Abstract
Cardiac dysfunction is a common cause of death among pediatric patients with mutations in the lysosomal hydrolase α-l-iduronidase (IDUA) gene, which causes mucopolysaccharidosis type I (MPS-I). The purpose of this study was to analyze adrenergic regulation of cardiac hemodynamic function in MPS-I. An analysis of murine heart function was performed using conductance micromanometry to assess in vivo cardiac hemodynamics. Although MPS-I (IDUA−/−) mice were able to maintain normal cardiac output and ejection fraction at baseline, this cohort had significantly compromised systolic and diastolic function compared with IDUA+/− control mice. During dobutamine infusion MPS-I mice did not significantly increase cardiac output from baseline, indicative of blunted cardiac reserve. Autonomic tone, measured functionally by β-blockade, indicated that MPS-I mice required catecholaminergic stimulation to maintain baseline hemodynamics. Survival analysis showed mortality only among MPS-I mice. Linear regression analysis revealed that heightened end-systolic volume in the resting heart is significantly correlated with susceptibility to mortality in MPS-I hearts. This study reveals that cardiac remodeling in the pathology of MPS-I involves heightened adrenergic tone at the expense of cardiac reserve with cardiac decompensation predicted on the basis of increased baseline systolic volumes.
Keywords: Hurler syndrome, cardiomyopathy, phosphorylation
mucopolysaccharidosis type I (MPS-I) is an autosomal recessive disorder caused by loss of function mutations in lysosomal hydrolase α-l-iduronidase (IDUA). Nearly 90 mutations have been identified in IDUA that give rise to the phenotypic continuum of MPS-I, the most severe form of which is known as Hurler syndrome (MPS-I) (2, 19, 28). The loss of functional IDUA protein results in a multisystem accumulation of glycosaminoglycan (GAG) substrates, dermatan sulfate, and heparan sulfate (20). Lysosomal accumulation of undegraded GAGs in affected cells leads to deficiencies in vital lysosomal functions, including recycling of nutrients and cellular membranes. This initiates a secondary cascade of downstream events, such as the accumulation of ganglioside GM2 and unesterified cholesterol, which further amplify the pathophysiological deficiencies in MPS-I.
As a consequence of IDUA deficiency, patients with MPS-I manifest numerous pathologies, including hepatosplenomegaly, dystosis multiplex, joint stiffness, hearing and visual abnormalities, cardiac valve dysfunction, cardiomyopathy, and mental retardation. In the worst cases, patients experience congestive heart failure and death within the first decade of life as a consequence of systemic organ dysfunction. Significant effort has been aimed at ameliorating MPS-I disease using cell transplantation, enzyme replacement, and, in preclinical studies, gene therapy (4, 18, 25). However, a great deal remains unknown regarding the natural history of MPS-I.
IDUA is the sole agent needed to correct MPS-I pathologies. IDUA delivery can be achieved by enzyme replacement therapy consisting of the exogenous administration of IDUA (16) or by the endogenous IDUA production from normal donor leukocytes that is possible after allogeneic hematopoietic cell transplantation (HCT) (14). HCT can be a life-saving measure for children with MPS-IH, and despite the significant morbidity from chemotherapy administered before HCT and the injury associated with immunologic graft-host rejection, more than 90% of children with MPS-IH survive long term when treated (3, 27, 30).
Cardiovascular disease is also a prominent feature of MPS-I (4–5, 15, 18, 23, 26). In MPS-I, cardiovascular pathologies include thickening of the mitral and aortic valves with regurgitation, hypertrophic cardiomyopathy, epicardial coronary artery occlusion, endocardial thickening, and dilated cardiomyopathy (9). Despite this observation, some of the most fundamental mechanisms involved in pathological cardiac compensation, such as adrenergic signaling, have not been analyzed.
In both acute and chronic cardiomyopathies, heart remodeling is a natural response involved in maintaining cardiac output (CO) (29). One of the major mechanisms by which this occurs is through catecholamine-mediated β-adrenergic signaling (29, 33). In the initial response to cardiac dysfunction, adrenergic stimulation provides a means of boosting cardiac performance. However, chronic adrenergic stimulation is often one of the causes of cardiac decompensation and heart failure (6). Studying the catecholamine regulation of cardiac function in disease states, such as MPS-I, can provide critical information about cardiac reserve and adrenergic tone, which are critical mediators of pump performance.
Numerous animal models have facilitated the study of pathologies and potential therapies for MPS-I, including the MPS-I cat (26), dog (24), and the mouse knockout model (IDUA−/−) (8, 15). However, to our knowledge, no cardiovascular studies have provided insight into catecholaminergic regulation of heart function in MPS-I animal models or patients. Based on the importance of adrenergic signaling in the regulation of heart function, we hypothesized that cardiac reserve and autonomic tone may be compromised in MPS-I hearts and contribute to the cardiomyopathic phenotype. This study provides a comprehensive analysis of real-time cardiac hemodynamics with a specific focus on adrenergic regulation of heart function within the context of MPS-I mice. These results may have significant implications for the therapeutic management of patients with MPS-I.
MATERIALS AND METHODS
Mice.
Adult IDUA gene-deletional mutant C57BL/6J mice (MPS-I), generated by homologous gene recombination and backcrossed to C57BL/6 for more than 12 generations, were obtained as offspring as previously described (8) and bred locally. Reverse-transcriptase polymerase chain reaction was performed on the founders of the C57BL/6J MPS-I mouse colony and on random offspring, all showing the expected genomic profile (8). All mice (MPS-I and IDUA+/−) used in this study were 8–13 mo of age. Control C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were housed and handled according to National Institutes of Health and University of Minnesota animal care guidelines. Experimental procedures were approved by University of Minnesota Institutional Animal Care and Use Committee. Only female mice were used in this study. The investigation conforms with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
In this study, mice heterozygous for the IDUA knockout allele (IDUA+/−) were used as controls. In this mouse model, IDUA+/− are equivalent to wild-type C57BL/6 mice in all measures of consequence associated with IDUA deficiency, including GAG accumulation (data not shown). As such, there is no known reported evidence of a haploinsufficiency phenotype associated with IDUA+/− mice. In addition, the obligatory parental heterozygotes of MPS-I patients are phenotypically normal. In terms of assays described in this study, hemodynamic performance of IDUA+/− mice are similar to that of C57BL/6 mice as previously reported by Palpant and colleagues (21, 22).
Conductance micromanometry.
Measurements of in vivo cardiovascular hemodynamics were obtained using conductance micromanometry as previously performed by this laboratory (11). Mice were anesthetized and ventilated via a tracheal cannulation connected to a pressure-controlled ventilator with 1% isoflurane at a peak inspiratory pressure of 15 cmH2O and a respiratory rate of 60 breaths/min. With the aid of a dissecting microscope, the heart was exposed via a thoracotomy. During surgical preparation some MPS-I mice repeatedly showed evidence of severe intractable arrhythmias or pump failure. This intractable cardiac dysfunction and subsequent decompensation resulted in two out of the eight deaths of MPS-I mice before data acquisition. For those that survived surgical preparation, a 1.4-Fr Millar pressure-volume catheter (SPR-839; Millar Instruments, Houston, TX) was then placed into the left ventricular (LV) chamber via an apical stab. LV pressure and volume measurements were collected at a sampling rate of 1 kHz. Data were analyzed with Ponemah software, P3 Plus (DSI International, St. Paul, MN). Transient inferior vena cava (IVC) occlusions were also performed to obtain the end-systolic and end-diastolic pressure-volume relationships. After we obtained the baseline hemodynamics (ventilated with isoflurane and O2), the mice received a continuous infusion of dobutamine (42 mg·kg−1·min−1) for 5 min. This was followed by an infusion of esmolol (250 μg·kg−1·min−1) for 6 min. IVC occlusions were performed at baseline, 4 min of dobutamine infusion, and 6 min of esmolol infusion. Data were acquired until the completion of the study or systolic pump failure. Cardiac pump failure was defined as the point when peak LV systolic pressure dropped below 40 mmHg. In these cases, the drug infusion was stopped and the mice were recovered to obtain instrument calibration. Aortic insufficiency is common in this murine strain, but no significant hemodynamic differences were observed in mice with and without aortic insufficiency.
Serum analysis.
MPS-I and IDUA+/− mice were anesthetized with isoflurane, and blood was collected by retro-orbital bleeding with 50 μl microcaps-EDTA (Drummond Scientific, Broomall, PA) into SAFE-T-FILL blood collection tubes coated with heparin (Ram Scientific, Yonkers, NY). Eyes were dressed with a drop of Triple Antibiotic HC Ointment (Phoenix Pharmaceutical, St. Joseph, MO). Whole blood analysis was carried out on a Hemavet HV850 (Drew Scientific, Waterbury, CT), analyzing 20 μl per sample as per the manufacturer's suggested protocol.
GAG quantification.
All mice assayed for tissue GAG content were perfused with 15 ml of PBS before the tissues were harvested. GAG was extracted from tissues by homogenization with a Bio-Gen PRO200 (PRO Scientific, Oxford, CT) for 2 min at 4°C in buffer containing 20 mM Tris (pH 8.0), 150 mM NaCl, 0.2% Triton X-100, and 5 mM EDTA. Homogenization was followed by vigorously vortexing the samples for 15 min at 4°C. Insoluble material was pelleted by centrifugation at 12,000 g for 10 min at 4°C, and the supernatant was transferred to a fresh tube. The total protein of tissue homogenates was measured by using the BCA Protein Assay kit (Thermo Fisher Scientific, Rockford, IL). Soluble GAG was measured by combining 50–300 μg tissue homogenate, diluted with PBS to 100 μl final volume, with an association reagent containing 32 mg/l 1,9-dimethylmethylene blue chloride (DMMB; Polysciences, Warrington, PA), 0.2 M GuHCl, 5% ethanol, 0.2% formic acid, and 2 g/l sodium formate (Thermo Fisher Scientific). This solution was vigorously shaken for 30 min at room temperature, and the formed GAG-DMMB complex was pelleted by centrifugation at 15,000 g for 15 min. The supernatant was discarded, and the GAG-DMMB pellets were solubilized in 1.0 ml of 50 mM sodium acetate (pH 6.8), 10% 1-propanol, and 4 M GuHCl (Thermo Fisher Scientific) by vigorously shaking for 15 min at room temperature. Reference standards of 1–7 μg HS (Sigma-Aldrich, St. Louis, MO) were prepared simultaneously with cell lysates. Absorbency was read at 656 nm (DMMB) and 540 nm (background) using a Synergy 2 plate reader (BioTek, Winooski, VT). All values were normalized to their respective amounts of total protein.
Western blot analysis.
For phospho-cardiac troponin I and actin Western blot analysis, the protein samples from MPS-I and heterozygous (IDUA+/−) mice were placed in Laemmli sample buffer in the presence of freshly added β-mercaptoethanol and heated at 70°C for 10 min. The proteins were separated by 12% NuPAG Novex Bis-Tris precast gels with MES Running buffer (Invitrogen, Carlsbad, CA). Separated proteins were transferred to nitrocellulose membrane and blocked with 5% milk (wt/vol in Tris-buffered saline-Tween-20) for 1 h. The blots were probed with rabbit polyclonal antibodies specific to phospho-troponin (cardiac serine-23/24) (1:1,000 dilution; Cell Signaling Technology, Danvers, MA) or mouse monoclonal anti-α-sarcomeric actin (clone 5C5 with 1:5,000 dilution). For phospho-phospholamban Western blot analysis, the protein samples were mixed with Laemmli sample buffer without 2-mercaptoethanol and without heating (native samples). The proteins were separated by 12% NuPAGE Novex Bis-Tris precast gels with MES Running buffer (Invitrogen). Blocked samples were probed with rabbit polyclonal antibodies specific to phospho-phospholamban (serine 16) (1:1,000 dilution; Upstate Biotechnology, Waltham, MA) or mouse monoclonal anti-α-sarcomeric actin (clone 5C5 with 1:5,000 dilution). In all cases the binding of primary antibodies was visualized by goat anti-rabbit (IRDye 800 conjugates) (Rockland Immuncohemicals, Gilbertsville, PA) or goat anti-mouse (Alexa fluor 680 conjugates, Invitrogen) secondary antibodies (1:5,000) and scanned with LI-COR Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Quantitative RT-PCR.
cDNA was generated from 1 μg of individual RNA from MPS-I (IDUA−/−) and IDUA+/− mice by reverse transcriptase reaction using TaqMan RT reagent kit (Applied Biosystems, Foster City, CA). All RT reactions were performed on the MJ Research PTC-200 thermocycler (Global Medical Instrumentation, Ramsey, MN). Real-time quantitative PCR was performed on cDNAs using the 2× SYBR green universal PCR master mix kit (Applied Biosystems) and run on Realplex master cycler epgradient (Eppendorf, Hauppauge, NY). All quantitative PCR assays were performed in duplicate on all heart samples from wild-type (n = 6), IDUA−/− (n = 10), and IDUA+/− (n = 6) mice and mice with transaortic constriction (positive control for heart failure) (n = 2). Cycling conditions consisted of 1 cycle of 95°C for 10 min and then 40 cycles of 95°C for 15 s, followed by 60°C for 1 min. The representative normalized average expression values from independent experiments are shown for each gene. Values are expressed as means ± SE for each group. Relative quantitation analysis of gene expression was conducted according to the 2−ΔΔCT method. GAPDH was used as an endogenous internal standard for gene expression analysis to determine the abundance of the amplified target gene within the same sample. After gene expression normalization, the values from each experimental group were normalized against wild-type control and are represented as the fold expression over wild-type. Primers were designed using PrimerSelect (DNASTAR Lasergene 7). All primers displayed single melting curve.
The following primers were used for quantitative RT-PCR: mouse atrial natriuretic factor (ANF)-forward, GCAGGGGCCGCACTTAGCTC; mouse ANF-reverse, CTGCGCCCTAGAGCACTGCC; mouse brain natriuretic peptide (BNP)-forward, AGCTGCTTGGGGAGGCGAGA; mouse BNP-reverse, GGGCCAGGCAATGATGCCGT; mouse α-skeletal actin-forward, ACGAGGCTGGCCCCTCCATT; mouse α-skeletal actin-reverse, AGAGAGCGCGAACGCAGACG; mouse β-myosin heavy chain (β-MHC)-forward, CTGAGCAGCTGGGCTCCACG; mouse β-MHC-reverse, CTCTAGCTGGGCGCGGAGGA; mouse GAPDH-forward, AACCCTGGACCACCCACCCC; and mouse GAPDH-reverse, TGTTGGGGGCCGAGTTGGGA.
Statistics.
All values are expressed as means ± SE. All single-group comparisons were analyzed by Student's t-test. All single variable multigroup comparisons were analyzed by one-way ANOVA with Tukey's post hoc analysis. Survival was analyzed by a Fisher exact test.
RESULTS
Baseline hemodynamic performance.
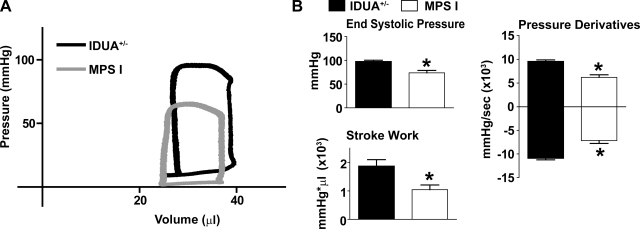
Conductance micromanometry was performed to assess real-time hemodynamic function. At baseline (raw traces: Fig. 1A), systolic function was significantly lower in MPS-I (IUDA−/−) mice based on measures of LV systolic pressure, stroke work (SW), and the positive derivative of pressure development (Fig. 1B). MPS-I mice also showed some evidence of diastolic dysfunction based on markedly reduced negative derivatives of pressure development (Fig. 1B). Derivative parameters provided measures of pressure development over time, indicating ventricular contractility (+dP/dt) and relaxation (−dP/dt). Significantly reduced LV end-diastolic pressure at baseline was also observed in MPS-I mice (Table 1). Despite these observations, LV volumes, ejection fraction, and CO were not significantly different from IDUA+/− mice at baseline (Table 1). These observations of reduced cardiac function in resting mice are consistent with previous reports using echocardiography and cardiac catheterization (20, 26).
Fig. 1.
Baseline hemodynamic function. A: representative raw pressure-volume loops of lysosomal hydrolase α-l-iduronidase (IDUA+/−; black) and mucopolysaccharidosis type I (MPS-I; gray) mice. B: mean hemodynamic data showing differences in cardiac performance at baseline. *P < 0.05 by t-test. Wild-type (WT), n = 7; MPS-I, n = 8.
Table 1.
Hemodynamic analysis at baseline and during catecholaminergic manipulation
| IDUA+/− | MPS-I | |
|---|---|---|
| Baseline | ||
| LVEDP, mmHg | 7.849 ± 1.311 | 4.367 ± 0.4815* |
| HR, beats/min | 530.7 ± 17.56 | 561.1 ± 10.68 |
| τ, s | 4.659 ± 0.5771 | 5.280 ± 0.5556 |
| Vmin, μl | 23.31 ± 4.523 | 19.27 ± 3.625 |
| Vmax, μl | 42.10 ± 3.546 | 35.71 ± 1.852 |
| SV, μl/beat | 23.49 ± 1.984 | 18.72 ± 2.232 |
| EF, % | 47.09 ± 2.791 | 53.05 ± 7.349 |
| CO, ml/min | 12.41 ± 1.016 | 10.55 ± 1.358 |
| PRSW, slope | 78.53 ± 14.06 | 61.30 ± 5.585 |
| Dobutamine | ||
| LVEDP, mmHg | 5.634 ± 0.6648 | 4.991 ± 0.7879 |
| HR, beats/min | 620.5 ± 4.890 | 566.2 ± 45.21 |
| τ, s | 3.189 ± 0.2825 | 7.731 ± 3.420 |
| Vmin, μl | 9.424 ± 2.146 | 9.051 ± 3.615 |
| SV, μl/beat | 33.58 ± 3.102 | 18.77 ± 4.006* |
| EF, % | 79.40 ± 4.534 | 63.21 ± 12.57 |
| PRSW, slope | 100.1 ± 6.286 | 94.20 ± 12.65 |
| Esmolol | ||
| LVEDP, mmHg | 9.128 ± 1.233 | 7.919 ± 0.4801 |
| HR, beats/min | 478.5 ± 12.61 | 450.6 ± 10.83 |
| Vmin, μl | 35.02 ± 1.783 | 29.26 ± 1.983 |
| Vmax, μl | 52.69 ± 3.357 | 41.74 ± 5.019 |
| SV, μl/beat | 18.49 ± 2.167 | 8.872 ± 1.841* |
| EF, % | 30.33 ± 1.369 | 20.55 ± 3.332* |
| PRSW, slope | 37.38 ± 2.640 | 19.02 ± 5.302* |
Values are means ± SE. IDUA, lysosomal hydrolase α-l-iduronidase; MPS-I, mucopolysaccharidosis type I; LVEDP, left ventricular end-diastolic pressure; HR, heart rate; τ, preload independent measure of ventricular isovolumic relaxation; Vmin and Vmax, minimum and maximum left ventricular volume, respectively; SV, stroke volume; EF, ejection fraction; CO, cardiac output; PRSW, preload recruitable stroke work.
P < 0.05 vs. IDUA+/− by t-test.
Hemodynamic function during catecholaminergic manipulation.
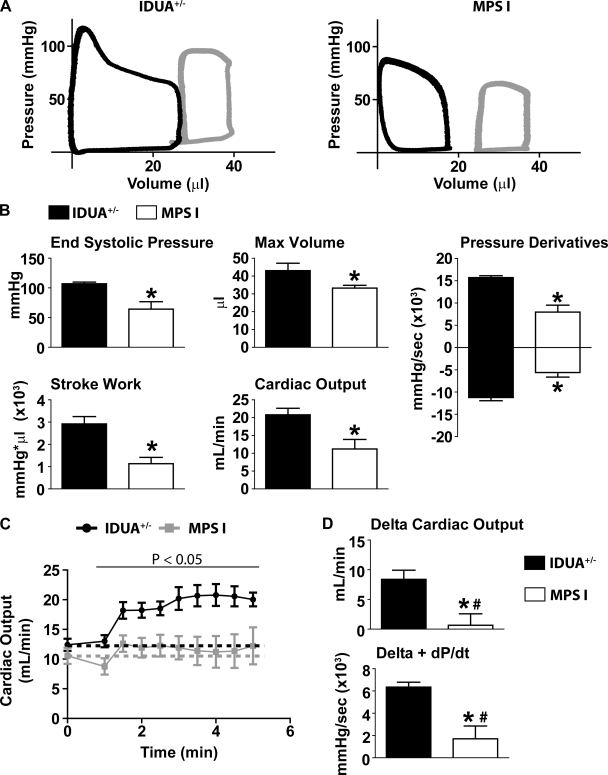
To determine cardiac responsiveness to catecholaminergic manipulation, mice were treated with dobutamine followed by esmolol. After steady-state cardiac performance was reached during dobutamine infusion (3 min), functionality was assessed between MPS-I and IDUA+/− mice (Fig. 2). Preload recruitable SW (PRSW), a load independent measure of systolic function measured as the slope of the SW and end-diastolic volume relationship, was acquired by IVC occlusion. Together with similar decreases in end-systolic volume (Vmin), the PRSW data showed that the intrinsic contractile performance of both MPS-I and IDUA+/− mice similarly increased during dobutamine infusion (Table 1). This indicates that, independent of preload and afterload, adrenergic signaling and myocardial catecholaminergic responsiveness was intact in MPS-I animals.
Fig. 2.
Hemodynamic function during dobutamine infusion. A: representative pressure-volume loops of IDUA+/− (left) and MPS-I (right) mice after 3 min of dobutamine. Gray loops, baseline; black loops, dobutamine. B: mean hemodynamic data showing differences in cardiac performance after 3 min of dobutamine. C: change in cardiac output during infusion of dobutamine. Dotted lines demarcate baseline values for each cohort (black, IDUA+/−; gray, MPS-I). D: mean hemodynamic values showing the change from baseline to steady-state dobutamine function (3 min). +dP/dt, pressure development over time, indicating ventricular contractility. *P < 0.05 by t-test. #Value not significantly different from zero based on Wilcoxon signed-rank post hoc analysis. IDUA+/−, n = 7; MPS-I, n = 8.
Hemodynamic measures of systolic function during dobutamine infusion (with preload and afterload effects contributing) showed evidence that MPS-I mice had significant contractile deficiencies as indicated by decreases in end-systolic pressure (ESP), the positive pressure derivative (+dP/dt), SW, and CO (Fig. 2, A and B). Furthermore, ventricular relaxation was significantly lower in MPS-I mice compared with IDUA+/− mice based on the measure of −dP/dt (Fig. 2B).
To determine the extent of cardiac reserve available in these mice, hemodynamic values were analyzed based on their delta change from baseline to 3 min of dobutamine infusion (Fig. 2, C and D). These data show that IDUA+/− mice appropriately enhance cardiac performance in response to adrenergic stimulation (Fig. 2, C and D). In marked contrast, during dobutamine infusion there was no significant difference from baseline values among the MPS-I cohort in measures of heart rate (561.1 ± 10.68 vs. 566.2 ± 45.21 beats/min; baseline vs. dobutamine; P > 0.05) and stroke volume (18.72 ± 2.232 vs. 18.77 ± 4.006 μl/beat; baseline vs. dobutamine; P > 0.05). As such, when compared with baseline, CO was not increased during dobutamine stimulation in MPS-I mice (Fig. 2, C and D). Another key marker of adrenergic responsiveness is the positive derivative of pressure development. This parameter did not significantly increase from baseline values in MPS-I mice during dobutamine infusion (Fig. 2D). Together, these results indicate that MPS-I mice showed a limited capacity to augment cardiac performance and increase CO beyond baseline levels in response to adrenergic stimulation.
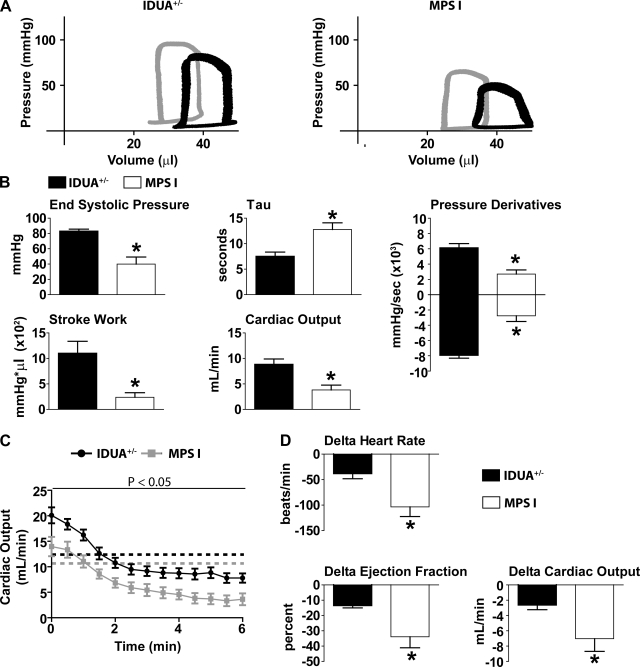
Immediately after dobutamine infusion, esmolol was infused to competitively block adrenergic receptors in the heart. β-Blockade reveals the autonomic tone understood here as the intrinsic contractile capacity of the heart in the absence of adrenergic stimulation (Fig. 3). MPS-I mice experienced significant cardiac decompensation in the absence of adrenergic support. Specifically, measures of systolic performance were markedly decreased in MPS-I mice compared with IDUA+/− mice (ESP, +dP/dt, SW, and CO) (Fig. 3, A and B). Significantly lower PRSW supports the conclusion of intrinsic myocardial systolic dysfunction in MPS-I mice compared with IDUA+/− mice (Table 1). Furthermore, diastolic dysfunction was manifest during esmolol infusion based on increased measures of τ, a preload independent measure of ventricular isovolumic relaxation, as well as a significantly lower −dP/dt in MPS-I mice compared with IDUA+/− mice (Fig. 3, A and B).
Fig. 3.
Hemodynamic function during esmolol infusion. A: representative pressure-volume loops of IDUA+/− (left) and MPS-I (right) mice after 5 min of esmolol. Gray loops, baseline; black loops, esmolol. B: mean hemodynamic data showing differences in cardiac performance after 5 min of esmolol. τ, preload independent measure of ventricular isovolumic relaxation. C: change in cardiac output from baseline to steady-state esmolol. Dotted lines demarcate baseline values for each cohort (black, IDUA+/−; gray, MPS-I). D: mean hemodynamic values showing the change from baseline to steady-state esmolol function (5 min). *P < 0.05 by t-test. IDUA+/−, n = 7; MPS-I, n = 6.
To determine the sympathetic tone required for normal cardiovascular function, the Δ change at 5 min of esmolol infusion compared with baseline was assessed (Fig. 3, C and D). These data showed that MPS-I and IDUA+/− mice are equally affected by β-blockade in the absolute change during the time course of infusion (Fig. 3C). However, when comparing steady-state function during esmolol infusion (5 min) relative to the original baseline, heart function revealed significant cardiac decompensation in MPS-I mice compared with IDUA+/− mice (Fig. 3D). Evidence of whole organ pump failure during β-blockade was shown by significant decreases (Δ from baseline) in heart rate, ejection fraction, and CO in MPS-I mice compared with IDUA+/− mice (Fig. 3D).
Hemodynamic decompensation in MPS-I mice.
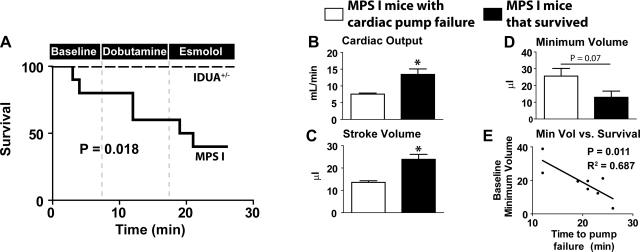
During the time course of this hemodynamic assay, MPS-I mice showed significantly increased susceptibility to mortality than IDUA+/− mice (P = 0.018) (Fig. 4A). An analysis of survival during this assessment showed no mortality among IDUA+/− mice. In contrast, MPS-I mice showed general susceptibility to cardiac decompensation during surgical preparations for pressure-volume analysis, with mortality occurring before data acquisition in two cases. During postmortem analysis, one MPS-I mouse had significant pulmonary venous aneurysm and died before data acquisition. Similar to other cardiomyopathic models (31), MPS-I mice also showed intolerance to adrenergic stimulation, with mortality occurring in two cases during dobutamine infusion. Furthermore, MPS-I mice without adrenergic tone experience pump failure as evidenced by the mortality occurring in two cases during esmolol infusion.
Fig. 4.
Analysis of survival and functional predictors of mortality. A: survival curve assessing mortality during surgical preparation and during catecholaminergic manipulation. Baseline refers to surgical placement of catheter and steady-state hemodynamic analysis. Subsequently, survival was assessed during dobutamine (time 0–4 min) and esmolol (time 4–10 min) infusion (IDUA+/− mice, n = 7; MPS-I mice, n = 10). B–D: comparison of baseline function in MPS-I mice that survived (n = 4) vs. MPS-I mice with cardiac pump failure (n = 4) during hemodynamic analysis. Baseline hemodynamic parameters analyzed include cardiac output (B), stroke volume (C), and minimum LV volume (Min Vol; D). E: linear regression analysis showing a statistically significant relationship between baseline minimum LV volume and time to cardiac pump failure (P = 0.011). *P < 0.05 by t-test.
The capacity to predict cardiac decompensation is a critical step toward a proper therapeutic management of heart diseases. Survival data were further analyzed to determine whether any baseline hemodynamic measures only found among the MPS-I cohort could be used to predict heart failure (Fig. 4B). Measures of contractility such as ESP and +dP/dt were not different among all MPS-I mice at baseline (data not shown). In contrast, MPS-I mice that experienced heart failure at any time during this hemodynamic study had reduced CO at baseline compared with MPS-I mice that survived the study (P < 0.05) (Fig. 4B). The basis for the observed difference in CO was manifest in a significant reduction in stroke volume (P < 0.05) as opposed to any difference in heart rate (Fig. 4C). Although statistical significance was not reached because of low numbers (n = 4 per group), the minimum volume reached during systole was greater in MPS-I mice with heart failure (P = 0.07) (Fig. 4D). Linear regression analysis was subsequently performed to determine whether LV minimum volume was statistically correlated with time to cardiac pump failure (Fig. 4E). This comparison showed a significant relationship between baseline LV end-systolic volume and time to death, with high baseline volumes correlating with increased susceptibility to cardiac pump failure (P = 0.011). This suggests a fundamental pathology in cardiac ejection as opposed to diastolic dysfunction. We acknowledge the limitations associated with a low number of animals in deriving this observation. However, we further note that a statistical significance was reached, revealing the significance of this comparison. Taken together, these data indicate that a decreased CO specifically attributed to an increased LV end-systolic volume significantly predicts the susceptibility to cardiac decompensation and pump failure in unstressed MPS-I mice.
Analysis of protein phosphorylation and heart failure markers.
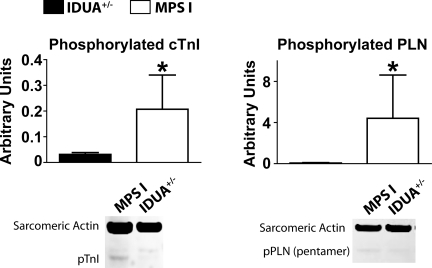
Adrenergic stimulation results in the phosphorylation of protein kinase A target proteins, including phospholamban and cardiac troponin I. The phosphorylation levels of these proteins are an indirect measure of adrenergic tone in hearts (10, 12, 17, 32). A biochemical analysis by Western blot analysis showed an increased baseline protein phosphorylation of phospholamban serine-16 and cardiac troponin I tandem serine-23/24 in hearts of MPS-I mice compared with IDUA+/− mice (Fig. 5). Taken together with the hemodynamic results, these data suggest that MPS-I mice have an increased baseline sympathetic tone compared with IDUA+/− mice.
Fig. 5.
Western blot analysis of phosphorylated troponin (p-TnI) serine-23/24 (left) and phospholamban (pPLN) serine-16 (right) show increased phosphorylation with the hearts of MPS-I mice compared with IDUA+/− mice. cTnI, cardiac TnI. *P < 0.05 by t-test. IDUA+/−, n = 5–8; MPS-I, n = 11–14.
To determine whether heart failure markers were increased in MPS-I hearts as observed with pathologies such as transaortic constriction, quantitative real-time PCR was carried out for transcripts of BNP, ANF, α-skeletal actin, and β-MHC (supplemental Fig. 1; note: supplemental figures may be found posted with the online version of this article). When compared with positive transaortic constriction controls, MPS-I hearts were not different from IDUA+/− hearts, showing no increase in transcripts for any of these heart failure markers.
Serum analysis showed that hemoglobin and hematocrit were not different between groups [MPS-I vs. IDUA+/−; Hb (in g/dl), 14.28 ± 0.75 vs. 15.17 ± 0.67; HCT (in %), 37.72 ± 2.11 vs. 40.03 ± 1.79, P > 0.05], indicating no polycythemia, anemia, or dehydration that could have confounded the cardiac studies. Consistent with previous studies of MPS-I tissues (4), the measures of GAGs from lung and liver from these mice showed a significant increase in MPS-I mice compared with IDUA+/− controls (P < 0.05) (supplemental Fig. 2).
DISCUSSION
Cardiovascular pathophysiological deficiencies contribute to the morbidity and mortality of patients with MPS-I. This study provides an assessment of cardiac hemodynamics in MPS-I mice with a specific focus on the catecholaminergic regulation of heart performance. Biochemical and functional data show that MPS-I mice have heightened cardiovascular adrenergic tone at baseline required for the maintenance of adequate heart performance. Under β-adrenergic stimulation (dobutamine) and blockade (esmolol), MPS-I mice experienced significant mortality compared with controls. It was further shown by linear regression analysis that cardiac pump failure in MPS-I mice was significantly correlated with heightened baseline end-systolic LV volume.
A key new finding of this study is the marked cardiac decompensation and death during dobutamine and esmolol stress in mice with MPS-I. This observation reveals a fundamental pathology in MPS-I hearts wherein heightened sympathetic drive acts as a compensatory bridge to survival. As such, MPS-I hearts lack cardiac reserve and undergo marked decompensation in the absence of catecholarminergic stimulus. Hemodynamic parameters were assessed to determine whether baseline cardiac function could predict cardiac pump failure in this cohort. These data show that there is a statistically significant correlation between increased end-systolic volume (thus reducing CO) and a heightened susceptibility to cardiac decompensation and pump failure in MPS-I hearts. Thus changes in cardiac geometry independent of changes in ventricular contractile or relaxation capacity (e.g., LV pressures or derivatives) predict cardiac decompensation in MPS-I hearts. This observation correlates with valvular disease, dilation of the LV outflow tract, and aortic insufficiency as a primary etiology for cardiovascular pathologies observed with MPS-I (4, 15, 26). Specifically, echocardiographic data have shown significant aortic regurgitation, long ejection times, and reductions in maximum blood flow velocity and peak pressure gradient across the aortic valve in murine MPS-I hearts (15). The development of fatal cardiac dysfunction is indicated by progressively reduced systolic ventricular ejection primarily because of aortic dysfunction involving noncompliant valves and enlarged outflow tract.
The cardiovascular dysfunction caused by MPS-I has long been established, and recent studies have begun to elucidate some of the underlying mechanisms by using various animal models (4, 15, 25–26). These studies have shown by histology and electron microscopy that MPS-I myocardium and valves are concentrically enlarged because of infiltration with highly vacuolated interstitial cells (4, 15). They also consistently report mitral and aortic insufficiency and reduced cardiac function by echocardiography (4, 15, 26) and cardiac catheterization (15). Despite these cardiomyopathic phenotypes, MPS-I mice containing indwelling radiotelemetry devices show normal blood pressure and heart rates compared with wild-type mice (15). This is consistent with observations in this study that show appropriate maintenance of CO under baseline conditions. Because of natural compensatory mechanisms invoked during the pathogenesis of acute and chronic heart disease (1, 13, 29, 34), it was hypothesized that a more precise analysis of cardiac function in the context of catecholaminergic stress might elucidate important information about heart performance in the context of MPS-I.
Catecholaminergic regulation of cardiac performance is one of the critical ways by which heart function is adjusted in response to whole animal physiological demands. In acute and chronic disease states, adrenergic stimulation of the heart has been shown to compensate for cardiovascular deficiencies, allowing for the maintenance of CO under resting conditions (7, 29, 31). In these contexts, heightened adrenergic tone eventually results in the loss of cardiac reserve, crippling the heart's capacity to respond to stress. With time, chronic adrenergic stimulation results in hemodynamic decompensation and heart failure (7). Manipulation of adrenergic signaling in the heart was used in this study to understand how MPS-I pathology affects catecholaminergic regulation of cardiac performance.
Biochemical assays assessing the protein phosphorylation of cardiac troponin I and phospholamban, as well as hemodynamic data acquired during esmolol infusion, are evidence that MPS-I mice have increased adrenergic tone under baseline conditions. As with other disease states (31), increased sympathetic tone may be required for functional compensation and long-term survival. The need for increased adrenergic tone could either be attributed to intrinsic contractile or extracardiac deficiencies. To address this question, load-independent measures of contractility were acquired by IVC occlusions during hemodynamic analysis. PRSW in MPS-I mice under baseline conditions and during dobutamine stimulation was not different than IDUA heterozygous controls. This suggests that variables independent of intrinsic contractile performance (e.g., myocardium) are, at least in part, responsible for the increased adrenergic tone in MPS-I hearts.
The data presented here indicate that differences in venous return (preload) may be one of the fundamental deficiencies of MPS-I. The observation that PRSW in MPS-I mice remains comparable with IDUA+/− mice at baseline and during dobutamine infusion indicates that other parameters of systolic and diastolic dysfunction (ESP, dP/dt, SV) may be decreased in MPS-I mice because of reduced cardiac preload. Based on the Frank-Starling principle that correlates load (myocardial stretch) with contractility (tension development), compromised venous return, and, consequently, reduced end-diastolic volume (Vmax) and LV pressure would explain, at least in part, the cardiac hemodynamic deficiencies of MPS-I mice.
In addition to a decreased end-diastolic pressure at baseline, evidence for venous pooling was indicated by reduced Vmax during dobutamine infusion. The maintenance of CO during sympathetic stimulation requires a concomitant increase in preload (not observed in MPS-I mice based on Vmax measurements), as well as an increase in intrinsic cardiac contractility (observed in MPS-I mice based on appropriate PRSW measurements). Beyond hemodynamic parameters, evidence for reduced venous capacitance was revealed in one MPS-I mouse with a severe pulmonary venous aneurysm. To our knowledge, this study provides the first evidence to suggest a vascular deficiency in Hurler syndrome. To further test the hypothesis that reduced venous capacitance contributes to the hemodynamic pathology of Hurler syndrome, other nonspecific adrenergic agonists such as isoprenaline should be used.
The ability for mammals to modulate heart rate is also a fundamental requirement of the stress pathway needed to maintain CO in response to increased peripheral metabolic demands. In this study, the lack of any change in heart rate in MPS-I mice during dobutamine infusion points to a conduction defect as well. This is consistent with a previous study using in vivo telemetry devices to measure ECG in unanesthetized freely moving animals (15). In this study, Jordan et al. (15) reported significantly reduced heart rate variability (coefficient of variation %), increased P-wave width, increased QRS-tri-wave width, and reduced S-wave amplitude in MPS-I mice compared with those of controls. When coupled with findings in the current report, these results strongly suggest a blunting of autonomic tone to the sinoatrial node as seen with parasympathetic blockade. These observations also indicate that concentric myocardial thickening because of increased interstitial cell infiltration may be causing mechanical-electrical discontinuity in the MPS-I heart. Although their intrinsic ventricular function appears intact, preload deficiencies and conduction defects in MPS-I mice prevent them from responding to increased metabolic demands during adrenergic stimulation.
In conclusion, this study provides the first data, to our knowledge, on the catecholaminergic regulation of heart performance in MPS-I. Cardiac decompensation is inevitable in MPS-I patients, and support of cardiac function by catecholamines is a necessary therapeutic modality. Consequently, these results reveal the need for further investigation into adrenergic regulation of heart performance in animal models and the MPS-I patients. The capacity to predict cardiac pump failure in unstressed hearts provides a major step forward in understanding the therapeutic management of any cardiac pathology. A key observation of this study was that reduced CO attributable to increased LV systolic volume in the unstressed heart significantly predicts pump failure in the MPS-I mouse. It remains to be seen whether reduced ventricular ejection secondary to progressive valvular insufficiency can act as a surrogate marker for heart failure in MPS-I patients. In addition to known pathophysiological indicators of MPS-I in the heart, these data also represent a starting point for further investigation into vascular dysfunction as it pertains to venous tone and cardiac preload. Taken together, this study provides insights into adrenergic dysregulation in MPS-I hearts and supports the conclusion that compensatory maladaptive cardiac remodeling of catecholaminergic signaling contributes to heart failure in MPS-I.
GRANTS
This study was supported by the Children's Cancer Research Fund and the American Heart Association (to J. Tolar); National Heart, Lung, and Blood Institute Grants R01-HL-49997 (to B. R. Blazer) and R01-HL-071016 and R01-HL-059301 (to J. M. Metzger); and the American Heart Association (to N. J. Palpant).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
Current address of N. J. Palpant: Univ. of Washington Medical School, Center for Cardiovascular Biology and Regenerative Medicine, 815 Mercer St., Seattle, WA, 98109.
REFERENCES
- 1. Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res 100: 1353–1362, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Beesley CE, Meaney CA, Greenland G, Adams V, Vellodi A, Young EP, Winchester BG. Mutational analysis of 85 mucopolysaccharidosis type I families: frequency of known mutations, identification of 17 novel mutations and in vitro expression of missense mutations. Hum Genet 109: 503–511, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Boelens JJ, Rocha V, Aldenhoven M, Wynn R, O'Meara A, Michel G, Ionescu I, Parikh S, Prasad VK, Szabolcs P, Escolar M, Gluckman E, Cavazzana-Calvo M, Kurtzberg J. Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with hurler syndrome. Biol Blood Marrow Transplant 15: 618–625, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Braunlin E, Mackey-Bojack S, Panoskaltsis-Mortari A, Berry JM, McElmurry RT, Riddle M, Sun LY, Clarke LA, Tolar J, Blazar BR. Cardiac functional and histopathologic findings in humans and mice with mucopolysaccharidosis type I: implications for assessment of therapeutic interventions in hurler syndrome. Pediatr Res 59: 27–32, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Braunlin EA, Berry JM, Whitley CB. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am J Cardiol 98: 416–418, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Brodde OE. Beta-adrenoceptor blocker treatment and the cardiac beta-adrenoceptor-G-protein(s)-adenylyl cyclase system in chronic heart failure. Naunyn Schmiedebergs Arch Pharmacol 374: 361–372, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Brodde OE, Bruck H, Leineweber K. Cardiac adrenoceptors: physiological and pathophysiological relevance. J Pharm Sci 100: 323–337, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Clarke LA, Russell CS, Pownall S, Warrington CL, Borowski A, Dimmick JE, Toone J, Jirik FR. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-l-iduronidase gene. Hum Mol Genet 6: 503–511, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Dangel JH. Cardiovascular changes in children with mucopolysaccharide storage diseases and related disorders—clinical and echocardiographic findings in 64 patients. Eur J Pediatr 157: 534–538, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Dash R, Kadambi VJ, Schmidt AG, Tepe NM, Biniakiewicz D, Gerst MJ, Canning AM, Abraham WT, Hoit BD, Liggett SB, Lorenz JN, Dorn GW, 2nd, Kranias EG. Interactions between phospholamban and β-adrenergic drive may lead to cardiomyopathy and early mortality. Circulation 103: 889–896, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, D'Alecy LG, Ingwall JS, Metzger JM. Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med 12: 181–189, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Frank K, Kranias EG. Phospholamban and cardiac contractility. Ann Med 32: 572–578, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Gerdes AM, Capasso JM. Structural remodeling and mechanical dysfunction of cardiac myocytes in heart failure. J Mol Cell Cardiol 27: 849–856, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K, James DC, Lucas CF, Rogers TR, Benson PF, Tansley LR, Patrick AD, Mossman J, Young EP. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet 2: 709–712, 1981 [DOI] [PubMed] [Google Scholar]
- 15. Jordan MC, Zheng Y, Ryazantsev S, Rozengurt N, Roos KP, Neufeld EF. Cardiac manifestations in the mouse model of mucopolysaccharidosis I. Mol Genet Metab 86: 233–243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld EF. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med 344: 182–188, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 66: 12–21, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Xu L, Hennig AK, Kovacs A, Fu A, Chung S, Lee D, Wang B, Herati RS, Mosinger Ogilvie J, Cai SR, Parker Ponder K. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mol Ther 11: 35–47, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Matte U, Yogalingam G, Brooks D, Leistner S, Schwartz I, Lima L, Norato DY, Brum JM, Beesley C, Winchester B, Giugliani R, Hopwood JJ. Identification and characterization of 13 new mutations in mucopolysaccharidosis type I patients. Mol Genet Metab 78: 37–43, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: The Metabolic Basis of Inherited Disease, edited by Scriver CR, Beaudet AL, Sly WS, Valle D. New York: McGraw Hill, 1997, p. 2465–2494 [Google Scholar]
- 21. Palpant NJ, D'Alecy LG, Metzger JM. Single histidine button in cardiac troponin I sustains heart performance in response to severe hypercapnic respiratory acidosis in vivo. FASEB J 23: 1529–1540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palpant NJ, Day SM, Herron TJ, Converso KL, Metzger JM. Single histidine-substituted cardiac troponin I confers protection from age-related systolic and diastolic dysfunction. Cardiovasc Res 80: 209–218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Renteria VG, Ferrans VJ, Roberts WC. The heart in the Hurler syndrome: gross, histologic and ultrastructural observations in five necropsy cases. Am J Cardiol 38: 487–501, 1976 [DOI] [PubMed] [Google Scholar]
- 24. Shull RM, Munger RJ, Spellacy E, Hall CW, Constantopoulos G, Neufeld EF. Canine alpha-l-iduronidase deficiency. A model of mucopolysaccharidosis I. Am J Pathol 109: 244–248, 1982 [PMC free article] [PubMed] [Google Scholar]
- 25. Sleeper MM, Fornasari B, Ellinwood NM, Weil MA, Melniczek J, O'Malley TM, Sammarco CD, Xu L, Ponder KP, Haskins ME. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation 110: 815–820, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Sleeper MM, Kusiak CM, Shofer FS, O'Donnell P, Bryan C, Ponder KP, Haskins ME. Clinical characterization of cardiovascular abnormalities associated with feline mucopolysaccharidosis I and VI. J Inherit Metab Dis 31: 424–431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med 350: 1960–1969, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abeysinghe S, Krawczak M, Cooper DN. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 21: 577–581, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev 79: 215–262, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Tolar J, Grewal SS, Bjoraker KJ, Whitley CB, Shapiro EG, Charnas L, Orchard PJ. Combination of enzyme replacement and hematopoietic stem cell transplantation as therapy for Hurler syndrome. Bone Marrow Transplant 41: 531–535, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther 15: 1086–1092, 2007 [DOI] [PubMed] [Google Scholar]
- 32. van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJ. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res 57: 37–47, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science 300: 1530–1532, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Zelarayan L, Gehrke C, Bergmann MW. Role of beta-catenin in adult cardiac remodeling. Cell Cycle 6: 2120–2126, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.