Abstract
The present study determined whether AMP-activated protein kinase (AMPK) regulates heme oxygenase (HO)-1 gene expression in endothelial cells (ECs) and if HO-1 contributes to the biological actions of this kinase. Treatment of human ECs with the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) stimulated a concentration- and time-dependent increase in HO-1 protein and mRNA expression that was associated with a prominent increase in nuclear factor-erythroid 2-related factor 2 (Nrf2) protein. Induction of HO-1 was also observed in rat carotid arteries after the in vivo application of AICAR. Induction of HO-1 by AICAR was blocked by the AMPK inhibitor compound C, the adenosine kinase inhibitor 5′-iodotubercidin, and by silencing AMPK-α1/2 and was mimicked by the AMPK activator A-769662 and by infecting ECs with an adenovirus expressing constitutively active AMPK-α1. AICAR also induced a significant rise in HO-1 promoter activity that was abolished by mutating the antioxidant responsive elements of the HO-1 promoter or by the overexpression of dominant negative Nrf2. Finally, activation of AMPK inhibited cytokine-mediated EC death, and this was prevented by the HO inhibitor tin protoporphyrin-IX or by silencing HO-1 expression. In conclusion, AMPK stimulates HO-1 gene expression in human ECs via the Nrf2/antioxidant responsive element signaling pathway. The induction of HO-1 mediates the antiapoptotic effect of AMPK, and this may provide an important adaptive response to preserve EC viability during periods of metabolic stress.
Keywords: vascular biology, metabolic stress, endothelium, AMP-activated protein kinase
amp-activated protein kinase (AMPK) is a ubiquitously expressed energy-sensing enzyme that functions as a protein serine/threonine kinase (17). AMPK exists as a heterotrimeric complex composed of α-, β-, and γ-subunits. The α-subunit of AMPK contains the catalytic domain and has two isoforms, α1 and α2, that are phosphorylated at Thr172 upon enzyme activation. Both α-isoforms of AMPK are expressed by endothelial cells (ECs); however, the predominant isoform is the α1-isoform (13, 43). In mammalian cells, AMPK is activated by increases in the AMP-to-ATP ratio, which occur in various stress conditions such as nutrient deprivation, prolonged exercise, hypoxia, ischemia, and heat shock (21). Binding of AMP to the α-subunit results in the partial allosteric activation of AMPK, and the enzyme becomes fully activated after the phosphorylation of Thr172 by AMPK kinases (13, 43). However, numerous physiological and pharmacologically relevant molecules are also capable of activating AMPK independent of changes in the AMP-to-ATP ratio (13, 14, 43). Once activated, AMPK coordinates a cellular program that prevents further ATP depletion by switching on catabolic pathways that generate ATP and turning off ATP-consuming anabolic pathways. AMPK mediates these effects through the direct phosphorylation of target proteins and by regulating gene expression (13, 17, 43).
A number of pharmacological activators of AMPK have been developed to probe AMPK function. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) is a well-established, cell-permeable activator of AMPK. Upon entering cells, AICAR is metabolized by adenosine kinase to 5-aminoimidazole-4-carboxamide, which mimics the effect of AMP on AMPK activation (7). More recently, the thienopyridone compound A-769662 has been identified as a potent and highly selective activator of AMPK. This small molecule directly activates AMPK in a manner similar to that of AMP, encompassing allosteric activation as well as protection from Thr172 dephosphorylation (16).
Although AMPK has traditionally been viewed as a modulator of metabolism, recent studies have demonstrated that AMPK also functions to regulate endothelial function. AMPK phosphorylates and activates endothelial nitric oxide (NO) synthase (eNOS), leading to the production of NO, a key modulator of vascular tone (5, 8, 27). Significantly, AMPK preserves EC function during periods of metabolic and inflammatory stress. AMPK suppresses reactive oxygen production and apoptosis in ECs exposed to high concentrations of glucose or free fatty acids and protects against oxidative EC injury (6, 20, 23, 24, 35). Furthermore, AMPK preserves EC viability during anoxia and is essential for angiogenesis in response to hypoxia (6, 29, 30). AMPK also exerts potent antiinflammatory effects by inhibiting TNF-α-mediated activation of NF-κB, the expression of adhesion receptors and chemokines, and leukocyte adhesion to ECs (12, 18). Moreover, we (14, 15) recently reported that AMPK activation limits postischemic leukocyte rolling and adhesion in the venular endothelium of mice. Although AMPK plays a critical role in promoting EC function during metabolic and inflammatory stress, the underlying mechanism responsible for these vasoprotective actions is not fully understood.
Heme oxygenase (HO)-1 is a highly inducible enzyme that degrades heme into equimolar amounts of carbon monoxide (CO), iron, and biliverdin (10). This oxidative reaction is inhibited by various metalloporphyrins, including tin protoporphyrin-IX (SnPP). The induction of HO-1 in vascular ECs serves an important cytoprotective role by catabolizing prooxidant heme to the antioxidant bile pigments biliverdin and bilirubin and by upregulating the expression of ferritin, which exerts an additional antioxidant effect by chelating iron. In addition, the generation of bilirubin and CO by HO-1 exerts potent antiapoptotic, anti-inflammatory, and angiogenic effects in ECs (2, 9, 10, 23).
Based on the findings that AMPK and HO-1 have similar effects on EC biology, we tested whether AMPK activation is functionally linked to HO-1 gene expression in the vascular endothelium. In particular, we examined whether AMPK modulates HO-1 gene expression in human arterial and venous ECs and human arterial smooth muscle cells (SMCs). To verify our in vitro experiments with cultured vascular cells, we also investigated whether the in vivo activation of AMPK influences the expression of HO-1 in rat carotid arteries. In addition, we identified the signaling pathway by which AMPK regulates HO-1 expression and determined whether HO-1 mediates the antiapoptotic effect of AMPK on ECs.
MATERIALS AND METHODS
Materials.
Streptomycin, penicillin, trypsin, SDS, gelatin, heparin, actinomycin D, cycloheximide, ceramide, propidium iodide, compound C, AICAR, methyl-l-arginine, Triton X-100, and medium 199 (M199) were from Sigma-Aldrich (St. Louis, MO). A-769662 was from Tocris Biosciences (Ellisville, MO), TNF-α was from Genzyme (Boston, MA), and SnPP was from Frontier Scientific (Logan, UT). A polyclonal HO-1 antibody was from StressGen Biotechnologies (Victoria, Canada), antibodies directed against AMPK-α, phospho-AMPK-α (Thr172), and phospho-acetyl-CoA carboxylate (ACC; Ser79) were from Cell Signaling Technology (Beverley, MA), and antibodies against nuclear factor-erythroid 2-related factor 2 (Nrf2) and β-actin were from Santa Cruz Biotechnologies (Santa Cruz, CA). Dowex 50W-X8 was from Bio-Rad Laboratories (Hercules, CA). [32P]dCTP (3,000 Ci/mmol) was from Amersham (Arlington Heights, IL), and l-[3H]arginine (43 Ci/mmol) was from Perkin Elmer (Boston, MA).
Cell culture.
Human umbilical vein ECs (HUVECs), human aortic ECs (HAECs), and human aortic SMCs (HASMCs) were purchased from Lonza (Allendale, NJ). ECs were serially cultured on gelatin-coated plates and grown in M199 supplemented with 20% bovine calf serum, 2 mM l-glutamine, 50 μg/ml EC growth factor, 90 μg/ml heparin, and 100 U/ml penicillin and streptomycin in an atmosphere of 95% air-5% CO2 (23). HASMCs were grown in M199 containing 20% bovine calf serum supplemented with 100 U/ml penicillin and streptomycin in an atmosphere of 95% air-5% CO2. HAECs and HASMCs were used between passages 4 and 8, whereas HUVECs were used between passages 6 and 14.
Small interfering RNA and infection with adenoviral vectors.
Gene expression was silenced using specific small interfering (si)RNAs targeting HO-1 (Dharmacon, Lafayette, CO) or AMPK-α1/2 (Santa Cruz Biotechnology). Transfection of cells with siRNA was carried out using a commercial transfection reagent (Invitrogen, Carlsbad, CA). A replication-defective adenovirus expressing a constitutively active AMPK mutant (AdAMPK-CA) was generously provided by Dr. Ming-Hui Zou (University of Oklahoma Health Sciences Center, Oklahoma City, OK). Cells were infected with the adenovirus expressing a constitutively active AMPK mutant (AdAMPK-CA) or an adenovirus expressing green fluorescent protein (AdGFP) at a multiplicity of infection of 20.
Western blot analysis.
Cells were lysed in sample buffer [125 mM Tris (pH 6.8), 12.5% glycerol, 2% SDS, 50 mM sodium fluoride, and trace bromophenol blue], and proteins were separated by SDS-PAGE. After transfer to nitrocellulose membrane, blots were blocked with PBS and nonfat milk (5%) and then incubated with antibodies directed against HO-1 (1:1,500), AMPK-α (1:500), phospho-AMPK (1:100), phospho-ACC (1:100), Nrf2 (1:100), or β-actin (1:2,000). Membranes were washed in PBS, incubated with horseradish peroxidase-conjugated goat anti-rabbit, rabbit anti-mouse, or donkey anti-goat antibodies, and developed with commercial chemoluminescence reagents (Amersham, Arlington Heights, IL). The expression of HO-1 protein was quantified by scanning densitometry and normalized with respect to β-actin.
Northern blot analysis.
HO-1 mRNA levels were determined by Northern blot analysis. Total RNA (30 μg) was loaded onto 1.2% agarose gels and fractionated by electrophoresis, and blots were transferred to Gene Screen Plus membranes (Perkin Elmer Life Sciences). Membranes were prehybridized for 4 h at 68°C in rapid hybridization buffer (Amersham) and then incubated overnight at 68°C in hybridization buffer containing [32P]DNA probes (1 × 108 counts/min) for HO-1 or 18S rRNA. DNA probes were generated by RT-PCR and labeled with [32P]dCTP using a random priming kit (Amersham). After hybridization, membranes were washed and exposed to X-ray film at −70°C, and HO-1 expression was quantified by scanning densitometry and normalized with respect to 18S rRNA.
Promoter analysis.
HO-1 promoter activity was determined using murine HO-1 promoter/firefly luciferase constructs (1 μg/ml) containing the wild-type enhancer (E1) coupled to a minimum HO-1 promoter as well as the mutant enhancer (M739) that had its three antioxidant responsive element (ARE) core sequences mutated. In some experiments, a plasmid expressing a dominant negative Nrf2 mutant (1 μg/ml) that had its transactivation domain deleted was used. All constructs were generously provided by Dr. Jawed Alam (The Ochsner Clinic Foundation, New Orleans, LA). A plasmid encoding Renilla luciferase (hRluc/TK, 0.02 μg/ml) was included in all samples to control for transfection efficiency. ECs were transfected using lipofectamine, cultured for 48 h, and then treated with AICAR. Cells were collected and lysed, and luciferase activity was measured using the Promega Dual-Light assay system and a Glomax luminometer (Promega, Madison, WI). Firefly luciferase activity was normalized with respect to Renilla luciferase.
AMPK activation.
AMPK activity was determined by Western blot analysis using phospho-specific antibodies directed against AMPK-α or ACC.
eNOS activation.
eNOS activity was determined by measuring the conversion of l-[3H]arginine to l-[3H]citrulline, as we have previously described (11). After the incubation of cells with l-[3H]arginine (2 μCi) for 4 h, l-[3H]arginine-containing media were removed, and cells were washed three times with PBS. Reactions were stopped by the addition of 300 μl of ice-cold Tris buffer containing Triton X-100 (0.1%), and cells were scrapped, vortexed, and centrifuged at 1,000 g for 1 min. Supernatants were then applied to a cation exchange resin (Dowex 50W-X8), and neutrally charged l-citrulline was eluted and measured for radioactivity.
Cell viability and caspase-3 activation.
Cell viability was determined by measuring the uptake of the membrane-impermeable stain trypan blue. Cells were treated with trypsin (0.25%), collected, and diluted (1:4) with trypan blue. Viable cells, which exclude trypan blue, were counted with a hemocytometer. Caspase-3 was monitored by measuring the cleavage of the p-nitroanilide-conjugated caspase-3 substrate DEVD. Briefly, cells were trypsinized, pooled with detached cells, washed in ice-cold PBS, and suspended in lysis buffer [50 mM HEPES (pH 7.5), 10% sucrose, and 0.1% Triton X-100] on ice for 10 min. After centrifugation at 13,000 g for 5 min at 4°C, supernatants were incubated with 50 μM DEVD, and absorbance was measured at 405 nm with a μQuant spectrophotometer (Bio-Tek Instruments, Winooski, VT).
Animal experiments.
Sprague-Dawley rats (400–450 g) were obtained from Charles River Laboratories (Wilmington, MA) and maintained on standard rat chow and water ad libitum with 12:12-h light-dark cycles. Animals were anesthetized (ketamine and xylazine, 1.0 ml/kg ip, Butler Schein Animal Health, Dublin, OH), the left carotid artery was isolated, and a local polymer-based delivery system was used to administer AICAR to the vessel wall, as we have previously described (33). The delivery system consisted of 200 μl of a 25% copolymer gel (PLF127, BASF, Florham Park, NJ) containing AICAR (1 mg) that was applied in a circumferential manner to the exposed adventitia of the carotid artery. A separate group of animals received an empty gel. After 24 h, animals were euthanized, and the left common carotid artery was excised, pulverized in liquid nitrogen, and processed for Western blot analysis. This investigation conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and was approved by the Institutional Animal Care and Use Committee of the University of Missouri.
Statistics.
Results are expressed as means ± SE. Statistical analyses were performed using a Student's two-tailed t-test or with ANOVA with post hoc Bonferroni testing when multiple groups were compared. P values of <0.05 were considered statistically significant.
RESULTS
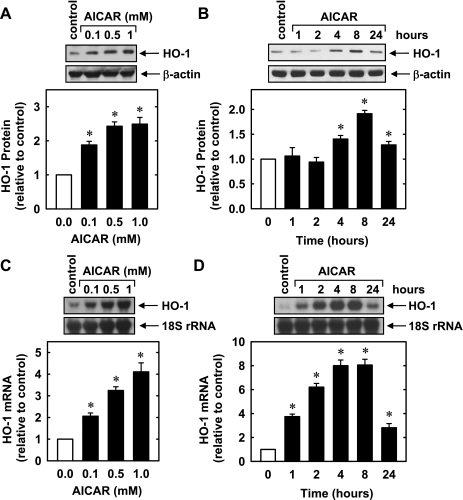
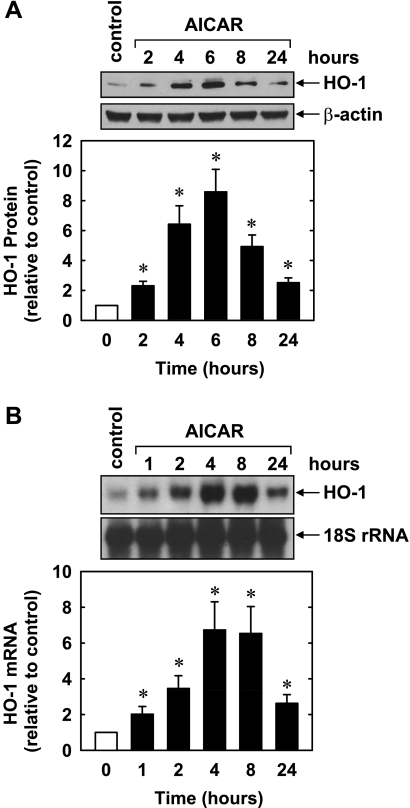
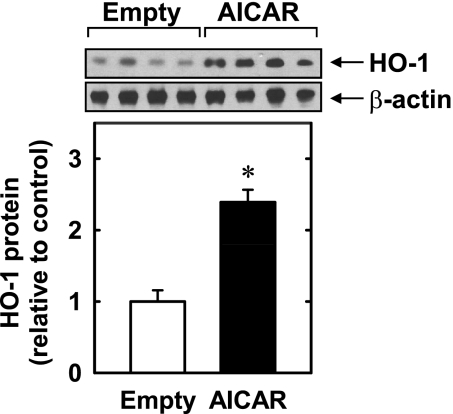
Treatment of HUVECs with AICAR (0.1–1.0 mM) stimulated a concentration- and time-dependent increase in HO-1 protein. An increase in HO-1 protein was detected after 8 h with 0.1 mM AICAR, and higher concentrations of AICAR showed a further increase in HO-1 protein (Fig. 1A). The induction of HO-1 protein was delayed, with a significant increase in HO-1 protein first detected at 4 h, peaked at 8 h, and returned toward baseline after 24 h of treatment (Fig. 1B). The induction of HO-1 protein by AICAR was associated with a concentration- and time-dependent rise in HO-1 mRNA that preceded the increase in HO-1 protein (Fig. 1, C and D). An increase in HO-1 mRNA was detected 1 h after AICAR exposure; transcript levels peaked between 4 and 8 h and remained elevated after 24 h of exposure. AICAR also stimulated a rise in HO-1 expression in HAECs (Fig. 2) and HASMCs (data not shown). Moreover, the perivascular administration of AICAR resulted in a significant increase in HO-1 protein expression in rat carotid arteries (Fig. 3).
Fig. 1.
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) stimulates heme oxygenase (HO)-1 protein and mRNA expression in human umbilical vein endothelial cells (HUVECs). A: concentration-dependent effect of AICAR (0.1–1.0 mM for 8 h) on HO-1 protein expression. B: time course of HO-1 protein expression after the administration of AICAR (0.5 mM). C: concentration-dependent effect of AICAR (0.1–1.0 mM for 4 h) on HO-1 mRNA expression. D: time course of HO-1 mRNA expression after the administration of AICAR (0.5 mM). HO-1 protein and mRNA expression were quantified by scanning densitometry, normalized with respect to β-actin or 18 S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE of 3–4 experiments. *Statistically significant effect of AICAR.
Fig. 2.
AICAR stimulates HO-1 protein and mRNA expression in human aortic ECs. A: time course of HO-1 protein expression after the administration of AICAR (0.5 mM). B: time course of HO-1 mRNA expression after the administration of AICAR (0.5 mM). HO-1 protein and mRNA expression were quantified by scanning densitometry, normalized with respect to β-actin or 18S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE of 3 experiments. *Statistically significant effect of AICAR.
Fig. 3.
AICAR stimulates HO-1 protein expression in blood vessels. HO-1 protein expression in rat carotid arteries treated with an empty gel or AICAR (1 mg)-containing gel for 24 h is shown. HO-1 protein expression was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SE of 4 measurements. *Statistically significant effect of AICAR.
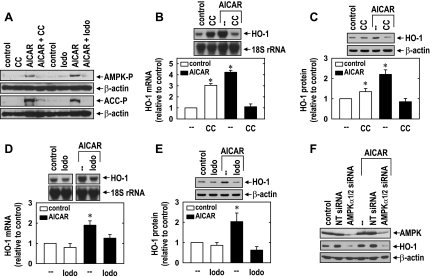
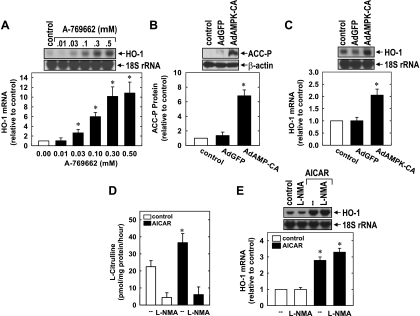
Treatment of HUVECs with AICAR (0.5 mM) resulted in the activation of AMPK, as reflected by the phosphorylation of AMPK and the AMPK substrate ACC (Fig. 4A). However, the AMPK inhibitor compound C (41) blocked the AICAR-induced phosphorylation of AMPK and ACC (Fig. 4A). Similarly, the adenosine kinase inhibitor 5′-iodotubercin (19), which blocks the intracellular conversion of AICAR to 5-aminoimidazole-4-carboxamide (required for AMPK activation), abrogated the activation of AMPK by AICAR (Fig. 4A). Incubation of cells with either compound C or 5′-iodotubercin prevented the AICAR-mediated induction of HO-1 mRNA and protein (Fig. 4, B–E). Interestingly, compound C stimulated a significant increase in HO-1 expression in the absence of AICAR, suggesting possible nonspecific actions by this inhibitor (Fig. 4, B and C). To further confirm the involvement of AMPK in the induction of HO-1 by AICAR, we knocked down the expression of both catalytic α-subunits of AMPK. Treatment of HUVECs with AMPK-α1/2 siRNA resulted in an ∼75% decline in AMPK-α1/2 protein (Fig. 4F). Moreover, the knockdown of AMPK-α1/2 suppressed basal HO-1 protein expression and abolished the induction of HO-1 protein by AICAR. In contrast, the nontargeting siRNA failed to block AMPK-α1/2 or HO-1 protein expression.
Fig. 4.
AICAR stimulates HO-1 expression in an AMP-activated protein kinase (AMPK)-dependent manner. A: inhibition of AICAR (0.5 mM for 4 h)-mediated AMPK activation, as reflected by the phosphorylation of AMPK (AMPK-P) and acetyl-CoA carboxylase (ACC-P) by compound C (CC; 20 μM) and iodotubercidin (Iodo; 0.3 μM). B: inhibition of AICAR-mediated HO-1 mRNA expression by CC. C: inhibition of AICAR-mediated HO-1 protein expression by CC. D: inhibition of AICAR-mediated HO-1 mRNA expression by Iodo. E: inhibition of AICAR-mediated HO-1 protein expression by Iodo. F: silencing of AMPK-α1/2 blocked AICAR-mediated HO-1 protein expression. Cells were incubated with CC (20 μM) or Iodo (0.3 μM), transfected with small interfering (si)RNA to AMPK-α1/2 (0.1 μM) or nontargeting siRNA (siNT; 0.1 μM), and then treated with AICAR (0.5 mM) for 8 h. HO-1 protein and mRNA expression were quantified by scanning densitometry, normalized with respect to β-actin or 18S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE of 3–5 experiments. *Statistically significant increase from control.
The ability of AMPK to induce HO-1 was also corroborated using the highly selective AMPK activator A-769662. Treatment of HUVECs with the AMPK activator A-769662 resulted in a pronounced increase in the expression of HO-1 mRNA (Fig. 5A). In addition, infection of HUVEC with AdAMPK-CA but not AdGFP stimulated AMPK activity and HO-1 mRNA expression (Fig. 5, B and C). Since AMPK results in the activation of eNOS and liberation of NO (5, 8, 27), which is a well-established inducer of HO-1 (26), the involvement of eNOS in the induction of HO-1 was also investigated. Consistent with previous reports (8, 27), we found that AICAR stimulated eNOS activity, and this could be prevented by the eNOS inhibitor methyl-l-arginine (Fig. 5D). However, treatment of HUVECs with methyl-l-arginine failed to inhibit the induction of HO-1 by AICAR (Fig. 5E).
Fig. 5.
Stimulation of HO-1 expression by the AMPK activator, A-769662, and an adenovirus expressing a constitutively active AMPK mutant (AdAMPK-CA). A: A-769662 stimulated HO-1 mRNA expression in a concentration-dependent manner. HUVECs were treated with A-769662 (0.01–0.05 mM) for 8 h. B: AdAMPK-CA but not adenovirus expressing green fluorescent protein (AdGFP) stimulated AMPK activity, as reflected by the phosphorylation of ACC. C: AdAMPK-CA stimulated HO-1 expression. HUVECs were infected with AdAMPK-CA or AdGFP at a multiplicity of infection of 20 for 2 days. D: AICAR stimulated endothelial nitric oxide synthase (eNOS) activity. Cells were pretreated with methyl-l-arginine (l-NMA; 1 mM) or vehicle for 30 min and then treated with AICAR (0.5 mM) for 4 h. E: effect of eNOS inhibition on AICAR-mediated HO-1 mRNA expression. Cells were pretreated with vehicle or l-NMA (1 mM) for 30 min and then treated with AICAR (0.5 mM) for 8 h. HO-1 protein and mRNA expression were quantified by scanning densitometry, normalized with respect to β-actin or 18S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE of 3–4 experiments. *Statistically significant increase from control.
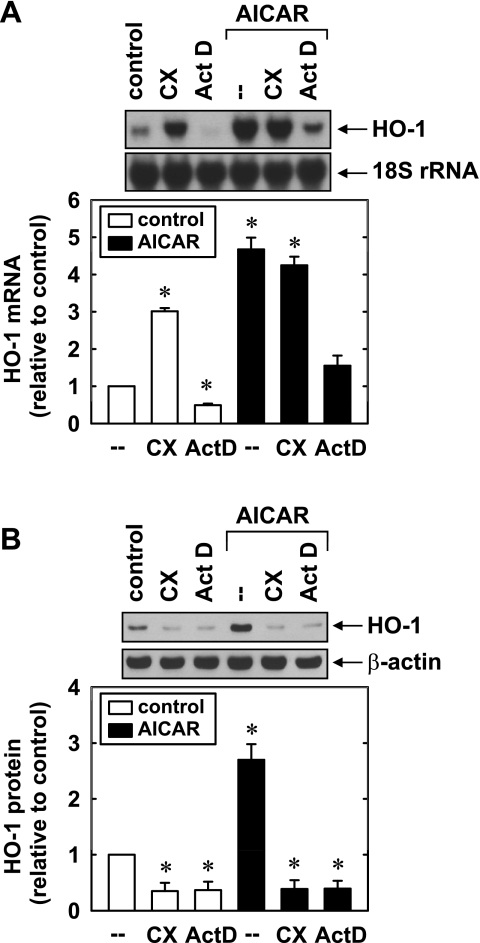
In subsequent experiments, we further explored the mechanism by which AMPK induces HO-1 gene expression. Incubation of HUVECs with the transcriptional inhibitor actinomycin D blocked the induction of HO-1 mRNA and protein by AICAR (Fig. 6, A and B). In contrast, the protein synthesis inhibitor cycloheximide had no effect on the increase in HO-1 mRNA, whereas it completely suppressing the elevation in HO-1 protein induced by AICAR. Actinomycin D also attenuated the basal expression of HO-1 mRNA, whereas cycloheximide significantly increased the basal HO-1 message. Both actinomycin D and cycloheximide reduced HO-1 protein levels in untreated cells.
Fig. 6.
AICAR-mediated HO-1 gene expression requires de novo RNA synthesis. A: effect of actinomycin D (Act D; 2 μg/ml) or cycloheximide (CX; 5 μg/ml) on the AICAR (0.5 mM for 4 h)-mediated increase in HO-1 mRNA. B: effect of Act D (2 μg/ml) or CX (5 μg/ml) on HOCl (0.5 mM for 8 h)-mediated increases in HO-1 protein. HO-1 protein or mRNA was quantified by scanning densitometry, normalized with respect to β-actin or 18S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SE; n = 4. *Statistically significant change in HO-1 expression.
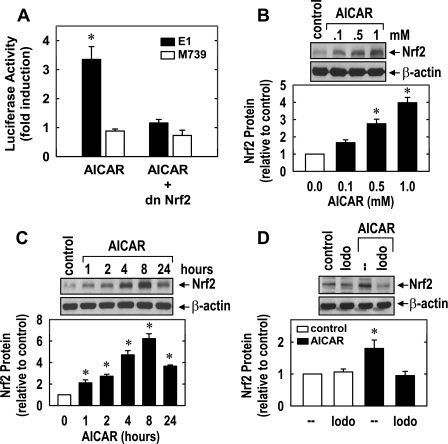
To determine whether the increased expression of HO-1 in response to AICAR involves the transcriptional activation of the gene encoding this protein, HUVECs were transiently transfected with a HO-1 reporter construct and promoter activity was monitored. Treatment of HUVECs with AICAR stimulated a threefold increase in HO-1 promoter activity that was abolished by mutating the AREs, suggesting that AICAR activates HO-1 gene transcription via AREs (Fig. 7A). Since the transcription factor Nrf2 plays a predominant role in ARE-mediated gene transcription, we determined whether Nrf2 mediates the activation of HO-1 by AICAR. Transfection of HUVECs with a dominant negative mutant of Nrf2 that had its activation domain deleted inhibited the AICAR-mediated increase in HO-1 promoter activity. In addition, AICAR resulted in a rapid concentration-dependent increase in Nrf2 protein beginning 1 h after drug treatment (Fig. 7, B and C). Significantly, the AICAR-mediated increase in Nrf2 protein expression was blocked by 5′-iodotubercidin, indicating that AMPK activity was necessary for the activation of Nrf2 (Fig. 7D).
Fig. 7.
AICAR stimulates HO-1 promoter activity and nuclear factor-erythroid 2-related factor 2 (Nrf2) expression in HUVECs. A: AICAR stimulated HO-1 promoter activity. Cells were cotransfected with a HO-1 promoter construct (E1) or a mutated HO-1 promoter construct (M739) and a Renilla luciferase construct, treated with AICAR (0.5 mM) for 8 h, and then analyzed for luciferase activity. In some experiments, a dominant negative mutant (dn)Nrf2 construct was cotransfected into cells. B: concentration-dependent effect of AICAR (0.1–1.0 mM for 8 h) on Nrf2 protein expression. C: time course of Nrf2 protein expression after the administration of AICAR (0.5 mM). D: Iodo inhibited AICAR-mediated Nrf2 protein expression. Cells were pretreated with Iodo (0.3 μM) for 30 min and then treated with AICAR (0.5 mM) for 8 h. Nrf2 protein expression was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SE of 3–6 experiments. *Statistically significant effect of AICAR.
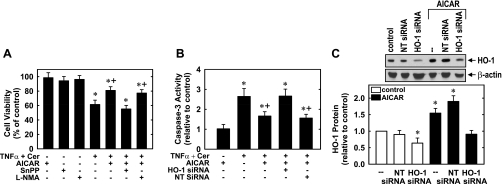
In a final series of experiments, the functional significance of the induction of HO-1 by AMPK was investigated. Treatment of HUVECs with TNF-α (100 ng/ml) in the presence of ceramide (25 μM), which sensitizes human ECs to TNF-α-mediated apoptosis (40), resulted in a significant decline in the number of viable cells and stimulated a marked increase in the activity of the proapoptotic effector caspase-3 (Fig. 8, A and B). Interestingly, pretreatment with AICAR (0.5 mM) for 8 h inhibited the cytokine-mediated loss of cell viability. However, the HO inhibitor SnPP reversed the cytoprotective property of AICAR, whereas the eNOS inhibitor methyl-l-arginine had no effect (Fig. 8A). In the absence of cytokine, AICAR, SnPP, or methyl-l-arginine had no effect on cell viability (Fig. 8A). AICAR also blocked cytokine-induced caspase-3 activation, and this was prevented by transfection of HUVECs with HO-1 siRNA, whereas the nontargeting siRNA had no effect (Fig. 8B). In the absence of cytokine, AICAR (Fig. 8B), HO-1 siRNA, or nontargeting siRNA (data not shown) had no effect on caspase-3 activity. Treatment of HUVECs with HO-1 siRNA abolished the induction of HO-1 by AICAR (Fig. 8C). In contrast, the nontargeting siRNA had no effect on AICAR-mediated increases in HO-1 protein expression, confirming the efficacy and selectivity of the HO-1 knockdown approach.
Fig. 8.
AICAR inhibits EC apoptosis in a HO-1-dependent manner. A: AICAR blocked cytokine-mediated loss of cell viability. HUVECs were pretreated with AICAR (0.5 mM) for 8 h and then exposed to TNF-α (100 ng/ml) and ceramide (Cer; 25 μM) for 24 h in the presence or absence of tin protoporphyrin-IX (SnPP; 10 μM) or l-NMA (1 mM). Results are means ± SE of 6 experiments. *Statistically significant effect of TNF-α (100 ng/ml) and Cer (25 μM); +statistically significant effect of AICAR. B: AICAR blocked cytokine-mediated activation of caspase-3. HUVECs were pretreated with AICAR (0.5 mM) for 8 h and then exposed to TNF-α (100 ng/ml) and Cer (25 μM) for 24 h in cells transfected with HO-1 siRNA (0.1 μM) or nontargeting (NT) siRNA (0.1 μM). Results are means ± SE of 6 experiments. *Statistically significant effect of TNF-α (100 ng/ml) and Cer (25 μM); +statistically significant effect of AICAR. C: HO-1 protein expression in cells treated with AICAR (0.5 mM for 8 h) and transfected with HO-1 siRNA (0.1 μM) or NT siRNA (0.1 μM). HO-1 protein was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SE; n = 4. *Statistically significant change in HO-1 protein expression.
DISCUSSION
In the present study, we identified AMPK as a novel inducer of HO-1 gene expression in the human vascular endothelium. Induction of HO-1 was observed after pharmacological or molecular activation of AMPK and was detected in both arterial and venous ECs. The AMPK-mediated induction of HO-1 occurred in an eNOS-independent fashion and was mediated by the Nrf2/ARE signaling pathway. Significantly, we also found that activation of AMPK inhibits cytokine-mediated EC death and caspase-3 activation and that HO-1 mediates this cytoprotective effect. Thus, the ability of AMPK to induce HO-1 gene expression may provide an important mechanism by which this kinase represses EC death during periods of metabolic and inflammatory stress.
Treatment of ECs with AICAR stimulates a concentration- and time-dependent increase in HO-1 protein and mRNA expression. The induction of HO-1 by AICAR is dependent on AMPK activity since two distinct pharmacological inhibitors of AMPK activation prevented the increase in HO-1 gene expression. Furthermore, knockdown of AMPK-α1/2 blocks the induction of HO-1 by AICAR. The ability of AMPK to stimulate HO-1 expression was also confirmed using the more selective AMPK activator A-769662 and by directly infecting ECs with an adenovirus expressing constitutively active AMPK-α1, the predominant isoform of AMPK present in human ECs (13, 43). However, the induction of HO-1 after AMPK activation is not restricted to the vascular endothelium. Aside from stimulating HO-1 expression in HUVECs and HAECs, activation of AMPK increases HO-1 expression in HASMCs. Significantly, the induction of HO-1 by AMPK was also observed in carotid arteries after the in vivo administration of AICAR, verifying our in vitro experiments with cultured vascular cells. Our finding that AMPK induces HO-1 expression in human vascular cells and rat arteries is consistent with a previous study (4) showing that AICAR stimulates HO-1 protein expression in rat pancreatic β-cells but contrasts with another study (35) where AICAR failed to induce HO-1 in porcine aortic ECs. Disparate results between these studies may reflect differences between animal species, culture conditions, and/or AICAR exposure times. Interestingly, several therapeutic agents that activate AMPK, including peroxisome proliferator-activated receptor agonists, statins, cilostazol, resveratrol, and curcumin, are known inducers of HO-1, raising the possibility that AMPK functions as a central signaling kinase to trigger HO-1 gene expression (42). Finally, the discovery that AMPK stimulates HO-1 expression adds to a growing list of antioxidant enzymes, including manganese SOD, catalase, γ-glutamylcysteine synthase thioredoxin, and mitochondrial uncoupling protein-2, that are induced by this kinase and highlights a critical role for AMPK in maintaining endothelial redox balance (6, 23, 24, 39).
The induction of HO-1 by AMPK is dependent on de novo RNA synthesis, as the transcriptional inhibitor actinomycin D blocked the induction of HO-1 mRNA. In contrast, stimulation of HO-1 mRNA was maintained in the presence of cycloheximide, indicating that AMPK-mediated HO-1 gene expression is independent of protein synthesis. Since AMPK activates eNOS and releases NO (5, 8, 27), which is a known inducer of HO-1 (26), we initially determined the involvement of this enzyme in mediating the induction of HO-1. Although the eNOS inhibitor methyl-l-arginine blocked the activation of eNOS by AICAR, methyl-l-arginine failed to prevent the induction of HO-1, indicating that AICAR-mediated increases in HO-1 expression are independent of eNOS activation. Transient luciferase reporter assays demonstrated that AMPK directly stimulates HO-1 promoter activity. However, the induction of HO-1 gene transcription requires the presence of AREs, since mutation of this responsive element abolished the stimulation of promoter activity by AMPK. While several transcription factors can bind to AREs, recent work has indicated that Nrf2 plays a predominant role in ARE-dependent HO-1 gene expression (1). In support of this, we observed that AICAR stimulates a rapid rise in Nrf2 protein. The induction of Nrf2 expression by AICAR requires AMPK activity since it was prevented by 5′-iodotubercidin. Furthermore, transfection of ECs with a dominant negative Nrf2 construct abolished the activation of HO-1 promoter activity in response to AICAR. Thus, the mobilization of Nrf2 plays an integral role in mediating HO-1 gene transcription by AMPK. The ability of AMPK to activate Nrf2 provides a novel pathway by which AMPK can regulate mammalian gene transcription. However, the mechanism by which AMPK activates Nrf2 is not known. Activation of Nrf2 is regulated by the cytosolic protein Keap1, which facilitates the degradation of Nrf2 via the proteasome. While AMPK may directly phosphorylate Nrf2, phosphorylation of Nrf2 plays a limited role in modulating Nrf2-dependent gene expression (37). Alternatively, AMPK has recently been shown to inhibit proteasomal activity, and this can result in the activation of Nrf2 (38). Consistent with this notion, proteasome inhibitors have been demonstrated to stimulate HO-1 gene expression by activating Nrf2 (36).
Recent studies have indicated that AMPK serves an important survival function in ECs. Activation of AMPK inhibits EC apoptosis in response to oxidative and metabolic stress and anoxia (6, 20, 23, 24, 30, 35). In the present study, we extend this concept to show that AMPK also protects ECs from inflammatory stress. In particular, activation of AMPK by AICAR attenuated EC death and caspase-3 activation in response to TNF-α and ceramide. Interestingly, the cytoprotection afforded by AICAR is mediated by HO-1, since inhibition of HO activity or deletion of HO-1 abolished the cytoprotective effect of AMPK. The cytoprotective action of HO-1 in ECs is likely mediated by CO since scavenging of CO with hemoglobin reversed the antiapoptotic effect of HO-1, whereas the exogenous administration of this gas mimicked the protection induced by HO-1 in response to a wide variety of proapoptotic stimuli, including TNF-α (2, 28). In contrast, inhibition of eNOS activity failed to reverse the protection afforded by AICAR. This latter finding is in agreement with a recent report (29) showing that AMPK protects anoxic ECs from apoptosis in an eNOS-independent fashion. Interestingly, while we show that AMPK stimulates the expression of HO-1, a recent report (32) found that the HO-1 reaction product CO activates AMPK in ECs, raising the possibility that a positive feedback loop exists between HO-1 and AMPK that may serve to amplify the cytoprotective effect of AMPK.
The ability of AMPK to stimulate HO-1 gene expression may be of pharmacological and therapeutic significance. Given our current finding that HO-1 contributes to the antiapoptotic action of AICAR, the induction of HO-1 by AICAR may underlie its ability to inhibit ischemia-reperfusion injury (22). Furthermore, we (14) recently reported that AICAR preconditioning prevents postischemic leukocyte rolling along and adhesion to the murine postcapillary venular endothelium in a manner that is dependent on HO activity, suggesting that HO-1 may also contribute to the antiadhesive actions of AICAR. Moreover, since HO-1 is able to normalize EC function and blood pressure under various pathological settings (10), the induction of HO-1 may also contribute to the restoration of endothelial function and blood pressure that is observed after chronic AMPK activation by AICAR in animal models of insulin resistance and hypertension (3, 35). Finally, our finding that AMPK upregulates HO-1 expression in vascular SMCs may also be of pharmacological relevance. In particular, the induction of HO-1 may participate in the AICAR-mediated inhibition of neointima formation after arterial injury given that HO-1 is a potent inhibitor of vascular SMC growth (3, 10, 34). Thus, the induction of HO-1 may contribute to the pleiotropic beneficial effects of AMPK activators in the circulation.
The present in vitro study provides strong evidence that AMPK promotes EC viability through the induction of HO-1. However, caution is required when extrapolating findings with cultured vascular cells to the intact animal. It will be important to confirm our findings using animal models of metabolic and/or inflammatory stress. In this regard, future experiments using HO-1-deficient mice will prove indispensible in establishing a role for HO-1 in mediating the biological actions of AMPK. Moreover, these animals may provide further insights into the degree and physiological significance of possible reciprocal cross-talk between the AMPK and HO-1 systems. However, the use of highly integrated pathophysiological animal models introduces further complexity. Aside from inducing AMPK activity, stimuli arising from metabolic stress may also directly induce HO-1 expression independent of AMPK. Here again, the use of animal models that lack the requisite proteins will allow one to discern the biological effects of AMPK and HO-1 as well as their regulatory interactions.
In conclusion, the present study demonstrates that AMPK induces HO-1 gene expression in human vascular cells and rat arteries via the Nrf2/ARE pathway. In addition, we found that AMPK inhibits cytokine-mediated EC apoptosis in an HO-1-dependent fashion. The ability of AMPK to stimulate HO-1 gene expression may provide a novel mechanism by which this kinase preserves EC viability and function during periods of metabolic and inflammatory stress.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-59976, HL-74966, HL-81720, and AA-14195.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 36: 166–174, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192: 1015–1026, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduced metabolic disturbances and lowers blood pressure in rats displaying features of insulin resistance syndrome. Diabetes 51: 2199–2206, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Cai Y, Martens GA, Hinke SA, Heimberg H, Pipeleers D, Van de Casteele M. Increased oxygen radical formation and mitochondrial dysfunction mediate beta cell apoptosis under conditions of AMP-activated protein kinase stimulation. Free Radic Biol Med 42: 64–78, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Chen Z, Peng CI, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JYJ. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase ser633. Circ Res 104: 496–505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colombo SL, Moncada S. AMPKα1 regulates the antioxidant status of vascular endothelial cells. Biochem J 421: 163–169, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells. Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulate nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55: 496–505, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MP. Stromal cell factor-1 promotes vascular angiogenesis via a heme oxygenase-1-dependent mechanism. J Exp Med 204: 605–618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med 10: 672–686, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durante W, Liao L, Iftikhar I, O'Brien WE, Schafer AI. Differential regulation of l-arginine transport and nitric oxide production by vascular smooth muscle and endothelium. Circ Res 78: 1075–1082, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Ewart MA, Kohlhaas CF, Salt IP. Inhibition of tumor necrosis factor α-stimulated monocyte adhesion to human aortic endothelial cells by AMP-activated protein kinase. Arterioscler Thromb Vasc Biol 28: 2255–2257, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Fisslhaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105: 114–127, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Gaskin FS, Kamada K, Yusof M, Durante W, Gross G, Korthuis RJ. AICAR preconditioning prevents postischemic leukocyte rolling and adhesion: role of KATP channels and heme oxygenase. Microcirculation 16: 167–176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaskin FA, Kamada K, Yusof M, Korthuis RJ. 5′-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol 292: H326–H332, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Goransson O, McBride A, Hawley SA, Ross FA, Shipiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem 282: 32549–32560, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardie DG, Carling D. The AMP-activated protein kinase-fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273l, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47: 1183–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Henderson JF, Paterson AR, Caldwell IC, Paul B, Chan MC, Lau KF. Inhibitors of nucleoside and nucleotide metabolism. Cancer Chemother Rep 23: 71–85, 1972 [PubMed] [Google Scholar]
- 20. Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 51: 159–167, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31: 162–168, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kingma JG, Jr, Simard D. Timely administration of AICA riboside reduces reperfusion injury in rabbits. Cardiovasc Res 28: 1003–1007, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55: 120–127, 2006 [PubMed] [Google Scholar]
- 24. Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, Coselli JS, Chen L, Wang XL, Zhang Y, Shen YH. Activation of AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes 58: 2246–2257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin CC, Liu XM, Peyton KJ, Wang H, Yang WC, Lin SJ, Durante W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol 28: 739–745, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu XM, Peyton KJ, Ensenat D, Wang H, Hannink M, Alam J, Durante W. Nitric oxide stimulates heme oxygenase-1 gene transcription via the Nrf2/ARE complex to promote vascular smooth muscle cell survival. Cardiovasc Res 75: 381–389, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morrow VA, Foufelle F, Connell JMC, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric oxide synthesis in human aortic endothelial cells. J Biol Chem 278: 31629–31639, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1: a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med 47: 1–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagata D, Kiyosue A, Takahashi M, Satonaka Tanaka K, Sata M, Nagano T, Nagai R, Hirati Y. A new constitutively active mutant of AMP-activated protein kinase inhibits anoxia-induced apoptosis of vascular endothelial cell. Hypertens Res 32: 133–139, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Nagata D, Mogi M, Walsh K. AMPK-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 278: 31000–31006, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Nagata D, Takeda R, Sata M, Satanaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-induced stimulated vascular smooth muscle cell proliferation. Circulation 110: 444–451, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Nizamutdinova IT, Kim YM, Kim HJ, Seo HG, Lee JH, Chang KC. Carbon monoxide (from CORM-2) inhibits high glucose-induced ICAM-1 expression via AMP-activated protein kinase and PPAR-γ activations in endothelial cells. Atherosclerosis 207: 405–411, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Peyton KJ, Ensenat D, Azam MA, Keswani A, Kannan S, Liu Xm Wang H, Tulis DA, Durante W. Arginase promotes neointima formation in rat injured carotid arteries. Arterioscler Thromb Vasc Biol 29: 488–494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peyton KJ, Reyna SV, Chapman GB, Ensenat D, Liu XM, Wang H, Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood 51: 441–446, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Schulz E, Dopheide J, Schuhmacher W, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr Suppression of JNK pathway by induction of a metabolic stress response prevents vascular injury. Circulation 118: 1347–1357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem 278: 2396–2402, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Sun Z, Huang Z, Zhang DD. Phosphorylation of Nrf2 at multiple sites by MAP kinases has limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One 4: e6588, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viana R, Aguado C, Esteban I, Moreno D, Viollet B, Knecht E, Sanz P. Role of AMP-activated protein kinase in autophagy and proteasome function. Biochem Biophys Res Commun 369: 964–968, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Xie Z, Zhang J, Wu J, Viollet B, Zhou MH. Up-regulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes 58: 1893–1901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Zheng L, Dengler TJ, Kluger MS, Madge LA, Schechner JS, Maher SE, Pober JS, Bothwell AL. Cytoprotection of human umbilical vein endothelial cells against apoptosis and CTL-mediated lysis provided by caspase-resistant Bcl-2 without alterations in growth or activation responses. J Immunol 164: 4665–4671, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou G, Sebhat IK, Zhang BB. AMPK activators: potential therapeutics for metabolic and other diseases. Acta Physiol (Oxf) 196: 175–190, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Zou MH, Wu Y. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol 35: 535–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]