Abstract
ATP-sensitive K+ (KATP) channels, composed of inward rectifier K+ (Kir)6.x and sulfonylurea receptor (SUR)x subunits, are expressed on cellular plasma membranes. We demonstrate an essential role for SUR2 subunits in trafficking KATP channels to an intracellular vesicular compartment. Transfection of Kir6.x/SUR2 subunits into a variety of cell lines (including h9c2 cardiac cells and human coronary artery smooth muscle cells) resulted in trafficking to endosomal/lysosomal compartments, as assessed by immunofluorescence microscopy. By contrast, SUR1/Kir6.x channels efficiently localized to the plasmalemma. The channel turnover rate was similar with SUR1 or SUR2, suggesting that the expression of Kir6/SUR2 proteins in lysosomes is not associated with increased degradation. Surface labeling of hemagglutinin-tagged channels demonstrated that SUR2-containing channels dynamically cycle between endosomal and plasmalemmal compartments. In addition, Kir6.2 and SUR2 subunits were found in both endosomal and sarcolemmal membrane fractions isolated from rat hearts. The balance of these KATP channel subunits shifted to the sarcolemmal membrane fraction after the induction of ischemia. The KATP channel current density was also increased in rat ventricular myocytes isolated from hearts rendered ischemic before cell isolation without corresponding changes in subunit mRNA expression. We conclude that an intracellular pool of SUR2-containing KATP channels exists that is derived by endocytosis from the plasma membrane. In cardiac myocytes, this pool can potentially play a cardioprotective role by serving as a reservoir for modulating surface KATP channel density under stress conditions, such as myocardial ischemia.
Keywords: cardiomyocytes, potassium channels, endocytosis, ischemia, electrophysiology, adenosine 5′-triphosphate-sensitive potassium channel, sulfonylurea receptor
atp-sensitive k+ (KATP) channels affect diverse physiological processes, ranging from the regulation of insulin secretion in pancreatic β-cells to the control of vascular tone. They act as metabolic sensors by controlling cellular excitability when opening in response to an impaired intracellular energetic status. The channel is composed of four inward rectifier K+ (Kir6) subunits and four sulfonylurea receptor (SUR) subunits (34). There are two Kir6 subfamily members, Kir6.1 and Kir6.2. There are also two types of sulfonylurea (SUR) subunits, SUR1 and SUR2, that belong to the ATP-binding cassette superfamily (32). SUR2 has two major functionally relevant splice variants, SUR2A and SUR2B (33). Various native KATP channels have different subunit compositions. Kir6.2 together with SUR1, for example, constitute the β-cell KATP channel (1, 31), whereas the cardiac and smooth muscle KATP channels are generally considered to consist of Kir6.2/SUR2A and Kir6.1/SUR2B subunit combinations, respectively (2, 26).
KATP channels protect against ischemia-reperfusion injury (12). Pharmacological approaches imply a role for mitochondrial KATP channels in protection and ischemic preconditioning (7). However, a definitive role for sarcolemmal KATP channels has also been demonstrated (29, 35, 36). The cellular mechanisms by which sarcolemmal KATP channels are protective may involve action potential shortening and prevention of intracellular Ca2+ overload. However, the molecular signals responsible for the protective effect are less well established. One possibility is the increased activity of available KATP channels mediated by intracellular protein kinase C (PKC) signaling pathways (15, 17). Another possibility is that the KATP channel density may be altered through transcriptional or posttranscriptional mechanisms (3). Posttranscriptional KATP channel regulatory mechanisms are not well characterized.
In addition to the role of SUR subunits in conferring distinct biophysical and pharmacological properties to the various types of KATP channels, they also affect channel subcellular trafficking. Both SURx and Kir6.x subunits contain specific amino acid sequences that regulate the trafficking of new synthesized KATP channels to the cell surface (38). To date, however, there has been no evidence to indicate that SUR1 and SUR2 subunits behave differently in this regard. Our data demonstrate that SUR1 subunits direct surface expression, whereas the SUR2 subunit, mostly found in muscle (5), directs KATP channel trafficking both to the surface and to intracellular compartments. Moreover, we demonstrate that the intracellular pool of KATP channels is mobile and has the ability to translocate to the sarcolemma (SL) after ischemic episodes to increase the KATP channel surface density. This ischemia-induced surface trafficking of KATP channels should be considered as a possible molecular mechanism for their protective role in postischemic damage.
MATERIALS AND METHODS
Plasmids.
pCS-MT-Kir6.1myc, pcDNA3-Kir6.2-hemagglutinin (HA), and a nonconductive mutant of mouse Kir6.2HA were constructed as previously described (28). pCI-Kir6.2-green fluorescent protein (GFP) (S65A) was from Dr. M. Takano (Jichi Medical School, Minamikawachi, Japan). Kir6.2-GFP(S65A)/SUR2A transfected cells exhibited KATP channel activity with biophysical properties and ATP-sensitivity indistinguishable from wild-type (not shown). Kir6.2HA+11 was a gift of Dr. L. Jan (University of California, San Francisco, CA), and hamster SUR1 cDNA was a gift of Dr. J. Bryan (Baylor College of Medicine). Both were subcloned into pcDNA3.1. Rat SUR2A in pCMV6 was provided by Dr. S. Seino (Kobe University Graduate School of Medicine, Kobe, Japan). pcDNA3-SUR2B (mouse) was a gift of Dr. J. Makielski (University of Wisconsin).
Cell culture, transfection, and immunofluorescence microscopy.
Cells were transfected with the KATP channel subunit cDNAs using lipofectamine plus (HEK-293T), lipofectamine 2000 (COS-1L and h9c2), or nucleofection (smooth muscle cells) (see supplemental methods; note: all supplemental material may be found posted with the online version of this article). For immunofluorescence microscopy, cells were pretreated with cycloheximide (100 μM), a protein synthesis inhibitor, for 4 to 5 h before fixation with paraformaldehyde and staining. Images were acquired using confocal microscopy.
Pulse-chase labeling and immunoprecipitation and antibody uptake experiments.
Cells were preincubated in methionine- and cysteine-free DMEM containing 15 mM HEPES and 5% dialyzed FBS and labeled with 250 μCi/ml [35S]Translabel (MP Biomedical) for 1 h. To chase, the cells were returned in growth medium supplemented with 5 mM methionine and cysteine. Immunoprecipitation was conducted with anti-myc antibodies, and the amount of Kir6.1myc in each sample was quantified by phosphorimaging after SDS-PAGE. Antibody uptake was conducted using the procedure described by Hu et al. (14) with modifications. The protocols used are described in detail in the supplemental methods.
Fractionation of cardiac tissue.
All animal procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of New York University School of Medicine. For experiments measuring KATP channel translocation in ischemic versus nonischemic rats, male Sprague-Dawley rats were overdosed with pentobarbital sodium and either euthanized immediately or 17–18 min after respiratory arrest. Membrane fractionation was performed using Optiprep gradients as described (21) with modifications (see supplemental methods). The plasma membrane and endosome-enriched fractions were subjected to Western blot analysis. The KATP subunit expression was normalized to that of the Na,K-ATPase.
Cardiac myocyte isolation and whole cell recordings.
Ventricular myocytes were isolated after enzymatic digestion of adult rat hearts. Some hearts were made globally ischemic before cell isolation by stopping flow for 20 min. Whole cell patch-clamp recording, mRNA isolation, and quantitative RT-PCR were performed as described previously (13, 37). The detailed procedures are given in the supplemental methods.
Data analysis.
Data are represented as means ± SE (n denoting the number of cells). Comparisons between groups were made using unpaired or paired Student's t-tests (SigmaStat, Systat Software) with a P value of <0.05 considered as statistically significant.
RESULTS
SUR2 subunits direct KATP channel trafficking to endosomal compartments.
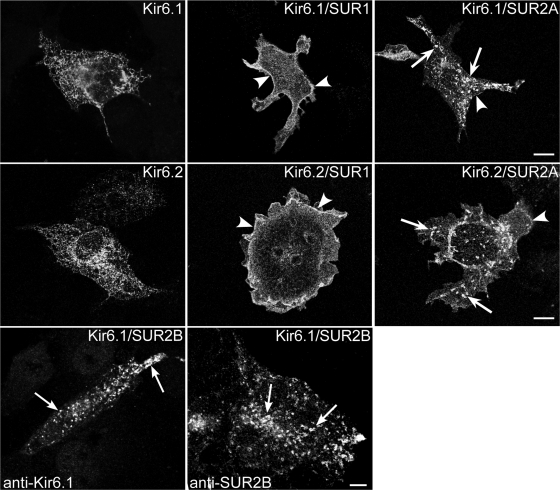
We investigated the subcellular trafficking patterns of KATP channels that contain either SUR1 or SUR2x subunits, initially using transfected cells. When COS-1L cells were transfected with epitope-tagged Kir6.1 or Kir6.2 cDNAs in the absence of an SUR subunit, the channel subunits were confined to the endoplasmic reticulum (ER) (Fig. 1) as confirmed by colocalization with the ER-specific marker ribophorin-2 (not shown). Consistent with the findings of others (38), we found predominant surface membrane labeling when coexpressing Kir6.1 or Kir6.2 together with SUR1 subunits (Fig. 1). However, when Kir6.x subunits were coexpressed with SUR2A subunits, a punctate intracellular staining pattern was observed in addition to the expression on the cell surface (Fig. 1). This subcellular localization pattern was also observed in other cell types (e.g., HEK-293T cells; supplemental Fig. S1) or when using untagged or GFP-tagged KATP channel constructs (e.g., Kir6.2-GFP; data not shown). Thus the vesicular staining pattern on SUR2A transfected cells did not depend on the nature of the Kir6.x subunit present, the cell type, or the method of detection.
Fig. 1.
Sulfonylurea receptor (SUR) 2 targets inwardly rectifying K+ (Kir) 6.1 and Kir6.2 to intracellular vesicles. COS-1L cells were transfected with plasmids encoding Kir6.1myc (top) or Kir6.2-hemagglutinin (HA) (middle) together with SUR1 or SUR2A or in the absence of SURx (with pcDNA3). Cells were pretreated with cycloheximide for 4 h before staining for immunofluorescence microscopy (IFM). When expressed alone, Kir6.1 or Kir6.2 staining was in a reticular pattern consistent with retention in the endoplasmic reticulum. When expressed together with SUR1, both Kir6.1 and Kir6.2 were predominantly localized on plasma membranes (arrowheads). When expressed with SUR2, both Kir6.1 and Kir6.2 were detected in intracellular vesicles (arrows) and on the plasma membrane. Bottom: COS-1L cells were transfected with a 1:9 (left) or a 1:1 (right) ratio of Kir6.1:SUR2B plasmid DNA and stained for IFM using goat anti-Kir6.1 or SUR2B antibodies. The untagged Kir6.1 subunit was localized to intracellular vesicles (arrows). SUR2B was also localized to intracellular vesicles. Figure panels have been adjusted for brightness and contrast. Bars = 10 μm.
We next investigated whether differences exist between SUR2A and SUR2B in terms of their subcellular trafficking; they differ from each other in the COOH-terminal 42 amino acids as a result of alternative splicing (33). In cells coexpressing Kir6.1/SUR2B subunits (the smooth muscle KATP channel subtype), both Kir6.1 and SUR2B subunits localized to intracellular vesicles (Fig. 1, bottom), suggesting trafficking to this subcellular compartment by the fully assembled heteromeric KATP channel. This finding demonstrates that SUR2B has the same trafficking properties as SUR2A.
To rule out the possibility that the experimental outcome was influenced by the ratio of subunits expressed, we investigated the effect of transfecting cells with different plasmid ratios. As the ratios of SURx to Kir6.1-encoding plasmids were increased over a 1,000-fold range, the localization changed from being exclusively in the ER (as expected from the limited SUR levels where few channels can be assembled) to a largely plasmalemmal pattern for SUR1-containing channels or to a predominant vesicular staining pattern for SUR2-containing channels (supplemental Fig. S2). Thus the localization was not dependent on the subunit ratio. We also investigated whether functional channels are needed for these unique localization patterns by using a nonconducting Kir6.2-AAA pore mutant subunit (37). However, the trafficking patterns were unperturbed (supplemental Fig. S3).
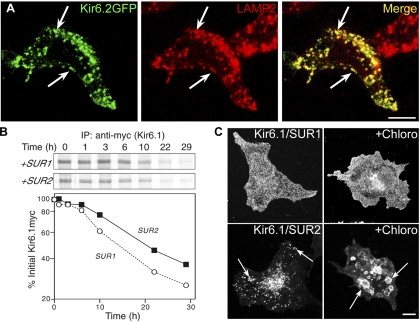
To determine the nature of the cellular organelles corresponding to the SUR2-containing vesicles, HEK-293T cells were transfected with Kir6.2-GFP and SUR2A cDNAs and stained with antibodies to human lysosomal-associated membrane protein-2 (LAMP2), a marker of endosomes and lysosomes (4). We found overlapping localization of LAMP2 and GFP (Fig. 2A), indicating that the SUR2-containing channels were targeted to the endosomal/lysosomal pathway. The vesicles generally did not correspond to those labeled with fluorescently tagged transferrin after a 15-min uptake period, indicating that KATP channels did not accumulate in early endosomes (supplemental Fig. S3). In addition, we did not observe any colocalization with mitotracker, a marker of mitochondria (supplemental Fig. S4).
Fig. 2.
Kir6.2/SUR2A channels localize to endosomes and lysosomes, but this does not affect the protein turnover rate. A: HEK-293T cells transfected with Kir6.2-green fluorescent protein (GFP)/SUR2A cDNAs were stained for IFM with rat anti-human lysosomal-associated membrane protein-2 (LAMP2) and rhodamine-conjugated anti-rat antibody. There was extensive overlap between the GFP signal and the staining for the lysosome/late endosome marker, LAMP2 (arrows). B: HEK-293T cells transfected with plasmids encoding Kir6.1myc and either SUR1 or SUR2A were subjected to pulse-chase labeling. After immunoprecipitation (IP), samples were subjected to SDS-PAGE (B, top), and the Kir6.1myc band was quantified and plotted as a function of the chase time (B, bottom). Depicted is 1 of 3 similar experiments. C: COS-1L cells transfected with Kir6.1myc and SUR1 or SUR2A cDNAs were pretreated with chloroquine (Chloro; 100 μM) and cycloheximide for 5 h before staining for IFM with anti-myc antibodies. The drug caused the intracellular vesicles to enlarge (arrows). Figure panels have been adjusted for brightness and contrast. Bars = 10 μm.
Turnover rate of KATP channel subunits.
The SUR2-dependent targeting to endosomes and lysosomes might be expected to accelerate channel turnover. However, the turnover rate of Kir6.1-myc subunits was comparable when expressed with SUR1 or SUR2A subunits as determined using a pulse-chase assay (the t1/2 was ∼20 and ∼15 h for SUR2 and SUR1-containing channels, respectively; Fig. 2B). We also examined whether the differences in channel trafficking could be explained by an accumulation of SUR2-containing channels in lysosomes as a consequence of slower degradation. The cells were incubated with chloroquine, which neutralizes lysosomal pH, thereby blocking degradation and causing an accumulation of proteins in endosomes and lysosomes (11). Because chloroquine treatment was not well tolerated in HEK-293T cells, this experiment was performed in COS-1L cells. As shown in Fig. 2C, chloroquine did not greatly affect the appearance of SUR1-containing KATP channels, which did not accumulate in vesicles. Kir6.1/SUR2A channels were still localized to vesicles, which were enlarged in size. The data are consistent with the notion that the subcellular localization of SUR2 channels is due to a unique mode of trafficking to endosomes and lysosomes.
Rapid internalization of Kir6.2/SUR2A KATP channels.
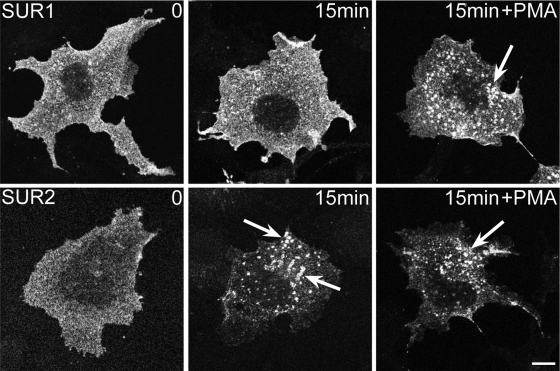
Previous reports have documented that KATP channels undergo endocytosis from the cell surface and that this process is greatly enhanced by the treatment of cells with phorbol 12-myristate 13-acetate (PMA), an activator of PKC (14, 22). To investigate whether there was an SUR-dependent difference in the rate of endocytosis, we used a Kir6.2 subunit containing an extracellular HA-epitope tag (Kir6.2HA+11). Live COS-1L cells were incubated with anti-HA antibodies, and the cells were either fixed immediately or after warming the cells to 37°C for 15 min. Internalization and vesicle trafficking were monitored by immunofluorescence microscopy. The SUR1-containing channels still predominantly localized at the cell surface, suggesting that the net uptake of the extracellular anti-HA antibody was slow (Fig. 3, top). By contrast, the extracellularly labeled SUR2-containing channels were initially observed at the surface, but by 15 min they were also observed in intracellular vesicles (Fig. 3, bottom), indicating that there was rapid and spontaneous antibody uptake. We also investigated the role of PKC activation. As previously reported (14), PMA dramatically accelerated the appearance of large labeled intracellular vesicles in SUR1-expressing cells (Fig. 3). However, this treatment had little effect in SUR2-expressing cells, most likely because of the already extensive internalization in the absence of PKC activation. The data demonstrate that SUR2-containing channels are present both on the cell surface and endosomal compartments and suggest that they dynamically cycle between these compartments.
Fig. 3.
Rapid internalization of SUR2-containing ATP-sensitive K+ (KATP) channels. COS1 cells transfected with plasmids encoding Kir6.2HA+11 and SUR1 (top) or SUR2A (bottom) were incubated for 2 h with anti-HA antibodies. Cells were warmed to 37°C for 15 min before fixation and staining with rhodamine-conjugated anti-rat antibodies. The anti-HA staining decorated the cell surface in SUR1-transfected cells, even after a 15-min incubation. By contrast, cell surface staining was initially observed in SUR2A-transfected cells, but vesicle staining was extensive after 15 min. Incubation of the cells with 100 nM PMA caused significant vesicle accumulation of antibody in SUR1-expressing cells but had little effect in SUR2-expressing cells. Figure panels have been adjusted for brightness and contrast. Bar = 10 μm.
SUR2-containing KATP channels localize to intracellular vesicles in smooth muscle and cardiac myocytes.
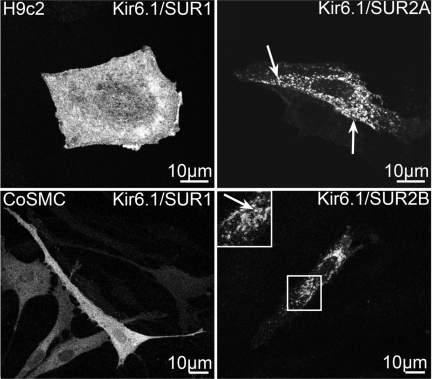
SUR2 subunits are natively found in cardiac and smooth muscle cells, which respectively express SUR2A and SUR2B subunits (26). We therefore investigated whether this unique trafficking mode is recapitulated when SUR2-containing KATP channels are exogenously expressed in primary human coronary artery smooth muscle cells or in h9c2 cells, a cardiac cell line derived from embryonic rat heart (18). As shown in Fig. 4, exogenously expressed SUR2-containing KATP channels targeted to intracellular vesicles in both cell types, whereas SUR1-containing channels were found primarily on the cell surface.
Fig. 4.
Differential trafficking of KATP channels in cardiac myocytes and coronary artery smooth muscle cells (CoSMC). H9c2 cells and CoSMCs were transfected with the indicated plasmid combinations. IFM was performed using an anti-myc antibody. Boxed regions are depicted in the inset. Labeled vesicles (arrows) were observed in cells transfected with SUR2. Figure panels have been adjusted for brightness and contrast.
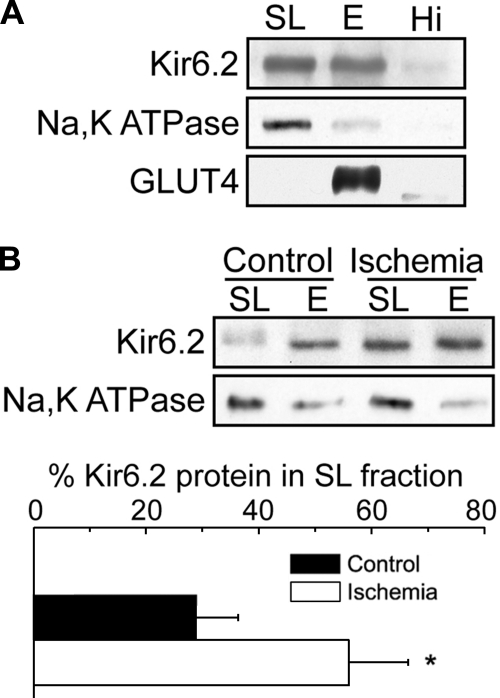
Rat heart KATP channels subunits translocate from endosomal membrane fractions to the SL following ischemia.
To further investigate whether native KATP channel subunits are present in endosomal compartments, we evaluated their distribution in membrane preparations obtained from rat hearts using density gradient centrifugation. The Kir6.2 subunit was detectable in both SL and endosome-enriched fractions (Fig. 5A), which were identified using antibodies to the Na,K-ATPase and GLUT4, respectively. The ER marker ribophorin was widely distributed but best represented in the highest density fraction (data not shown), in contrast to Kir6.2. To examine the pathophysiological relevance of the endosomal localization of KATP channels in ventricular tissue, we subjected hearts to global ischemia before cell fractionation. The amount of Kir6.2 present in the SL fraction increased markedly after ischemia relative to the endosome-enriched fractions (Fig. 5B). Comparable results were obtained for SUR2A recovered in the SL fraction (23 vs. 58% after ischemia; data not shown). Similar results have been reported in isolated myocytes (3) and in Langendorff-perfused rat hearts (9).
Fig. 5.
Increase in cardiac myocyte sarcolemmal KATP channels following global ischemia. A: cell fractionation was performed on rat ventricular tissue from nonischemic and ischemic rats using Optiprep gradient centrifugation. The sarcolemmal fraction (SL = 0/5% interface), endosomal fraction (E = 5/15% interface), and a loose pellet representing the highest density material (Hi) were subjected to Western blot analysis. B: representative Western blot of the SL and E fractions of membranes prepared from nonischemic control and ischemic rat hearts. The results from 3 experiments were quantified and plotted below as means ± SE. *P < 0.05. Figure panels have been adjusted for brightness and contrast.
Increased sarcolemmal KATP channel density following ischemia.
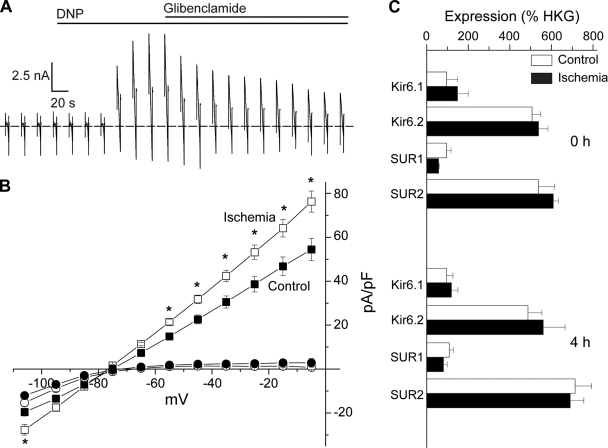
A translocation of KATP channels to the SL after ischemia would be expected to lead to an increased KATP channel density. To measure this directly, we next performed patch-clamp recordings using ventricular myocytes isolated from rat hearts that were rendered globally ischemic before the cell isolation procedure. They were compared with myocytes isolated from nonischemic hearts. In each group, myocytes were isolated from both the left and right ventricular free walls. There were no differences between the groups in cell capacitance (Table 1). Current-voltage relationships were recorded using slow voltage ramps. The current-voltage relationships were similar between groups as demonstrated by the absence of differences in the zero-current potential and currents recorded at −5 mV. Dinitrophenol (DNP) was used to maximally activate KATP channel current. The application of DNP (100 μM) led to a large outward shift of membrane current at voltages positive to the K+ equilibrium potential (Ek), which reached to a plateau within 1 to 2 min (Fig. 6A). The reversal potential of the DNP-activated current was close to the Ek (not shown) and was partially blocked by glibenclamide (2 μM), consistent with prior observations of an incomplete inhibition of KATP channels by glibenclamide in metabolically impaired cells (10). Whereas the currents before the application of DNP were not significantly different between the ischemic and the nonischemic groups, DNP induced a larger current density in the ischemic group compared with the nonischemic group (Table 1, and Fig. 6B), indicating that there was an increase in functional KATP channels. We also evaluated mRNA expression by quantitative RT-PCR in the control and postischemic cardiomyocytes (Fig. 6C). There were no statistically significant changes in mRNA levels of any of the KATP channel subunits, suggesting the involvement of posttranscriptional mechanisms in the increased current density. The magnitude of the enhanced KATP channel current density after ischemia (∼40%) was similar to the degree of KATP channel subunit sarcolemmal translocation. Although we cannot rule out other posttranscriptional events, our data are in support of a model where SUR2-containing KATP channels translocate from endosomal compartments to the SL during stress events, such as myocardial ischemia, which leads to an increased number of sarcolemmal channels that may participate in protection from stress responses.
Table 1.
Electrical properties of ventricular cardiomyocytes isolated from ischemic rat hearts
| Current Density at −5 mV, pA/pF |
||||||
|---|---|---|---|---|---|---|
| n | Cm, pF | Ei=0, mV | PreDNP | DNP | Glibenclamide | |
| Control | ||||||
| LV | 15 | 151.4 ± 12.17 | −74.8 ± 0.97 | 1.4 ± 2.74* | 51.1 ± 5.25† | 26.2 ± 4.61* |
| RV | 6 | 140.8 ± 20.31 | −75.4 ± 0.96 | −0.3 ± 0.38* | 62.8 ± 12.21 | 20.4 ± 6.55* |
| Ischemia | ||||||
| LV | 15 | 136.4 ± 10.83 | −76.6 ± 0.65 | 0.7 ± 0.23* | 70.9 ± 5.04† | 32.4 ± 5.39* |
| RV | 7 | 125.2 ± 20.82 | −77.6 ± 1.06 | 1.7 ± 2.50* | 87.5 ± 9.76 | 33.4 ± 9.19* |
Values are means ± SE and are shown for nonischemic (control) and postischemic (ischemia) myocytes from the left (LV) or right (RV) ventricular free wall. Measurements include membrane capacitance (Cm), the zero-current voltage of the current-voltage relationship (Ei =0), and peak steady-state current density before (preDNP) and after (DNP) application of 100 μM dinitrophenol or glibenclamide (2 μM).
P < 0.05 compared with DNP group (paired t-test);
P < 0.05 comparing control and ischemic groups (t-test). There were no significant differences between LV and RV myocytes in either group.
Fig. 6.
Increase in sarcolemmal KATP channel density in ventricular myocytes isolated from ischemic rat hearts. A and B: ventricular myocytes were isolated from nonischemic or ischemic rat hearts and subjected to whole cell patch clamping with a ramp protocol. A: representative current trace is depicted. The maximal KATP channel current was estimated as the steady-state peak current after dinitrophenol (DNP) application. The dotted line indicates 0 current. B: current-voltage relationships of current densities before (circles) and after (squares) DNP application for cells in the nonischemic (filled symbols; n = 21) and ischemic (open symbols; n = 22) groups (left and right ventricular myocyte data were pooled). *P < 0.05. C: real-time RT-PCR analysis of indicted channel subunit mRNA expression in cardiac myocytes at 0 and 4 h after isolation from nonischemic (control) and ischemic hearts. Data (means ± SE) are expressed relative to housekeeping genes. Primer sequences are in the online supplement (Table S1).
DISCUSSION
Subcellular localization of KATP channels.
KATP channels were first discovered as ATP-gated K+-selective channels present in isolated cardiac myocytes (27), and the sarcolemmal presence of KATP channels has since then been confirmed by many others. The surface topology of KATP channels has been mapped with high-resolution scanning ion conductance microscopy, and they were found to be organized in clusters and anchored in the Z-grooves of the SL (20). This is in keeping with the staining pattern observed using antibodies directed against the Kir6.2 subunit, which has been found to localize along Z-lines of isolated rat cardiac myocytes (25), suggesting an enrichment of KATP channels in t-tubular structures. Collectively, these data provide clear evidence for the presence of sarcolemmal KATP channels. Cell fractionation approaches have provided evidence that cardiac KATP channel subunits are also present in endosomal fractions (9) where they may function as a reservoir. The specific signaling mechanisms for the localization of KATP channels to these subcellular fractions have not been identified. Our data provide novel mechanistic insight by demonstrating that the subtype of SUR subunit present in the KATP channel complex is responsible for its specific subcellular localization. Specifically, we show that, independent of the cell type, SUR2-containing KATP channels localize to endosomal and lysosomal vesicles in transfected cells, whereas SUR1 consistently directed expression predominantly to the plasma membrane. The presence of SUR2-containing channels in endosomal/lysosomal compartments is not indicative of a degradation pathway since the protein turnover rate is similar when comparing SUR1 and SUR2-containing channels. Moreover, Western blot analysis of rat cardiac membrane fractions indicated that Kir6.2 and SUR2x subunits are both present in sarcolemmal as well as endosomal compartments. Collectively, these data indicate that KATP channels are not only expressed at the SL but are also present in endosomal compartments. The presence of a dynamic endosomal pool of SUR2-containing KATP channels suggests that SUR1 and SUR2 have evolved specialized trafficking and membrane regulatory pathways.
The dynamic nature of sarcolemmal KATP channel expression.
KATP channel subunit expression is regulated at the transcriptional and/or translational level, for example as evidenced by alterations in their expression levels after hypoxia or ischemia (16, 24). Our data, along with previously published findings (3, 9), suggest an additional mechanism by which the functional sarcolemmal pool of KATP channels is regulated. The picture that emerges is one of continuous and rapid cycling of KATP channels from endosomal to membrane locations, with the balance between these compartments being shifted by events such as ischemia. Evidence favoring this mechanism of regulating the KATP channel surface density includes our data demonstrating that extracellularly labeled SUR2-containing channels are rapidly internalized. A rapid internalization of KATP channels, and their appearance in rab7-positive endosomes, has also been previously observed in COS-7 cells treated with PMA. This effect was blocked by the PKC inhibitor chelerythrine (14), which suggested an obligatory role of PKC activation in channel cycling. However, there is some evidence that PKC may actually promote cardiac myocyte KATP channel translocation from endosomal to sarcolemmal compartments instead of causing internalization (9). Since our data were obtained under basal conditions and in the absence of exogenous stimuli, the role or PKC in KATP channel internalization and cycling is not apparent in our studies. Although internalization and recycling of Kir6.2/SUR1 channels can occur (23), our results are consistent with a model for a trafficking mechanism, whereby both types of assembled channels are transported via the secretory pathway to the plasma membrane, where SUR2-containing channels are more rapidly and dynamically cycled to endosomal and lysosomal compartments. In isolated myocytes subjected to a hypoxia protocol, inhibitors of protein trafficking were shown to reduce the appearance of KATP channels at the SL (3). It would be of interest to determine whether a similar effect can be demonstrated in intact heart tissue as the model would predict.
Increased sarcolemmal KATP channel density after myocardial ischemia.
What might be the function of an intracellular pool of KATP channels? One possibility is that the endosomal organelles might require KATP channels to regulate their ionic milieu and/or function. Our cell fractionation and patch-clamp studies point to an additional function as a reservoir of channels available for recruitment to the SL after stress conditions, such as ischemia. A similar conclusion has been reached by others when describing sarcolemmal translocation after ischemia in Langendorff-perfused male rat hearts (9) and increased surface density of KATP channels with “preconditioning” protocols in isolated cardiomyocytes (3). Of particular interest is our novel observation that the increased KATP channel current density was sustained (i.e., the patch-clamp experiments were performed several hours after ischemia). This result raises the possibility that, once initiated, the sarcolemmal translocation of KATP channels is maintained. Several molecular mechanisms may be responsible, including changes in gene expression. Kir6.2 has been reported to be significantly downregulated after ischemia-reperfusion, whereas the upregulation of Kir6.1 and SUR subunits occurred with cardiac remodeling during the weeks and months after an ischemic event (16). We did not observe such changes most likely because of the short (6 h) postischemic period. Overall, mRNA expression of KATP channel subunits was remarkably stable, which suggests that the sarcolemmal translocation observed in our studies may be a short-term adaptive response to protect against the ischemic insult. It would be of interest to explore the time course of the sarcolemmal translocations and upregulation of sarcolemmal KATP channel density to examine whether it may partly account for the sustained protection of ischemic preconditioning that is induced by short ischemic periods. The molecular mechanism(s) by which the KATP channel translocates to the SL after an ischemic episode is also worth investigating. It is possible that the dynamics of endosomal/sarcolemmal cycling process are altered. However, it is also possible that the sarcolemmal stability of KATP channels is increased. A potential candidate for mediating this process is ankyrin-B, a peripheral membrane protein that helps anchor plasma membrane proteins to the cytoskeleton and interacts with Kir6.2 (19). In any event, since the activity of sarcolemmal KATP channels in cardiac myocytes is known to be important in protection from the effects of ischemia, the recruitment of KATP channels from intracellular compartments is likely to play a significant contributory role in this process.
GRANTS
These studies were supported by National Institutes of Health Grants HL-064838, HL-093563, and HL-085820 (to W. A. Coetzee) and DK-067283 (to M. J. Rindler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Aguilar-Bryan L, Bryan J, Nakazaki M. Of mice and men: K(ATP) channels and insulin secretion. Recent Prog Horm Res 56:47–68, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60:667–687, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Budas GR, Jovanovic S, Crawford RM, Jovanovic A. Hypoxia-induced preconditioning in adult stimulated cardiomyocytes is mediated by the opening and trafficking of sarcolemmal KATP channels. FASEB J 18:1046–1048, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol 101:85–95, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes 45:1439–1445, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Coetzee WA. Regulation of ATP sensitive potassium channel of isolated guinea pig ventricular myocytes by sarcolemmal monocarboxylate transport. Cardiovasc Res 26:1077–1086, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol 62:79–109, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Crane A, Aguilar-Bryan L. Assembly, maturation, and turnover of K(ATP) channel subunits. J Biol Chem 279:9080–9090, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Edwards AG, Rees ML, Gioscia RA, Zachman DK, Lynch JM, Browder JC, Chicco AJ, Moore RL. PKC-permitted elevation of sarcolemmal KATP concentration may explain female-specific resistance to myocardial infarction. J Physiol 587:5723–5737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Findlay I. Sulphonylurea drugs no longer inhibit ATP-sensitive K+ channels during metabolic stress in cardiac muscle. J Pharmacol Exp Ther 266:456–467, 1993 [PubMed] [Google Scholar]
- 11. Gottlieb TA, Gonzalez A, Rizzolo L, Rindler MJ, Adesnik M, Sabatini DD. Sorting and endocytosis of viral glycoproteins in transfected polarized epithelial cells. J Cell Biol 102:1242–1255, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grover GJ. Protective effects of ATP-sensitive potassium-channel openers in experimental myocardial ischemia. J Cardiovasc Pharmacol 24, Suppl 4: S18–S27, 1994 [PubMed] [Google Scholar]
- 13. Harrell MD, Harbi S, Hoffman JF, Zavadil J, Coetzee WA. Large-scale analysis of ion channel gene expression in the mouse heart during perinatal development. Physiol Genomics 28:273–283, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron 38:417–432, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hu K, Li GR, Nattel S. Adenosine-induced activation of ATP-sensitive K+ channels in excised membrane patches is mediated by PKC. Am J Physiol Heart Circ Physiol 276:H488–H495, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Isidoro Tavares N, Philip-Couderc P, Papageorgiou I, Baertschi AJ, Lerch R, Montessuit C. Expression and function of ATP-dependent potassium channels in late post-infarction remodeling. J Mol Cell Cardiol 42:1016–1025, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Ito K, Sato T, Arita M. Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels during reoxygenation in guinea-pig ventricular myocytes. J Physiol 532:165–174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res 98:367–381, 1976 [DOI] [PubMed] [Google Scholar]
- 19. Kline CF, Hund TJ, Mohler PJ. Ankyrin regulates KATP channel membrane trafficking and gating in excitable cells. Channels (Austin) 4:55–57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Korchev YE, Negulyaev YA, Edwards CR, Vodyanoy I, Lab MJ. Functional localization of single active ion channels on the surface of a living cell. Nat Cell Biol 2:616–619, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Lei B, Lionetti V, Young ME, Chandler MP, d'Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36:567–576, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Mankouri J, Taneja TK, Smith AJ, Ponnambalam S, Sivaprasadarao A. Kir6.2 mutations causing neonatal diabetes prevent endocytosis of ATP-sensitive potassium channels. EMBO J 25:4142–4151, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control K(ATP) channel surface density. J Biol Chem 285:5963–5973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melamed-Frank M, Terzic A, Carrasco AJ, Nevo E, Avivi A, Levy AP. Reciprocal regulation of expression of pore-forming KATP channel genes by hypoxia. Mol Cell Biochem 225:145–150, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol 5:1, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 305:147–148, 1983 [DOI] [PubMed] [Google Scholar]
- 28. Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, Rosner E, Kaneko M, Manaris T, Holmes TC, Coetzee WA. Is the molecular composition of K(ATP) channels more complex than originally thought? J Mol Cell Cardiol 33:1541–1546, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Rajashree R, Koster JC, Markova KP, Nichols CG, Hofmann PA. Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal KATP channels. Am J Physiol Heart Circ Physiol 283:H584–H590, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Seino S. Physiology and pathophysiology of K(ATP) channels in the pancreas and cardiovascular system: a review. J Diabetes Complications 17:2–5, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81:133–176, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol 39:51–60, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol 110:655–664, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland AK, Shen M, Sakamoto K, Tian R, Jovanovic A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol 210:224–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest 109:509–516, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tong X, Porter LM, Liu G, Dhar-Chowdhury P, Srivastava S, Pountney DJ, Yoshida H, Artman M, Fishman GI, Yu C, Iyer R, Morley GE, Gutstein DE, Coetzee WA. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol Heart Circ Physiol 291:H543–H551, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22:537–548, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.