Abstract
Endothelial dysfunction is now considered an important early event in the development of atherosclerosis, which precedes gross morphological signs and clinical symptoms. The assessment of flow-mediated dilation (FMD) was introduced almost 20 years ago as a noninvasive approach to examine vasodilator function in vivo. FMD is widely believed to reflect endothelium-dependent and largely nitric oxide-mediated arterial function and has been used as a surrogate marker of vascular health. This noninvasive technique has been used to compare groups of subjects and to evaluate the impact of interventions within individuals. Despite its widespread adoption, there is considerable variability between studies with respect to the protocols applied, methods of analysis, and interpretation of results. Moreover, differences in methodological approaches have important impacts on the response magnitude, can result in spurious data interpretation, and limit the comparability of outcomes between studies. This review results from a collegial discussion between physiologists with the purpose of developing considered guidelines. The contributors represent several distinct research groups that have independently worked to advance the evidence base for improvement of the technical approaches to FMD measurement and analysis. The outcome is a series of recommendations on the basis of review and critical appraisal of recent physiological studies, pertaining to the most appropriate methods to assess FMD in humans.
Keywords: methodology, recommendations, shear rate, vascular function, atherosclerosis, cardiovascular risk, endothelial function
in general terms, flow-mediated dilatation (FMD) can describe any vasodilatation of an artery following an increase in luminal blood flow and internal-wall shear stress (Fig. 1). However, the term has conventionally come to describe subtle variations of the technique introduced by Celermajer, Deanfield, and colleagues in the Lancet in 1992 (14). This landmark paper introduced an approach involving assessment of peripheral conduit artery diameter following a period of distal limb ischemia. The Celermajer/Deanfield approach was based on several key studies and observations. Nobel prize-winning experiments by Furchgott (33) established that the endothelium produces a labile vasodilator substance, whereas animal studies established that FMD in arteries was dependent on the presence of an intact endothelial lining (93, 108) and that shear stress-sensitive ion channels existed in endothelial cells (16, 63, 83). Rubanyi, Vanhoutte, and colleagues (102) indicated that, in response to flow, the endothelium released a substance that possessed the characteristics of Furchgott's endothelium-derived relaxing factor, later identified as nitric oxide (NO) (78), and in situ studies using NO antagonists decreased FMD (17, 48). Subsequent animal studies consolidated the link between increases in flow, wall shear stress, endothelial NO synthase expression, and NO bioactivity (6, 123). The accumulated evidence strongly suggested that flow-associated shear was the physiological stimulus to endothelium-mediated vasodilation in vivo. In humans, Vallance, Collier, and Moncada (124) established that NO production occurs basally and in response to pharmacological stimulation, whereas increases in flow-associated shear subsequent to differing periods of arterial occlusion induced vasodilation of large conduit arteries (3, 80, 104, 107). Although Celermajer, Deanfield, et al. (14) provided no direct evidence that their FMD technique induced dilation that could be blocked by NO antagonists, they reasonably assumed from the evidence available at the time that the dilator response was likely to be endothelium dependent and NO mediated.
Fig. 1.
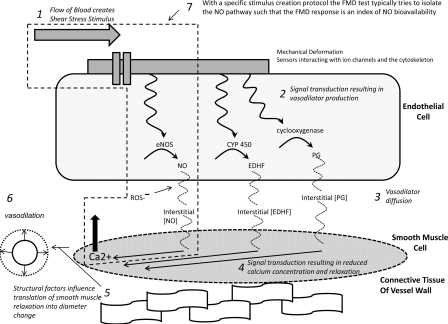
Schematic representation of steps involved in flow-mediated dilation (FMD) generation from the initiation of the shear-stress stimulus (step 1) to the resultant vessel diameter change (step 6). Blood flow-associated shear stress is sensed by deformation of mechanosensitive structures at the cell membrane. These structures could include the glycocalyx, the primary cilia, and mechanosensitive ion channels (19). Shear-stress mechanotransduction activates a signaling cascade that results in vasodilator production (step 2). The vasodilators produced and predominantly involved in FMD appear to depend on the nature of the shear-stress stimulus (100) and the endothelial phenotype (96). Vasodilators must diffuse from the endothelial cell into the smooth muscle cell (step 3). Nitric oxide (NO) may react with reactive oxygen species (ROS), decreasing its bioavailability (103). In the vascular smooth muscle, vasodilators trigger a signaling cascade that results in a lowering of calcium concentration and vasorelaxation (step 4). Vessel wall structural factors (e.g., relative proportions of collagen and elastin or wall-to-lumen ratio) may influence the diameter change that results from a given degree of smooth muscle relaxation (69) (step 5). FMD is quantified as the change in vessel diameter from baseline dimensions before the application of the shear-stress stimulus (step 6). Given the established vasoprotective properties of NO, FMD is often intended as an index of NO bioavailability (37, 100). Thus efforts have been directed at identifying specific protocols and stimulus profiles that are able to isolate the NO pathway (step 7). Examining vasodilatory responses to exogenous nitroglycerin can isolate and interrogate function downstream of the endothelium. EDHF, endothelial-derived hyperpolarizing factor; PG, vasodilatory prostaglandins; CYP 450, cytochrome P450; eNOS, endothelial NO synthase.
Studies performed after the Celermajer and Deanfield paper largely confirmed the assumption that their FMD technique was NO dependent. Joannides et al. (52) published evidence that radial artery dilation (FMD = 3.6%) following 3 min of ischemia was converted to constriction (−2.8%) in the presence of NO blockade with N-monomethyl-l-arginine (52). Mullen et al. (79) found that NO blockade decreased the radial artery FMD response to 5-min ischemia from ∼5.3% to 0.7%, with no difference in the hyperemic blood-flow stimulus, making it unlikely that stimulus magnitude reductions explained the FMD response abolition. However, 15 min of ischemia induced a radial FMD response, which was not affected by NO blockade (79). This supported the notion that the duration of ischemia was an important determinant of the mechanisms responsible for the subsequent vasodilator responses (79). More recently, the superficial femoral artery FMD, induced by a 5-min cuff occlusion period, was found to be largely NO dependent (61). Therefore, most (52, 61, 68, 79), but not all (96), studies suggest that FMD can be substantially attenuated by NO blockade. Taken together, these physiological studies generally reinforced the validity of the approach introduced by Celermajer and Deanfield as an endothelium-dependent and NO-specific index of endothelial function (14).
FMD has become popular in clinically orientated studies, in part, because it strongly predicts cardiovascular events in patients with established cardiovascular disease (Table 1). These studies generally indicate that FMD provides independent prognostic information, which may exceed the predictive value of traditional risk factors (Table 1). Studies that have examined the prognostic role of FMD in asymptomatic subjects have suggested a more modest association (31, 32, 132), and it has been suggested that FMD may become less predictive in older individuals in whom arterial distensibility may be limited (129, 133) (Figs. 1–4). In summary, FMD appears to be predictive of cardiovascular events in asymptomatic subjects and those with established cardiovascular diseases. FMD appears at least as predictive as traditional risk factors (Table 1), a conclusion supported by a recent meta-analysis (49). Moreover, a change in FMD may also provide important prognostic information in humans (Table 1).
Table 1.
Endothelial function as predictor of prognosis
| FMD As A Predictor |
|||||||
|---|---|---|---|---|---|---|---|
| Authors | Journal | Year | N | Group | Subjects with CVD or CVD Risk | Healthy Subjects | Change in FMD |
| Shechter et al. | IJC | 2009 | 435 | men and women | P (future CVD) | ||
| Kitta et al. | JACC | 2009 | 251 | CAD | single FMD: NP (future events) FMDs across time: IP (future events) | FMD increase after 6 or 26 mo ∼fewer events | |
| Yeboah et al. | Circ | 2009 | 3026 | men and women | IP (future CVD) | ||
| Rossi et al. | JACC | 2008 | 2264 | women | P (future CVD) | ||
| Yeboah et al. | Circ | 2007 | 2791 | men and women | IP (future CVD) | ||
| Shimbo et al. | Atheroscl | 2007 | 842 | men and women | P (future CVD in lowest tertiles) | ||
| Suessenbacher et al. | Vasc Med | 2006 | 68 | CAD | single FMD: NP (future events) | FMD increase after 14 or 44 mo ∼fewer events | |
| Karatzis et al. | AJC | 2006 | 98 | ACS | P (future events) | ||
| Patti et al. | Circ | 2005 | 136 | CAD | IP (stent restenosis) | ||
| Meyer et al. | JACC | 2005 | 75 | CHF | IP (deterioration and death) | ||
| Frick et al. | JACC | 2005 | 398 | Chest pain | NP (future events) | ||
| Fischer et al. | EHJ | 2005 | 67 | CHF | IP (survival) | ||
| Fichtlscherer et al. | Circ | 2004 | 198 | ACS | Endothelial function increase after 8 wk ∼fewer ACS | ||
| Fathi et al. | JACC | 2004 | 444 | CAD and healthy | IP (in higher risk patients) | NP (in no/low risk subjects) | |
| Gocke et al. | JACC | 2003 | 199 | PVD | IP (future events) | ||
| Brevetti et al. | Circ | 2003 | 131 | PVD | Low FMD: IP | ||
| Chan et al. | JACC | 2003 | 106 | CAD | IP (FMD/GTN) | FMD decrease across time ∼future events | |
| Modena et al. | JACC | 2002 | 400 | Hypertension | P (future events) | FMD increase after drug treatment ∼fewer events | |
| Neunteufl et al. | AJC | 2000 | 73 | Chest pain | IP (future events) | ||
NP, no predictor; P, predictor; IP, independent predictor; CAD; coronary artery disease, ACS, acute coronary syndrome; CHF, chronic heart failure; PVD, peripheral vascular disease; FMD, flow-mediated dilation; GTN, glyceryl trinitrate; IJC, International Journal of Cardiology; JACC, Journal of the American College of Cardiology; Circ, Circulation; Atheroscl, Atherosclerosis; Vasc Med, Vascular Medicine; AJC, The American Journal of Cardiology; EHJ, European Heart Journal.
Fig. 4.
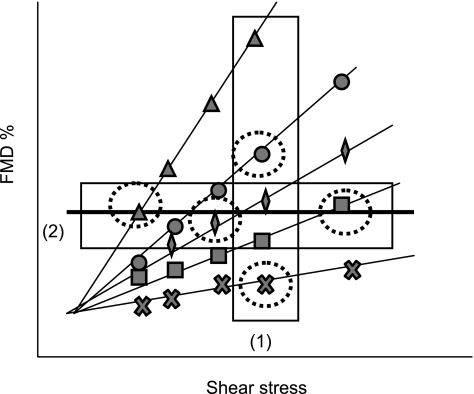
Hypothetical data in which no between-subject relationship between shear and FMD is observed despite the presence of (variable) within-subject shear-FMD relationships. Thin solid lines represent within-subject shear-FMD relationships when exposed to 5 different shear-stress stimuli. Circled points represent the shear-stress stimulus and FMD response in each subject during a single reactive hyperemia test. The thick solid line represents the between-subject shear-FMD relationship. Box 1 represents a selection of data in which subjects received the same shear-stress stimulus. In this selection it is straight-forward to conclude that differences in the FMD response represent biological variability in steps 2-4 described Fig. 1. Box 2 represents a selection of data in which subjects have the same FMD response but experienced very distinct shear-stress stimuli. In this data selection the subjects have differences in endothelial function, and only the variable shear-stress stimulus has allowed them to experience a similar FMD response. This highlights the importance of determining an effective method to account for the stimulus magnitude.
The FMD technique has increasingly been applied in physiological studies to examine the mechanisms that underlie the acute or chronic impact of stimuli that alter vascular function and risk (e.g., exercise training, smoking, hypercholesterolemia, hypertension) (20, 26, 34, 41, 54, 81, 101, 134) or to study hemodynamic effects on the vasculature in vivo (85, 87, 98, 115, 120, 121, 131). Consequently, the FMD test represents an important tool to improve our physiological insight and understanding of mechanisms that alter endothelial and vascular function. It is clear, however, that minor changes in the methodological approach can critically impact the nature and magnitude of the FMD response (10, 25, 79). Whereas previous guidelines made important contributions to standardizing the technical approach and setting minimum standard requirements for FMD measurement (18, 38), recent studies have identified important physiological and technical issues that can impact the validity, reproducibility, and interpretation of FMD studies (5, 8, 10, 40, 45, 55, 70, 85, 86, 90, 97, 99, 100, 113, 117, 126). Given the widespread use of FMD, these issues merit detailed discussion. This review results from discussion between several distinct research groups who have independently worked to provide an evidence base and physiological background for the improvement of the practical guidance and technical approaches to FMD measurement and analysis.
Technical Issues Pertaining to Duplex Ultrasound Assessment of FMD
Diameter and velocity assessment.
High-resolution B-mode ultrasound has become the research tool of choice for measuring conduit artery diameter for FMD assessment. The main advantages of B-mode ultrasound are that it is relatively cost effective, noninvasive, portable, and reproducible when following appropriate training and experience (22–24, 130). The main challenge with B-mode imaging is to identify clear vascular boundaries. Imaging of a blood vessel in the longitudinal plane allows visualization of the double lines of Pignoli (92), distinguishable demarcated boundaries that allow for precise diameter measurement (≤0.05 mm) (130) by automated edge-detection software.
Because of the recent acknowledgment of the importance of quantifying shear stress during the FMD responses (86, 99, 100), duplex ultrasound for simultaneous acquisition of B-mode diameter and pulsed-wave Doppler velocity signals is recommended where available. An important limitation of duplex ultrasound is that the same transducer is employed to detect signals for both the Doppler frequency shift as well as the arterial diameter, which have competing requirements for optimal data acquisition. B-mode echoes are of greater intensity with perpendicular incidence of the ultrasound beam to the vessel orientation (90 degrees), whereas optimal pulsed-wave Doppler signals require parallel incidence with the direction of blood flow (0 degrees) (62, 82, 118). Consequently, a compromise must be reached to uphold fundamental principles and assumptions for both modalities as well as to minimize the loss of signal quality (82).
Modern duplex ultrasound systems incorporate a narrow Doppler beam aperture that can be steered 20–30° of center of the B-mode imaging beam. This ensures that measurable Doppler shifts are achievable at an approximate angle of 60° between the Doppler beam and the vessel orientation, while maintaining optimal B-mode imaging. For clinical ultrasound, approximation of the Doppler beam-vessel angle ≤60° in relation to the direction of blood flow allows estimation of blood-flow velocity within reasonable levels of measurement error (94) and with adequate quality (46, 62). Because the error associated with incorrect estimation of insonation angle increases exponentially with angles >60° (62, 82, 118), we recommend an insonation angle of ≤60° when velocity assessment is used for shear rate calculation. However, insonation angles above 60° may be validly used under circumstances where extensive flow calibrations have been undertaken (98). In all circumstances, the angle used should be reported in the methods section.
Analysis of velocity signal.
Blood-flow velocity can be calculated using the peak (peak Doppler shifts) or mean velocity (intensity weighted mean of all Doppler shifts). The peak-velocity approach measures the fastest moving blood cells, located in the center of the vessel. It is assumed that half the peak velocity is representative of the mean velocity (67). The intensity-weighted mean-velocity approach involves estimating mean velocity from all of the Doppler shifts measured across the cross section of the vessel, from the slower outer lamina to the faster central layers of flow. This latter approach can be limited by incomplete sampling of Doppler shifts across the full width of the artery by the current linear array transducers (118). Because of the narrow Doppler beams, the slower moving peripheral lamellae from the lateral aspects of the artery are not taken into account, even if the Doppler sample gate is widely spaced to encompass the near and far walls of the artery (118). This can overestimate velocity by up to 33% (29).
Both methods of velocity assessment have their merits and disadvantages and do not appear to be interchangeable (30). This should be appreciated when comparing absolute blood flow/velocity values between studies that have adopted different approaches. Moreover, it is advisable to choose a single method within a study and also preferably between studies from the same laboratory. Claims of assessment of absolute blood flow should only be made where ultrasound machines and analysis methods have been validated against phantom artery preparations or string phantoms (130).
From velocity and diameter data, shear rate can be calculated. In most studies shear rate is calculated rather than shear stress, as it is generally assumed that blood viscosity does not differ substantially between individuals and/or groups or after interventions (11, 35, 86). In a recent study, it was demonstrated that the addition of viscosity measurements does not have a significant impact on shear stress calculations and does not alter the interpretation of the FMD results (86). Because different approaches have been adopted to calculate shear rate (90), caution is warranted when comparing shear rate data between studies. We recommended using identical settings within and between studies from one laboratory (23). In addition, calculations should all be clearly described in the methods sections given the fact that different methods can impact on absolute values as explained above.
Methodological Considerations Pertaining to FMD Assessment
Subject preparation.
FMD can be influenced by dietary intake (7), recent aerobic or resistance exercise (20, 41, 75, 120), caffeine and alcohol ingestion (44, 88), and supplement/medication use (39, 72, 111). We therefore recommend assessing FMD when subjects are fasted and have avoided exercise, caffeine, alcohol, drugs, stimulants, and medications for a consistent period of time (at least 6 h) to minimize the effect of these confounding factors (Table 2). In the case of clinical populations in whom medication use cannot be avoided, tests should be conducted after a standardized period of time following medication and a careful history of medication use and timing should be collected. Time of day at which assessments are made may also affect FMD (53, 84, 112). It is recommended that tests be conducted at a similar time of day for repeat assessments, and, for between-group studies, assessment times should be standardized.
Table 2.
Recommendations for FMD assessment to examine a largely nitric oxide-mediated, endothelium-dependent vasodilation of a conduit artery in humans
| Methodological and Technical Guidelines |
|---|
| Subject Preparation |
| - Rest in a quiet, preferably darkened room for a period of ≥20 min before assessment. |
| - Supine posture (i.e., the imaged artery should not be substantially above or below heart level). |
| - Tests should be standardized and, for multiple tests, conducted at a similar time of day. |
| - Cuff must be placed distal to the imaged artery and inflated for 5 min. |
| - Subjects must be fasted for ≥6 h. |
| - Subjects must avoid exercise or food/drinks that contain caffeine or alcohol for ≥8 h. |
| - Careful history should be taken regarding the use/timing of drugs because some drugs have an effect. |
| - Premenopausal women should be assessed on days 1-7 of the menstrual cycle. |
| Protocol |
| - Baseline diameter must be examined before cuff inflation for a period of at least 1 min. |
| - Present absolute baseline diameter should be in results section. |
| - Measurement of postdeflation diameter should start before cuff release. |
| - Measurements should be performed for ≥3 min in upper limb arteries and ≥5 min in lower limb arteries. |
| Technique |
| - Continuous measurement of velocity and diameter using duplex ultrasound should be performed. |
| - Blood velocity should be assessed using an insonation ≤60°. |
| - Use the same angle within a study and study group (and report angle). |
| - B-mode images with a probe of ≥7.5 MHz should be used (and report probe details). |
| Analysis |
| - Continuous edge detection and wall tracking should be used to capture true peak diameter and for calculation of shear rate. |
| - Peak velocity outer envelope assessment is recommended for analysis of the Doppler signal. |
| - Automated mathematical algorithms should be used to calculate the peak diameter. |
| - Present the FMD response in absolute (in mm) and relative (in %) change. |
| - The relevant shear-rate stimulus (area-under-the-curve until peak diameter) must be presented. |
| - The use of ratio normalization (e.g., FMD/shear) is currently unresolved, and at this time no recommendation to use such normalization can be provided. |
Because acute sympathetic nervous system activation and ambient temperature can alter FMD (27, 45, 70, 126), testing should be conducted in a quiet (preferably darkened), temperature-controlled thermoneutral room after the subject has been resting quietly. Finally, premenopausal women should be assessed in a standardized phase of the menstrual cycle (ideally days 1–7, when concentrations of circulating female sex hormones are lowest) (42, 127).
Protocol: cuff position.
The importance of cuff position (distal or proximal to ultrasound measurement site) has been examined in various studies (8, 25). Small changes to the placement (8, 25) of the cuff can alter the contribution of different vasoactive substances to FMD [e.g., nitric oxide (25, 60), endothelium-derived hyperpolarizing factor (47), and prostaglandins (25, 60)]. It may also alter the shear magnitude and/or transmural pressure response, resulting in a different FMD stimulus (1, 25, 91). A 5-min cuff occlusion distal to the ultrasound probe placed on the brachial artery was associated with an ∼7% FMD response, which was abolished by NO blockade (25). However, when the cuff was placed above the ultrasound probe, the ∼12% FMD response was only partially decreased by NO blockade (i.e., to ∼7.5%) (25). These data indicate that cuff placement may influence the nature of the FMD response, and the dilation of arteries within the ischemic territory may be affected by dilators other than NO and also by myogenic responses. We therefore recommend cuff occlusion below the imaged artery to ensure maximal dependence of the vasodilator response on the endothelium and endothelium-derived NO.
Analysis: edge detection and wall tracking.
Initial studies using the FMD technique relied on manual assessment of vessel diameters using visual inspection of single frames and placement of ultrasonic calipers at discrete points along the long axis of the B-mode image (13, 14). This method of manual assessment is highly operator dependent and subject to significant observer error (38, 74, 95, 130). Computer-assisted analysis, utilizing edge-detection and wall-tracking software, permits multiple measurements along the vessel wall. Studies comparing the validity and reproducibility of computerized edge-detection and wall-tracking systems demonstrate significantly lower intraobserver variation for the automated systems than for the manual technique (95, 109, 130). Therefore, validated, accurate, and reproducible edge-detection and wall-tracking systems should be used to improve the validity of the FMD measurements.
Protocol: assessment of peak diameter.
In the first studies using FMD, peak artery diameter was assessed on a single frame at 60 s postdeflation (Fig. 2) (13, 14). The reason for defining 60 s as the appropriate time to take a peak diameter measure dates back to the original work of Celermajer, Deanfield et al. (14). Although this approach is still used (43, 57, 128), recent papers have questioned the validity of this technique to detect the “true” peak diameter (10, 114).
Fig. 2.
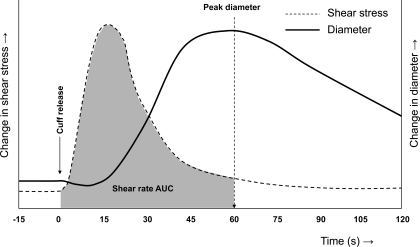
Schematic presentation of diameter and shear-stress (or rate) responses following cuff deflation in response to a 5-min ischemic stimulus. The gray area represents the relevant shear rate area-under-the-curve (AUC) that is believed to be the main stimulus for the peak diameter.
PEAK DIAMETER IN THE BA.
It was recently demonstrated that calculating FMD on the basis of the 60-s value can lead to a 25–40% underestimation of the true FMD in humans (10) (Fig. 3). Also, a predetermined time window (e.g., 50–70 or 70–90 s) can result in an underestimate of the true maximal FMD (10). This may lead to a systemic reduction in the effect size of interventions and result in type II statistical errors (71). Because the time to peak diameter differs between (10, 71, 85) and within (50) groups, measurement of time to peak diameter as a potential simple marker of risk has attracted interest. However, recent data have been interpreted as indicating that the time to peak diameter is at least partially NO independent and that the time to peak diameter does not appear to be a useful adjunctive measure of endothelial health (71). These studies, nonetheless, endorse continuous measurement of arterial diameter responses during FMD. On the basis of available data, an assessment period of at least 180 s for the brachial artery seems warranted when assessing the FMD, with most peak measurements occurring in the first 120 s after cuff release (10). Further work remains to determine the determinants and utility of time to peak diameter measurement.
Fig. 3.
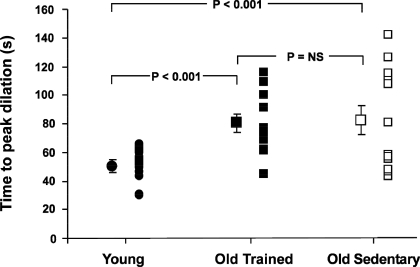
Mean and individual brachial artery diameter time to peak dilation following a period of 5 min of forearm ischemia in healthy young (n = 12, •), old fit (n = 12, ▪), and old unfit (n = 12, □) subjects. Error bars represent standard error of the mean. [Adapted from Black et al. (10).]
IDENTIFICATION OF PEAK DIAMETER: TIMING OF DIAMETER MEASUREMENTS AND SIZE OF TIME BINS.
The 2002 guidelines recommended measurement of the brachial diameter at end diastole to limit the influence of potential differences in vascular compliance on diameter measurements (18). However, interpretation of recent data indicates that FMD (and nitroglycerin-mediated dilation) measurements using an average of the vessel diameter over the entire cardiac cycle yield equivalent results over a wide range of vascular compliance (55, 85).
Another aspect of FMD analysis is the identification of the peak dilation. For current, automated diameter analysis systems, various time bins have been used to average the peak diameter and subsequently calculate FMD, varying from 3 s up to 10 s (10, 28, 39, 41, 85, 86). Shorter time bins (e.g., 1 s) will likely result in greater peak diameter and FMD, whereas longer bins (e.g., 10 s) will result in a lower FMD. A recent study reported that the FMD is significantly lower when using a 10-s time bin compared with 3- or 5-s time bins (38). In light of these data, we recommend that laboratories apply a consistent time-bin methodology and report the method used.
OTHER CONDUIT ARTERIES.
An increasing number of studies are examining endothelial function in conduit arteries such as the posterior tibial (56, 110), popliteal (9, 87, 89, 119), superficial femoral (13, 21, 116, 131), deep femoral (131), and common femoral (114) arteries. These studies have largely assumed that, when adopting the typical FMD methodological approach, the NO-dependent nature is comparable to that observed in the radial artery (61, 68, 79). However, confirmatory evidence to this effect exists only for the superficial femoral artery (61). Because endothelial NO synthase content is heterogeneous throughout the arterial tree (64), the relative contribution of NO to FMD may differ between conduit vessels. In addition, arteries in the legs demonstrate a significantly later peak than those in the arms (114). This means that the 3-min postdeflation time window for the brachial artery is unlikely to capture the peak diameter in arteries of different size, location, and structure. This should be taken into consideration when examining the dilator response in arteries of different size. Therefore, we recommend a 5-min time window to capture the peak diameter in arteries other than the brachial artery. Further work is necessary to establish the NO dependence of FMD responses in conduit vessels other than the radial and superficial femoral arteries.
Protocol: assessment of baseline diameter.
The FMD response is characteristically presented as a change from baseline diameter. In the classic approach (11), baseline diameter has been defined as the diameter preceding cuff inflation, and this remains the most frequently adopted method in the literature (16, 30, 69, 93, 98, 109). This approach has been used in studies of prognosis (n = ∼10,000, Table 1), such that data indicating that FMD is clinically relevant fundamentally assume that FMD is calculated using a precuff inflation baseline.
Some recent physiological studies have assessed baseline diameter at the end of the cuff inflation (20, 72, 73, 79, 81). The rationale for this is that restoration of the occlusion-induced change in diameter represents an integrative part of the FMD response itself. Some studies suggest that radial artery vasoconstriction occurs during distal cuff inflation (36, 125), whereas recent studies examining the brachial diameter during occlusion demonstrate conflicting results (89, 99, 117, 125). One recent study directly compared the impact of using precuff inflation vs. end-of-cuff inflation diameter on brachial artery FMD (95). The brachial artery diameter during cuff inflation was significantly larger than that assessed preinflation, consequently leading to a significantly different FMD. More importantly, this effect of cuff inflation on the baseline diameter differed across different age groups. The typical age-related reduction in FMD was not found when the baseline diameter preceding cuff deflation was used (95). Given that the impact of occlusion on arterial diameter differs among populations (95), we recommend using the preocclusion diameter as the baseline value.
Shear stimulus and the FMD response.
Several studies have demonstrated, using various manipulations of the shear-stress stimulus within subjects, that exposure to shear stress leads to diameter increases in a dose-dependent fashion (8, 65, 85, 86, 99). The close relationship between a change in shear stimulus and a change in diameter indicates that the shear stimulus is the eliciting stimulus for artery dilation during the FMD response (76). When shear stress increases, this signal is transduced by the endothelial cells and stimulates the release of vasoactive substances, which then act on the vascular smooth muscle (4, 59). The magnitude of dilation is therefore determined by 1) the characteristics of the shear-stress stimulus (e.g., amount, pattern), 2) the transduction of the vasodilator response to the smooth muscles, 3) the response of the smooth muscle to a given vasodilator signal through changes in calcium concentrations, and 4) the resulting diameter change, which may be affected by structural characteristics of the vessel wall (58, 66). Steps 2-4 are the source of biological variability and result in between-subject response differences (Fig. 1). The purpose of the FMD test is to interrogate these biological differences to identify endothelial function.
WHAT PORTION OF THE REACTIVE HYPEREMIA PROFILE IS RELEVANT TO FMD-RESPONSE DEVELOPMENT.
The postdeflation shear profile is transient in nature, and there is a significant delay between the peak shear rate and the peak diameter (18) (Fig. 2). This raises questions about which part of the reactive hyperemia shear profile represents the relevant stimulus for dilation. Both postdeflation peak shear rate and the entire stimulus-until-peak diameter have been hypothesized to contribute to the FMD-response magnitude (99, 105). Pyke and Tschakovsky (99) were the first to truly examine this matter and performed a series of experiments in which they independently manipulated the peak or the total shear-rate stimulus. These experiments demonstrated that the total, rather than peak, shear rate determines the magnitude of the FMD response (99). This finding was recently confirmed by others (86).
It is also important to mention that it is not possible, using some machines, to simultaneously have active B-mode and pulsed-wave velocity windows in duplex mode. This, along with the fact that simultaneous “live” velocity and diameter measures can degrade B-mode image resolution in older machines, has led to the practice of recording velocity for an initial period (e.g., 30 s) followed by a switch to diameter measurement to capture the peak diameter. In this case, it is possible to miss the true peak diameter, and shear rate can only be calculated for the period of time during which velocity measures are recorded. The validity of this approach to shear calculation rests on whether there is a similar correlation between FMD and the shear-stress stimulus calculated up to, e.g., 30 s, compared with the individualized time-to-peak diameter. In this context, one recent study suggested that a good correlation existed between shear rate calculated to 30 s, 60 s, and to the individual peak diameter (113). Therefore, measuring the first 30 s of the shear-stress stimulus may represent a valid alternative under some circumstances, but the optimal approach must be to continuously collect high-resolution Doppler and B-mode images to the time of peak diameter for the calculation of shear.
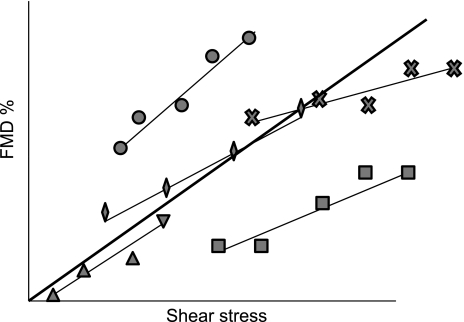
RELATIONSHIP BETWEEN SHEAR STRESS AND FMD: BETWEEN-SUBJECT COMPARISONS.
In contrast to the within-subject studies (8, 65, 86, 99), the relationship between shear and FMD may be weak when between-subject comparisons are undertaken (Figs. 4 and 5). For example, a recent study found a correlation between total shear rate area-under-the-curve and FMD in young adults (r2 = ∼0.15), whereas no correlation was evident in either children or older adults (113). This does not undermine the role of shear stress as the stimulus for FMD but primarily illustrates the importance of biological variability in the FMD response to shear stress attributable to variability in the above steps 2-4 (Fig. 1). It can be appreciated that different individuals may have the same FMD even though they have experienced substantially different shear-stress stimuli (or vice versa, Fig. 4). A conclusion that similar FMD in this situation reflects similar endothelial function could be potentially misleading, as would a conclusion that different FMD is attributable to different shear stress. Given the importance of shear rate, we recommend measuring and presenting the shear stimulus until peak diameter (85, 86, 99).
Fig. 5.
For any normalization approach to be appropriate, the covariate (i.e., shear) should be at least moderately correlated to the outcome (i.e., FMD) (5). Thin solid lines represent hypothetical within-subject shear-FMD relationships, where the dilation is presented to 5 incremental shear-stress stimuli. The good within-subject shear-FMD relationship suggests that normalization is appropriate when using a within-subject comparison in this data set. The thick solid line represents the between-subject correlation fitted across the mean values for the 5 subjects. A between-subject correlation is present in this data set, which is a necessary assumption for normalization in cross-sectional studies. Interindividual variation is expected in 1) the response of shear to the hyperemic stress, 2) the slope of within-subject relationship, and 3) the intercept of within-subject relationship.
FMD ANALYSIS: STIMULUS NORMALIZATION.
The FMD test elicits a reactive hyperemia stimulus, which is variable between subjects (51, 86, 97) and determined by several factors (15, 77, 106, 122). Consequently, when distinct FMD responses are observed between groups or individuals, it may be unclear whether this is attributable to differences in biological variability in endothelial function per se or differences in the magnitude of reactive hyperemia (Fig. 5). This raises the issue of how to account for the magnitude of the stimulus when interpreting FMD results.
On the basis of the relationship between FMD and eliciting shear-rate stimuli within young healthy subjects described above (8, 65, 86, 99), researchers began normalizing the FMD responses by dividing the FMD by shear rate (21, 86, 89). However, important questions have since arisen regarding the validity of this approach (5, 40). When adopting this type of normalization, there must be at least a moderate correlation between shear and FMD in each particular research setting (2, 5). This correlation should also be relatively stable in all the groups or experimental conditions that are examined in any study (5, 40). If the relationship between shear and FMD is weak and/or inconsistent between study groups, then shear normalization may be misleading and decrease statistical power (5). Whereas a strong relation between the “dose” of shear rate and the FMD is observed within young subjects (97, 99, 100), a relatively weak relation has been found between young healthy subjects (114), with no relation reported in other groups (113) (Figs. 4 and 5).
Even if there is a moderate to strong relationship between shear and FMD, as suggested by within-subject comparisons, the accuracy of normalization depends on the characteristics of this relationship in each research setting (2, 5). Relevant assumptions for the use of ratios indicate that normalization is valid if 1) the relationship between both parameters is linear, 2) the intercept for the regression slope of this relationship is zero, 3) data (including residuals) are normally distributed, 4) variances are similar between groups, and 5) the ratio does not lead to spurious correlations with other variables (2) (Fig. 5). A recent study found that all assumptions for reliable use of FMD/shear ratios were violated in the comparison of FMD between samples of boys, young men, and older men (5). Logarithmic transformation of shear rate and FMD improved adherence to assumptions in this particular study (5), but it was recommended that this and other methods of normalization, such as analysis of covariance (5, 40) or allometric scaling (5), should be further investigated before widespread use. In essence, the accuracy of FMD normalization via simple division by shear rate depends on the shear-FMD relationship being at least moderately strong and consistent between groups or conditions. If these conditions are not met, this process will be inconsistently applied across these groups and conditions (2), leading to inaccurate conclusions (5).
SUMMARY: SHEAR STIMULUS AND THE FMD RESPONSE.
Taken together, there is a physiological and mechanistic basis for considering the impact of shear rate when interpreting FMD responses because increased shear is the stimulus for FMD (76). However, there are various factors that can influence the transduction of shear stress into conduit artery dilation. These factors may be both methodological [e.g., cuff position, duration of shear, and duration of ischemia (7, 8, 25, 79, 86)] and physiological [e.g., arterial stiffness, flow pattern, and blood viscosity (12, 73)] and have not been fully described or accounted for. Moreover, there is evidence both for (85) and against (5, 113, 114) using a ratio normalization of the dilatory response to shear rate. In addition, the relationship between shear rate and FMD may not be linear, and the mathematical assumptions necessary to normalize FMD to shear are invalidated in certain study populations (5). On the basis of this current evidence, we endorse measuring shear and acknowledge it as the eliciting stimulus for FMD. Researchers should ideally report the total shear-rate stimulus and, if necessary, investigate the relationship between it and the dilatory response in their publications. However, the use of ratio normalization (FMD/shear) is presently unresolved, and at this time it is not possible to recommend a method for correcting for differences in shear. Further research will be necessary to 1) accurately quantify the shear response to occlusion (to improve and validate estimation of the shear stimulus) and 2) determine how best to account for the shear stimulus in relation to the conduit artery dilation for widespread utility of the FMD procedure.
Recommendations for FMD Assessment and Future Perspectives
The FMD technique will soon enter its third decade as a research tool in humans. There are many attractive reasons to pursue this technique, including the fact that it provides a noninvasive and direct measure of artery function and health in vivo. There is increasing evidence that FMD provides valuable and independent prognostic information in humans. However, different methodological approaches limit its validity, comparability, and its potential use as a clinical and physiological research tool. In addition, improving understanding of the physiological and technical principles underpinning the FMD technique will improve its application and interpretation of (patho)physiological changes that may occur between groups or after interventions. Performing and reporting FMD in a manner consistent with the physiology of the response to shear stress will ultimately improve the accuracy of FMD measurement for prediction of future clinical risk and as a methodological investigative tool. This review has provided an updated physiological rationale for the techniques employed in FMD assessment. In addition, several unresolved issues in the practice of FMD were highlighted, and it is expected that this will stimulate further work aimed at improving what has become an extremely popular research and clinical measurement tool.
GRANTS
D. J. Green received funding from the National Heart Foundation of Australia and the Australian Research Council. D. H. J. Thijssen is supported by the Dutch Heart Foundation (E. Dekker stipend). M. E. Widlansky is supported by K23HL089326, AHA-ASP MGrant-in-Aid 10GRNT3880044, and the Greater Milwaukee Foundation. M. E. Tschakovsky is supported by funding from the Natural Science and Engineering Research Council of Canada. R. A. Harris is supported by a Scientific Development Grant (10SDG3050006) from the American Heart Association. B. Parker is supported by NIH 1R01HL098085-01. J. Padilla is supported by the NIH T32 AR048523. K. E. Pyke is supported by funding from the Natural Science and Engineering Research Council of Canada.
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Agewall S, Doughty RN, Bagg W, Whalley GA, Braatvedt G, Sharpe N. Comparison of ultrasound assessment of flow-mediated dilatation in the radial and brachial artery with upper and forearm cuff positions. Clin Physiol (Oxf) 21: 9–14, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord 19: 644–652, 1995 [PubMed] [Google Scholar]
- 3. Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation 79: 93–100, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J 73: 1983–1992, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Atkinson G, Batterham AM, Black MA, Cable NT, Hopkins ND, Dawson EA, Thijssen DH, Jones H, Tinken TM, Green DJ. Is the ratio of flow-mediated dilation and shear rate a statistically sound approach to normalization in cross-sectional studies on endothelial function? J Appl Physiol 107: 1893–1899, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Berdeaux A, Ghaleh B, Dubois-Randé JL, Vigué B, La Rochelle CD, Hittinger L, Giudicelli JF. Role of vascular endothelium in exercise-induced dilation of large epicardial coronary arteries in conscious dogs. Circulation 89: 2799–2808, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Berry KL, Skyrme-Jones RAP, Meredith IT. Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery flow-mediated dilation. Clin Sci 99: 261–267, 2000 [PubMed] [Google Scholar]
- 8. Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol 286: H442–H448, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Boot CR, Groothuis JT, Van Langen H, Hopman MT. Shear stress levels in paralyzed legs of spinal cord-injured individuals with and without nerve degeneration. J Appl Physiol 92: 2335–2340, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Califano JP, Reinhart-King CA. Exogenous and endogenous force regulation of endothelial cell behavior. J Biomech 43: 79–86, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 24: 1468–1474, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Noninvasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Chironi G, Craiem D, Miranda-Lacet J, Levenson J, Simon A. Impact of shear stimulus, risk factor burden and early atherosclerosis on the time-course of brachial artery flow-mediated vasodilation. J Hypertens 26: 508–515, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest 88: 1663–1671, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooke JP, Stamler J, Andon N, Davies PF, McKinley G, Loscalzo J. Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Physiol Heart Circ Physiol 259: H804–H812, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract 6: 16–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dawson EA, Whyte GP, Black MA, Jones H, Hopkins N, Oxborough D, Gaze D, Shave RE, Wilson M, George KP, Green DJ. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol 105: 1562–1568, 2008 [DOI] [PubMed] [Google Scholar]
- 21. de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol 287: H374–H380, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Donald AE, Charakida M, Cole TJ, Friberg P, Chowienczyk PJ, Millasseau SC, Deanfield JE, Halcox JP. Non-invasive assessment of endothelial function: which technique? J Am Coll Cardiol 48: 1846–1850, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Donald AE, Charakida M, Falaschetti E, Lawlor DA, Halcox JP, Golding J, Hingorani AD, Smith GD, Deanfield JE. Determinants of vascular phenotype in a large childhood population: the Avon Longitudinal Study of Parents and Children (ALSPAC). Eur Heart J 31: 1502–1510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol 51: 1959–1964, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001 [PubMed] [Google Scholar]
- 26. Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation 99: 3227–3233, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290: H1446–H1453, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol 571: 661–668, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evans DH, McDicken WN. Doppler Ultrasound: Physics, Instrumentation, and Signal Processing. Chichester, NY: Wiley, 2000 [Google Scholar]
- 30. Evans DH, Schlindwein FS, Levene MI. The relationship between time averaged intensity weighted mean velocity, and time averaged maximum velocity in neonatal cerebral arteries. Ultrasound Med Biol 15: 429–435, 1989 [DOI] [PubMed] [Google Scholar]
- 31. Fathi R, Haluska B, Isbel N, Short L, Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol 43: 616–623, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Frick M, Suessenbacher A, Alber HF, Dichtl W, Ulmer H, Pachinger O, Weidinger F. Prognostic value of brachial artery endothelial function and wall thickness. J Am Coll Cardiol 46: 1006–1010, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 34. Ghiadoni L, Huang Y, Magagna A, Buralli S, Taddei S, Salvetti A. Effect of acute blood pressure reduction on endothelial function in the brachial artery of patients with essential hypertension. J Hypertens 19: 547–551, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Gnasso A, Carallo C, Irace C, Spagnuolo V, De Novara G, Mattioli PL, Pujia A. Association between intima-media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation 94: 3257–3262, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol 51: 1953–1958, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1233–1234; discussion 1237–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol 107: 445–453, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Harris RA, Padilla J. Proper “normalization” of flow-mediated dilation for shear. J Appl Physiol 103: 1108; author reply 1109, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Harris RA, Padilla J, Hanlon KP, Rink LD, Wallace JP. The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity 16: 578–584, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92: 3431–3435, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 45: 1441–1448, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Hijmering ML, de Lange DW, Lorsheyd A, Kraaijenhagen RJ, van de Wiel A. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: a small trial in healthy volunteers. Neth J Med 65: 29–35, 2007 [PubMed] [Google Scholar]
- 45. Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Hoskins PR, Thrush A, Martin K, Whittingham TA. Diagnostic Ultrasound: Physics and Equipment. Cambridge, UK: Cambridge University, 2002 [Google Scholar]
- 47. Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 280: H2462–H2469, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Hutcheson IR, Griffith TM. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol Heart Circ Physiol 261: H257–H262, 1991 [DOI] [PubMed] [Google Scholar]
- 49. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Irace C, Tschakovsky ME, Carallo C, Cortese C, Gnasso A. Endothelial dysfunction or dysfunctions? Identification of three different FMD responses in males with type 2 diabetes. Atherosclerosis 200: 439–445, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Ishibashi Y, Takahashi N, Shimada T, Sugamori T, Sakane T, Umeno T, Hirano Y, Oyake N, Murakami Y. Short duration of reactive hyperemia in the forearm of subjects with multiple cardiovascular risk factors. Circ J 70: 115–123, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 53. Jones H, Green DJ, George K, Atkinson G. Intermittent exercise abolishes the diurnal variation in endothelial-dependent flow-mediated dilation in humans. Am J Physiol Regul Integr Comp Physiol 298: R427–R432, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Karatzi K, Papamichael C, Karatzis E, Papaioannou TG, Stamatelopoulos K, Zakopoulos NA, Zampelas A, Lekakis J. Acute smoke-induced endothelial dysfunction is more prolonged in smokers than in non-smokers. Int J Cardiol 120: 404–406, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Kizhakekuttu TJ, Gutterman DD, Phillips SA, Jurva JW, Arthur EI, Das E, Widlansky ME. Measuring FMD in the brachial artery: How important is QRS-gating? J Appl Physiol 109: 959–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kobayashi N, Tsuruya Y, Iwasawa T, Ikeda N, Hashimoto S, Yasu T, Ueba H, Kubo N, Fujii M, Kawakami M, Saito M. Exercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremities. Circ J 67: 505–510, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Koh KK, Quon MJ, Lee Y, Han SH, Ahn JY, Chung WJ, Kim JA, Shin EK. Additive beneficial cardiovascular and metabolic effects of combination therapy with ramipril and candesartan in hypertensive patients. Eur Heart J 28: 1440–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 260: H862–H868, 1991 [DOI] [PubMed] [Google Scholar]
- 59. Koller A, Kaley G. Role of endothelium in reactive dilation of skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 259: H1313–H1316, 1990 [DOI] [PubMed] [Google Scholar]
- 60. Koller A, Sun D, Huang A, Kaley G. Corelease of nitric oxide and prostaglandins mediates flow-dependent dilation of rat gracilis muscle arterioles. Am J Physiol Heart Circ Physiol 267: H326–H332, 1994 [DOI] [PubMed] [Google Scholar]
- 61. Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kremkau F. Diagnostic Ultrasound: Principles and Instruments. St. Louis, MO: Elsevier Saunders, 2002 [Google Scholar]
- 63. Lansman JB, Hallam TJ, Rink TJ. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? Nature 325: 811–813, 1987 [DOI] [PubMed] [Google Scholar]
- 64. Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol Heart Circ Physiol 284: H1307–H1312, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Leeson P, Thorne S, Donald A, Mullen M, Clarkson P, Deanfield J. Non-invasive measurement of endothelial function: effect on brachial artery dilatation of graded endothelial dependent and independent stimuli. Heart 78: 22–27, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 259: 381–392, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Li S, Hoskins PR, Anderson T, McDicken WN. Measurement of time averaged mean velocity during pulsatile flow using time-averaged maximum frequency of Doppler ultrasound waveforms. Ultra Med Biol 19: 105–113, 1993 [DOI] [PubMed] [Google Scholar]
- 68. Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol 78: 1210–1214, 1996 [DOI] [PubMed] [Google Scholar]
- 69. Lind L. Arterial compliance influences the measurement of flow-mediated vasodilation, but not acetylcholine-mediated forearm blood flow. The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis 190: 212–215, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Lind L, Johansson K, Hall J. The effects of mental stress and the cold pressure test on flow-mediated vasodilation. Blood Press 11: 22–27, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Liuni A, Luca MC, Lisi M, Dragoni S, Di Stolfo G, Mariani JA, Uxa A, Gori T, Parker JD. Observations of time-based measures of flow-mediated dilation of forearm conduit arteries: Implications for the accurate assessment of endothelial function. Am J Physiol Heart Circ Physiol 299: H939–H945, 2010 [DOI] [PubMed] [Google Scholar]
- 72. Magen E, Viskoper JR, Mishal J, Priluk R, London D, Yosefy C. Effects of low-dose aspirin on blood pressure and endothelial function of treated hypertensive hypercholesterolaemic subjects. J Hum Hypertens 19: 667–673, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Malik AR, Kondragunta V, Kullo IJ. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension 51: 1512–1518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mancini GB, Yeoh E, Abbott D, Chan S. Validation of an automated method for assessing brachial artery endothelial dysfunction. Can J Cardiol 18: 259–262, 2002 [PubMed] [Google Scholar]
- 75. McGowan CL, Levy AS, Millar PJ, Guzman JC, Morillo CA, McCartney N, Macdonald MJ. Acute vascular responses to isometric handgrip exercise and effects of training in persons medicated for hypertension. Am J Physiol Heart Circ Physiol 291: H1797–H1802, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Melkumyants AM, Balashov SA, Khayutin VM. Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc Res 23: 741–747, 1989 [DOI] [PubMed] [Google Scholar]
- 77. Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44: 134–139, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Moncada S, Radomski MW, Palmer RM. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol 37: 2495–2501, 1988 [DOI] [PubMed] [Google Scholar]
- 79. Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001 [DOI] [PubMed] [Google Scholar]
- 80. Nabel EG, Selwyn AP, Ganz P. Large coronary arteries in humans are responsive to changing blood flow: an endothelium-dependent mechanism that fails in patients with atherosclerosis. J Am Coll Cardiol 16: 349–356, 1990 [DOI] [PubMed] [Google Scholar]
- 81. Neunteufl T, Priglinger U, Heher S, Zehetgruber M, Soregi G, Lehr S, Huber K, Maurer G, Weidinger F, Kostner K. Effects of vitamin E on chronic and acute endothelial dysfunction in smokers. J Am Coll Cardiol 35: 277–283, 2000 [DOI] [PubMed] [Google Scholar]
- 82. Oates C. Cardiovascular Haemodynamics and Doppler Waveforms Explained. Cambridge, UK: Cambridge University, 2001 [Google Scholar]
- 83. Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature 331: 168–170, 1988 [DOI] [PubMed] [Google Scholar]
- 84. Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation 109: 2507–2510, 2004 [DOI] [PubMed] [Google Scholar]
- 85. Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Adjusting flow-mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res 46: 592–600, 2009 [DOI] [PubMed] [Google Scholar]
- 86. Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus (Abstract). Cardiovasc Ultrasound 6: 44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009 [DOI] [PubMed] [Google Scholar]
- 88. Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (Lond) 109: 55–60, 2005 [DOI] [PubMed] [Google Scholar]
- 89. Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol 291: H3043–H3049, 2006 [DOI] [PubMed] [Google Scholar]
- 90. Parker BA, Trehearn TL, Meendering JR. Pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol 107: 1357–1359, 2009 [DOI] [PubMed] [Google Scholar]
- 91. Peretz A, Leotta DF, Sullivan JH, Trenga CA, Sands FN, Aulet MR, Paun M, Gill EA, Kaufman JD. Flow mediated dilation of the brachial artery: an investigation of methods requiring further standardization (Abstract). BMC Cardiovasc Disord 7: 11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 74: 1399–1406, 1986 [DOI] [PubMed] [Google Scholar]
- 93. Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986 [DOI] [PubMed] [Google Scholar]
- 94. Polak JF. Peripheral Vascular Sonography: A Practical Guide. Baltimore, MD: Williams and Wilkins, 2004 [Google Scholar]
- 95. Preik M, Lauer T, Heiss C, Tabery S, Strauer BE, Kelm M. Automated ultrasonic measurement of human arteries for the determination of endothelial function. Ultraschall Med 21: 195–198, 2000 [DOI] [PubMed] [Google Scholar]
- 96. Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol 298: H119–H126, 2010 [DOI] [PubMed] [Google Scholar]
- 97. Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97: 499–508, 2004 [DOI] [PubMed] [Google Scholar]
- 98. Pyke KE, Hartnett JA, Tschakovsky ME. Are the dynamic response characteristics of brachial artery flow-mediated dilation sensitive to the magnitude of increase in shear stimulus? J Appl Physiol 105: 282–292, 2008 [DOI] [PubMed] [Google Scholar]
- 99. Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007 [DOI] [PubMed] [Google Scholar]
- 100. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rognmo O, Bjornstad TH, Kahrs C, Tjonna AE, Bye A, Haram PM, Stolen T, Slordahl SA, Wisloff U. Endothelial function in highly endurance-trained men: effects of acute exercise. J Strength Cond Res 22: 535–542, 2008 [DOI] [PubMed] [Google Scholar]
- 102. Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986 [DOI] [PubMed] [Google Scholar]
- 103. Rush JW, Denniss SG, Graham DA. Vascular nitric oxide and oxidative stress: determinants of endothelial adaptations to cardiovascular disease and to physical activity. Can J Appl Physiol 30: 442–474, 2005 [DOI] [PubMed] [Google Scholar]
- 104. Schretzenmayr A. Uber kreislaufregulatorische Vorgange an den groben Arterien bei Muskelarbeit. Pflügers Arch 232: 743–748, 1933 [Google Scholar]
- 105. Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J Am Coll Cardiol 38: 1859–1865, 2001 [DOI] [PubMed] [Google Scholar]
- 106. Silber HA, Ouyang P, Bluemke DA, Gupta SN, Foo TK, Lima JA. Why is flow-mediated dilation dependent on arterial size? Assessment of the shear stimulus using phase-contrast magnetic resonance imaging. Am J Physiol Heart Circ Physiol 288: H822–H828, 2005 [DOI] [PubMed] [Google Scholar]
- 107. Sinoway LI, Hendrickson C, Davidson WR, Jr, Prophet S, Zelis R. Characteristics of flow-mediated brachial artery vasodilation in human subjects. Circ Res 64: 32–42, 1989 [DOI] [PubMed] [Google Scholar]
- 108. Smiesko V, Kozik J, Dolezel S. Role of endothelium in the control of arterial diameter by blood flow. Blood Vessels 22: 247–251, 1985 [PubMed] [Google Scholar]
- 109. Sonka M, Liang W, Lauer RM. Automated analysis of brachial ultrasound image sequences: early detection of cardiovascular disease via surrogates of endothelial function. IEEE Trans Med Imaging 21: 1271–1279, 2002 [DOI] [PubMed] [Google Scholar]
- 110. Stoner L, Sabatier MJ, Mahoney ET, Dudley GA, McCully KK. Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury. Spinal Cord 45: 49–56, 2007 [DOI] [PubMed] [Google Scholar]
- 111. Taneva E, Borucki K, Wiens L, Makarova R, Schmidt-Lucke C, Luley C, Westphal S. Early effects on endothelial function of atorvastatin 40 mg twice daily and its withdrawal. Am J Cardiol 97: 1002–1006, 2006 [DOI] [PubMed] [Google Scholar]
- 112. ter Avest E, Holewijn S, Stalenhoef AF, de Graaf J. Variation in non-invasive measurements of vascular function in healthy volunteers during daytime. Clin Sci (Lond) 108: 425–431, 2005 [DOI] [PubMed] [Google Scholar]
- 113. Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. Does arterial shear explain the magnitude of flow-mediated dilation? A comparison between young and older humans. Am J Physiol Heart Circ Physiol 296: H57–H64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol 295: H1927–H1934, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009 [DOI] [PubMed] [Google Scholar]
- 116. Thijssen DH, de Groot PC, Smits P, Hopman MT. Vascular adaptations to 8-week cycling training in older men. Acta Physiol (Oxf) 190: 221–228, 2007 [DOI] [PubMed] [Google Scholar]
- 117. Thijssen DH, van Bemmel MM, Bullens LM, Dawson EA, Hopkins ND, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. The impact of baseline diameter on flow-mediated dilation differs in young and older humans. Am J Physiol Heart Circ Physiol 295: H1594–H1598, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thrush A, Hartshorne T. Peripheral Vascular Ultrasound. How, Why and When. New York: Churchill Livingstone, 2004 [Google Scholar]
- 119. Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586: 5003–5012, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 122. Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1237; discussion 1237–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 123. Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol 281: H1380–H1389, 2001 [DOI] [PubMed] [Google Scholar]
- 124. Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2: 997–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 125. Weissgerber TL, Davies GA, Tschakovsky ME. Low flow-mediated constriction occurs in the radial but not the brachial artery in healthy pregnant and nonpregnant women. J Appl Physiol 108: 1097–1105, 2010 [DOI] [PubMed] [Google Scholar]
- 126. Widlansky ME, Vita JA, Keyes MJ, Larson MG, Hamburg NM, Levy D, Mitchell GF, Osypiuk EW, Vasan RS, Benjamin EJ. Relation of season and temperature to endothelium-dependent flow-mediated vasodilation in subjects without clinical evidence of cardiovascular disease (from the Framingham Heart Study). Am J Cardiol 100: 518–523, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab 86: 5389–5395, 2001 [DOI] [PubMed] [Google Scholar]
- 128. Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 115: 3086–3094, 2007 [DOI] [PubMed] [Google Scholar]
- 129. Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol 45: 1987–1993, 2005 [DOI] [PubMed] [Google Scholar]
- 130. Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001 [DOI] [PubMed] [Google Scholar]
- 131. Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006 [DOI] [PubMed] [Google Scholar]
- 132. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]
- 133. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang X, Zhao SP, Li XP, Gao M, Zhou QC. Endothelium-dependent and -independent functions are impaired in patients with coronary heart disease. Atherosclerosis 149: 19–24, 2000 [DOI] [PubMed] [Google Scholar]