Abstract
The Na,K-ATPase is a ubiquitous transmembrane pump and a specific receptor for cardiac glycosides such as ouabain and digoxin, which are used in the management of congestive heart failure (CHF). A potential role for these so-called endogenous cardiotonic steroids (CS) has been explored, and it has become apparent that such compounds are elevated and may play an important role in a variety of physiological and pathophysiological conditions such as hypertension and CHF. Recent evidence suggests that the Na,K-ATPase may act as a signal transducer upon CS binding and induce nonproliferative cardiac growth, implicating a role for endogenous CS in the development of cardiac hypertrophy and progressive failure of the heart. In the present study, we tested whether hypertrophic responses to pressure overload would be altered in mutant mice that specifically express ouabain-sensitive or ouabain-resistant α1- and α2-Na,K-ATPase subunits, as follows: α1-resistant, α2-resistant (α1R/Rα2R/R); α1-sensitive, α2-resistant (α1S/Sα2R/R); and α1-resistant, α2-sensitive (α1R/Rα2S/S, wild-type). In α1S/Sα2R/R mice, pressure overload by transverse aortic coarctation induced severe left ventricular (LV) hypertrophy with extensive perivascular and replacement fibrosis at only 4 wk. Responses in α1R/Rα2S/S and α1R/Rα2R/R mice were comparatively mild. Mutant α1S/Sα2R/R mice also had LV dilatation and depressed LV systolic contractile function by 4 wk of pressure overload. In separate experiments, chronic Digibind treatment prevented the rapid progression of cardiac hypertrophy and fibrosis in α1S/Sα2R/R mice. These data demonstrate that mice with a ouabain-sensitive α1-Na,K-ATPase subunit have a dramatic susceptibility to the development of cardiac hypertrophy, and failure from LV pressure overload and provide evidence for the involvement of endogenous CS in this process.
Keywords: cardiotonic steroids, fibrosis, transverse aortic coarctation, heart failure
despite continued advances in the prevention and treatment of cardiovascular disease, heart failure remains a major public health problem with increasing prevalence and mortality rates (4). Present therapeutic options for heart failure combine neurohormonal antagonism with diuretics and digitalis drugs, the latter of which are part of a larger class of so-called cardiotonic steroids (CS) that includes digoxin, ouabain, and marinobufagenin (1, 13, 41). Although the therapeutic actions of these CS compounds are attributed to its specific inhibition of the catalytic α-subunit of the Na,K-ATPase (NKA) and the subsequent elevation in intracellular Ca2+ levels, recent studies have demonstrated that binding of exogenous CS to the α-subunit of the NKA may directly initiate a variety of intracellular signaling cascades, independent of changes in transport activity. This dual role for CS was demonstrated in isolated neonatal rat cardiac myocytes, where therapeutic concentrations of ouabain were found to regulate transcription of several cardiac growth-related genes and thereby induce hypertrophic growth (34, 43). Subsequent studies on isolated heart and neonatal rat cardiac myocyte preparations have specifically implicated the involvement of the Src/epidermal growth factor receptor and phosphatidylinositol-3 kinase/Akt signaling cascades as downstream targets of the steroid-sodium pump interaction (17, 18, 30).
The concept of an endogenous sodium pump inhibitor with similar properties to therapeutic cardioactive steroids emerged over 30 years ago, when two separate groups first documented the presence of endogenous digoxin-like immunoreactive substances in the bovine hypothalamus (11, 20). Similar substances with specific cross reactivity to digoxin antibodies and sodium pump inhibitory activity were later isolated from plasma of volume-expanded dogs (16). Subsequently, several groups were successful in the isolation and purification of two distinct classes of endogenous CS, cardenolides and bufadienolides, from multiple mammalian tissues (2, 14, 19, 28, 29). Importantly, clinical studies have identified above normal circulating levels of ouabain/digitalis-like factor in diverse pathological conditions (2, 3, 12, 15, 27, 35). Circulating CS levels are strongly correlated with the extent of left ventricular (LV) remodeling and impairment of LV function, implying a definitive pathophysiological role for this new class of steroid hormones.
In this study, we sought to specifically determine whether endogenous CS compounds interact with specific cardiac NKA α-isoforms and thereby mediate hypertrophic growth of the heart in response to induced chronic mechanical stress. We used knock-in mutant mice in which the α1- or α2-NKA subunits were selectively sensitive or resistant to CS to study the progression of cardiac remodeling in response to pressure overload by surgically induced transverse aortic coarctation (TAC).
MATERIALS AND METHODS
Animal model.
Experiments were carried out with mutant mice expressing NKA isoforms with altered ouabain sensitivities including, α1-sensitive/α2-resistant (α1S/Sα2R/R), α1-resistant/α2-sensitive (α1R/Rα2S/S, wild-type), and α1-resistant/α2-resistant mice (α1R/Rα2R/R) mice. Mice with ouabain-sensitive α1-isoform and/or ouabain-sensitive α2-isoform were generated by amino acid substitutions at positions 111 and 122 in the first extracellular loop of the α-subunit, as previously described (5, 6). Animals were obtained from two established colonies at the University of Cincinnati, both of which were on a mixed 129SvJ and Black Swiss background. The colony of single-mutant α1R/Rα2R/R mice were maintained by mating heterozygous male and female animals (α1R/Rα2S/R × α1R/Rα2S/R) (5). The colony of double-mutant (α1S/Sα2R/R) mice were maintained by mating homozygous double-mutant animals. Breeding pairs were periodically backcrossed to a parallel subcolony of wild-type mice to sustain a consistent genetic background between mutant and wild-type mice. The wild-type animals used in these studies were obtained from both colonies and show no differences in any observed measurements. Genotypes were determined by PCR analysis of DNA from tail biopsies, as described. All experiments were approved by the University of Cincinnati Institutional Animal Care and Use Committee in accordance with established guidelines.
Surgical model of cardiac hypertrophy.
Pressure overload of the LV was induced by TAC in 2–3-mo-old mice of each genetic background as described (39). Mice were anesthetized with isoflurane (1.5–2.5% in 100% oxygen) and intubated. A midsternal incision was made, and the transverse aortic segment was dissected. The aorta was ligated between the innominate and left common carotid arteries by tying a 7-0 silk suture around a 27-gauge needle placed on top of the transverse segment of the aorta. The needle was then removed, leaving the intact suture to produce a stenosis of uniform diameter. Sham-operated mice underwent a similar procedure except that the suture was pulled around the transverse aorta and left untied. Perioperative mortality from the procedure (sham and TAC) averaged ∼10% and was not different between the genotypes. All mice were weighed and monitored daily until terminal experimental procedures were performed. Preliminary observations for this study indicated that TAC α1S/Sα2R/R mice rapidly progress into heart failure at 4–5 wk postsurgery. Because these mice showed clear signs of congestive heart failure (CHF) (chest fluid accumulation, congested lungs, enlarged hearts, and atrial thrombus formation) within 2–4 wk of TAC, all terminal measurements were made at 4 wk.
Echocardiography.
Animals were anesthetized with 1.0–1.5% isoflurane, and two-dimensional guided M-mode echocardiography was performed at 4 wk post-TAC on all mice using a SONOS 5500 Ultrasound system equipped with a 13-MHz transducer (Hewlett-Packard, Palo Alto, CA). All measurements were done from leading edge to leading edge according to the American Society of Echocardiography guidelines (38). M-mode measurements were used to determine LV end-diastolic diameter (LVEDD), LV posterior and anterior wall thickness (PWTh and AWth) during diastole, and LV end-systolic diameter (LVESD) over three consecutive cardiac cycles. LV mass was calculated according to uncorrected cube assumptions with some modifications using the equation, LV mass (in mg) = 1.055[(LVEDD + LVPWTh + LVAWth)3 − LVEDD3], where 1.055 is the specific gravity of myocardium (36). Percentage of ejection fraction (%EF) was calculated using the formula %EF = (LVED volume − LVES volume)/LVED volume.
In vivo hemodynamic measurements.
Transstenotic pressure gradient was determined in all mice that underwent TAC or sham surgery by simultaneous pressure recording from both right and left carotid arteries. Mice were anesthetized with an intraperitoneal injection of ketamine (50 μg/g body wt) and inactin (thiobutabarbital, 100 μg/g body wt; Sigma, St. Louis, MO). The left carotid artery was cannulated with fluid-filled polyethylene tubing and connected to a low-compliance pressure transducer (COBE Cardiovascular, Arvada, CO). A high-fidelity, 1.4-French Millar Mikro-Tip transducer (Model SPR-671; Millar Instruments, Houston, TX) was inserted into the right carotid artery for measurement of right carotid artery blood pressure and then advanced into the LV to monitor cardiac performance, as previously described (31, 33). At the end of the experiment, hearts were excised, carefully dissected free of adherent tissue, blotted dry, and weighed. Hearts were then processed for further analysis as described.
Morphological and histological evaluation.
Hearts were fixed in 10% buffered formalin solution and paraffin embedded. Transverse sections of 4 μm of thickness were obtained at the midventricle level and stained with hematoxylin and eosin (H and E) for examination of gross appearance. Masson's trichrome counterstained with hematoxylin was used to examine fibrosis by quantitative morphometry. Images were captured using a Olympus Q Color-5 camera and processed using Q capture Pro software. To assess myocyte cross-sectional area, images were taken at 200× from two random fields (2,560 × 1,920 pixels; 323.89 μm × 431.40 μm) from the epicardial region (from 4 sections of each heart, for a total of 8 images per heart). Myocyte diameter was measured at the level of the nucleus of myocardial cells of each section using NIH Image J software. To quantify global fibrosis (interstitial and perivascular), we used custom-designed software programmed in IDL (IDL 6.2; ITT Visual Information Solutions, Boulder, CO) that allows quantification of the blue collagen pixels as a percentage of the total number of pixels (https://irc.cchmc.org/research/cardiac.php).
Immunoblot analysis.
Following endpoint functional assessment, hearts were excised promptly from anesthetized mice, rinsed in ice-cold phosphate-buffered saline, frozen in liquid N2, and stored at −80°C. Frozen hearts were manually pulverized in liquid N2 and homogenized in buffer containing 10 mM Tris (pH 7.4), 1 mM EDTA, 2 mM dithiothreitol, 0.25% Nonidet P-40, protease inhibitor cocktail (P-8340, Sigma) and phosphatase inhibitor cocktail (P-5726, Sigma). Protein concentration in each heart homogenate was measured using Coomassie protein assay reagent (Pierce, Rockford, IL). Western blot analysis was performed as described previously with minor modifications (23). In brief, protein samples were boiled for 10 min in loading buffer [250 mM Tris (pH 6.8), 10% SDS, 50% glycerol, and 0.05% (wt/vol) bromphenol blue] and separated by electrophoresis in 8% (for α1- and α2-NKA) or 12% polyacrylamide gels (for Src). The separated proteins were transferred to nitrocellulose membranes and blocked in 5% nonfat dry milk in 20 mM Tris, pH 7.6, 150 mM NaCl, and 0.01% Tween 20 (TBST) for 1 h at room temperature. The blots were incubated in TBST containing α1-isoform-specific monoclonal antibody (1:1,000, α6F; University of Iowa Developmental Studies Hybrid Bank, Iowa City, IA) and α2-isoform-specific rabbit monoclonal HERED antibody (1:500) at 4°C overnight. After incubation with peroxidase-conjugated goat anti-mouse IgG antibody for α1 (1:8,000; KPL, Gaithersburg, MD) and peroxidase-conjugated rabbit IgG for α2 (1:8,000; Cell Signaling Technology, Beverly, MA), immunoreactivity was visualized following treatment with LumiGlo chemiluminiscent reagents (KPL), and Biomax ML and MR films (Eastman Kodak, Rochester, NY) were used to develop autoradiograms of probed membranes. Equal protein loading was confirmed following reprobing of the membranes with αS-actin-specific mouse monoclonal antibody (1:10,000, Sigma). For determination of activated Src protein levels, membranes were first probed for phosphorylated Src at tyrosine 416 using rabbit polyclonal IgG (1:1,000, Cell Signaling) and then stripped and reprobed for total Src protein (1:1,000, Cell Signaling).
Chronic Digibind infusion.
In a separate series of experiments, we compared the progression of hypertrophy in α1S/Sα2R/R and α1R/Rα2R/R mice in the presence of an anti-digoxin Fab fragment (Digibind; GlaxoSmithKline, Philadelphia, PA), which binds to and sequesters digoxin-like molecules (8, 37). At 2 wk post-TAC, Digibind was administered at a rate of 50 μg/day via a jugular catheter connected to a subcutaneously implanted osmotic minipump (Alzet 2002; Durect, Cupertino, CA). Terminal functional measurements and postmortem morphometric and histological analyses were performed at the 4-wk endpoint as described.
Statistical analysis.
Statistical analysis was performed using a one-way ANOVA or mixed, two-way ANOVA (SigmaStat; Systat Software, San Jose, CA). Where necessary, post hoc comparisons of individual values with correction for multiple comparisons were accomplished using the Tukey's test (26). Data are presented as means ± SE, and differences were presumed significant at P < 0.05.
RESULTS
Accelerated pressure overload-induced cardiac hypertrophy in α1S/Sα2R/R mice.
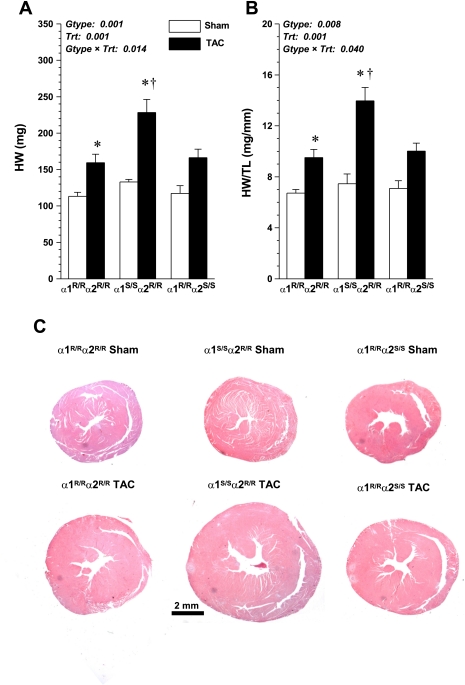
Figure 1, A and B, shows absolute and normalized cardiac gravimetric data in sham and TAC mice and demonstrates that the hypertrophic response to pressure overload was markedly augmented in the hearts of α1S/Sα2R/R mice compared with both α1R/Rα2R/R and α1R/Rα2S/S mice, which were not different from each other. Cross-sectional whole mounts of hearts at 4 wk post-sham and post-TAC surgery are shown in Fig. 1C. There were no differences in ventricular wall thickness of sham mice of all three genotypes. In TAC mice, profound LV and RV wall thickening was seen in α1S/Sα2R/R mice, whereas only slight thickening of the LV was apparent in α1R/Rα2R/R and α1R/Rα2S/S mice. Because the degree of hypertrophic growth is directly dependent on the magnitude of the pressure overload, we also measured the pressure gradient across the aortic constriction in these same animals at the end of the experiment (at week 4) and found that there were no significant differences between the groups (α1R/Rα2R/R, 55 ± 5; α1S/Sα2R/R, 53 ± 8; α1R/Rα2S/S, 72 ± 7). Indeed, the gradient in the α1S/Sα2R/R mice, which had the highest degree of hypertrophy, had the lowest pressure gradient. We are therefore confident that the enhanced myocardial growth in these mice was not attributable to a greater degree of pressure overload.
Fig. 1.
Comparison of cardiac weight in sham ouabain α1-resistant, α2-resistant (α1R/Rα2R/R) (n = 6), sham α1-sensitive, α2-resistant (α1S/Sα2R/R) (n = 6), sham α1-resistant, α2-sensitive (α1R/Rα2S/S) (n = 6), transverse aortic coarctation (TAC) α1R/Rα2R/R (n = 8), TAC α1S/Sα2R/R (n = 10), and TAC α1R/Rα2S/S mice (n = 5) at 4 wk postsurgery; values are means ± SE. A: whole heart weight (HW). B: heart weight normalized to tibia length (TL). C: transverse sections of hearts. Main effects and interaction terms from two-way ANOVA are shown in each panel. Gtype, genotype; Trt, treatment. Post hoc comparisons were as follows: *significant effect vs. respective sham (P < 0.05); †significant difference compared with TAC α1R/Rα2S/S and TAC α1R/Rα2R/R (P < 0.001).
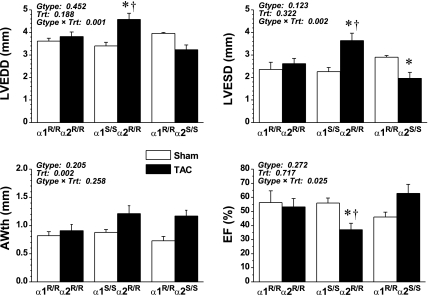
Echocardiographic indices of the LV structure and function in sham and TAC animals are shown in Fig. 2. At 4 wk, sham-operated mice of all three genotypes did not differ echocardiographically. Consistent with postmortem gravimetric data, in vivo echocardiographic measurements of LVEDD and LVESD revealed cardiac enlargement in α1S/Sα2R/R mice but not in α1R/Rα2R/R or α1R/Rα2S/S mice. AWth was modestly increased in all three groups (treatment effect P = 0.002, interaction P = 0.258).
Fig. 2.
Echocardiography measures of left ventricular (LV) structure and function of sham α1R/Rα2R/R (n = 5), sham α1S/Sα2R/R (n = 6), sham α1R/Rα2S/S (n = 3), TAC α1R/Rα2R/R (n = 5), TAC α1S/Sα2R/R (n = 6), and TAC α1R/Rα2S/S mice (n = 5) compared at 4 wk. values are means ± SE. Results from two-way ANOVA are shown in each panel. Post hoc comparisons are as follows: *significant difference vs. corresponding sham (P < 0.05); †significant difference vs. TAC α1R/Rα2S/S and TAC α1R/Rα2R/R (P < 0.05). LVEDD, LV end-diastolic diameter; LVESD, LV end-systolic diameter; AWth: LV anterior wall thickness; EF: ejection fraction.
Diminished LV function in α1S/Sα2R/R mice following TAC.
We assessed LV function at 4 wk post-TAC in terminal experiments using a closed-chest, catheter-based approach, and results are shown in Table 1. No differences were observed in hemodynamic and cardiac performance indices between the three sham-treated groups. Following 4 wk of pressure overload, all three groups demonstrated evidence of compromised hemodynamic function, but the changes in the α1S/Sα2R/R mice were most severe. Left carotid mean arterial pressure, downstream of the aortic constriction, was lowest in the TAC α1S/Sα2R/R mice. The rate of LV contraction and relaxation (dP/dtmax and dP/dtmin) tended to be lower in α1S/Sα2R/R mice, but differences were not significant, owing largely to the high degree of variability observed in these mice. The time constant of relaxation (τ) was also elevated to a greater extent in the TAC α1S/Sα2R/R mice compared with the other two TAC groups. These data indicate substantial degrees of systolic and diastolic dysfunction in α1S/Sα2R/R mice after only 4 wk of pressure overload. Ejection-phase indices of cardiac performance, determined echocardiographically under isoflurane anesthesia, also suggested significant deficits in cardiac contraction in the α1S/Sα2R/R mice compared with TAC α1R/Rα2R/R and α1R/Rα2S/S mice.
Table 1.
Cardiac function in anesthetized mice
| α1R/Rα2R/R |
α1S/Sα2 R/R |
α1R/Rα2S/S |
||||
|---|---|---|---|---|---|---|
| Sham (n = 6) | TAC (n = 8) | Sham (n = 5) | TAC (n = 8) | Sham (n = 3) | TAC (n = 5) | |
| Heart Rate, bpm | 480 ± 39 | 455 ± 19 | 434 ± 17 | 410 ± 16 | 396 ± 11 | 412 ± 12 |
| LC MAP, mmHg | 89 ± 3 | 72 ± 4* | 84 ± 4 | 63 ± 4* | 72 ± 2 | 75 ± 3 |
| LC SP, mmHg | 103 ± 4 | 77 ± 4* | 92 ± 4 | 74 ± 6* | 83 ± 3 | 84 ± 5 |
| LVSP, mmHg | 104 ± 3 | 133 ± 7* | 101 ± 3 | 122 ± 12 | 96 ± 3 | 162 ± 9*† |
| dP/dtmax, mmHg/s | 8013 ± 602 | 6750 ± 657 | 6665 ± 147 | 5454 ± 2026 | 7430 ± 589 | 8443 ± 533 |
| dP/dtmin, mmHg/s | −10028 ± 953 | −7881 ± 1146 | −8475 ± 588 | −5999 ± −2308 | −8796 ± 434 | 8781 ± −892 |
| τ, ms | 10 ± 1.0 | 14 ± 1.0* | 11 ± 1.0 | 17 ± 2.0*† | 11 ± 0.5 | 11 ± 1.0 |
| LVEDP, mmHg | 2.4 ± 0.5 | 3.1 ± 1.4 | 2.3 ± 1.0 | 5.0 ± 1.5 | 5.9 ± 3.3 | 5.3 ± 0.9 |
| EF, % | 56 ± 7 | 53 ± 6 | 56 ± 4 | 37 ± 5*† | 46 ± 3 | 63 ± 7 |
| FS, % | 35 ± 7 | 32 ± 4 | 34 ± 3 | 21 ± 3*† | 27 ± 2 | 40 ± 5 |
| Vcf, cm/s | 6.6 ± 1.0 | 6.6 ± 0.9 | 6.2 ± 0.5 | 3.8 ± 0.7*† | 4.4 ± 0.4 | 8.0 ± 1.1* |
Values are expressed as means ± SE. Data are from mutant mice that specifically express ouabain-sensitive or ouabain-resistant α1- and α2-Na,K-ATPase subunits, as follows: α1-resistant, α2-resistant (α1R/Rα2R/R); α1-sensitive, α2-resistant (α1S/Sα2R/R); and α1-resistant, α2-sensitive (α1R/Rα2S/S, wild-type). TAC, transverse aortic coarctation; LC MAP, left carotid mean arterial pressure; LC SP, left carotid systolic pressure; LV SP, left ventricular systolic pressure; dP/dtmax and dP/dtmin, average maximum and minimum values of the first derivative of left ventricular pressure; τ, time constant of isovolumetric pressure decay; LVEDP, left ventricular end-diastolic pressure; EF, ejection fraction; FS, fractional shortening; Vcf, velocity of circumferential shortening.
P < 0.05 vs. corresponding sham;
P < 0.05 vs. TAC value in other two groups.
These data demonstrate that ouabain sensitivity of the α1-NKA in the mouse confers a marked inclination for the development of cardiac hypertrophy and dysfunction in response to chronic pressure overload. Importantly, these data further indicate that this inclination is not conferred by ouabain sensitivity of the α2-NKA because no decremental changes were observed in the α1R/Rα2S/S mice (wild-type) compared with α1R/Rα2R/R mice. In the remainder of our experiments, therefore, we have limited the presentation of our findings to comparisons between α1S/Sα2R/R mice and α1R/Rα2R/R mice so that observed differences may be ascribed specifically to effects of α1 ouabain sensitivity, without confounding influences of α2 ouabain sensitivity.
Increased fibrosis and cellular hypertrophy in α1S/Sα2R/R mice.
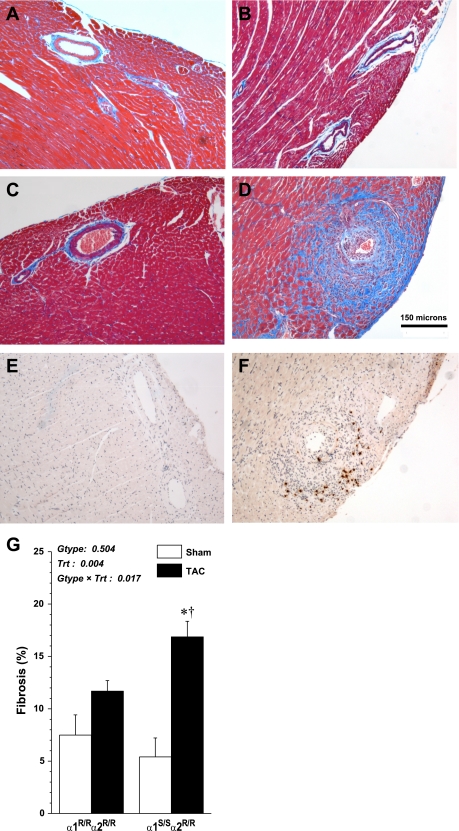
Results from histopathological evaluation of cardiac sections are illustrated in Fig. 3, A–D. Whereas there were no differences in sham mice in response to pressure overload, there was greater perivascular and interstitial fibrosis in α1S/Sα2R/R hearts compared with α1R/Rα2R/R hearts. Quantification of total collagen content in heart sections by digital image analysis is shown in Fig. 3G. TAC α1S/Sα2R/R hearts had a significant increase in total collagen accumulation compared with TAC α1R/Rα2R/R hearts (17% ± 1.4 vs. 12% ± 1). We also performed immunohistochemistry on cardiac tissue of TAC-operated α1S/Sα2R/R and α1R/Rα2R/R hearts using anti-major basic protein 1, which confirmed perivascular eosinophil accumulation (Fig. 3, E and F).
Fig. 3.
Masons trichrome-stained sections at 4 wk after sham and TAC surgery. Heart sections showing degree of collagen accumulation; original magnification 100×. A: sham α1R/Rα2R/R. B: sham α1S/Sα2R/R. C: TAC α1R/Rα2R/R. D: TAC α1S/Sα2R/R. Immunohistochemistry on heart sections showing eosinophil accumulation in TAC α1R/Rα2R/R (E) and TAC α1S/Sα2R/R (F). G: quantification of total collagen content of heart sections: measurements from 4 sections per heart were averaged and then used to calculate mean values (± SE; n = 4 per group). Results from two-way ANOVA are shown. *Significant effect vs. corresponding sham (P < 0.001); †significant effect vs. TAC α1R/Rα2R/R (P < 0.05).
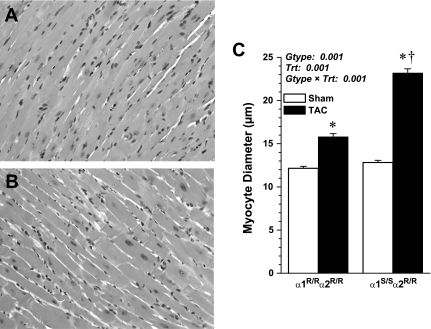
Myocardial cell diameter was measured from H and E-stained heart sections, as shown in Fig. 4, A and B, and summary data are shown in Fig. 4C. Sham-operated α1R/Rα2R/R and α1S/Sα2R/R mice had similar myocyte diameter, and TAC mice of both genotypes had significantly larger myocardial cells at 4 wk post-TAC compared with corresponding shams. However, TAC α1S/Sα2R/R myocytes were significantly larger compared with TAC α1R/Rα2R/R (23.18 ± 0.5 μm vs. 15.78 ± 0.37 μm, P < 0.001; Fig. 4C) with enlarged nuclei confirming augmented myocardial cell hypertrophy. Quantitative analysis of myocyte cross-sectional area of epicardial cells also confirmed greater myocyte hypertrophy in TAC α1S/Sα2R/R mice compared with TAC α1R/Rα2R/R mice (562.1 ± 17.2 vs. 952.5 ± 26.4 μm2, P < 0.001).
Fig. 4.
Representative photomicrographs of hematoxylin and eosin-stained LV sections from TAC α1R/Rα2R/R (A) and TAC α1S/Sα2R/R (B); original magnification 200×. C: summary data of myocyte diameter measurements (n = 2–3 hearts and 50–60 measurements/group); values are means ± SE. Results from two-way ANOVA are shown. Post hoc comparisons are as follows: *P < 0.001 vs. corresponding sham; †P < 0.001 vs. α1R/Rα2R/R.
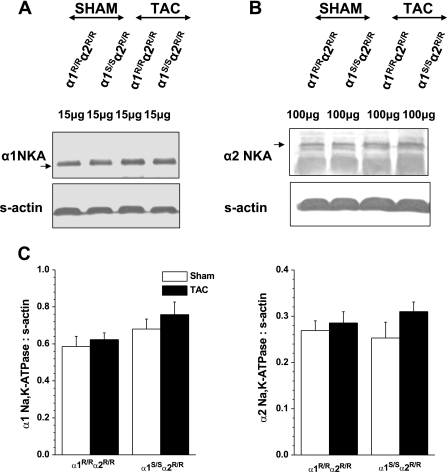
NKA-α1 and -α2 isoform levels were not altered at 4 wk post-TAC.
Western blot analyses were carried out on whole tissue homogenates to determine whether cardiac α1- and α2-NKA isoform proteins were differentially expressed in response to pressure overload in the α1S/Sα2R/R and α1R/Rα2R/R mice. Figure 5 shows immunoblots and densitometry analysis for α1- and α2-NKA isoform expression. At 4 wk post-TAC, there were no significant differences in protein expression levels of both NKA isoforms. There were also no detectable differences in phosphorylated Src at tyrosine 416 between TAC α1S/Sα2R/R and α1R/Rα2R/R mice (data not shown).
Fig. 5.
Expression of Na,K-ATPase (NKA) α-isoforms in hearts of α1R/Rα2R/R (n = 3) and α1S/Sα2R/R (n = 3) mice, 4 wk after sham and TAC surgery. Proteins were resolved by 8% polyacrylamide gel electrophoresis. Immunoblots for α1-NKA (A) and α2-NKA (B) and densitometry analyses (C) are shown. Values are means ± SE.
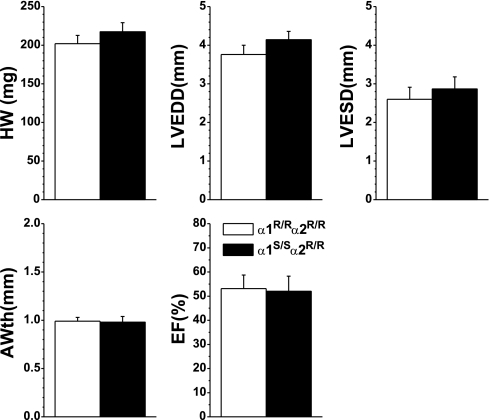
Digibind attenuated cardiac myocyte hypertrophy and fibrosis in TAC α1S/Sα2R/R mice.
To further examine whether the observed differences in cardiac hypertrophy were attributable to alterations in the response to endogenous CS, we treated mice with Digibind for the final 2 wk of the 4-wk TAC protocol. At 4 wk, the heart weights of TAC + Digibind-treated α1S/Sα2R/R and α1R/Rα2R/R mice were not different from each other, as shown in Fig. 6. In contrast to prior data from untreated TAC mice, both groups showed a modest but equivalent degree of hypertrophy, and the degree of hypertrophy in the TAC + Digibind α1S/Sα2R/R was less than in the untreated TAC α1S/Sα2R/R mice (see Fig. 1). In vivo echocardiographic measurements also support the finding that Digibind treatment normalized the degree of hypertrophic growth between α1R/Rα2R/R and α1S/Sα2R/R mice.
Fig. 6.
Echocardiography measures of LV structure of Digibind-treated TAC α1R/Rα2R/R (n = 6) and TAC α1S/Sα2R/R mice (n = 7), compared at 4 wk. Values are means ± SE.
DISCUSSION
CS have been identified as a new class of steroid hormones that mediate hypertrophic growth in cardiac tissue on the basis of primarily in vitro studies utilizing neonatal cardiomyocytes (21, 22, 34) and have been shown to produce mild hypertrophic response in vivo in rats during extended periods of treatment (24, 25, 40). However, a direct demonstration of the relevance of the NKA isoforms in mediating the cardiovascular effects of endogenous CS in vivo has been lacking. Because rats, like mice, express a ouabain-resistant α1-NKA isoform, it is reasonable to speculate that the hypertrophic response observed previously is mediated primarily through the α2-isoform. Alternatively, it is possible that the levels of CS achieved in these studies were sufficient to interact with the α1-NKA to some degree. Using mutant mice expressing ouabain-resistant or ouabain-sensitive α1- and/or α2-NKA subunits, we explored the role of these isoforms in mediating the progression of cardiac structural and functional changes in response to TAC. Our findings indicate that α1-NKA ouabain sensitivity confers a marked susceptibility for the development of cardiac hypertrophy and fibrosis in response to pressure overload.
We have previously demonstrated that genetic alteration of the so-called ouabain binding site of the NKA α-subunit does not alter baseline cardiac function or structure but produces dramatic changes in the physiological effects of exogenously administered cardenolides such as ouabain and digoxin (5–7) as well as of bufadienolides such as marinobufagenin (42). Additionally, it has been demonstrated that the cardiac glycoside binding site of the NKA plays an important role in blood pressure regulation in response to endogenous ligands in the context of adrenocorticotropin hormone-induced hypertension (7, 8, 32). We show here that mice with a ouabain-sensitive α1-NKA have a greater tendency to develop pressure overload-induced hypertrophy compared with both WT and α1R/Rα2R/R mice, which express a ouabain-resistant α1-isoform.
In most strains of mice, LV dysfunction and pulmonary congestion occur around 12 wk after onset of pressure overload. By contrast, most of the α1S/Sα2R/R mice showed overt signs of heart failure (decompensation) beginning at 3–4 wk. By echocardiography, ejection phase indices of LV function were significantly depressed in α1S/Sα2R/R mice, as indicated in Fig. 2 and Table 1. Postmortem analysis confirmed severe hypertrophy with LV dilatation and perivascular and interstitial fibrosis. Although pressure-dependent indices of LV contractility measured by LV catheterization (i.e., dP/dtmax and dP/dtmin) were not significantly lower in the TAC α1S/Sα2R/R, this appears to be attributable to the high degree of variability that occurred in this group. In fact, of the eight mice evaluated in this group, three appeared to be severely decompensated (dP/dt < 4,500 mmHg/s), whereas three others were relatively normal (dP/dt > 7,000 mmHg/s); two were midrange. This range of values would suggest that these mice decompensate very quickly between 4 and 5 wk of pressure overload.
Previous studies have suggested that exogenous application of CS can alter expression levels of NKA isoforms both in vitro and in vivo (17, 24, 25). In the present study, however, we found no discernable differences in α1- or α2-NKA protein levels in whole cardiac cell extracts between TAC α1S/Sα2R/R and α1R/Rα2R/R mice in response to pressure overload. Furthermore, extensive studies on rat neonatal cardiac myocytes have shown that ouabain can activate the Src/MAPK pathway via the NKA, which leads to the induction of growth-related genes. We did not see a significant difference in Src protein activation in heart extracts of TAC α1S/Sα2R/R and α1R/Rα2R/R mice after 4 wk of pressure overload. This may not be surprising, however, because this pathway is generally regarded as an early-response signal transduction pathway, and it may have been differentially activated in the α1S/Sα2R/R mice only during the early phases of pressure overload.
We used the digoxin-immune Fab Digibind, which is used to sequester endogenous and exogenous circulating CS to specifically examine whether the accelerated hypertrophy and fibrosis observed in the pressure overload α1S/Sα2R/R mice can be attributed to elevated levels of circulating CS. Digibind treatment successfully attenuated myocyte hypertrophy and cardiac fibrosis in TAC α1S/Sα2R/R mice, supporting a specific role for endogenous CS in the augmented hypertrophic response seen under chronic pressure overload. With regard to the severity of the fibrosis in the α1S/Sα2R/R mice, it has been previously reported that administration of marinobufagenin can induce myocardial collagen expression, both in vitro and in vivo, and that this may occur through a PKCδ-dependent signaling pathway, possibly involving the transcription factor Friend leukemia integration-1 (9, 10). The presence of perivascular inflammation in cardiac tissue of TAC α1S/Sα2R/R mice might indicate that endogenous CS are involved in an inflammatory process leading to fibrosis in cardiac tissue. It is further possible that accelerated cardiomyocyte growth is secondary to increased myocardial wall stress imparted by the increased fibrosis.
The outcome of this study has larger implications in terms of the potential role of endogenous CS in human CHF because humans express sensitive α1- and α2-NKA isoforms. These data may suggest that the ouabain-resistant α1-isoform normally expressed in rats and mice may represent a trait for cardiomyopathy resistance. As several clinical studies have identified a positive correlation with circulating concentrations of these endogenous factors with cardiac dysfunction, hypertrophy, and arterial hypertension, it may be necessary to consider this potential resistance in future studies using these species as models.
GRANTS
This work was supported by National Institutes of Health Grants DK57552 (J. N. Lorenz), HL66062 and HL28573 (to J. B. Lingrel), and by American Heart Association Predoctoral Fellowship 09PRE2260930 (to A. N. Wansapura).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Michelle Nieman for technical expertise and advice in conducting these experiments.
REFERENCES
- 1. Akera T, Brody TM. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev 29: 187–220, 1977 [PubMed] [Google Scholar]
- 2. Bagrov AY, Fedorova OV, Dmitrieva RI, Howald WN, Hunter AP, Kuznetsova EA, Shpen VM. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension 31: 1097–1103, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Balzan S, Neglia D, Ghione S, D'Urso G, Baldacchino MC, Montali U, L'Abbate A. Increased circulating levels of ouabain-like factor in patients with asymptomatic left ventricular dysfunction. Eur J Heart Fail 3: 165–171, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation 113: 799–805, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, Lingrel JB. The α2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem 278: 53026–53034, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The α1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem 279: 54053–54061, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Dostanic-Larson I, Lorenz JN, Van Huysse JW, Neumann JC, Moseley AE, Lingrel JB. Physiological role of the α1- and α2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol 290: R524–R528, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci USA 102: 15845–15850, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, Kim EG, Smaili S, Periyasamy SM, Hariri IM, Fedorova L, Liu J, Wu L, Kahaleh MB, Xie Z, Malhotra D, Fedorova OV, Kashkin VA, Bagrov AY, Shapiro JI. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension 49: 215–224, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Elkareh J, Periyasamy SM, Shidyak A, Vetteth S, Schroeder J, Raju V, Hariri IM, El-Okdi N, Gupta S, Fedorova L, Liu J, Fedorova OV, Kahaleh MB, Xie Z, Malhotra D, Watson DK, Bagrov AY, Shapiro JI. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. Am J Physiol Renal Physiol 296: F1219–F1226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fishman MC. Endogenous digitalis-like activity in mammalian brain. Proc Natl Acad Sci USA 76: 4661–4663, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fridman AI, Matveev SA, Agalakova NI, Fedorova OV, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous ligand of alpha-1 sodium pump, is a marker of congestive heart failure severity. J Hypertens 20: 1189–1194, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Gheorghiade M, Pitt B. Digitalis Investigation Group (DIG) trial: a stimulus for further research. Am Heart J 134: 3–12, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Goto A, Ishiguro T, Yamada K, Ishii M, Yoshioka M, Eguchi C, Shimora M, Sugimoto T. Isolation of a urinary digitalis-like factor indistinguishable from digoxin. Biochem Biophys Res Commun 173: 1093–1101, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Gottlieb SS, Rogowski AC, Weinberg M, Krichten CM, Hamilton BP, Hamlyn JM. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation 86: 420–425, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Gruber KA, Whitaker JM, Buckalew VM., Jr Endogenous digitalis-like substance in plasma of volume-expanded dogs. Nature 287: 743–745, 1980 [DOI] [PubMed] [Google Scholar]
- 17. Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem 275: 27832–27837, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem 277: 18694–18702, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA 88: 6259–6263, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haupert GT, Jr, Sancho JM. Sodium transport inhibitor from bovine hypothalamus. Proc Natl Acad Sci USA 76: 4658–4660, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang L, Kometiani P, Xie Z. Differential regulation of Na/K-ATPase α-subunit isoform gene expressions in cardiac myocytes by ouabain and other hypertrophic stimuli. J Mol Cell Cardiol 29: 3157–3167, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Huang L, Li H, Xie Z. Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J Mol Cell Cardiol 29: 429–437, 1997 [DOI] [PubMed] [Google Scholar]
- 23. James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB. Identification of a specific role for the Na,K-ATPase α2 isoform as a regulator of calcium in the heart. Mol Cell 3: 555–563, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Kennedy DJ, Elkareh J, Shidyak A, Shapiro AP, Smaili S, Mutgi K, Gupta S, Tian J, Morgan E, Khouri S, Cooper CJ, Periyasamy SM, Xie Z, Malhotra D, Fedorova OV, Bagrov AY, Shapiro JI. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am J Physiol Renal Physiol 294: F450–F454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 47: 488–495, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Kusuoka H, Hoffman JI. Advice on statistical analysis for Circulation Research. Circ Res 91: 662–671, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kuznetsova T, Manunta P, Casamassima N, Messaggio E, Jin Y, Thijs L, Richart T, Fagard RH, Bianchi G, Staessen JA. Left ventricular geometry and endogenous ouabain in a Flemish population. J Hypertens 27: 1884–1891, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Laredo J, Hamilton BP, Hamlyn JM. Ouabain is secreted by bovine adrenocortical cells. Endocrinology 135: 794–797, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Lichtstein D, Samuelov S. Endogenous ‘ouabain like’ activity in rat brain. Biochem Biophys Res Commun 96: 1518–1523, 1980 [DOI] [PubMed] [Google Scholar]
- 30. Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol 293: C1489–C1497, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Lorenz JN. A practical guide to evaluating cardiovascular, renal, and pulmonary function in mice. Am J Physiol Regul Integr Comp Physiol 282: R1565–R1582, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Lorenz JN, Loreaux EL, Dostanic-Larson I, Lasko V, Schnetzer JR, Paul RJ, Lingrel JB. ACTH-induced hypertension is dependent on the ouabain-binding site of the α2-Na+-K+-ATPase subunit. Am J Physiol Heart Circ Physiol 295: H273–H280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorenz JN, Robbins J. Measurement of intraventricular pressure and cardiac performance in the intact closed-chest anesthetized mouse. Am J Physiol Heart Circ Physiol 272: H1137–H1146, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Peng M, Huang L, Xie Z, Huang WH, Askari A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J Biol Chem 271: 10372–10378, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Pierdomenico SD, Bucci A, Manunta P, Rivera R, Ferrandi M, Hamlyn JM, Lapenna D, Cuccurullo F, Mezzetti A. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am J Hypertens 14: 44–50, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Pollick C, Hale SL, Kloner RA. Echocardiographic and cardiac Doppler assessment of mice. J Am Soc Echocardiogr 8: 602–610, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Pullen MA, Brooks DP, Edwards RM. Characterization of the neutralizing activity of digoxin-specific Fab toward ouabain-like steroids. J Pharmacol Exp Ther 310: 319–325, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 39. Sakata Y, Hoit BD, Liggett SB, Walsh RA, Dorn GW., 2nd Decompensation of pressure-overload hypertrophy in Gαq-overexpressing mice. Circulation 97: 1488–1495, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Skoumal R, Szokodi I, Aro J, Foldes G, Gooz M, Seres L, Sarman B, Lako-Futo Z, Papp L, Vuolteenaho O, Leppaluoto J, DeChatel R, Ruskoaho H, Toth M. Involvement of endogenous ouabain-like compound in the cardiac hypertrophic process in vivo. Life Sci 80: 1303–1310, 2007 [DOI] [PubMed] [Google Scholar]
- 41. The Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 336: 525–533, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Wansapura AN, Lasko V, Xie Z, Fedorova OV, Bagrov AY, Lingrel JB, Lorenz JN. Marinobufagenin enhances cardiac contractility in mice with ouabain-sensitive α1 Na+-K+-ATPase. Am J Physiol Heart Circ Physiol 296: H1833–H1839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie ZJ, Wang YH, Ganjeizadeh M, McGee R, Jr, Askari A. Determination of total (Na+-K+)-ATPase activity of isolated or cultured cells. Anal Biochem 183: 215–219, 1989 [DOI] [PubMed] [Google Scholar]