-linking reagents can potentially resolve the dissociation concerns, preventing the undesired extravasation and extending the circulating half-life.
Glutaraldehyde cross-linking is known to be a highly nonspecific process that involves most of the lysine residues distributed on the surface of the protein and is widely used to polymerize Hb (15, 18). In the United States, there are two glutaraldehyde polymerized Hbs undergoing phase III clinical trials: Hemopure (HBOC-201, OPK Biotech, Cambridge, MA) and Polyheme (Northfield Laboratories, Evanston, IL). Hemopure is a polymerized bovine Hb (PBH) with a P50 of 38 mmHg and a molecular weight ranging between 130 and 500 kDa (10). Polyheme is a glutaraldehyde cross-linked pyridoxalated human Hb with a P50 of 30 mmHg and a molecular weight ranging between 128 and 400 kDa (15). Although polymerized Hbs have demonstrated potential, they, like other blood substitutes, are vasoconstrictive predominantly from scavenging of nitric oxide (NO) by plasma ferrous (Fe2+) heme. NO scavenging by acellular Hb occurs via two reactions: an oxidative reaction of NO with oxyHb (NO dioxygenase) and NO binding to deoxyHb (34). A third reaction of NO with oxyHb, postulated to be physiologically important, is the binding of NO to the strongly conserved β-chain cysteine amino acid at position 93 (39). In this context, a minimal concentration of acellular Hb is sufficient to distort the NO diffusion field (26). Decreasing the intracellular NO concentration limits the activation of the NO receptor soluble guanylate cyclase (sGC), resulting in decreased production of the intracellular second messenger cGMP. Endothelial NO is synthesized from l-arginine by endothelial NO synthase (eNOS) in endothelial cells in a process regulated by shear stress, oxygen conditions, and inflammation (35). This pathway is the most important for vascular smooth muscle relaxation, and, therefore, decreasing activity results in vasoconstriction. Hypertension is the most frequent manifestation of vasoconstriction and appears to be the main unwanted side effect of many Hb solutions.
NO supplementation has many complications as well and is short lived due to the rapid scavenging rate of NO by acellular Hb and the Hb within erythrocytes (27). Currently, the clinical therapeutic potential of pure NO has been explored via inhaled NO gas from pressurized tanks. Alternatives for intravascular NO therapy include formulations based on compounds containing either NO or NO precursors in a stable form, which typically lack the capacity for controlled and sustained delivery (20). Our current platform uses a sol-gel precursor in conjunction with a glass-forming combination of chitosan and polyethylene glycol to form a fine powder composed of nanoparticles upon drying via lyophilization (11). Chitosan is a linear polysaccharide and is insoluble in water, and, by incorporating polyethylene glycol in the particles, the hydration rate can be controlled. This hydrogel-glass hybrid material retains NO in a stable form when dry, releases NO upon exposure to moisture, and is stable over long-term dry storage. Since a thermally driven redox reaction involving nitrite is used to generate NO, the formed NO is retained within the nanoparticles as long as they are anhydrous. NO release kinetics after hydration are controlled by modifications of the described components, with minimal changes of release properties during long-term dry storage (11). Based on our previous results (5), the release of NO from the NO-releasing nanoparticles (NO-np) measured with a NO chemiluminescence analyzer is stable (0.08 μmol NO/mg/h) over 8 h in PBS solution. In vivo, the systemic effects on mean arterial pressure (MAP), microvascular effects on blood vessel diameter, and exhaled NO are reduced to 3 h (5).
In the present study, the central objective was to establish the efficacy of NO-np as a means to restore smooth muscle NO bioavailability and therefore combat the vasoconstriction and hypertension induced by the NO scavenging of PBH (Oxyglobin, OPK Biothech; approved in the United States and the European Union for veterinary use). The potential benefit of combining NO-np with a glutaraldehyde-PBH infusion was investigated to determine the potential hemodynamic improvements as a result of NO-np coadjunct therapy. It was hypothesized that NO-np would counter both the systemic hypertension and decrease vasoconstrictor effects of PBH infusion, improving systemic and microvascular function. This study was performed using the hamster window chamber model to concurrently access systemic and microvascular parameters. PBH was infused at a stock concentration (13 g/dl) in a volume equal to 10% of the animal estimated blood volume (7% body wt), and intravascular NO supplementation was provided with NO-np (10 and 20 mg/kg body wt). Nanoparticles free of NO were used as control. This report provides in vivo experimental evidence that intravascular exogenous supplementation of NO using NO-np can reduce the hypertension and vasoconstriction induced by acellular Hb scavenging of NO. Given the critical importance of NO in regulating pathophysiological states involving the vasculature, controlled and sustained intravascular NO delivery could profoundly impact the current treatment of hemolytic, metabolic, and endothelial dysfunction-induced vascular disease states.
METHODS
Animal preparation.
Investigations were performed in 50- to 65-g male golden Syrian hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal skinfold chamber window. Animal handling and care followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. The hamster chamber window model is widely used for microvascular studies in the unanesthetized state, and the complete surgical techniques have been described in detail elsewhere (7, 8). The window chamber preparation was allowed at least 2 days for recovery before the preparation was assessed under a microscope for any signs of edema, bleeding, or unusual neovascularization. Animals were anesthetized again, and arterial and venous catheters filled with a heparinized saline solution (30 IU/ml) were implanted. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion criteria.
Animals were suitable for the experiments if 1) systemic parameters were within normal range, namely, heart rate (HR) > 340 beat/min, MAP > 80 mmHg, systemic Hct > 45%, and arterial Po2 > 50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under ×650 magnification did not reveal signs of edema or bleeding. Hamsters are a fossorial species with a lower arterial Po2 than other rodents due to their adaptation to a subterranean environment.
Synthesis of NO-np and control nanoparticles.
The synthesis of NO-np has been recently reported (11). Briefly, a hydrogel-glass composite was synthesized using a mixture of tetramethylorthosilicate, polyethylene glycol, chitosan, glucose, and sodium nitrite in 0.5 M sodium phosphate buffer (pH 7). Nitrite was reduced to NO within the matrix because of the glass properties of the composite affecting redox reactions initiated with thermally generated electrons from glucose. After the redox reaction, the ingredients were combined and dried using a lyophilizer, resulting in a fine powder composed of nanoparticles containing NO. Once exposed to an aqueous environment, the hydrogel properties of the composite allowed for the opening of water channels inside the particles, facilitating the release of trapped NO over extended time periods. Control nanoparticles (control-np), free of NO inside, were produced identically but without nitrite.
Systemic parameters.
MAP and HR were continuously recorded (MP 150, Biopac Systems, Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes (25 μl, ∼50% of the heparinized glass capillary tube was filled). Hb content was determined spectrophotometrically from a single drop of blood (B-Hemoglobin, Hemocue, Stockholm, Sweden).
Blood chemistry and biophysical properties.
Arterial blood was collected in heparinized glass capillaries (0.05 ml) and immediately analyzed for arterial Po2, arterial Pco2, and pH (Blood Chemistry Analyzer 248, Bayer, Norwood, MA).
metHb measurement.
Blood was collected into microhematocrit tubes and divided for whole blood and plasma metHb determination. Plasma Hb was obtain by centrifugation. metHb was established according to Winterbourn (41). Calibration was ensured using standard levels at 5.2%, 2.6%, and 1.m% MetHb (RNA Medical, Bayer Diagnostics, Medfield, MA).
Plasma nitrite/nitrate.
Blood samples were collected from the carotid artery and centrifugedd to separate RBCs and plasma. Plasma proteins were removed by adding equal volumes of methanol and centrifuged at 15,000 rpm for 10 min. Concentrations of nitrite/nitrate in the supernatant were measured with a NOx analyzer (ENO-20, Eicom, Kyoto, Japan). This analyzer combines the Griess method and HPLC.
Microvascular experimental setup.
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded and then fixed to the microscopic stage of a transillumination intravital microscope (BX51WI, Olympus, New Hyde Park, NY). Animals were given 20 min to adjust to the change in the tube environment before measurements. The tissue image was projected onto a charge-coupled device camera (COHU 4815) connected to a videocassette recorder and viewed on a monitor. Measurements were carried out using a ×40 (LUMPFL-WIR, numerical aperture: 0.8, Olympus) water-immersion objective. The same sites of study were followed throughout the experiment so that comparisons could be directly made to baseline levels.
Microhemodynamics.
Arteriolar and venular blood flow velocities were measured online using the photodiode cross-correlation method (21) (Photo Diode/Velocity Tracker model 102B, Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to vessel size to obtain mean RBC velocity (28). A video image-shearing method was used to measure vessel diameter (D) (22). Blood flow (Q) was calculated from the measured values as follows: Q = π × V(D/2)2. Changes in arteriolar and venular diameter from baseline were used as indicators of a change in vascular tone. This calculation assumes a parabolic velocity profile and has been found to be applicable to tubes of 15- to 80-μm internal diameters and for Hct in the range of 6–60% (28).
Functional capillary density.
Functional capillaries, defined as those capillary segments that have a RBC transit of at least a single RBC in a 30- to 45-s period, in 10 successive microscopic fields were assessed, totaling a region of 0.46 mm2. Each field had between two and five capillary segments with RBC flow. Functional capillary density (FCD; in cm−1), i.e., the total length of RBC perfused capillaries divided by the area of the microscopic field of view, was evaluated by measuring and adding the length of capillaries that had RBC transit in the field of view. The relative change in FCD from baseline levels, after each intervention, is indicative of the extent of the change in capillary perfusion.
PBH infusion.
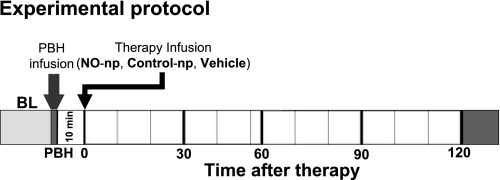
An equivalent volume to 10% of the animal blood volume (calculated as 7% of the animal body wt) of PBH (13 g/dl, Oxyglobin, OPK Biotech) was infused via the jugular vein at a rate of 0.1 ml/min (top load). Figure 1 shows the experimental protocol.
Fig. 1.
Experimental protocol. The volume of infused polymerized bovine Hb (PBH) was estimated as 10% of the animal's blood volume. Therapies to reverse hemodynamics complications induced by nitric oxide (NO) scavenging by PBH were based on NO-releasing nanoparticles (NO-np; at two doses based on the animal's body weight: 10 and 20 mg/kg), control nanoparticles (control-np; particles free of NO inside), and only the vehicle (deoxygenated saline). All therapies were infused 10 min after the end of the infusion of PBH. The time points reported are baseline (BL), at the end of the infusion of PBH (PBH), and the time relative to the time after infusion of the therapies.
NO-np infusion.
NO-np was infused 10 min after the end of the PBH infusion. Nanoparticles were suspended in deoxygenated saline and infused in a volume of 50 μl (equivalent to <2% of the animal's blood volume) via the jugular vein at a rate lower than 100 μl/min. Figure 1 shows the experimental protocol.
Experimental groups.
After the infusion of PBH, animals were randomly divided into four experimental groups. Groups were named based on the type of nanoparticles used and the concentration infused, i.e., therapy of 10 mg/kg NO-np [NO-np (10 mg/kg)], therapy of 20 mg/kg NO-np [NO-np (20 mg/kg)], therapy of 20 mg/kg control-np [Control-np (20mg/kg)], and only the vehicle solution [vehicle (no nanoparticles)]. The two concentrations of NO-np were used to study intravascular NO dose effects. One concentration of control-np (20 mg/kg) was used because there was no expected effect in NO bioavailability from these particles in circulation. A vehicle group was included (50 μl deoxygenated saline) to establish the effects of the nanoparticles.
Data analysis.
Results are presented as means ± SD. Data within each group were analyzed using ANOVA for repeated measurements (Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunns multiple-comparison test. Data between groups were analyzed using two-way ANOVA nonparametric repeated measurements, and, when appropriate, post hoc analyses were performed using Bonferroni tests. The Grubbs' method was used to assess closeness for all measured parameters values at baseline and after the infusion of PBH. Microhemodynamic measurements were compared with baseline levels obtained before the experimental procedure. A ratio of 1.0 signifies no change from baseline, whereas lower and higher ratios are indicative of changes proportionally lower and higher than baseline (i.e., 1.5 would mean a 50% increase from the baseline level). The same vessels and functional capillary fields were followed so that direct comparisons to their baseline levels could be performed, allowing for more robust statistics for small sample populations. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, San Diego, CA). Changes were considered statistically significant if P < 0.05.
RESULTS
Twenty hamsters were used in the study. All animals tolerated the experimental protocol and were divided into the following four groups: NO-np (10 mg/kg) (n = 5, 65.2 ± 4.3 g); NO-np (20 mg/kg) (n = 5, 65.2 ± 4.6 g); control-np (20 mg/kg) (n = 5, 66.8 ± 5.9 g); and vehicle (n = 5, 64.6 ± 5.5 g). All animals included in the study passed the Grubbs' testm ensuring that all measured parameter values at baseline were within a similar population (P < 0.05).
Blood gas chemistry.
Blood chemistry results are shown in Table 1. The infusion of PBH increased total and acellular Hb in all groups. PBH-induced vasoconstriction treatment with NO-np increased total and acellular metHb compared with control-np and vehicle proportionally to the dose of NO-np used and in a time-dependent fashion. metHb was measured up to 4 h after the infusion in some of the animals treated with NO-np, and acellular and total metHb seemed to increase until 3 h, corresponding to the previously observed intravascular release of NO for NO-np (5). In all animals treated with NO-np, PBH was more sensitive to oxidation than Hb contained in the RBC. NO-np treatment also increased plasma nitrite and nitrate compared with baseline, control-np, and vehicle. No significant changes in blood gas parameters were measured.
Table 1.
Systemic parameters
| NO-np (10 mg/kg body wt) |
NO-np (20 mg/kg body wt) |
Control-np (20 mg/kg body wt) |
Vehicle |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 h | 2 h | 1 h | 2 h | 1 h | 2 h | 1 h | 2 h | |
| Total Hb, g/dl | 14.8 ± 0.4 | 16.5 ± 0.5* | 16.3 ± 0.4* | 16.7 ± 0.4* | 16.4 ± 0.4* | 16.6 ± 0.4* | 16.4 ± 0.5* | 16.7 ± 0.5* | 16.6 ± 0.5* |
| Acellular Hb, g/dl | 1.9 ± 0.3* | 1.7 ± 0.2* | 1.8 ± 0.2* | 1.6 ± 0.2* | 1.8 ± 0.3* | 1.7 ± 0.2* | 2.0 ± 0.3* | 1.8 ± 0.2* | |
| Total metHb, % | |||||||||
| Acellular metHb, % | 14 ± 3†§ | 19 ± 3†§ | 31 ± 4†¶§ | 46 ± 5†‡§ | 3 ± 1 | 5 ± 1 | 2 ± 1 | 4 ± 1 | |
| Nitrite, nM | 421 ± 32 | 923 ± 209*†§ | 1133 ± 235*†§ | 1039 ± 246*†§ | 1412 ± 331*†§ | 391 ± 88 | 366 ± 56 | 355 ± 47 | 384 ± 41 |
| Nitrate, μM | 1.3 ± 0.3 | 3.2 ± 0.9*†§ | 3.6 ± 0.7*†§ | 4.1 ± 1.2*†§ | 4.6 ± 1.3*†§ | 1.1 ± 0.3 | 1.5 ± 0.3 | 1.2 ± 0.3 | 1.4 ± 0.3 |
| Arterial Po2, mmHg | 57.6 ± 5.1 | 64.1 ± 6.2 | 63.2 ± 5.9 | 62.2 ± 4.8 | 65.1 ± 6.2 | 56.2 ± 6.2 | 59.2 ± 6.6 | 52.2 ± 7.2 | 51.2 ± 7.8 |
| Arterial Pco2, mmHg | 52.8 ± 5.3 | 48.2 ± 6.0 | 49.3 ± 6.0 | 47.1 ± 6.2 | 47.1 ± 5.6 | 53.2 ± 6.0 | 47.2 ± 7.0 | 53.2 ± 6.4 | 51.7 ± 5.9 |
| Arterial pH | 7.338 ± 0.020 | 7.331 ± 0.026 | 7.331 ± 0.019 | 7.325 ± 0.022 | 7.328 ± 0.022 | 7.344 ± 0.027 | 7.341 ± 0.025 | 7.343 ± 0.029 | 7.341 ± 0.024 |
Values are means ± SD. Baseline includes all the animals.
NO-np, nitric oxide-releasing nanoparticles; control-np, control nanoparticles; vehicle, deoxygenated saline; metHb, fraction of total Hb converted to metHb.
P < 0.05 compared with baseline;
P < 0.05 compared with next time point;
P < 0.05 compared with control-np at the identical concentration;
P < 0.05 compared with vehicle;
P < 0.05 compared with 10 mg/kg NO-np.
MAP and HR.
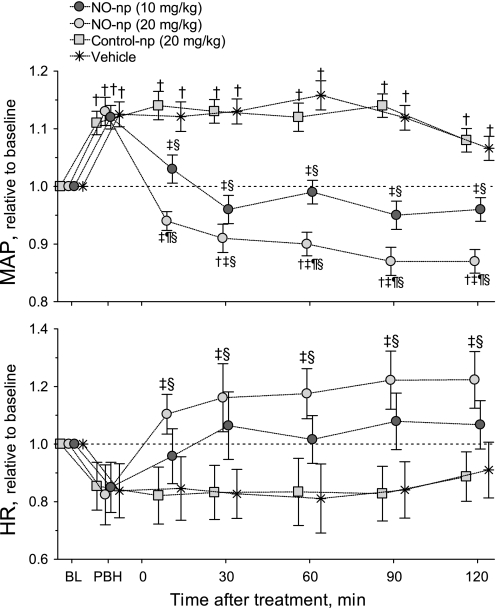
Figure 2 shows changes in MAP and HR relative to baseline for all groups. The infusion of PBH increased MAP in all groups (absolute baseline values are also provided). Treatment with 10 mg/kg NO-np decreased MAP compared with control-np and vehicle, and the higher dose of NO-np (20 mg/kg) decreased MAP compared with baseline and treatments with control-np and vehicle. The decrease of PBH-induced hypertension with NO-np extended throughout the observation period (2 h after the infusion of NO-np). Treatment with 20 mg/kg NO-np increased HR compared with control-np and vehicle, whereas the low dose of NO-np (10 mg/kg) did not have any impact on HR.
Fig. 2.
Changes of mean arterial pressure (MAP) and heart rate (HR) relative to BL. NO-np reversed the hypertension induced by PBH over the 2-h observation time. Control-np did not have any effect on MAP or HR. †P < 0.05 compared with BL; ‡P < 0.05 compared with control-np at the same time point; ¶P < 0.05 compared with 10 mg/kg NO-np; §P < 0.05 compared with vehicle. BL MAP values (means ± SD) were as follows: NO-np (10 mg/dl), 112 ± 6 mmHg; NO-np (20 mg/dl), 108 ± 8 mmHg; control-np (20 mg/dl), 114 ± 5 mmHg; and vehicle, 108 ± 7 mmHg. Baseline HR values (means ± SD) were as follows: NO-np (10 mg/dl), 433 ± 23 beats/min; NO-np (20 mg/dl), 428 ± 28 beats/min; control-np (20 mg/dl), 437 ± 25 beats/min; and vehicle, 425 ± 26 beats/min. Note that symbols for each experimental group have been artificially displaced at each time point for clarity. Control-NO, −4 min; NO-np (20 mg/kg), −1 min; NO-np (10 mg/kg), +1 min; and vehicle, +4 min.
Arteriolar vessel diameter and perfusion.
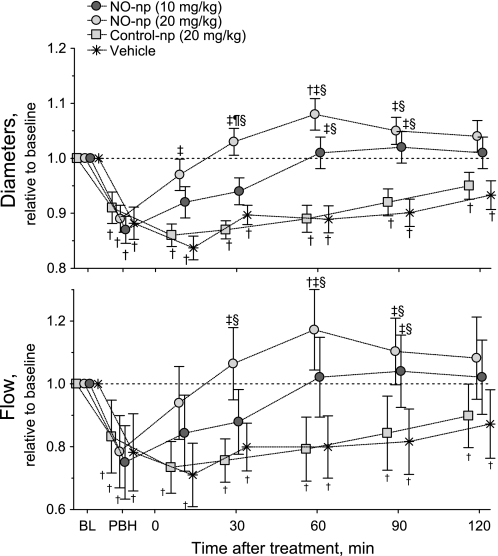
Figure 3 shows changes in arteriolar diameter and blood flow relative to baseline in all groups (absolute baseline values are also provided). The infusion of PBH produced significant vasoconstriction in all groups, and treatment with NO-np was able to restore arteriolar diameter. Diameters of groups treated with control-np and vehicle remained constricted over 2 h. Arteriolar flow decreased from baseline after the infusion of PBH, and NO-np corrected the decrease in arteriolar blood flow. Treatment with NO-np demonstrated significantly higher flows compared with control-np and vehicle.
Fig. 3.
Changes in microvascular hemodynamics (arteriolar vessel diameter and blood flow) relative to BL. NO-np reversed PBH-induced vasoconstriction over the 2-h observation time. Arteriolar blood flows were significantly decreased by PBH, and treatment with NO-np restored/increased blood flows. †P < 0.05 compared with BL; ‡P < 0.05 compared with control-np at the same time point; ¶P < 0.05 compared with 10 mg/kg NO-np; §P < 0.05 compared with vehicle. BL diameters (means ± SD) in arterioles were as follows: NO-np (10 mg/dl), 66 ± 8 μm, n = 24; NO-np (20 mg/dl), 62 ± 6 μm, n = 22; control-np (20 mg/dl), 60 ± 9 μm, n = 25; and vehicle, 66 ± 8 μm, n = 24. BL red blood cell velocities (means ± SD) in arterioles were as follows: NO-np (10 mg/dl), 4.6 ± 0.5 mm/s, n = 24; NO-np (20 mg/dl), 4.3 ± 0.6 mm/s, n = 22; control-np (20 mg/dl), 4.6 ± 0.6 mm/s, n = 25; and vehicle, 4.7 ± 0.6 mm/s, n = 24. BL blood flows (means ± SD) in arterioles were as follows: NO-np (10 mg/dl), 12.8 ± 3.2 nl/s, n = 24; NO-np (20 mg/dl), 11.1 ± 3.6 nl/s, n = 22; control-np (20 mg/dl), 13.0 ± 3.2 nl/s, n = 25; and vehicle, 14.2 ± 3.9 nl/s, n = 24. Note that symbols for each experimental group have been artificially displaced at each time point for clarity. Control-NO, −4 min; NO-np (20 mg/kg), −1 min; NO-np (10 mg/kg), +1 min; and vehicle, +4 min.
FCD.
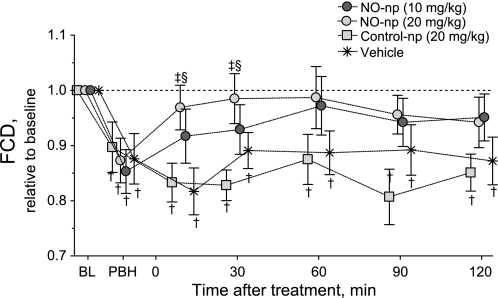
Figure 4 shows changes in FCD relative to baseline for all groups (absolute baseline values are also provided). FCD was significantly reduced from baseline after the infusion of PBH. Treatment with NO-np restored FCD to baseline levels. The reduction in FCD was sustained over the entire observation period for groups treated with control-np and vehicle.
Fig. 4.
Changes in functional capillary density (FCD) relative to baseline. NO-np restored FCD to BL levels, after FCD was decreased by the infusion of PBH. Conversely, control-np did not have any effect on FCD. †P < 0.05 compared with BL; ‡P < 0.05 compared with control-np at the same time point; §P < 0.05 compared with vehicle. BL FCD (means ± SD) were as follows: NO-np (10 mg/dl), 107 ± 11 cm−1; NO-np (20 mg/dl), 115 ± 10 cm−1; control-np (20 mg/dl), 102 ± 8 cm−1; and vehicle, 102 ± 6 cm−1. Note that symbols for each experimental group have been artificially displaced at each time points for clarity. Control-np, −4 min; NO-np (20 mg/kg), −1 min; NO-np (10 mg/kg), +1 min; and vehicle, +4 min.
DISCUSSION
The present study shows that increases in systemic blood pressure and peripheral vasoconstriction associated with an infusion of PBH can be reversed through the administration of NO-np. The results are consistent with a mechanism that involves restoring intravascular NO concentrations. If indeed the reversal is due to replenishing the NO scavenged by PBH, then the results support the infusion of NO-np in situations during which vascular NO bioavailability is decreased, such as acellular Hb transfusions or hemolysis. Therefore, NO-np can prevent or limit or simply counteract NO scavenging-induced hypertension and vasoconstriction complications by replacing the NO consumed by acellular Hb. The sustained release of NO from NO-np is reflected by the continued reversal of the hypertension and vasoconstriction compared with changes produced by control-np. Dose-dependent concentrations of exogenous NO released by NO-np were evidenced by the significant decrease in blood pressure, vasodilation, and acellular metHb formation. Acellular and total metHb formation was also time dependent in both groups that received NO-np. metHb was measured up to 4 h after the infusion in some of the animals treated with NO-np (data not included in the results), and acellular and total metHb seemed to increase until 3 h, corresponding to the previously observed intravascular release of NO for NO-np. Furthermore, these data support the generally accepted concept that PBH-induced hypertension and vasoconstriction are due to NO scavenging. This biological process is translated clinically as elevation of blood pressure, reduction of cardiac output, and increase of peripheral vascular resistance.
An additional hypothesis for vasoconstriction induced by acellular Hb solutions involves oxygen autoregulation (19). The theory proposed is that vascular smooth muscle cells act as oxygen sensors, regulating vessel diameter to adjust blood flow based on tissue oxygen metabolic demands. In the case of acellular Hb solutions, they enhance oxygen delivery by passive diffusion and convective transport of oxygen bound to Hb (42). However, as long as oxygen is mostly consumed outside the vessel wall, and the diffusion distances are the same before and after acellular Hb solution infusion, and the flux of oxygen through the vessel wall changes minimally. In the case that all the acellular oxyHb remains in the luminal space, the oxygen flux through the vessel wall is determined by the oxygen tension gradient and remains unaffected by the presence of acellular Hb. According to the Stokes-Einstein relation, facilitated diffusion is a function of the size of the oxyHb molecule and the viscosity of the Hb solution, and it is limited to the luminal space (42). Our study is not consistent with the observed vasoactivity changes being explained by the pure oxygen autoregulation theory, because PBH delivered similar amounts of oxygen to arterioles in animals treated with vehicle or control-np. On the other hand, treatment with NO-np increased perfusion, which increased the oxygen delivery capacity. The observed oxidation of PBH into metHb by NO released from NO-np decreases the PBH oxygen carrying capacity; however, within the first 30 min after treatment with 10 mg/kg NO-np, oxidized PBH was <10%, which makes an oxygenation-dependent explanation for the mechanism unlikely.
A study (43) in eNOS-deficient mice has suggested that the scavenging of endothelium-derived NO is an important mechanism responsible for the vasoconstriction observed after the administration of PBH. These results are limited, because as endothelium-derived NO is absent, and the parameter used to address vascular tone changes (MAP) is already affected. The lack of a further increase on pressure upon the infusion of PBH does not prove any causality. Additionally, knockout, transgenic, and gene-targeted models should be interpreted cautiously, as when a gene is deleted, there are sometimes developmental defects. Our results with normal healthy animals prove the link between NO and vascular complications of PBH. In this case, the hypertension and vasoconstriction resulting from large polymeric Hb solutions are clearly due to depletions of NO synthesized by the endothelium (43). A study by Yu et al. (44) demonstrated that inhaled gaseous NO prevented pulmonary and systemic hypertension induced by PBH infusion, although a large fraction of the observed effects were on pulmonary hemodynamics. Any effects beyond the lung vasculature during NO inhalation can only be attributed NO-related metabolites (such as nitrite). On the other hand, nitrite can be a unique vasodilator, in part due to NO production through the allosterically regulated nitrite reductase activity of Hb (31). Other strategies to attenuate NO scavenging by acellular Hbs include sodium nitrite as a means for compensating NO scavenging as result of hemolysis (31) and during resuscitation with HBOCs (38). Another approach contemplated to overcome the NO scavenging effects is to coinfuse S-nitrosohemoglobin along with an unmodified HBOC, which seems to be effective under acute hypoxic, but not normoxic, conditions (23). Since NO synthesized by endothelial cells is the major source of NO to the vasculature (26), we focused our research on developing an intraluminal source of NO. The present results suggest that a fraction of the intravascular NO released by NO-np diffuses abluminally to reach the smooth muscle of the microvessel of peripheral tissues, an effect that cannot be expected from inhaled NO. Moreover, as intraluminal NO is expected to be extremely low, a minor increase in intraluminal NO affects the diffusion gradients of endothelium-derived NO, thus allowing the endothelium-derived NO to diffuse further into the parenchymal tissues.
PBH is a nonspecific glutaraldehyde polymerized Hb, and, when used to resuscitate from hemorrhagic shock, it restores blood pressure by increasing vascular resistance (6). Katz et al. (24) used nitroglycerin coadministered with PBH to reduce vasoconstriction during resuscitation from hemorrhagic shock in a swine model, and they were able to obtain a transient decrease in constriction. This result can be easily forecasted, as nitroglycerine is an organic nitrate that releases NO from a three-electron reduction process. The reducing agents involved in nitroglycerine bioactivation are based on specific enzymes, with the main limitation that all organic nitrates lose their effectiveness by continuous use (so-called nitrate tolerance) (13). The proposed mechanisms for nitrate tolerance include depletion of tissue thiols, desensitivity of sGC, or increase in the breakdown of cGMP by a paradoxical upregulation of phosphodiesterases (PDEs) (25). Additionally, the short half-life of nitroglycerin and other NO donors results in harmful or ineffective NO levels, as their optimal dose is difficult to determine. On the other hand, NO-nps are novel NO generators without the organic nitrite limitations, because they do not require external reducing agents. During NO-np production, nitrite is reduced to NO through a sugar-mediated thermal reduction, and the pure NO remains trapped in the particles until they are hydrated. Therefore, the amount of NO released by NO-np is independent of catalytic agents. Only during sGC desensitization (mechanism of NO tolerance) can we expect to see a decreased responsiveness to NO-np.
Other alternatives to prevent the hypertension and vasoconstriction induced by PBH include coadjunct therapy with sildenafil, a PDE5 inhibitor. The proposed mechanism, as mentioned above, is to enhance the vasodilatory effects of the endogenously generated NO by limiing cGMP breakdown, inhibition of which appears capable of preventing some of the negative hemodynamic changes induced by PBH alone (14). A combination of PDE5 inhibitors with the administration of exogenous NO from NO-np will increase the efficacy of a given dose of NO-np, decreasing the amount of NO-np needed to counteract the hemodynamic consequences of PBH and increasing the clinical scenarios where PDE5 inhibition may be applicable. The potential therapeutic collaboration between sildenafil (or another PDE5 inhibitor) and NO-np has been shown to produce minor effects on MAP but significant vasodilation (5). Thus, their coadmintration improves blood flow and permits the enhancement of oxygen delivery to tissues, even without the need to increase the oxygen carrying capacity (5). Several other strategies have been explored to reduce NO scavenging by acellular Hb solutions; a variety of molecular modifications and efforts to create modified Hb through recombinant technology have been undertaken, with varying degrees of success (36). Unfortunately, these efforts are often expensive, which raises questions regarding the cost of a commercial scale up of the process. The use of NO-np, possibly in combination with a PDE5 inhibitor, should increase the overall efficacy of any of the acellular Hb-based approaches.
The compartmentalization of Hb within the erythrocyte limits scavenging of NO through the NO dioxygenase reaction. During intravascular hemolysis, NO physiology is disrupted by the release of acellular Hb, leading to enhanced scavenging of NO by the released Hb, resulting in vasoconstriction. This hemolysis-triggered vasoconstriction is similar in origin to what occurs through the NO scavenging appreciated during the administration of acellular HBOCs. Our findings demonstrate that NO-np can prevent/reverse the negative effects of acellular Hb and therefore should function to produce vasodilation during acute or chronic hemolysis. The levels of acellular Hb after the infusion of PBH were comparable with those seen in hemolysis resulting from sickle cell vasoocclusive crisis (37) and during other clinically relevant human hemolytic conditions (e.g., cardiopulmonary bypass, hemoglobinuria, alloimmune hemolysis, etc) (33). All of these conditions have been associated with progressive vasculopathy and hypertension and are associated with systemic NO scavenging by acellular Hb (32). NO nanoparticles may have a therapeutic role in minimizing the vascular toxicities of more severe episodes of intravascular hemolysis, such as erythrocyte parasitic diseases. In these scenarios, the ability of NO to attenuate the pathophysiological effects of acellular Hb has been amply demonstrated, but a practical mechanism to administer NO remains to be defined (16). NO-nps are unique vasodilators under normal physiological conditions (5), and, in the presence of acellular Hb, they can minimize the expected vascular adverse events.
It should be noted that the model in the present study is one in which vascular volume is actually added to an isovolemic animal. The hypervolemic infusion model used to evaluate vasoconstrictive responses induced by PBH has all the regulatory mechanisms responsible for vasoconstrictive responses, fully represented compared with the clinically relevant conditions where HBOCs are expected to be used (e.g., exchange transfusion and resuscitation from hemorrhagic shock). However, metHb formation, inherent to the NO reaction with Hb, can limit the oxygen carrying benefits of PBH; therefore, an appropriate balance among the anemic conditions, supplemental oxygen carrying capacity, and exogenous NO from NO-np needs to be defined for each condition to attain the maximal benefit. Mechanistically, as the acellular Hb and NO-np are in the intravascular compartment, a significant fraction of the NO released by NO-np is consumed by Hb, both acellular and inside the erythrocytes. This NO consumption due to the reactions between NO and Hb is responsible for the increase in metHb. Only a fraction of the NO released by NO-np is accountable for restoring the effects of the physiologically synthesized NO consumed by acellular Hb. However, as more acellular Hb is oxidize to metHb, the intravascular NO scavenging is reduced, and it is possible that more NO released from NO-np reaches the extravascular compartment. Moreover, the pure NO released by NO-np is capable of inducing oxidative and covalent modification of protein amino acid residues, such as S-nitrosation, the addition of a nitroso group to a thiol to form nitrosothiols.
In conclusion, this study has provided new data suggesting that the negative hemodynamic conditions resulting from reduced NO availability can be prevented/reversed by NO-np. NO-np was highly effective in restoring systemic and microvascular hemodynamics after the PBH infusion. The physiological responses to intravascular infusion of NO-np are consistent with a sustained release of physiologically relevant levels of NO, as reflected by the of reversal the PBH-induced increase on blood pressure and vascular constriction. Until now, both topical and injection experiments have failed to show any toxicity attributable to NO-nps (5). However, future studies to address the biodistribution, clearance, and biocompatibility of NO-nps are essential to determine the true clinical relevance. Additionally, studies in other animal models, and under other conditions such as low volume/pressure or hypoxia, would be of interest in determining the extended value of combined NO-np and plasma expander therapy using other HBOCs. Given the importance of NO in such a wide range of health issues, there has been enormous growth in the use of exogenous NO to examine its effects in cells, tissues, whole animals, and humans.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL062354 and P01-HL071064 and FJC: a Foundation of Philanthropic Funds. A. J. Friedman was supported by research grants from the American Society for Dermatologic Surgery Cutting Edge Program, the La-Roche Posay North American Foundation, and the Women's Dermatologic Society.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Froilan P. Barra and Cynthia Walser for surgical preparation of the animals.
REFERENCES
- 1. Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov 3: 152–159, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Amberson WR, Jennings JJ, Rhode CM. Clinical experience with hemoglobin-saline solutions. J Appl Physiol 1: 469–489, 1949 [DOI] [PubMed] [Google Scholar]
- 3. Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol 34: 34–40, 1997 [PubMed] [Google Scholar]
- 4. Bunn HF, Esham WT, Bull RW. Renal handling of hemoglobin. I. Glomerular filtration. J Exp Med 129: 909-&, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long lived circulating nanoparticles. Free Rad Med Biol 49: 530–508, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabrales P, Tsai AG, Intaglietta M. Polymerized bovine hemoglobin can improve small-volume resuscitation from hemorrhagic shock in hamsters. Shock 31: 300–307, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol Heart Circ Physiol 246: H508–H517, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Endrich B, Asaishi K, Götz A, Messmer K. Technical report–a new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med 177: 125–134, 1980 [DOI] [PubMed] [Google Scholar]
- 9. Finucane ML, Slovic P, Mertz CK. Public perception of the risk of blood transfusion. Transfusion 40: 1017–1022, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Freilich D, Pearce LB, Pitman A, Greenburg G, Berzins M, Bebris L, Ahlers S, McCarron R. HBOC-201 vasoactivity in a phase III clinical trial in orthopedic surgery subjects–extrapolation of potential risk for acute trauma trials. J Trauma Injury Infect Crit Care 66: 365–376, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, Friedman JM. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 19: 12–20, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Gilligan DR, Altschule MD, Katersky EM. Studies of hemoglobinemia and hemoglobinuria produced in man by intravenous injection of hemoglobin solutions. J Clin Invest 20: 177–187, 1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gori T, Parker JD. The puzzle of nitrate tolerance: pieces smaller than we thought? Circulation 106: 2404–2408, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Gotshall RW, Hamilton KL, Foreman B, van Patot MC, Irwin DC. Glutaraldehyde-polymerized bovine hemoglobin and phosphodiesterase-5 inhibition. Crit Care Med 37: 1988–1993, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gould SA, Moore EE, Hoyt DB, Burch JM, Haenel JB, Garcia J, DeWoskin R, Moss GS. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg 187: 112–120, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med 12: 1417–1422, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Greenburg AG. Benefits and risks of blood transfusion in surgical patients. World J Surg 20: 1189–1193, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Greenburg AG, Kim HW. Use of an oxygen therapeutic as an adjunct to intraoperative autologous donation to reduce transfusion requirements in patients undergoing coronary artery bypass graft surgery. J Am Coll Surg 198: 373–384, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Guyton AC, Langston JB, Navar G. Theory for renal autoregulation by feedback at the juxtaglomerular apparatus. Circ Res Suppl 15: 187–197, 1964 [PubMed] [Google Scholar]
- 20. Homer K, Wanstall J. In vitro comparison of two NONOates (novel nitric oxide donors) on rat pulmonary arteries. Eur J Pharmacol 356: 49–57, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Intaglietta M, Silverman NR, Tompkins WR. Capillary flow velocity measurements in vivo and in situ by television methods. Microvasc Res 10: 165–179, 1975 [DOI] [PubMed] [Google Scholar]
- 22. Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res 5: 309–312, 1973 [DOI] [PubMed] [Google Scholar]
- 23. Irwin D, Buehler PW, Alayash AI, Jia Y, Bonventura J, Foreman B, White M, Jacobs R, Piteo B, TissotvanPatot MC, Hamilton KL, Gotshall RW. Mixed S-nitrosylated polymerized bovine hemoglobin species moderate hemodynamic effects in acutely hypoxic rats. Am J Respir Cell Mol Biol 42: 200–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katz LM, Manning JE, McCurdy S, Sproule C, McGwin G, Jr, Moon-Massat P, Cairns CB, Freilich D. Nitroglycerin attenuates vasoconstriction of HBOC-201 during hemorrhagic shock resuscitation. Resuscitation 81: 481–487, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC, Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation 104: 2338–2343, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Lancaster JR. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci USA 91: 8137–8141, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide 1: 18–30, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res 15: 93–101, 1978 [DOI] [PubMed] [Google Scholar]
- 29. Marcucci C, Madjdpour C, Spahn DR. Allogeneic blood transfusions: benefit, risks and clinical indications in countries with a low or high human development index. Br Med Bull 70: 15–28, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Maton A, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD. Human Biology and Health. Englewood Cliffs, NJ: Prentice Hall, 1993 [Google Scholar]
- 31. Minneci PC, Deans KJ, Shiva S, Zhi H, Banks SM, Kern S, Natanson C, Solomon SB, Gladwin MT. Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis. Am J Physiol Heart Circ Physiol 295: H743–H754, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest 115: 3409–3417, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naumann HN, Diggs LW, Barreras L, Williams BJ. Plasma hemoglobin and hemoglobin fractions in sickle cell crisis. Am J Clin Pathol 56: 137–147, 1971 [DOI] [PubMed] [Google Scholar]
- 34. Patel RP. Biochemical aspects of the reaction of hemoglobin and NO: implications for Hb-based blood substitutes. Free Radic Biol Med 28: 1518–1525, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Qiu W, Kass DA, Hu Q, Ziegelstein RC. Determinants of shear stress-stimulated endothelial nitric oxide production assessed in real-time by 4,5-diaminofluorescein fluorescence. Biochem Biophys Res Commun 286: 328–335, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Reid TJ. Hb-based oxygen carriers: are we there yet? Transfusion 43: 280–287, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez C, Vitturi DA, He J, Vandromme M, Brandon A, Hutchings A, Rue LW, 3rd, Kerby JD, Patel RP. Sodium nitrite therapy attenuates the hypertensive effects of HBOC-201 via nitrite reduction. Biochem J 422: 423–432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Stramer SL, Hollinger FB, Katz LM, Kleinman S, Metzel PS, Gregory KR, Dodd RY. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion 49: 1S–235S, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Winterbourn CC. Reaction of superoxide with hemoglobin. In: CRC Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC, 1985, p. 137–141 [Google Scholar]
- 42. Wittenberg JB. The molecular mechanism of hemoglobin-facilitated oxygen diffusion. J Biol Chem 241: 104–114, 1966 [PubMed] [Google Scholar]
- 43. Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation 117: 1982–1990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu B, Volpato GP, Chang K, Bloch KD, Zapol WM. Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide. Anesthesiology 110: 113–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]