Abstract
2-Aminoethoxydiphenyl borate (2-APB) analogs are potentially better vascular gap junction blockers than others widely used, but they remain to be characterized. Using whole cell and intracellular recording techniques, we studied the actions of 2-APB and its potent analog diphenylborinic anhydride (DPBA) on vascular smooth muscle cells (VSMCs) and endothelial cells in situ of or dissociated from arteriolar segments of the cochlear spiral modiolar artery, brain artery, and mesenteric artery. We found that both 2-APB and DPBA reversibly suppressed the input conductance (Ginput) of in situ VSMCs (IC50 ≈ 4–8 μM). Complete electrical isolation of the recorded VSMC was achieved at 100 μM. A similar gap junction blockade was observed in endothelial cell tubules of the spiral modiolar artery. Similar to the action of 18β-glycyrrhetinic acid (18β-GA), 2-APB and DPBA depolarized VSMCs. In dissociated VSMCs, 2-APB and DPBA inhibited the delayed rectifier K+ current (IK) with an IC50 of ∼120 μM in the three vessels but with no significant effect on Ginput or the current-voltage relation between −140 and −40 mV. 2-APB inhibition of IK was more pronounced at potentials of ≤20 mV than at +40 mV and more marked on the fast component than on the slow component, which was mimicked by 4-aminopyridine but not by tetraethylammonium, nitrendipine, or charybdotoxin. In contrast, 18β-GA caused a linear inhibition of IK between 0 to +40 mV, which was similar to the action of tetraethylammonium or charybdotoxin. Finally, the 2-APB-induced inhibition of electrical coupling and IK was not affected by the inositol 1,4,5-trisphosphate receptor antagonist xestospongin C. We conclude that 2-APB analogs are a class of potent and reversible vascular gap junction blockers with a weak side effect of voltage-gated K+ channel inhibition. They could be gap junction blockers superior to 18β-GA only when Ca2+-actived K+ channel inhibition by the latter is a concern but inositol 1,4,5-trisphosphate receptor and voltage-gated K+ channel inhibitions are not.
Keywords: gap junction, cochlea, diphenylborinic anhydride, 18β-glycyrrhetinic acid, xestospongin C
gap junctions play a key role in the development, structure, and physiology of blood vessels, including the inner ear artery (16, 19, 32, 52). For instance, the maintenance and regulation of vascular tone rely on gap junction-mediated interactions among vascular smooth muscle cells (VSMCs) and endothelial cells (ECs) (15, 53). Vasoactive agents, such as ACh, substance P, and bradykinin, cause a primary hyperpolarization in ECs and a secondary hyperpolarization in SMCs via so-called EDHF as well as nitric oxide and prostaglandin production (5). Studies (19, 52) on various vascular preparations have suggested that gap junction coupling, K+, cytochrome P-450 products (e.g., epoxyeicosatrienoic acids), etc. act as EDHFs, but, among them, gap junction coupling appears to be the major and universal mechanism. In the cochlear spiral modiolar artery (SMA), the secondary hyperpolarization in VSMCs is mainly (60%) mediated by gap junctions (32). Gap junction malfunctions have been implicated in many diseases, including those of the cardiovascular, skin, and auditory systems (16, 44, 47). Gap junction blockers and activators are important tools in characterizing these intercellular mechanisms and pathologies as well as for treatment of these diseases (10, 14).
Several groups of compounds have been shown to inhibit gap junction communication (48), including long-chain alcohols (35), gaseous anesthetics (62), glycyrrhetinic acid (GA) derivatives (11, 21), fenamates (26), and connexin (Cx)-mimic peptides (36). Unfortunately, the great majority of these compounds have shown a limited and variable effect in blocking gap junctions in the vessels of different organs and species. The variable effectiveness could be due to the molecular heterogeneity of the channel proteins (Cx) of the gap junctions (16, 24, 27, 44). Moreover, these agents have been found to exert more or fewer side effects on nonjunctional membrane channels (7, 58). Therefore, there is a pressing need for specific, potent, and reversible gap junction blockers.
Using cellular electrophysiological analysis, we (21) have previously demonstrated that 18β-GA at 30 μM completely blocked the electrical coupling among VSMCs and ECs in the cochlear artery (SMA) along with a mild inhibition on the delayed rectifier K+ current (IK) and noted it as the best-known vascular gap junction blocker. Another GA compound, 18α-GA, was half as potent in blocking gap junctions, with a similar nonjunctional side effect.
2-Aminoethoxydiphenyl borate (2-APB) and its analogs have recently been reported to reversibly inhibit gap junction-mediated electrical coupling in cultured cells (2, 25) and chemical communication between cultured or retinal cells (48, 54). Bai et al. (2) reported that 2-APB potently blocks gap junctions formed by Cx36 and Cx50 proteins (IC50: 3–3.7 μM) but not gap junctions of Cx45, Cx46, and Cx43 (IC50: 18–52 μM). More importantly, 2-APB was found to directly act on the channel protein (54). Therefore, 2-APB and its analogs may be better gap junction blockers than 18β-GA in small arteries and arterioles, considering that all other known vascular gap junction blockers, including GA compounds, are unlikely to act directly on Cx proteins (54). In this regard, 2-APB at a low concentration (10 μM) has been used to block electrocoupling-mediated EDHF and secondary relaxation in rabbit iliac arteries (13, 20). However, the potency of 2-APB on gap junction and nonjunctional channels in native vascular tissues remains to be characterized.
The present study not only determined the potency and efficacy for 2-APB and its potent analog diphenylborinic anhydride (DPBA) on junctional and nonjunctional conductances in native arteriolar cells but also compared them with those of 18β-GA (21). These comparative results yielded a proper strategy in selecting the best vascular gap junction-blocking agent among these two classes of compounds. The results provide a scientific basis for rational data interpretation in vascular research where these compounds are applied. Portions of these results have appeared in two meeting abstracts (40, 41).
METHODS
Preparation of arteriolar segments of the SMA, brain artery, and mesenteric artery.
Guinea pigs (250–450 g) were killed by exanguination under deep general anesthesia by an intramuscular injection of an anesthetic mixture (1 ml/kg) of ketamine (500 mg), xylazine (20 mg), and acepromazine (10 mg) dissolved in 8.5 ml water. The SMA was isolated as previously described (34). The whole length of the SMA was dissected from the cochlea. Brain arteriolar (BA) segments were harvested from the branches of anterior inferior cerebellar artery in the pia. The mesenteric artery (MA) and its branches were harvested from the upper ileum mesentery. Further manual cleaning of connective tissues was performed with the vessels immerged in Krebs solution composed of (in mM) 125 NaCl, 5 KCl, 1.6 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 20 NaHCO3, and 7.5 glucose and saturated with 95% O2-5% CO2 at 35°C (pH 7.4). Animal procedures were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University.
The SMA or other arteriole branches were sectioned into 3- to 4-mm-long segments for conventional intracellular recordings. For whole cell recordings of SMCs in situ of the vessel segments (21), a short length of the vessel [∼0.4 mm long, outer diameter (OD): 30–50 μm] was transferred to a glass-bottomed petri dish filled with a normal external solution composed of (in mM) 138 NaCl, 5 KCl, 1.6 CaCl2, 1.2 MgCl2, 5 Na-HEPES, 6 HEPES, and 7.5 glucose with pH 7.4 and an osmolarity of 300 mosM. The preparation was secured in the dish by platinum strips on each end and digested with collagenase A (1.5 mg/ml, Roche) dissolved in the normal external solution at 37°C for 15 min. After complete washout of the enzyme, the vessel was further cleaned of its adventitial tissue. The petri dish containing the preparation was placed onto the stage of an inverted microscope (Axiovert 35, Zeiss) equipped with a camera and micromanipulators (MX7600R and MX10L, Siskiyou Design Instruments). The vessel segment and the electrode pipette were visualized at a magnification of 10 × 20 or 10 × 40 with the DIC function on.
Dissociation of SMCs and ECs.
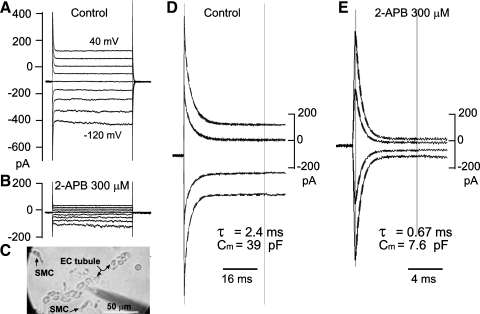
Dissociated VSMCs and ECs were prepared from the SMA or arteriolar branches of the BA or MA of guinea pigs. The cleaned arterioles were incubated for 20 min in a low-Ca2+ buffer solution containing (in mM): 142 NaCl, 5 KCl, 0.05 CaCl2, 1 MgCl2, 4 Na-HEPES, 5 HEPES (pH 7.2), and 7.5 glucose. The arterioles were then cut into 1-mm-long segments and digested for 20–25 min at 37°C with a buffer solution containing papain (1.5 mg/ml), collagenase A (2 mg/ml), BSA (3.75 mg/ml), and DTT (0.3 mg/ml). After centrifugation (67 g for 5 min) and replacement of the supernatant with enzyme-free buffer three times, the preparation was triturated with a pasteur pipette. The cell-rich suspension was transferred to a petri dish with a coverslip bottom coated with poly-l-lysine. Once the dissociated cells attached to the glass bottom, the dish was mounted onto the inverted microscope (Axiovert 35, Zeiss) and perfused with the normal extracellular solution (see above) for whole cell recordings. VSMCs were identified by their characteristic spindle shape (see Fig. 7C) (4, 21, 50). ECs were identified in the same dish by their oval shape with a shorter diameter (≥8 μm) combined with their characteristic membrane response: a hyperpolarization or an outward current response to 3 μM ACh, which was distinct from that of VSMCs (Table 1 vs. Table 2) (21). There was a chance that 5–10 ECs would remain joined as a tubule after dispersion of the SMA segments. These ECs in tubules could be identified for certain by the morphology visualized under a DIC microscope (see Fig. 7C).
Fig. 7.
2-APB suppressed the gap junction coupling with little other membrane effects in ECs tubules from the SMA. A and B: current traces elicited by 500-ms step commands in the absence (A) and presence (B) of 2-APB from a cell in a tubule of ECs (C; the electrode pipette points to a cell other than that in A). D and E: representation of the initial part of A and B, respectively, but with traces of −100-, −60-, −40-, and 20-mV steps removed and the time scale expanded for clarity. Note that single-term exponential function fit well with the transients in both the absence and presence of 2-APB. Rinput increased from 334 MΩ to 1.7 GΩ, suggesting that 300 μM 2-APB completely isolated the recorded cell in the control condition, under which the cell appeared tightly coupled to at least four other cells. The EC showed an obvious inward, but not outward, rectification in the voltage range tested.
Table 1.
Membrane properties of smooth muscle cells in the whole cell configuration in situ of short segments of the SMA, BA, and MA and in dispersed status
| Cells In Situ of the Vessel Segment |
Dissociated Single Cells |
|||||
|---|---|---|---|---|---|---|
| Membrane Property | SMA | BA | MA | SMA | BA | MA |
| Input resistance, MΩ | 557 ± 57 | 480 ± 54.1 | 306 ± 38.9* | 3,744 ± 241 | 3,603 ± 237 | 2,827 ± 161‡§ |
| n | 34 | 23 | 32 | 29 | 50 | 50 |
| Input conductance, nS | 2.36 ± 0.19 | 2.82 ± 0.34 | 4.57 ± 0.51* | 0.30 ± 0.02 | 0.34 ± 0.02 | 0.42 ± 0.03†§ |
| n | 34 | 23 | 32 | 29 | 50 | 50 |
| Input capacitance, pF | 67 ± 14.8 | 107 ± 27.5 | 240 ± 75.4‡ | 6.13 ± 0.19 | 9.03 ± 0.65‡ | 13.3 ± 0.42‡§ |
| n | 23 | 11 | 15 | 55 | 16 | 10 |
Values are means ± SE; n, number of cells. Input resistance and input conductance were measured between −60 and −40 mV. Input capacitance was measured by a step from −40 to −100 mV. SMA, spiral modiolar artery; BA, brain artery; MA, mesenteric artery.
P < 0.01, comparison between the MA and SMA or BA;
P < 0.05 and
P < 0.01, comparison between the SMA and BA or MA;
P < 0.05, comparison between the BA and MA.
Table 2.
Membrane properties of endothelial cells from the SMA
| Membrane Property | Cells In Situ of a Tubule | Dissociated Single Cells |
|---|---|---|
| Input resistance, MΩ | 279 ± 64.5 | 982 ± 109 |
| n | 5 | 9 |
| Input conductance, nS | 4.1 ± 1.03 | 1.14 ± 0.14 |
| n | 5 | 9 |
| Input capacitance, pF | 296 ± 166 | 17.7 ± 4.9 |
| n | 5 | 8 |
Values are means ± SE; n, number of cells. Input resistance and input conductance were measured between −40 and −20 mV (see Fig. 7). Input capacitance was measured by a step from −40 to 20 mV.
Tight-seal whole cell recordings.
The specimen was continuously superfused with the normal external solution (0.2 ml/min) at room temperature (22–25°C). Conventional whole cell recordings were performed using an Axopatch 1D amplifier (Molecular Devices). Recording pipettes were pulled by a Sutter Instruments P-2000 puller. The pipette had a tip of ∼1-μm OD and a resistance of ∼5 MΩ after being filled with an internal solution containing (in mM) 130 K-gluconate, 10 NaCl, 2.0 CaCl2, 1.2 MgCl2, 10 HEPES, 5 EGTA (118 nM free Ca2+), and 7.5 glucose adjusted to pH 7.2 and to an osmolarity of 290 mosM. Pipette capacitance was well compensated after a gigaseal with the cell was achieved. Membrane currents or voltage signals were low-pass filtered at 1 or 10 kHz (−3 dB); data were recorded on a computer equipped with a Digidata 1322A AD interface and pCLAMP 9.2 software (Axon Instruments) at sampling intervals of 10, 20, or 100 μs. A Minidigi digitizer and Axoscope 9.2 software (Axon Instruments) were used to simultaneously carry out gap-free recording at a sampling interval of 50 ms.
The transient current passing the membrane input capacitance (Cinput) was routinely uncompensated to monitor and calculate the access resistance (Ra) and other membrane parameters online or offline. The offline calculation was done by an exponential fit to capacitive current transients and by commonly used equations (1, 21, 39). Cinput for in situ cells was calculated according to the following equation: C = Q/V, where the charge (Q) was obtained by a three- or four-term exponential fit to the current transient elicited by a voltage step (V; in mV) (Fig. 1C and Supplemental Material, Supplemental Fig. 1).1 The voltage clamping error introduced by the current (I) passing the access resistance was corrected offline according to the following equation: Vm = Vc − I × Ra (where Vm is the actual clamping membrane voltage and Vc is the apparent command voltage) except where noted otherwise. Leak subtraction was done offline when appropriate.
Fig. 1.
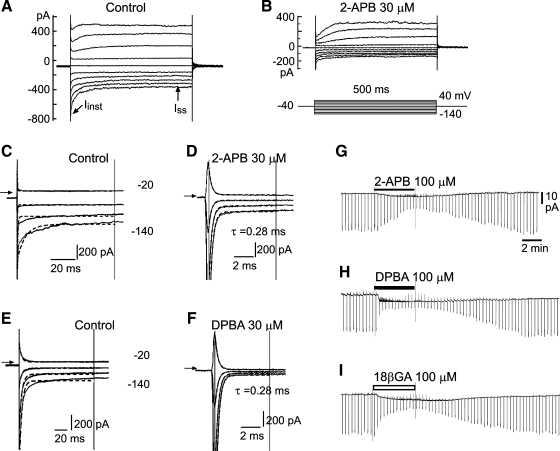
2-Aminoethoxydiphenyl borate (2-APB) and its analog diphenylborinic anhydride (DPBA) inhibit the electrical coupling of smooth muscle cells (SMCs) in situ of arteriolar segments. A and B: whole cell current traces induced by voltage steps (inset in B) in 20-mV increments before (A) and during (B) application of 30 μM 2-APB to the spiral modiolar artery (SMA) segment. Note that 2-APB caused a substantial reduction in the steady-state current (Iss), reflecting an increase in membrane input resistance (Rinput) from 0.37 to 0.87 GΩ in this cell. C and D: time scale-expanded presentation of the initial part of A and B, respectively (some traces were omitted for clarity), showing that the current transients in the control (C) fitted poorly (r ≤ 0.90) with a single-term exponential function (dashed lines between two cursors) but fitted well with a three-term exponential function (see Supplemental Fig. 1). In the presence of 2-APB (D; r > 0.96), a single-term exponential function fitted well with the current transient. τ, Time constant for the bottom trace. The arrows indicate the zero current level. E and F: whole cell currents showing that 30 μM DPBA caused another cell to increase in Rinput from 0.55 to 2.1 GΩ and that there was a change of capacitive transient decay from a multiple-term to a single-term exponential fashion. The calculated membrane input capacitance was 131, 95, 6.1, and 6.4 pF for the cells in C, E, D, and F, respectively. The results shown in A and E were from different cells in situ of a brain artery (BA). G–I: gap-free traces showing the time course of input conductance changes by 2-APB, DPDA, and, for comparison, 18β-glycyrrhetinic acid (18βGA) in a vascular SMC (VSMC) of the mesenteric artery (MA). The downward deflections indicate the amplitudes of Iss induced by a 2-s step from a holding potential of −40 to −100 mV. Note that DPBA exhibited faster washin effects than the other two agents.
Intracellular recordings.
Conventional intracellular recordings were conducted from cells of the SMA, BA, and MA as previously described (33). Briefly, a 2- to 5-mm-long segment of the SMA or other arteriole branches of 40–80 μm in OD was pinned with minimum stretch to the silicon rubber layer (Sylgard 184, Dow Corning) in the bottom of the bath chamber (volume: 0.5 ml) and continuously superfused with Krebs solution at 35°C. The glass microelectrode was filled with 2 M KCl and had a resistance of 60–150 MΩ. Intracellular impalement was obtained at the adventitial surface of the vessel with a micromanipulator (MP-1, Narishige). The transmembrane potential and injected current were simultaneously monitored with a NPI preamplifier (SEC10-LX, NPI) and recorded with a computer equipped with pCLAMP8 software (Axon Instruments) at sampling intervals of 0.1, 0.5, or 10 ms. The resting potential (RP) was normally determined 5 min after the initial voltage jump at the penetration and checked by the voltage jump at the withdrawal of the electrode.
Drug application and statistics.
Drugs were applied by superfusion via an array of capillary inlets near the preparation in the dish. The solution flowing over the preparation could be switched to one that contained drug(s) or one of different ionic composition by shifting the inlets without change in flow speed. The drugs used in this study included ACh, 4-aminopyridine (4-AP), tetraethylammonium (TEA), 2-APB, DPBA, and xestospongin C (XeC) (all from Sigma Research Biochemicals) as well as 18β-GA (MP Biomedicals). The compounds 2-APB and 18β-GA were dissolved in DMSO as stock solutions before being further diluted with normal Krebs solution or HEPES external solution to final concentrations. DMSO in the final solutions was ≤0.1%, which alone showed no detectable effect on the membrane voltage or current. Statistical values are expressed as means ± SE.
RESULTS
General findings.
Intracellular recordings were made from >100 cells with resting potentials of −61 ± 2.3 mV (n = 65), −69 ± 2.1 mV (n = 32), and −72 ± 1.9 mV (n = 25) in the SMA, BA, and MA, respectively. As we previously reported (34), the RP of SMA cells showed a robust bimodal distribution with a border at approximately −60 mV and mean RP values of low- and high-RP cells at −39.2 ± 1.28 mV (n = 23) and −73.3 ± 1.58 mV (n = 33), respectively. Cells from the BA and MA showed a less prominent bimodal distribution (40).
Whole cell recordings were made on in situ and dissociated VSMCs of the SMA, BA, and MA from ∼70 guinea pigs. Step and ramp voltage commands from a holding potential of −40 mV were routinely applied to determine the membrane properties of the cell. The current transients during the voltage steps showed a time course that fitted poorly to a single-term exponential function in cells in situ of all the three vessels (Fig. 1, A, C, and E) but fitted well to a three- or four-term exponential function (Supplemental Fig. 1). This implicated a multiple membrane source in the charging circuit, suggesting the existence of electrical coupling among multiple cells in the vessel. On the other hand, dissociated VSMCs from any kind of vessel showed a step-induced capacitive current that was fast in decay and fitted well with a single-term exponential function (see below). We took the slope conductance (or resistance) between −60 and −40 mV in the whole cell current-voltage (I-V) curve as the measurement of input conductance [Ginput; the reciprocal of input resistance (Rinput)] to minimize the implication of rectification. We used the current transient elicited by the voltage step from −40 to −100 mV for Cinput estimation to standardize the measurements.
Data of Rinput, Ginput, and Cinput of in situ and dissociated VSMCs from the three arterioles are shown in Table 1. There were several findings of note. First, the Ginput of in situ cells was 7.87, 8.29, and 10.9 times that of dissociated cells in the SMA, BA, and MA, respectively, indicative of a tighter electrical coupling in the MA than in the SMA and BA. Second, the Cinput of in situ cells was 11, 12, and 18 times that of the respective dissociated cells in the SMA, BA, and MA, further supporting a tighter coupling in the MA than in the other two vessels. Third, either the Ginput or Cinput of dissociated VSMCs was significantly different among the three vessels with an order of MA > BA > SMA, which was consistent with the visual impression of the cell sizes of the vessels.
The I-V relation of the whole cell current of either in situ or dissociated VSMCs showed a prominent outward rectification when the cell was depolarized beyond −40 mV but typically exhibited only a small or no inward rectification at negative potentials lower than −60 mV under the condition of normal 5 mM K+ extracellular solution and high-K+ internal solution (Fig. 1, A and B). A small but discernible inward rectification was seen in 15 of 159 dissociated VSMCs from all 3 vessels. The inward rectification was facilitated by high extracellular K+ (50 mM) and blocked by 100 μM Ba2+ in cells either in situ or dissociated (n = 10; see also Ref. 40), indicating its mediation by an inward rectifier K+ (Kir) channel (31).
Dissociated ECs and tubules composed of 5–10 or more ECs were identified occasionally in the dispersed SMA suspension but were very rarely in dispersed preparations of the other two arterioles. The identified ECs, either in a tubule or in dispersed status, frequently showed (7 of 9 cells) a robust inward rectification but little, if any, outward rectification (see Fig. 7), which was consistent with a previous report (9) on identified ECs acutely dissociated from the rat small MA. EC membrane properties are shown in Table 2. Of note, Ginput and Cinput of the dissociated single EC were significantly larger than those of the dissociated VSMC (Table 1 vs. Table 2), consistent with the morphological observation that average single ECs were significantly larger than VSMCs.
2-APB and DPBA block the electrical coupling of in situ SMCs.
Application of 30–100 μM 2-APB or DPDA caused a significant attenuation of the steady-state current amplitude induced by voltage steps from the holding potential of −40 mV in cells in situ of any of the three vessel segments (Figs. 1B and 2), indicating an increase in Rinput or a reduction in Ginput. Ginput inhibition was detectable within 0.5 min and reached a steady state within ∼5 min for 100 μM 2-APB (Fig. 1, G–I). The inhibition was fully reversed by an ∼10-min wash with drug-free bath solution. DPBA acted faster, with full Ginput inhibition within 2 min and complete washout in ∼10 min. In comparison, 18β-GA (100 μM) showed an action with washin and washout effects similar to those of 2-APB (21).
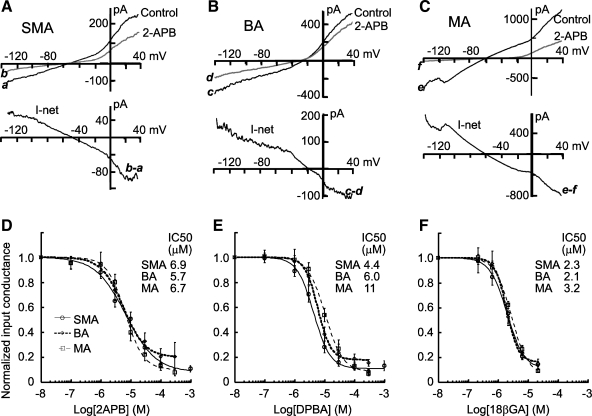
Fig. 2.
Concentration-dependent inhibition by 2-APB, DPBA, and 18β-GA of the membrane input conductance (Ginput) of the three vessels. A–C: exemplary ramp whole cell current-voltage (I-V) curves in the absence and presence of 30 μM 2-APB in cells in situ of the SMA, BA, and MA (top) as well as the respective 2-APB-induced net current I-V curves (bottom). The access resistance was not compensated. Note that 2-APB caused a reduction of the slope conductance in cells of all three vessels. Ginput was reduced from 1.04, 4.04, and 7.96 nS to 0.24, 0.78, and 0.24 nS in A, B, and C, respectively. D–F: plots of Ginput against the concentrations of the three compounds. Ginput was normalized to the control value of each cell. IC50 values resulted from fitting the data with the following modified Hill equation: y = (1 − C)/[1 + (x/Ki)h] + C, where C is the theoretical residual conductance in supramaximal concentrations and h is the Hill coefficient. The differences of the IC50 values or the residual Ginput (10–17%) were not statistically significant (P > 0.05) between any two compounds for each kind of vessel and between any two vessels for each compound. All data points are from 6–12 cells except for the points of 300 and 1,000 μM, where 2–4 cells were tested.
We compared the Ginput blocking potency of these two compounds, and with that of 18β-GA, among the three vessels. As shown in Fig. 2, the I-V curve slope was reduced in the voltage range (−140 to 40 mV) tested in all three vessels, and the 2-APB- or DPBA-induced net current showed an approximately linear I-V relation with a reversal potential (−33 ± 2.4 mV) very close to the RP (zero current potential) of the recorded cell (−32 ± 2.2 mV, n = 21, P > 0.05 by paired t-test ). This suggested that the Ginput reduction by 2-APB and DPBA was mainly due to a blockade of gap junction-mediated electrical coupling, as was the case with 18β-GA (21).
Ginput inhibition by 2-APB or DPBA was concentration dependent in all three vessels with IC50 values of ∼8 and 6 μM, respectively, which were not significantly different between these two compounds (Fig. 2) or between any two of the three vessels. However, the IC50 values were approximately three times higher than those for 18β-GA (Fig. 2, D--F), although all of the differences were not statistically significant (P > 0.05 by Student's t-test). In the presence of 100 μM 2-APB and DPBA, Rinput of VSMCs in the three vessels increased from several hundred megaohms to the order of a few gigohms, values similar to those of dissociated VSMCs (Fig. 2 and Table 3 vs. Table 1). Furthermore, the voltage step-induced capacitive transients became well fitted with a single-term exponential function in 100 μM of either compound. The current decay time constant and capacitance were similar to those of dissociated VSMCs for each vessel type (P > 0.05, n ≥ 6; Fig. 1 and Table 3 vs. Table 1). Taken together, these data indicated that a complete electrical isolation of the recorded VSMC could generally be achieved at ≥100 μM of either compound.
Table 3.
Membrane actions of 2-APB and DPBA on in situ vascular smooth muscle cells
| 2-APB (100 μM) |
DPBA (100 μM) |
|||||
|---|---|---|---|---|---|---|
| Control | In treatment | Change, % | Control | In treatment | Change, % | |
| SMA | ||||||
| Input resistance, MΩ | 584 ± 106 | 4,164 ± 400† | 908 ± 137 | 571 ± 114 | 4,937 ± 741† | 982 ± 107 |
| n | 13 | 12 | ||||
| Input conductance, nS | 2.24 ± 0.26 | 0.24 ± 0.033† | −86 ± 2.1 | 2.6 ± 0.43 | 0.26 ± 0.43* | −88 ± 1.4 |
| n | 13 | 12 | ||||
| Input capacitance, pF | 55.6 ± 9.5 | 6.01 ± 0.29† | −89.2 ± 3.1 | 71.5 ± 22.5 | 5.82 ± 0.19† | −91.1 ± 1.2 |
| 11 | 10 | |||||
| BA | ||||||
| Input resistance, MΩ | 316 ± 30 | 1,835 ± 219† | 631 ± 177 | 683 ± 92 | 3,703 ± 367† | 559 ± 141 |
| n | 6 | 8 | ||||
| Input conductance, nS | 3.89 ± 1.0 | 0.57 ± 0.076† | −80 ± 6.1 | 1.7 ± 0.26 | 0.27 ± 0.02† | −81 ± 3.7 |
| n | 6 | 8 | ||||
| Input capacitance, pF | 91.4 ± 26.8 | 6.7 ± 0.61† | −90.4 ± 2.9 | 116.2 ± 41.7 | 7.12 ± 0.7† | −89.5 ± 3.3 |
| n | 4 | 5 | ||||
| MA | ||||||
| Input resistance, MΩ | 360 ± 114 | 2,967 ± 644† | 884 ± 238 | 380 ± 81 | 3,336 ± 479† | 1,190 ± 479 |
| n | 6 | 12 | ||||
| Input conductance, nS | 4.31 ± 1.0 | 0.44 ± 0.15* | −87 ± 3.2 | 4.7 ± 1.2 | 0.31 ± 0.04* | −87 ± 3.4 |
| n | 6 | 12 | ||||
| Input capacitance, pF | 281 ± 131 | 9.52 ± 1.29† | −81.8 ± 9.3 | 193 ± 61.3 | 9.58 ± 0.9† | −89.8 ± 4.29 |
| n | 7 | 7 | ||||
Values are means ± SE; n, number of cells. Input resistance and input conductance were measured between −60 and −40 mV. Input capacitance was measured by a step from −40 to −100 mV. 2-APB, 2-aminoethoxydiphenyl borate; DPBA, diphenylboronic anhydride.
P < 0.05 and
P < 0.01, comparison between the control and treatment (paired t-test).
2-APB and DPBA, like 18β-GA, depolarize arteriolar cells.
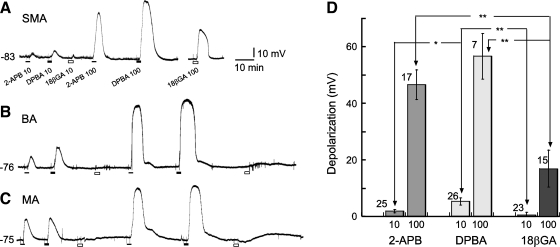
Conventional intracellular recordings on MA cells revealed that bath application of 1–1,000 μM 2-APB or DPBA, like 18β-GA, depolarized arteriolar cells in a roughly concentration-dependent manner (Fig. 3). The depolarization by 2-APB or DPBA of up to 1 mM was small (1–10 mV) in cells with a low RP (approximately −40 mV), whereas it varied greatly from cell to cell and from application to application in cells with a high RP (approximately −75 mV). In the latter cases, the depolarization often suddenly became very large, as if it had reached a threshold of regeneration. This phenomenon has been explained by a regenerative inactivation of the Kir channel according to its unique voltage dependency (34). Because of this phenomenon, our effort to determine the EC50 of the concentration-depolarization responses for these drugs was not successful. Figure 3 shows the depolarizations by the three drugs at 10 and 100 μM in the three vessels, demonstating that the depolarizing efficacy (amplitude) of the same concentration of 2-APB was slightly weaker than that of DPBA but that both agents were significantly stronger than 18β-GA.
Fig. 3.
2-APB and DPBA, like 18β-GA, cause a concentration-dependent depolarization in arteriolar cells. A–C: representative gap-free records of the depolarizations by two concentrations of the three agents in the three different vessels. The resting membrane potential of each cell is shown on the left of the traces. D: column graph presentation of the three compound-induced depolarizations in SMA cells, suggesting that 2-APB depolarized the vascular cells with an efficacy slightly lower than that of DPDA but higher than that of 18β-GA. Numbers of cells tested are indicated near the error bars. *P < 0.05; **P < 0.01.
Application of 100 μM 2-APB or DPBA also induced a small (1–10 mV) but statistically significant depolarization (4.1 ± 1.3 mV, n = 6, and 6.7 ± 2.2 mV, n = 10, respectively, both P < 0.05) in the zero current potential of in situ or dissociated cells under the whole cell configuration (Fig. 4, A and B). The difference between the magnitudes of 2APB- and DPBA-induced depolarizations was not statistically significant.
Fig. 4.
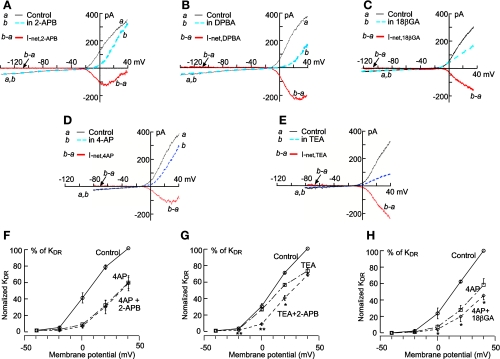
2-APB and DPBA cause an inhibition of the outward rectification that is mimicked and masked by 4-aminopyridine (4-AP) rather than by tetraethylammonium (TEA). A–E: ramp-constructed whole cell I-V curves from dissociated individual VSMCs from the BA in the absence and presence of 100 μM 2-APB, DPBA, 30 μM 18β-GA, 1 mM 4-AP, and 1 mM TEA. Note that the robust outward rectification was reduced by 2-APB and DPBA more pronouncedly at +20 mV than at +40 mV (A and B). Accordingly, the I-V curve of the net current (b − a) showed a U-shaped inward current between −30 and +40 mV but little net current between −140 and −30 mV. In contrast, I-V curves of the 18β-GA-induced net current (b − a) exhibited an almost linear inhibition between −10 and +40 mV. In comparison, the net current I-V curve of 1 mM 4-AP (D) qualitatively resembled those of 2-APB and DPBA, whereas the net current I-V curve of TEA resembled that of 18β-GA. F–H: I-V plots of step-induced Iss mean amplitudes [delayed rectifier K+ (KDR) current (IK); see also Fig. 5] (n = 3–6 for each point). Note that the effects of 2-APB on IK were nullified by the presence of 10 mM 4-AP at all voltage levels (F), whereas 2-APB inhibition of IK at −20 to 20 mV remained prominent in the presence of 1 mM TEA (G: *P < 0.05 and **P < 0.01 by paired t-test). 18β-GA inhibited IK further at 0 to 40 mV in the presence of 10 mM 4-AP (H).
Channel mechanism underlying 2-APB- and DPBA-induced depolarization: a comparison with 18β-GA-induced depolarization.
The possible channel mechanism underlying 2-APB- and DPBA-induced depolarization was investigated by whole cell voltage-clamp experiments on dissociated VSMCs and, in a few cases, on ECs.
The results shown in Fig. 4 demonstrate that both 2-APB and DPBA (100 μM) caused an inhibition of the outward rectification in the whole cell steady-state I-V curve while exerting no significant effect at potentials negative to −30 mV. The inhibition became weaker when the command voltage approached +40 mV. Accordingly, the 2-APB net current I-V curve between −30 and 40 mV exhibited a typical U-shape, whereas that of DPBA always had a shorter right arm of the U-segment (Fig. 4, A vs. B). This type of inhibition on outward rectification was mimicked by 4-AP (Fig. 4D), a widely used inhibitor selective for voltage-gated K+ (Kv) channels in VSMCs (31, 45), but was not simulated by TEA (Fig. 4E), an inhibitor quite selective for large-conductance Ca2+-activated K+ (BKCa) channels (45); TEA exerted almost constant proportional inhibition on the outward rectifier current in the voltage range tested (−20 to 40 mV; Fig. 4E).
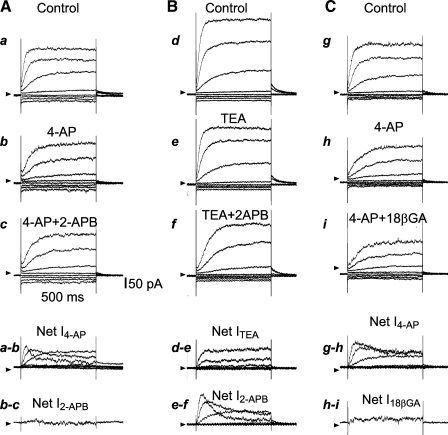
Furthermore, the 2-APB-induced inhibition of the rectification exhibited a time-dependent characteristic that was mimicked and masked by 4-AP but not by TEA (31, 45). The results shown in Fig. 5 demonstrate that the outward rectifier current activated by depolarizing steps developed with a delay in time, the typical kinetics of IK. Both 4-AP and 2-APB inhibited IK, and the inhibition was more pronounced on the early than on the late component of IK in the +20- and +40-mV step traces. Such kinetics are very different from those of currents blocked by the Ca2+-activated K+ (KCa) channel blocker TEA (1 mM) or charybdotoxin (ChTX; 50 nM; Figs. 5, A and B, and 6A and Supplemental Fig. 2). In the presence of 10 mM 4-AP, 2-APB induced little further change in IK in the entire pulse duration and voltage range tested. On the other hand, in the presence of 1 mM TEA or 50 nM ChTX, 2-APB elicited a significant further inhibition in the voltage range between −20 and 20 mV, with a time/voltage dependence similar to the actions of 2-APB or 4-AP used alone (Figs. 4G and 5B).
Fig. 5.
2-APB and 18β-GA inhibit IK differently in its time course. A–C: representative current traces elicited by steps from −40 to −100 mV through +40 mV in 20 increments in 3 different dissociated VSMCs of the BA (top 3 rows) and the drug-sensitive net currents (bottom 2 rows). Note that at +40 mV, 10 mM 4-AP mainly suppressed the early component of IK (A,a and b, and C,g and h). The addition of 100 μM 2-APB caused little further inhibition of IK (A), but the addition of 100 μM 18β-GA induced an additional small, but significant, inhibition on IK (C,h and i; see also Fig. 4). Compared with 4-AP, 1 mM TEA suppressed IK during all the 500-ms step durations (C). The addition of 2-APB caused an inhibition that was more pronounced on the early component than on the late component of IK at +40 mV (B,e and f), which resembled the effect of 4-AP alone (A,a and b).
Fig. 6.
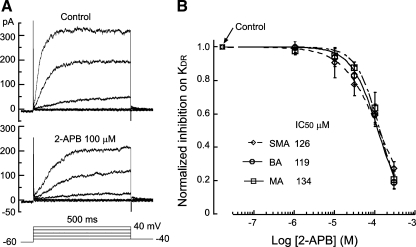
2-APB inhibits IK concentration dependently in three vessels with a similar potency. A: representative whole cell current traces from a dissociated VSMC from the BA in the absence (control) and presence of 100 μM 2-APB. The bottom traces show the voltage step protocol applied. B: collective data plots of mean steady-state amplitudes of IK at 20 mV of the three vessels. The IC50 values revealed by Hill equation fit were not significantly different among the three vessels (P > 0.05). n = 6 for all data points except those of 1 and 300 μM concentrations, where n = 3 or 4.
Interestingly, the inhibition by TEA or ChTX of IK was very similar to that by 30 μM 18β-GA, i.e., both 18β-GA and TEA suppressed both the early and late components of IK throughout the activation voltage up to +40 mV (Fig. 4, C and E; see also Ref. 21). In the presence of 1 mM TEA, 18β-GA caused a small further inhibition of IK (n = 5; Supplemental Fig. 3), whereas in the presence of 4-AP, 18β-GA induced a significant further inhibition of IK (Figs. 4H and 5C).
Finally, in the presence of 50 nM ChTX, a more specific blocker of BKCa and intermediate-conductance KCa (IKCa) channels, 2-APB (100 μM) exhibited an action (n = 4) similar to that of 2-APB alone, whereas 18β-GA (30 μM) caused little further inhibition of IK (n = 3).
Using the leak-subtracted steady-state amplitude of IK at +20 mV as an index, we determined the concentration-inhibition relation of the drugs (Fig. 6). The IC50 values of 2-APB were 126, 119, and 134 μM in VSMCs from the SMA, BA, and MA, respectively. The IC50 values of DPBA were 122, 116, and 102 μM and the IC50 values of 18β-GA were 48, 41, and 65 μM in VSMCs from the SMA, BA, and MA, respectively. IC50 differences among the vessels for each drug were not statistically significant (P > 0.05). The IC50 values of 18β-GA were significantly smaller than those of 2-APB or DPBA (P < 0.05).
The membrane effects of 2-APB and DPBA on ECs dissociated from the SMA were also analyzed with whole cell recordings. ECs in a tubule typically showed a single-term exponential relaxation with a long time constant (2–7 ms) in its step-induced capacitive transient (Fig. 7). The calculated Cinput was 5- to 20-fold larger than that of the dissociated single EC (Table 2), and the mean Rinput was less than one-third of the single EC, consistent with the electrical property of multiple cells that were tightly electrocoupled with each other. In the presence of 100–300 μM 2-AB, Cinput and Ginput were both reduced to the value for a single EC (≤15 pF and ≤1.1 nS, respectively, n = 4). In the single EC, 100 μM 2-APB or DPBA caused no significant change in the whole cell I-V relation between −140 and 40 mV (n = 3).
Possible implication of inositol 1,4,5-trisphosphate receptors in 2-APB-induced depolarization and gap junction blockade.
2-APB has long been known to block the inositol 1,4,5-trisphosphate receptor (IP3R) and store-operated Ca2+ entry (42). These two Ca2+ signaling pathways are functional mechanisms in VSMCs and ECs (18, 30, 33, 38, 46). Two compounds, nitrendipine and XeC, which act on two different IP3R signaling steps, were tested. First, nitrendipine was used to block IKCa channels, which are opened by IP3R activation and the resulting Ca2+ release (18, 33). In intracellular recordings from intact arteriolar segments, nitrendipine alone did not significantly change RPs in cells from all three vessel tested (n = 12 for each vessel; see also Ref. 33), suggesting that the existence of background IP3R activation was negligible in these in vitro arterioles. This is consistent with most reports by others on in vitro arterial preparations (see the discussion). Whole cell recordings showed that nitrendipine had no significant effect on Rinput and Cinput in in situ cells from the BA (n = 6).
Second, XeC (3 μM), a selective IP3R blocker (17), caused neither a significant change in the membrane potential of cells from the three vessels (n = 3–6 for each kind of arteriole) nor a significant change in the I-V curve in the whole cell configuration of dissociated VSMCs (n = 3–5 for each arteriole; Supplemental Fig. 4). Finally, in the presence of XeC, 2-APB-induced inhibition of Rinput and Cinput in in situ cells from the BA and MA were not significantly affected (n = 4 and 6; Supplemental Fig. 4).
DISCUSSION
The present study not only extends a previous analysis (2) of a gap junction blockade actions of 2-APB and its analog DPBA in cultured cells to three native vascular preparations but, for the first time, also characterizes the nonjunctional membrane effects in VSMCs and ECs. The main findings were as follows: 1) 2-APB and DPBA were potent drugs that block vascular gap junction-mediated electrical coupling, but their IC50 values (∼6 μM) were about threefold that of 18β-GA (Fig. 2; see also Ref. 21); 2) 2-APB and DPBA inhibited IK in VSMCs with an IC50 value of ∼ 130 μM, which was also about three times that of 18β-GA; 3) 2-APB and DPBA inhibition of IK was mainly on Kv channels, whereas that of 18β-GA was mainly on BKCa channels; and 4) the junctional and nonjunctional actions of 2-APB observed did not involve IP3R antagonism.
This study further demonstrated that whole cell recordings from SMCs that remain embedded in arteriolar segments could be a unique and feasible method for studying various arteriolar preparations beyond the few cases previously reported (21, 22, 50, 51, 58–60). Using a proper concentration of these gap junction blockers, we could achieve a complete electrical isolation of the cells in situ of a vessel segment, but a nonjunctional side effect should also be considered for data interpretation. Based on the data that the IC50 value was ∼20 times higher for nonjunctional than for junctional inhibition, all three compounds tested should have value as gap junction blockers. Moreover, we demonstrated a feasibility of determining the junctional inhibition of 2-APB in EC tubules of the SMA. This EC tubule preparation could be a useful approach toward a better understanding of the intercellular communication between ECs of microvessels beyond the SMA, as the EC tube preparation has also been successfully made from hamster cremaster muscle arterioles for patch-clamp recording and Ca2+-imaging experiments (6).
Gap junction blockade of 2-APB and DPBA versus 18β-GA.
The blocking effect on gap junction coupling of 2-APB (100 μM) and DPDA (100 μM) was first indicated by an increase in Rinput or a decrease in Ginput of in situ VSMCs to the values of freshly dissociated VSMCs of the respective arteriole (Figs. 1 and 2 and Table 3), suggesting a complete electrical isolation of the recorded VSMC in situ. Second, both the Cinput value and single exponential decay of the step-induced capacitive current in the presence of 2-APB or DPBA (e.g., Fig. 1, D and F) indicated a single cell RC circuit load, confirming the full electrical isolation of the recorded cell in situ (12, 39). Taken together, these data suggest that at ≥100 μM, 2-APB or DPBA made a full electrical isolation of the cells in the arteriole segment.
We demonstrated that 2-APB and DPBA had similar potency and efficacy in blocking vascular gap junctions with IC50 values of 4.4–8 μM (Fig. 2), which were very close to IC50 values determined for blockade of electrical coupling of cultured normal rat kidney cells (5.7 μM) and human embryonic kidney-293 cells (10.3 μM) (25). The IC50 values of 2-APB on vascular gap junctions indicated a more potent blockade than that on Cx43-formed gap junctions (51.6 μM) but a less potent blockade than that on gap junctions of Cx36 or Cx50 in neuroblastoma cells (IC50: 3–3.7 μM) (2). Since VSMCs express Cx43 and, occasionally, Cx37 and Cx45, and ECs express Cx37, Cx40, and Cx43 (16), our value of IC50 may reflect that heteromeric and heterotypic constructions of gap junctions formed by Cx43 and other Cx are common in arterioles. In blocking gap junctions, 2-APB and DPBA showed an IC50 value about threefold higher than that of 18β-GA in the three arterioles (Fig. 2; see also Ref. 21). The homogeneous sensitivity to these gap junction inhibitors among the three arterioles may reflect that these arterioles have identical molecular construction of their gap junctions.
It has been reported that DPBA showed a 1.3–1.5 times larger maximal inhibition than 2-APB on hemichannels formed by Cx26/Cx32 and Cx32 in transfected Hela cells (54). Our data from some cells did show that DPBA exerted a stronger and faster inhibition on gap junctions than 2-APB (Fig. 1), although the collective data means did not exhibit a statistically significant difference.
We did not determine the potency of 2-APB and DPBA in blocking the myoendothelial coupling. This was partially because the experimental configuration did not allow the pure measurement of myoendothelial coupling without implicating other intercellular couplings in the vessel (21). Instead, we found it possible to test their actions on the gap junction coupling between ECs in several EC tubules from dispersed SMA segments (Fig. 7). Despite the fact that the low availability of such tubules prevented us from determining IC50 values, 100–300 μM 2-APB always caused a complete isolation of the recorded EC, indicating that 2-APB might have an IC50 value on the same order as what we determined in Fig. 2, where the configuration of the measurement should normally implicate gap junctions between cells within the muscle layer and the endothelial layer as well as between these two layers.
Nonjunctional membrane actions of 2-APB and DPBA versus 18β-GA.
Our data identified that the nonjunctional action of 2-APB and DPBA on VSMCs was primarily an inhibition of Kv channels (Figs. 4–6 and Supplemental Fig. 2). First, the inhibition of outward rectification became less pronounced at depolarized voltages beyond +10 or +20 mV (i.e., seen as the positive slope arm of the U-shaped net current). This pattern of inhibition was mimicked by the Kv channel blocker 4-AP but not by the KCa blockers TEA or ChTX. Inhibition of Kv channels by 2-APB was further supported by the differential sensitivity of the kinetic phases of IK activation. At strong depolarization steps, 2-APB, like 4-AP but not TEA, inhibited the early (fast) component more strongly than the late (slow) component (Fig. 5). It is known that vascular Kv channels exhibit a reduced conductance in strong depolarization steps, especially in the late phase of the depolarization (8, 45). This feature has been attributed to robust influx of Ca2+ at this condition: the resulting submembrane Ca2+ surge inhibits Kv channels. Moreover, the inhibition of Kv channels, not KCa channels, was substantiated by the finding that when KCa channels were suppressed by TEA or ChTX, 2-APB inhibition on IK remained intact (Fig. 5B). These results are one step further from previous observations showing that 2-APB inhibited depolarization-activated, but unclassified, K+ channels in Limulus ventral photoreceptors (56) and arteriolar VSMCs in spontaneous hypertensive rats (61) and the 2-APB increased the excitability of pyramidal neurons (23). Finally, 2-APB appeared to have no effect on Kir channel-mediated inward rectification in dissociated ECs. Unfortunately, we failed to confirm this result in isolated VSMCs, as the inward rectifier K+ current was rarely observed (15 of 159 cells tested). More rigorous tests are needed to determine the action of 2-APB analogs on vascular Kir channels.
In comparison, 18β-GA caused an inhibition of IK that was mimicked and largely attenuated by TEA and ChTX but not by 4-AP (Figs. 4 and 5 and Supplemental Fig. 3). Neither 18β-GA nor TEA induced a net current with a positive slope segment in their I-V curves (Fig. 4, C and E). In addition, 18β-GA inhibited step-activated IK in both the early and late phases, which was similar to the effect of TEA or ChTX (Fig. 5, B and C), suggesting that 18β-GA inhibited mainly KCa channels rather than Kv channels.
Lack of IP3R involvement in the junctional and nonjunctional actions of 2-APB.
The observed inhibitory actions of 2-APB on gap junction coupling and nonjunctional Kv channels are unlikely related to the well-known antagonisms of 2-APB on the IP3R and store-operated Ca2+ entry (3, 23, 42). The reasons are as follows: first, these Ca2+ mobilization mechanisms are normally activated, if any, at a negligibly low level in in vitro vascular preparations until they are stimulated by strong sheer stress (37, 49), by vasoactive agents such as norepinephrine, ATP (30), and ACh (18, 32), and by hypoxia (57). As usual, the in vitro small artery preparations used in this study showed no discernible background IP3R activation and Ca2+ mobilization, which was indicated by no membrane potential responses to IKCa channel blockers (nitrendipine, etc.) and to the selective IP3R antagonist XeC. Any inhibition of the negligible background IP3R activation by 2-APB would be expected to cause little effect on cytosolic Ca2+ and Ca2+-modulated channels. Second, even if any IP3R antagonism had occurred in the presence of 2-APB, it would have decreased cytosolic Ca2+, and the latter was expected to enhance rather than inhibit gap junction coupling (43) and Kv channels (8, 45). Finally, the selective IP3R inhibitor XeC caused no alteration in the 2-APB-induced inhibition of electrocoupling and IK.
Kv channel inhibition by 2-APB is a novel finding, but its underlying mechanism remains to be determined. 2-APB has been reported to activate transient receptor potential (TRP)V1, TRPV2, and TRPV3 channels expressed in human embryonic kidney-293 cells (28), and VSMCs express TRPV1 and TRPV2 channels, which are highly permeable to Ca2+ (29, 55). It would be interesting to test whether activation of these TRPV channels is responsible for the 2-APB inhibition of Kv channels.
Conclusions.
In summary, our data demonstrated that 100 μM 2-APB or DPBA near fully isolated in situ VSMCs electrically in the arterioles of the SMA, BA, and MA with a mild nonjunctional depolarizing action via inhibition of the delayed rectifier Kv channel. The potency of both the junctional and nonjunctional actions of 2-APB or DPBA was about one-third of that of 18β-GA, but the latter showed a weaker depolarizing efficacy. Therefore, 2-APB or DPBA could be a better tool than 18β-GA for blocking vascular gap junctions only when the inhibition of IP3R and store-operated Ca2+ entry are not a concern but KCa channel inhibition is. With these agents, voltage-clamp investigation of ion channels in cells under nondispersed conditions is possible, while IK inhibition needs to be taken into consideration.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants R01-DC-004716 (to Z.-G. Jiang), DC-00105 (to A. L. Nuttali), and P30-DC-005983 as well as by National Natural Science Foundation of China Grants 30900490 (to K. T. Ma) and 3040043 and 30960417 (to J.-Q. Si).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Takatoshi Karasawa, Janice Moore, and Lisa Belair for reading the manuscript.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Armstrong CM, Gilly WF. Access resistance and space clamp problems associated with whole-cell patch clamping. Methods Enzymol 207: 100–122, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB). J Pharmacol Exp Ther 319: 1452–1458, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16: 1145–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bradley KK, Jaggar JH, Bonev AD, Heppner TJ, Flynn ER, Nelson MT, Horowitz B. Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells. J Physiol 515: 639–651, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Cohen KD, Jackson WF. Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells. Microcirculation 12: 169–182, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol 531: 359–373, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys 42: 167–195, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Crane GJ, Walker SD, Dora KA, Garland CJ. Evidence for a differential cellular distribution of inward rectifier K channels in the rat isolated mesenteric artery. J Vasc Res 40: 159–168, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Daleau P. Lysophosphatidylcholine, a metabolite which accumulates early in myocardium during ischemia, reduces gap junctional coupling in cardiac cells. J Mol Cell Cardiol 31: 1391–1401, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther 246: 1104–1107, 1988 [PubMed] [Google Scholar]
- 12. de Roos AD, van Zoelen EJ, Theuvenet AP. Determination of gap junctional intercellular communication by capacitance measurements. Pflügers Arch 431: 556–563, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Edwards DH, Chaytor AT, Bakker LM, Griffith TM. Modulation of gap-junction-dependent arterial relaxation by ascorbic acid. J Vasc Res 44: 410–422, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Eloff BC, Gilat E, Wan X, Rosenbaum DS. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction: evidence supporting a novel target for antiarrhythmic therapy. Circulation 108: 3157–3163, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 19: 277–284, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Figueroa XF, Isakson BE, Duling BR. Vascular gap junctions in hypertension. Hypertension 48: 804–811, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19: 723–733, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Ganitkevich VY, Isenberg G. Effect of membrane potential on the initiation of acetylcholine-induced Ca2+ transients in isolated guinea pig coronary myocytes. Circ Res 78: 717–723, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol 141: 881–903, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Griffith TM, Chaytor AT, Bakker LM, Edwards DH. 5-Methyltetrahydrofolate and tetrahydrobiopterin can modulate electrotonically mediated endothelium-dependent vascular relaxation. Proc Natl Acad Sci USA 102: 7008–7013, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guan BC, Si JQ, Jiang ZG. Blockade of gap junction coupling by glycyrrhetinic acids in guinea pig cochlear artery: a whole-cell voltage- and current-clamp study. Br J Pharmacol 151: 1049–1060, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guibert C, Beech DJ. Positive and negative coupling of the endothelin ETA receptor to Ca2+-permeable channels in rabbit cerebral cortex arterioles. J Physiol 514: 843–856, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagenston AM, Rudnick ND, Boone CE, Yeckel MF. 2-Aminoethoxydiphenyl-borate (2-APB) increases excitability in pyramidal neurons. Cell Calcium 45: 310–317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation 15: 503–514, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harks EG, Camina JP, Peters PH, Ypey DL, Scheenen WJ, van Zoelen EJ, Theuvenet AP. Besides affecting intracellular calcium signaling, 2-APB reversibly blocks gap junctional coupling in confluent monolayers, thereby allowing measurement of single-cell membrane currents in undissociated cells. FASEB J 17: 941–943, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP. Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther 298: 1033–1041, 2001 [PubMed] [Google Scholar]
- 27. Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys 34: 325–472, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-Aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem 279: 35741–35748, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res 99: 119–131, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100: 246–254, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Jackson W. Potassium channels in the peripheral microcirculation. Microcirculation 12: 113–127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang ZG, Nuttall AL, Zhao H, Dai CF, Guan BC, Si JQ, Yang YQ. Electrical coupling and release of K+ from endothelial cells co-mediate ACh-induced smooth muscle hyperpolarization in guinea-pig inner ear artery. J Physiol 564: 475–487, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang ZG, Shi X, Guan BC, Zhao H, Yang YQ. Dihydropyridines inhibit ACh-induced hyperpolarization in cochlear artery via blockade of intermediate conductance calcium activated potassium channels. J Pharmacol Exp Ther 320: 544–551, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Jiang ZG, Si JQ, Lasarev MR, Nuttall AL. Two resting potential levels regulated by inward rectifying potassium channels in guinea pig cochlea spiral modiolar artery. J Physiol 537: 829–842, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnston MF, Simon SA, Ramon F. Interaction of anaesthetics with electrical synapses. Nature 286: 498–500, 1980 [DOI] [PubMed] [Google Scholar]
- 36. Kwak BR, Jongsma HJ. Selective inhibition of gap junction channel activity by synthetic peptides. J Physiol 516: 679–685, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwan HY, Leung PC, Huang Y, Yao X. Depletion of intracellular Ca2+ stores sensitizes the flow-induced Ca2+ influx in rat endothelial cells. Circ Res 92: 286–292, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Leung FP, Yung LM, Yao X, Laher I, Huang Y. Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol 153: 846–857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Arch 411: 137–146, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Ma KT, Yang Y, Guan BC, Karasawa T, Shi XR, Jiang ZG. Heterogeneous expression of inward rectifier K+-channels (Kir) may contribute to distinct modes of resting membrane potentials among the cochlear, brain and mesenteric arterioles (Abstract). Assoc Res Otolaryngol Midwint Meet Abstr 31: 211–212, 2008 [Google Scholar]
- 41. Ma KT, Yang YQ, Jiang ZG. 2-Aminoethoxydiphenyl borate (2-APB) inhibits gap junction coupling and delayed rectifier K+-channels in guinea-pig arterioles in the cochlea and other beds (Abstract). Assoc Res Otolaryngol Midwin Res Meet Abstr 32: 276, 2009 [Google Scholar]
- 42. Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122: 498–505, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Matchkov VV, Gustafsson H, Rahman A, Briggs Boedtkjer DM, Gorintin S, Hansen AK, Bouzinova EV, Praetorius HA, Aalkjaer C, Nilsson H. Interaction between Na+/K+-pump and Na+/Ca2+-exchanger modulates intercellular communication. Circ Res 100: 1026–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol 127: 2516–2524, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Palmada M, Schmalisch K, Bohmer C, Schug N, Pfister M, Lang F, Blin N. Loss of function mutations of the GJB2 gene detected in patients with DFNB1-associated hearing impairment. Neurobiol Dis 22: 112–118, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Pan F, Mills SL, Massey SC. Screening of gap junction antagonists on dye coupling in the rabbit retina. Vis Neurosci 24: 609–618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prasad AR, Logan SA, Nerem RM, Schwartz CJ, Sprague EA. Flow-related responses of intracellular inositol phosphate levels in cultured aortic endothelial cells. Circ Res 72: 827–836, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Quinn K, Beech DJ. A method for direct patch-clamp recording from smooth muscle cells embedded in functional brain microvessels. Pflügers Arch 435: 564–569, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Quinn K, Guibert C, Beech DJ. Sodium-potassium-ATPase electrogenicity in cerebral precapillary arterioles. Am J Physiol Heart Circ Physiol 279: H351–H360, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Sandow SL. Factors, fiction and endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol 31: 563–570, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Segal S. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Tao L, Harris AL. 2-Aminoethoxydiphenyl borate directly inhibits channels composed of connexin26 and/or connexin32. Mol Pharmacol 71: 570–579, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Deshpande M, Payne R. 2-Aminoethoxydiphenyl borate inhibits phototransduction and blocks voltage-gated potassium channels in Limulus ventral photoreceptors. Cell Calcium 32: 209–216, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Welsh DJ, Scott P, Plevin R, Wadsworth R, Peacock AJ. Hypoxia enhances cellular proliferation and inositol 1,4,5-triphosphate generation in fibroblasts from bovine pulmonary artery but not from mesenteric artery. Am J Respir Crit Care Med 158: 1757–1762, 1998 [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto Y, Fukuta H, Nakahira Y, Suzuki H. Blockade by 18β-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. J Physiol 511: 501–508, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamamoto Y, Klemm MF, Edwards FR, Suzuki H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol 535: 181–195, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yamazaki J, Kitamura K. Cell-to-cell communication via nitric oxide modulation of oscillatory Cl− currents in rat intact cerebral arterioles. J Physiol 536: 67–78, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Y, Gao YJ, Zuo J, Lee RM, Janssen LJ. Alteration of arterial smooth muscle potassium channel composition and BKCa current modulation in hypertension. Eur J Pharmacol 514: 111–119, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Zygmunt PM, Hogestatt ED. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br J Pharmacol 117: 1600–1606, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]