Abstract
Ablating insulin receptors in cardiomyocytes causes subendocardial fibrosis and left ventricular (LV) dysfunction after 4 wk of transverse aortic constriction (TAC). To determine whether these maladaptive responses are precipitated by coronary vascular dysfunction, we studied mice with cardiomyocyte-restricted knock out of insulin receptors (CIRKO) and wild-type (WT) TAC mice before the onset of overt LV dysfunction. Two weeks of TAC produced comparable increases (P < 0.05 vs. respective sham) in heart weight/body weight (mg/g) in WT-TAC (8.03 ± 1.14, P < 0.05 vs. respective sham) and CIRKO-TAC (7.76 ± 1.25, P < 0.05 vs. respective sham) vs. WT-sham (5.64 ± 0.11) and CIRKO-sham (4.64 ± 0.10) mice. In addition, 2 wk of TAC were associated with similar LV geometry and function (echocardiography) and interstitial fibrosis (picrosirius red staining) in CIRKO and WT mice. Responses to acetylcholine (ACh), NG-monomethyl-l-arginine (l-NMMA), and sodium nitroprusside (SNP) were measured in coronary arteries that were precontracted to achieve ∼70% of maximal tension development using the thromboxane A2 receptor mimetic U-46619 (∼3 × 10−6 M). ACh-evoked vasorelaxation was absent in WT-TAC but was present in CIRKO-TAC albeit reduced relative to sham-operated animals. l-NMMA-evoked tension development was similar in vessels from CIRKO-TAC mice but was lower (P < 0.05) in WT-TAC animals vs. the respective sham-operated groups, and SNP-evoked vasorelaxation was similar among all mice. Thus estimates of stimulated and basal endothelial nitric oxide release were better preserved in CIRKO vs. WT mice in response to 2 wk of TAC. These findings indicate that maladaptive LV remodeling previously observed in CIRKO-TAC mice is not precipitated by coronary artery dysfunction, because CIRKO mice exhibit compensatory mechanisms (e.g., increased eNOS transcript and protein) to maintain coronary endothelial function in the setting of pressure overload.
Keywords: nitric oxide, blood vessel, mice, cardiac hypertrophy, endothelium-dependent vasorelaxation
diabetic patients with cardiovascular disease (CVD) have a worse prognosis than nondiabetic individuals who develop CVD (7). Even when the extent of coronary artery disease and infarct size are accounted for, diabetic patients have a higher incidence of heart failure and mortality in the setting of acute coronary syndromes or revascularization procedures. Although the precise mechanism responsible for the poor outcome of diabetic patients in these situations is unclear, insulin resistance and hyperglycemia may each play significant roles.
Both cardiac myocytes and vascular endothelial cells may be adversely affected by diabetes, particularly in the setting of pathological stresses such as ischemia or pressure overload. Hyperglycemia and altered insulin signaling may independently or collectively have negative consequences in the vasculature (3, 5, 14, 21). In addition, growing evidence suggests that cardiac myocytes may affect the coronary vasculature through paracrine mechanisms (12).
To elucidate the adverse vascular effects that are directly related to impaired myocardial insulin signaling, we generated mice with cardiomyocyte-restricted knock out of the insulin receptor (CIRKO) (1). Previously, we examined the role of cardiomyocyte insulin signaling in left ventricular (LV) remodeling following LV pressure overload (8), isoproterenol infusion (12), and after coronary artery ligation (16). In the first investigation, the degree of LV hypertrophy evoked by 4 wk of transverse aortic constriction (TAC) was similar in CIRKO and wild-type (WT) mice, but a maladaptive pattern of LV remodeling was evident only in CIRKO animals (8). In this regard, CIRKO hearts exhibited LV dilation and systolic dysfunction in response to TAC. Furthermore, gross histology showed that subendocardial fibrosis was more severe in hearts from CIRKO-TAC vs. WT-TAC mice. In the second study, 5 days of isoproterenol infusion produced similar LV hypertrophy between groups, but interstitial and subendocardial fibrosis was more pronounced in CIRKO mice (12). Third, adverse LV remodeling was accelerated in CIRKO hearts following coronary artery ligation (16).
Fibrosis in a subendocardial distribution in the setting of TAC raised the possibility of ischemic injury in a watershed vascular territory. Diffuse subendocardial ischemia could result from reductions of resting coronary blood flow or, more likely, from reduced coronary flow reserve during periods of increased myocardial demand. Either of these conditions could occur from endothelial and/or vascular smooth muscle dysfunction. Therefore, we tested the hypothesis that endothelial and/or vascular smooth muscle dysfunction exist in coronary arteries from CIRKO mice during the development of cardiac hypertrophy.
METHODS
All protocols used in this study were approved by the Animal Use and Care Committee at the University of Utah and conformed to the guidelines set by the American Physiological Society and Animal Welfare Act.
General procedures.
Male CIRKO mice (sham = 12, TAC = 11) and their WT littermates (sham = 14, TAC = 14) weighing 20–30 g (12–15 wk of age) were housed under controlled temperature (23°C) and light conditions (12:12-h light-dark cycle) and received food and water ad libitum. Two weeks after completing TAC or sham surgery (8), echocardiography was performed (1, 4, 8). Later (24 h), mice were anesthetized (2–5% isoflurane), the chest was opened, and the heart was excised and immediately immersed in iced physiological saline solution (PSS; contents described below). The apical portion of the heart was carefully separated from the base using a scalpel, placed in formalin, and later prepared as appropriate to assess fibrosis and endothelial nitric oxide (NO) synthase (eNOS) immunostaining (see Histology) (8, 12). Next, the coronary arteries were isolated from the base while it remained immersed in iced PSS and were used to measure reactivity (see Vascular function). Remaining tissue from the base was snap-frozen in liquid nitrogen for later RNA extraction, quantitative RT-PCR, and Western immunoblotting (described below). Finally, the thoracic aorta and femoral artery, which were immersed in iced PSS during the coronary artery dissections, were isolated and used to measure vascular reactivity (6, 9, 19, 21–23).
TAC or sham surgery.
The minimally invasive TAC procedure has been described and validated previously (8). In brief, a vascular clip (Hemoclip Plus; Weck Closure Systems, Research Triangle Park, NC) was placed around the aortic arch between the inominate and the left common carotid arteries and clamped to a prespecified diameter (27 G).
Echocardiography.
After TAC or sham surgery (2 wk), mice were anesthetized with isoflurane and imaged in the left lateral decubitus position with a linear 13-MHz probe (General Electric; Vivid V echocardiograph) as previously described (1, 4, 8, 9, 21). Echocardiography was performed on 8 of 14 WT-sham and WT-TAC mice, 6 of 12 CIRKO-sham mice, and 6 of 11 CIRKO-TAC mice.
Vascular function.
Following echocardiography (24 h), mice were anesthetized deeply with 3–5% isoflurane, and the heart was excised, trimmed of adherent tissue, and weighed. Next, a scalpel was used to divide the heart approximately midway between the base and apex. The base of the heart was immersed in iced PSS and used to obtain arteries for vascular function studies. Two segments of the anterior descending coronary artery were dissected free and mounted on a wire-type myograph. The myograph was submerged in a temperature-controlled, 8-ml tissue “bath” containing oxygenated (95% O2-5% CO2) PSS (pH ∼7.35–7.45). Two segments each of thoracic aorta and femoral artery, i.e., vessels not exposed to pressure overload, also were dissected free, mounted on myographs, and studied. After the arteries were mounted, tissue baths were warmed gradually to 37°C over 30 min at 0 mg tension. During this time and throughout each experiment, the pH and temperature of all buffer solutions was checked at 30-min intervals, and contents of the tissue bath were exchanged at 15-min intervals. Experimental protocols specific to each vessel type are described.
Coronary arteries.
When the vessel chamber reached 37°C, tension was increased manually by 25 mg every 2 min to 100 mg. Later (30 min), internal circumference-active tension curves were generated to determine the vessel diameter that evoked the greatest tension development (Lmax) to 100 mM potassium chloride (KCl). After vessels were precontracted to ∼70% of Lmax tension using the thromboxane A2 receptor mimetic 9,11-dideoxy-9α,11α-methanoepoxy-prostaglandin-F2α (U-46619; ∼3 × 10−6 M), dose-response curves to acetylcholine (ACh, 10−8 to 10−4 M) were performed in the absence and presence of NG-monomethyl-l-arginine (l-NMMA, 10−3 M). Vasocontractile responses to U-46619 (10−8 to 10−5 M) and KCl (10–100 mM) were assessed to evaluate receptor and non-receptor-mediated vasoconstriction, respectively. Vasocontractile responses to l-NMMA (10−3 M) were performed after vessels were precontracted with U-46619 to estimate basal NO production (10). Finally, sodium nitroprusside (SNP, 10−9 to 10−4 M) dose-response curves were performed to determine endothelium-independent vasorelaxation. Each protocol was separated by ∼30 min.
Femoral arteries and aortas.
Vascular function studies using murine arteries from these two regions have been described (21, 25, 26). All tension data were recorded continuously by a computer through an analog-to-digital interface card (Biopac Systems, Santa Barbara, CA) that allowed for subsequent off-line quantitative analyses. Vascular function studies were performed on 1–3 arteries from 14 WT-sham and WT-TAC mice, 12 CIRKO-sham mice, and 11 CIRKO-TAC mice.
Histology.
After remaining in formalin for 24 h, the apical portion of six hearts from each group was transferred to 75% ethanol, embedded in paraffin, and processed for histology. Three short-axis 5-μm-thick sections from each heart were stained with picrosirius red to determine the percent fibrosis in each field using NIH Image J software (Bethesda, MD). These sections were deparaffinized, rehydrated, treated with primary anti-eNOS antibody followed by biotinylated secondary antibody, and percent eNOS expression per unit tissue area was quantified from fluorescent images using Metamorph software (Sunnyvale, CA). Twenty fields of view per tissue section were used for each analysis. Therefore, data were obtained from 20 fields of view × 3 tissue sections × 6 hearts per group.
RNA extraction and quantitative RT-PCR.
Gene expression was analyzed as previously described (15, 21). Briefly, total RNA was extracted from hearts with Trizol reagent (Invitrogen, Carlsbad, CA), and 3 μg of RNA were reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen) (17). The resulting cDNA were subjected to quantitative real-time RT-PCR on an ABI Prism 7900HT instrument (Applied Biosystems, Foster City, CA) in 384-well plate format using SYBR Green as a probe and a ROX internal reference dye (Invitrogen). Primers were selected from PrimerBank or designed using Primer3Plus and the appropriate GenBank reference sequence (18, 24). All reactions were performed in triplicate. Relative quantification was performed by interpolating crossing-point data on an independent standard curve. Expression of each gene was corrected to the housekeeping gene peptidylprolyl isomerase A (cyclophilin). Results were normalized to the control group (WT-sham = 1.0). Primer sequences used were [accession no. and forward and reverse primers (5′-3′)] as follows: nitric oxide synthase 3 (NM_008713; forward gaccctcaccgctacaacat; reverse gctcattttccaggtgcttc); connective tissue growth factor (Ctgf; NM_010217; forward ggagtgggtgtgtgacgag; reverse cattggtaactcgggtggag); vascular endothelial growth factor (Vegfa; NM_001025250; forward ccaaagccagcacataggag; reverse ccgggatttcttgcgctttc); and peptidylprolyl isomerase also known as cyclophilin (NM_008907; forward agcactggagagaaaggatttgg; reverse tcttcttgctggtcttgccatt).
Western immunoblotting.
Myocardial tissue was homogenized and centrifuged for 20 min at 13,800 rpm at 4°C. Supernatants were separated into aliquots for subsequent protein assay (bicinchoninic acid method) and immunoblotting and stored at −80°C. Equal amounts of protein were suspended in loading buffer (Noves Tris-Glycine SDS sample Buffer X2; Invitrogen) containing β-mercaptoethanol (1:20) and incubated for 5 min at 95–100°C. Proteins then were resolved (110 V) in a SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore Immobilon-P Transfer membrane; Millipore, Billerica, MA) at 4°C. Following transfer, the membranes were blocked with 5% nonfat dry milk or 5% BSA in Tris-buffered saline with 0.1% Tween 20 for 60 min at room temperature. Blocked membranes were probed with primary antibodies for total eNOS (BD Transduction Laboratories, Franklin Lakes, NJ) and hypoxia-inducible factor-1α (HIF-1α; Santa Cruz Biotechnology, Santa Cruz, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technology, Danvers, MA) was used as a loading control. After being washed (3 × 10 min), membranes were incubated with secondary antibodies conjugated with Alexa Fluor Dyes (Invitrogen) for 60 min at room temperature. After washing (3 × 10 min), densitometry was obtained and quantified (Odyssey V3.0.16; Licor, Lincoln, NE). To determine the fold change in protein expression from WT-sham vs. the three treatments (i.e., WT-TAC, CIRKO-sham, and CIRKO-TAC), the following approach was used, using eNOS as an example. The densitometric ratios for eNOS to GAPDH were determined for WT-sham animals, and group mean averages were calculated and normalized to one by dividing each ratio by the group mean value. Next, the ratio of eNOS to GAPDH was determined for each treatment group and divided by the group mean eNOS to GAPDH in WT-sham mice and expressed as fold change relative to WT-sham (2, 15, 21, 27).
Preparation of isolated cardiac myocytes and ventricular homogenates for immunoblotting.
Two WT-sham and two CIRKO-sham mice were injected intraperitoneally with 100 U heparin. Later (30 min), animals were anesthetized with 1.5 mg chloral hydrate intraperitoneally, and the heart was excised and placed in iced NPSS. The aorta was cannulated and perfused retrogradely at ∼60 mmHg for 8–10 min with collagenase buffer (contents described below). After being minced, the heart myocytes were dissociated by sequential washing in buffer with gradually increasing calcium concentration until a final concentration of 1 mM was achieved. The cells then were gently pelleted by centrifugation, resuspended in modified DMEM, and plated in laminin-coated tissue culture wells (1). Total eNOS expression was determined in cardiomyocytes and remaining ventricular homogenates using procedures described earlier.
Isolated working hearts.
Coronary flow (ml/min) was assessed in isolated working hearts from additional WT and CIRKO mice 14 days after the sham or TAC procedure and perfused with 5.0 mM glucose and 0.4 mM palmitate under conditions as previously described by us (1, 11).
Drugs and solutions.
All chemicals were obtained from Sigma Chemical (St. Louis, MO) unless noted otherwise. PSS contained (in mM) 125 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 18 NaHCO3, 0.026 Na2EDTA, and 11.2 glucose. ACh, SNP, l-NMMA, phenylephrine, and KCl were prepared daily from stock solutions using distilled deionized water. Collagenase buffer contained (in mM) 126 NaCl, 4.4 KCl, 1.0 MgCl2, 0.025 CaCl2, 4.0 NaHCO3, 10.0 HEPES, 5.5 glucose, 30.0 2,3-butanedione monoxime, 1.8 pyruvate, pH 7.3, and 0.9 mg/ml type 1 collagenase (1). Krebs-Henseleit-bicarbonate contained (in mM) 118.5 NaCl, 25.0 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 0.5 EDTA, and 11.0 glucose. The latter solution was gassed with 95% O2-5% CO2 and supplemented with 0.4 mM palmitate bound to 3% BSA and 11 mM glucose (1).
Genotyping.
CIRKO mice were generated by crossing mice that were homozygous for a floxed insulin receptor allele in which loxP sites flank exon 4 of the insulin receptor gene (IRlox/lox) with IRlox/lox transgenic mice in which cardiac-specific expression of cre recombinase was driven by the α-myosin heavy chain (α-MHC) promoter. CIRKO mice have the genotype Cre-IRlox/lox. Genotyping was performed using PCR for α-MHC Cre as previously described (1).
Statistical analyses.
Data are presented as means ± SE. Significance was accepted when P < 0.05. Comparison of one end point among four groups (e.g., heart weight-to-body weight ratio) was made using a one-way ANOVA. Tukey post hoc tests were performed when significant main effects were obtained. Comparison of multiple time points (i.e., different drug doses) within and among groups (e.g., vascular relaxation) was made using a two-way repeated-measures ANOVA. Tukey post hoc tests were performed when significant main effects were obtained.
RESULTS
Cardiac remodeling after TAC.
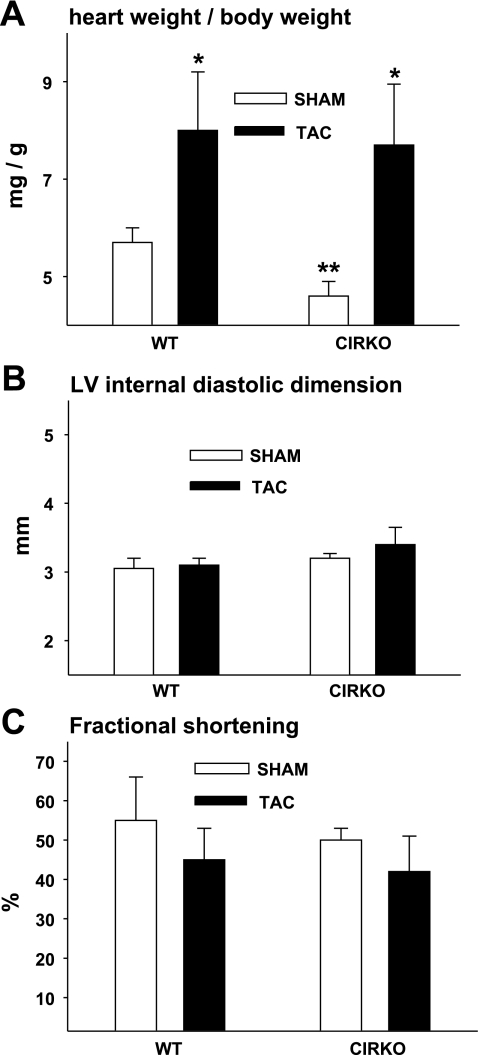
As we have observed previously (8), heart weight was lower in CIRKO-sham vs. WT-sham mice (Table 1). Two weeks of TAC increased heart weight (44 and 34% vs. respective sham-operated controls) and heart weight/body weight to the same degree in CIRKO and WT mice (Table 1 and Fig. 1A). Importantly, the pattern of LV remodeling in response to 2 wk of TAC was similar between CIRKO and WT mice. For example, LV internal diastolic dimension, LV internal systolic dimension, fractional shortening, and interventricular septum thickness were not different between CIRKO-TAC and WT-TAC animals (Table 1 and Fig. 1, B and C). These findings allowed us to test the hypothesis that coronary endothelial and/or vascular smooth muscle dysfunction exists in CIRKO mice during the development of cardiac hypertrophy and precedes the onset of overt cardiac dysfunction.
Table 1.
Characteristics of WT and CIRKO mice 2 wk after sham or TAC surgery
| WT |
CIRKO |
|||
|---|---|---|---|---|
| Sham | TAC | Sham | TAC | |
| Mouse characteristics | ||||
| n | 14 | 14 | 12 | 11 |
| Body wt, g | 27.6 ± 1.3 | 26.1 ± 0.9 | 29.0 ± 1.8 | 26.4 ± 2.5 |
| Heart wt, mg | 154 ± 8 | 206 ± 26* | 134 ± 7† | 193 ± 28* |
| Echocardiographic measures | ||||
| n | 8 | 8 | 6 | 6 |
| IVSd, mm | 1.01 ± 0.06 | 1.27 ± 0.06* | 0.87 ± 0.08 | 1.16 ± 0.06* |
| IVSs, mm | 1.76 ± 0.11 | 1.85 ± 0.07 | 1.60 ± 0.11 | 1.75 ± 0.14 |
| LVIDs, cm | 1.41 ± 0.19 | 1.73 ± 0.12 | 1.62 ± 0.11 | 2.03 ± 0.35 |
| PWd, mm | 0.98 ± 0.07 | 1.1 ± 0.08 | 0.80 ± 0.07 | 0.88 ± 0.05 |
| PWs, mm | 1.39 ± 0.08 | 1.54 ± 0.06 | 1.31 ± 0.10 | 1.35 ± 0.08 |
| EF, % | 89 ± 4 | 82 ± 4 | 88 ± 2 | 79 ± 6 |
Values are means ± SE; n, no. of animals. WT, wild-type mice; CIRKO, mice with cardiac myocyte-restricted knockout of the insulin receptor; TAC, transverse aortic constriction to produce pressure overload. Measurements were performed on anesthetized mice; IVS, interventricular septum; LVID, left ventricular internal dimension; PW, posterior wall; EF, ejection fraction; d, diastole; s, systole.
P < 0.05 vs. respective sham;
P < 0.05 vs. WT sham.
Fig. 1.
Echocardiographic measurements and heart weights. A: heart weight-to-body weight ratios were greater in wild-type (WT) mice and mice with cardiac selective deletion of the insulin receptor (CIRKO) that were exposed to 14 days of transverse aortic constriction (TAC) vs. a sham operation (sham). *P < 0.05, sham vs. TAC; **P < 0.05, CIRKO vs. similarly treated WT. B: left ventricular (LV) internal diastolic dimension in sham and TAC mice was similar between groups. C: fractional shortening in sham and TAC mice was similar between groups. The no. of mice/group = 8 (WT-sham and WT-TAC) and 6 (CIRKO-sham and CIRKO-TAC).
ACh-induced vasorelaxation.
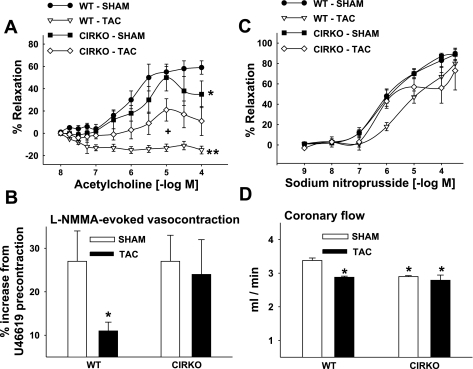
Characteristics of coronary arteries from the four groups of mice are shown in Table 2. ACh-evoked vasorelaxation was generally similar between the sham-operated groups except for the highest dose (10−4 M ACh) which produced greater vasorelaxation in WT vs. CIRKO mice (Fig. 2A). Maximal vasorelaxation, however, was not different in vessels from WT-sham (58 ± 9%) and CIRKO-sham (46 ± 13%) animals. Thus loss of insulin signaling in the heart in the absence of pressure overload does not influence this estimate of endothelium-dependent function. Contrary to our initial hypothesis, endothelial dysfunction produced by pressure overload was less severe in CIRKO-TAC vs. WT-TAC mice. Specifically, while ACh-evoked relaxation observed in U-46619-precontracted vessels was absent in coronary arteries from WT-TAC mice, vasorelaxation in CIRKO-TAC mice was only reduced by ∼50% compared with CIRKO-sham animals (21 ± 9 vs. 46 ± 13%). Pretreatment with l-NMMA abolished ACh-evoked vasorelaxation in all groups (data not shown), indicating that the vasorelaxant response in this set of experiments was solely due to stimulated NO production.
Table 2.
Vessel characteristics from WT and CIRKO mice 2 wk after sham or TAC surgery
| WT |
CIRKO |
|||
|---|---|---|---|---|
| Sham (n = 14) | TAC (n = 14) | Sham (n = 12) | TAC (n = 11) | |
| Coronary artery | ||||
| Start width, μm | 105 ± 7 | 121 ± 12 | 107 ± 7 | 107 ± 4 |
| Lmax width, μm | 323 ± 14 | 340 ± 21 | 333 ± 18 | 377 ± 17 |
| Length, μm | 1,330 ± 60 | 1,288 ± 71 | 1,287 ± 71 | 1,261 ± 71 |
| Aorta | ||||
| Start width, μm | 388 ± 20 | 357 ± 10 | 417 ± 17 | 358 ± 37 |
| Width at 1,500 mg tension, μm | 947 ± 20 | 957 ± 19 | 921 ± 10 | 942 ± 32 |
| Length, μm | 1,800 ± 53 | 1,771 ± 29 | 1,700 ± 43 | 1,725 ± 21 |
Values are means ±SE; n, no. of animals. Start width, internal diameter at 0 mg tension; Lmax width, width at diameter that evoked the greatest tension development to 100 mM KCl; 1-3 segments of coronary artery and 2 segments of aorta were examined per animal.
Fig. 2.
Coronary vascular reactivity and blood flow. A: acetylcholine-evoked vasorelaxation in WT and CIRKO mice 14 days after the sham or TAC procedure. *P < 0.05, CIRKO-sham vs. WT-sham at 10−4 M; **WT-TAC vs. all groups at the 7 highest doses; +CIRKO-TAC vs. CIRKO-sham at 10−5 M. B: coronary artery vasocontraction in response to NG-monomethyl-l-arginine (l-NMMA) after precontraction using 9,11-dideoxy-9α,11α-methanoepoxy-prostaglandin-F2α (U-46619). *P < 0.05 vs. all. C: sodium nitroprusside-evoked vasorelaxation was generally similar among groups. The no. of mice (and coronary segments studied) was 14 (and 28) for WT-sham; 14 (and 35) for WT-TAC; 12 (and 24) for CIRKO-sham; and 11 (and 22) for CIRKO-TAC. D: coronary flow (ml/min) was greater in hearts from WT vs. CIRKO-sham mice. TAC reduced coronary flow in hearts from WT but not CIRKO mice. *P < 0.05, TAC vs. sham; **P < 0.05, WT-sham vs. CIRKO-sham. The no. of hearts used for coronary flow studies was 20 for WT-sham and 12 each for WT-TAC, CIRKO-sham, and CIRKO-TAC. Results represent means ± SE.
Basal NO production estimated from l-NMMA administration.
The magnitude of vasocontraction in response to administering the eNOS inhibitor l-NMMA to precontracted arteries estimates basal NO production (10). WT-TAC mice showed evidence of reduced basal NO production. Unexpectedly, no difference in l-NMMA-induced contraction existed between vessels from CIRKO-TAC and CIRKO-sham animals (Fig. 2B).
Vasocontraction and smooth muscle function.
Non-receptor-mediated (i.e., KCl) and receptor-mediated (i.e., U-46619) vasocontractile responses were similar among groups (data not shown). To assess smooth muscle function independent from endothelial effects, the direct acting NO donor SNP was used. Maximal SNP-induced vasorelaxation (89 ± 7, 81 ± 10, 90 ± 4, and 82 ± 16%; Fig. 2C) was not different among WT-sham, WT-TAC, CIRKO-sham, and CIRKO-TAC mice, respectively.
Coronary flow.
In sham hearts, coronary flow was higher in WT vs. CIRKO hearts. TAC resulted in decreased coronary flows in WT mice. By contrast, TAC was not associated with any further decline in coronary flow in CIRKO-TAC vs. CIRKO-Sham (Fig. 2D).
Control experiments using arteries distal to the aortic arch.
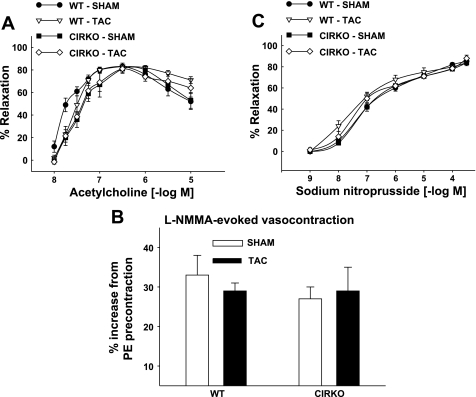
In this model of LV pressure overload, only the proximal aorta and coronary arteries are directly exposed to increased pressure. All vessels distal to the constriction have normal or low pressure. To distinguish between direct effects of pressure overload and indirect effects of cardiac dysfunction (or other sequelae of TAC), segments of thoracic aorta and femoral arteries that were not exposed to pressure overload served as “control” vessels. In aortas we observed that: 1) maximal ACh-evoked vasorelaxation (80 ± 4, 85 ± 3, 83 ± 7, and 82 ± 7; Fig. 3A); 2) maximal l-NMMA-evoked vasocontraction (33 ± 5, 27 ± 3, 29 ± 2, and 29 ± 7%; Fig. 3B); 3) KCl- and phenylephrine-induced vasocontraction (data not shown); and 4) maximal SNP-induced vasorelaxation (90 ± 2, 87 ± 3, 86 ± 1, and 93 ± 4%; Fig. 3C) were similar among WT-sham, WT-TAC, CIRKO-sham, and CIRKO-TAC mice, respectively. The pattern of results among groups for femoral arteries was similar to aorta (data not shown). Hence, the abnormalities of endothelial function were only seen in vessels directly exposed to higher pressures.
Fig. 3.
Aortic reactivity. Acetylcholine-evoked vasorelaxation (A), l-NMMA-evoked vasocontraction after precontraction using phenylephrine (PE; B), and sodium-nitroprusside evoked vasorelaxation (C) were similar among WT and CIRKO mice 14 days after the sham or TAC procedure. Results are means ± SE from 2 aortic segments/mouse from 14 WT-sham, 14 WT-TAC, 12 CIRKO-sham, and 11 CIRKO-TAC mice.
Assessment of fibrosis.
Interstitial fibrosis as measured from picrosirius red staining was similar between WT-TAC (3.8 ± 0.7%) and CIRKO-TAC (3.0 ± 0.7%) mice. There were no differences in the quantity of endocardial vs. epicardial fibrosis.
Assessment of eNOS distribution: Immunostaining.
eNOS immunostaining was present in 18.4 ± 7.4, 15.1 ± 3.6, and 12.7 ± 5.3% of total tissue area of sections obtained from the endocardium, midmyocardium, and epicardium, respectively, of WT-TAC mice and 13.8 ± 1.5, 16.0 ± 2.3, and 10.7 ± 2.3%, respectively, of CIRKO-TAC mice (P = not significant). These data indicate no differences exist concerning transmural eNOS expression in CIRKO vs. WT mice 14 days following TAC.
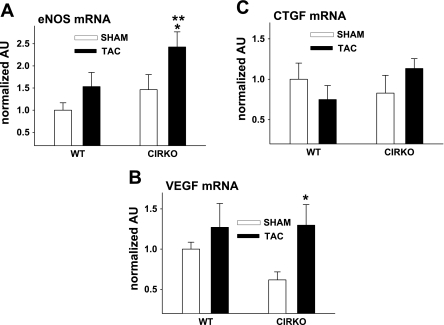
Assessment of eNOS, VEGF, and CTGF mRNA in heart homogenates.
eNOS (Fig. 4A) and VEGF (Fig. 4B) mRNA were greater in hearts from CIRKO-TAC vs. CIRKO-sham mice. A nonsignificant trend (P = 0.09) existed for eNOS mRNA to be higher in hearts from CIRKO-TAC vs. WT-TAC mice. Consistent with the findings from picrosirius red staining, CTGF (Fig. 4C) mRNA was similar among groups.
Fig. 4.
mRNA in heart homogenates. Endothelial nitric oxide synthase (eNOS; A) and vascular endothelial growth factor (VEGF; B) mRNA were greater (*P ≤ 0.05) in hearts from CIRKO-TAC vs. CIRKO-sham mice. A trend existed (**P = 0.09) for higher eNOS mRNA in hearts from CIRKO-TAC vs. WT-TAC mice. Connective tissue growth factor (CTGF; C) mRNA was similar among groups. Results are means ± SE from 4 WT-sham, 5 WT-TAC, 7 CIRKO-sham, and 5 CIRKO-TAC mice.
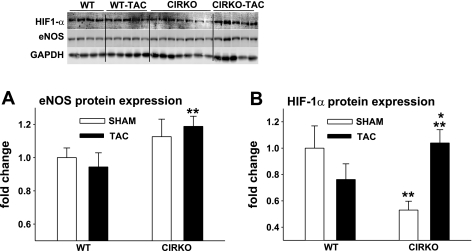
Assessment of eNOS and HIF-1α protein expression.
eNOS (Fig. 5A) and HIF-1α (Fig. 5B) protein expression were greater in ventricular homogenates from CIRKO-TAC vs. WT-TAC mice. HIF-1α was higher in CIRKO-TAC vs. CIRKO-sham animals. HIF-1α was lower in tissue from CIRKO-sham vs. WT-sham mice.
Fig. 5.
Protein expression in heart homogenates. eNOS (A) and hypoxia-inducible factor-1α (HIF-1α; B) protein expression were greater (**P < 0.05) in ventricular homogenates from CIRKO-TAC vs. WT-TAC mice. HIF-1α was higher (*P < 0.05) in CIRKO-TAC vs. CIRKO-sham animals. HIF-1α was lower (**P < 0.05) in tissue from CIRKO-sham vs. WT-sham mice. Representative immunoblots are shown using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control. Results are means ± SE from 4 WT-sham, 5 WT-TAC, 7 CIRKO-sham, and 5 CIRKO-TAC mice.
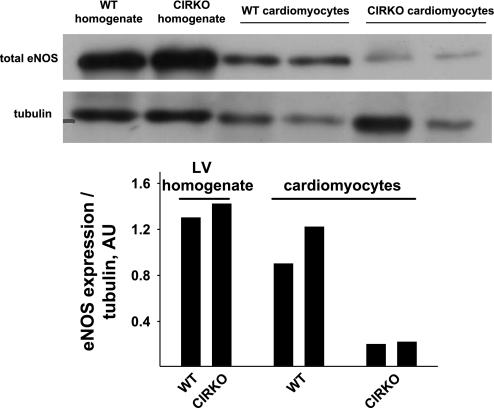
Assessment of eNOS protein expression in ventricular homogenates and cardiac myocytes.
eNOS protein was similar in ventricular homogenates from two WT-sham and two CIRKO-sham mice. eNOS protein was approximately fivefold greater in cardiomyocytes from WT vs. CIRKO mice (Fig. 6).
Fig. 6.
Relative eNOS protein levels in isolated cardiomyocytes vs. heart homogenates. eNOS protein was similar in ventricular homogenates from 2 WT-sham and 2 CIRKO-sham mice. eNOS protein was ∼5-fold greater in cardiomyocytes from WT vs. CIRKO mice. AU, arbitrary units.
DISCUSSION
Previous work showed that CIRKO mice exhibit a maladaptive pattern of LV remodeling, subendocardial fibrosis, and reduced systolic function in response to 4 wk of TAC compared with WT animals (8). Arterial dysfunction might cause repetitive demand ischemia in the watershed region of the left ventricle in CIRKO mice and precipitate the previously described cardiac phenotype. In the present study, we sought to determine whether coronary endothelial and/or vascular smooth muscle dysfunction precedes these myocardial abnormalities in CIRKO mice with LV pressure overload. Our strategy was to examine arterial function in CIRKO and WT mice when TAC-induced cardiac hypertrophy was present, but before the maladaptive pattern of cardiac remodeling developed.
Coronary endothelial dysfunction is less severe in CIRKO vs. WT mice after TAC.
Reactivity of isolated coronary arteries from both sham-operated groups was similar. Both CIRKO and WT mice had evidence of coronary endothelial dysfunction after TAC, whereas vascular smooth muscle function was not changed. Unexpectedly, the coronary endothelial dysfunction was less pronounced in CIRKO-TAC mice. Specifically, estimates of stimulated (i.e., ACh-evoked) and basal (i.e., l-NMMA-evoked) NO release were less severely impaired in coronary arteries from CIRKO vs. WT mice exposed to 14 days of pressure overload. In keeping with our vascular function results, we also observed that coronary flow was reduced less in isolated perfused hearts from CIRKO-TAC vs. WT-TAC mice. Thus the pattern of vascular dysfunction and coronary flow reduction in CIRKO mice exposed to 14 days of TAC was congruent. Because arterial dysfunction was less severe in CIRKO vs. WT mice after TAC, it is unlikely that the maladaptive cardiac remodeling that develops when CIRKO mice are subjected to longer-term (i.e., 4 wk) pressure overload via TAC is precipitated by impaired coronary vasorelaxation.
Other mechanisms to explain adverse LV remodeling in CIRKO mice with LV pressure overload.
An alternative mechanism potentially responsible for the maladaptive cardiac phenotype that develops in CIRKO vs. WT mice exposed to 4 wk of TAC might involve a reduction in capillary density. We have reported this mechanism might be operative when CIRKO mice are infused with isoproterenol for 5 days (12). Isoproterenol is thought to induce cardiac hypertrophy and injury mainly via overstimulation of β-adrenergic signaling pathways rather than through a hemodynamic mechanism. As such, the nature of cardiac injury in the isoproterenol model is fundamentally different from that evoked by LV pressure overload (i.e., the present study). Interestingly, VEGF, HIF-1α, and eNOS mRNA and eNOS protein were lower in hearts from CIRKO vs. WT mice in that study. In contrast, we found that, relative to the respective sham-operated group, 14 days of TAC increased VEGF and eNOS mRNA and HIF-1α protein expression in hearts from CIRKO but not WT mice. These data, together with those concerning vascular function and coronary flow, suggest that CIRKO mice might have compensatory mechanisms that facilitate adequate coronary perfusion during the early (i.e., 14 days) development of pressure overload cardiac hypertrophy. These compensatory changes likely are insufficient in response to longer (i.e., 4 wk) exposure to increased afterload.
Biochemical mechanisms of compensation in the coronary vasculature of CIRKO mice.
The mechanisms accounting for the relative preservation of endothelium-dependent relaxation in CIRKO mice with LV pressure overload are of interest. First, we determined whether the contribution to total eNOS protein from different cell types was the same between groups. Total eNOS protein was assessed in ventricular homogenates (which include all cell types present in the heart) and isolated cardiomyocytes, the latter serving as a preparation that should be relatively free from vascular endothelial cells. While total eNOS protein from ventricular homogenates was similar between WT and CIRKO mice, eNOS protein was approximately fivefold greater in isolated cardiomyocytes from WT vs. CIRKO mice. By inference, the contribution to total eNOS from other cell types, most likely endothelial cells, must be greater in CIRKO vs. WT mice. Upregulation of eNOS in vascular endothelial cells might explain, at least in part, the relative vascular protection we observed in CIRKO vs. WT mice exposed to 14 days of TAC. Second, we explored whether differences existed between groups concerning TAC-induced alterations of eNOS transcript and protein. Consistent with the aforementioned observation, TAC increased eNOS mRNA relative to sham-operated animals in CIRKO but not WT mice. Furthermore, eNOS protein was greater in ventricular homogenates from CIRKO-TAC vs. WT-TAC mice. Thus our data all point to a preferential increase in eNOS in CIRKO mice after 14 days of TAC. Third, to determine whether greater relaxation in vessels from CIRKO-TAC vs. WT-TAC mice was secondary to upregulation of other endothelial-derived substances (e.g., hyperpolarizing factors), we performed ACh dose-response curves before and after NOS inhibition. In both groups, ACh-evoked vasorelaxation was abolished completely in the presence of l-NMMA. Based on this finding, it seems likely that the relatively enhanced endothelium-dependent vasorelaxation is almost exclusively related to NO availability.
Insulin receptors are located in the endothelial layer of blood vessels, and their stimulation evokes arterial vasodilation and facilitates blood flow (13, 14, 21). Although insulin receptors are deleted from cardiomyocytes of CIRKO mice, they are present in other cell types, i.e., endothelial cells, vascular smooth muscle cells, and fibroblasts. Systemic glucose homeostasis is normal in CIRKO mice (1, 8). Because the present experiments were performed in the absence of exogenous insulin, it is unlikely that compensatory increases in insulin sensitivity of endothelial cells could account for our findings.
In summary, maladaptive cardiac remodeling that develops in CIRKO mice after long-term (i.e., 4 wk) pressure overload is not secondary to impaired epicardial coronary artery function present after 14 days of pressure overload. In fact, CIRKO mice appear to have several compensatory mechanisms that help limit reductions in coronary perfusion operative at 14 days (development phase) but not after 4 wk (sustained phase) of TAC. It is possible that impaired myocardial insulin signaling contributes to increased injury and adverse remodeling primarily via mechanisms that are intrinsic to the myocyte, e.g., decreased metabolic reserve.
GRANTS
Student support was provided, in part, by: the American Heart Association (AHA), Western States Affiliate, Undergraduate Student Summer Research Program; the American Physiological Society Undergraduate Student Summer Research Program; and the University of Utah Undergraduate Research Opportunities Program. This work was funded, in part, by National Heart, Lung, and Blood Institute (NHLBI) Grants RO1 HL-070070 and UO1 HL-087947 to E. D. Abel who is an Established Investigator of the AHA, the University of Utah College of Health, the University of Utah Research Foundation, a Western States Affiliate Grant-In-Aid 06-55222Y, and NHLBI Grant R15 HL-091493-01 to J. D. Symons.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The assistance of Heather Theobald (RT-PCR analyses and mouse colonies) was greatly appreciated.
Preliminary data were presented at the American Heart Association Annual General Meeting (20).
REFERENCES
- 1. Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 109: 629–639, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boudina S, Bugger H, Sena S, O'Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119: 1272–1283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Carlstrom J, Symons JD, Wu TC, Bruno RS, Litwin SE, Jalili T. A quercetin supplemented diet does not prevent cardiovascular complications in spontaneously hypertensive rats. J Nutr 137: 628–633, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108: 1341–1348, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ensunsa JL, Symons JD, Lanoe L, Schrader H, Keen CL. Reduction of arginase activity via dietary manganese deficiency: effects on arterial function. Exp Biol Med 229: 1143–1153, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Fein FS, Sonnenblick EH. Diabetic cardiomyopathy. Cardiovasc Drugs Ther 8: 65–73, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol 285: H1261–H1269, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Jalili T, Carlstrom J, Kim S, Freeman D, Jin H, Wu TC, Litwin SE, Symons J. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J Cardiovasc Pharmacol 47: 531–541, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Lefer AM, Ma XL. Decreased basal nitric oxide release in hypercholesterolemia increases neutrophil adherence to rabbit coronary artery endothelium. Arterioscler Thromb 13: 771–776, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Mazumder PK, O'Neil BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53: 2366–2374, 2004 [DOI] [PubMed] [Google Scholar]
- 12. McQueen AP, Zhang D, Hu P, Swenson L, Yang Y, Zaha VG, Hoffman JL, Yun UJ, Chakrabarti G, Wang Z, Albertine KH, Abel ED, Litwin SE. Contractile dysfunction in hypertrophied hearts with deficient insulin receptor signaling: possible role of reduced capillary density. J Mol Cell Cardiol 39: 882–892, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care 10: 523–530, 2007 [DOI] [PubMed] [Google Scholar]
- 15. O'Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, Guo A, Zaha VG, Davis DK, Schell JC, Boudina S, Wayment B, Litwin SE, Shioi T, Izumo S, Birnbaum MJ, Abel ED. A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab 6: 294–306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sena S, Hu P, Zhang D, Wang X, Wayment B, Olsen C, Avelar E, Abel ED, Litwin SE. Impaired insulin signaling accelerates cardiac mitochondrial dysfunction after myocardial infarction. J Mol Cell Cardiol 46: 910–918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sena S, Rasmussen IR, Wende AR, McQueen AP, Theobald HA, Wilde N, Pereira RO, Litwin SE, Berger JP, Abel ED. Cardiac hypertrophy caused by peroxisome proliferator- activated receptor-gamma agonist treatment occurs independently of changes in myocardial insulin signaling. Endocrinology 148: 6047–6053, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Spandidos A, Wang X, Wang H, Dragnev S, Thurber T, Seed B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance (Abstract). BMC Genomics 9: 633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Symons JD, Hayashi Y, Ensunsa JL. Improved coronary vascular function evoked by high-intensity treadmill training is maintained in arteries exposed to ischemia and reperfusion. J Appl Physiol 95: 1638–1647, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Symons JD, Hu P, Abel ED, Litwin SE. Coronary arteries in mice with cardiac selective deletion of the insulin receptor are resistant to dysfunction evoked by pressure overload (Abstract). Circulation 112: 2005 [Google Scholar]
- 21. Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to eNOS in the regulation of endothelial function and blood pressure. Circ Res 104: 1085–1094, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Symons JD, Mullick AE, Ensunsa JL, Ma AA, Rutledge JC. Hyperhomocysteinemia evoked by folate-depletion: effects on coronary and carotid arterial function. Arterioscler Thromb Vasc Biol 22: 772–780, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Symons JD, Rutledge JC, Simonsen U, Pattathu RA. Vascular dysfunction produced by hyperhomocysteinemia is more severe in the presence of low folate. Am J Physiol Heart Circ Physiol 290: H181–H191, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3 1. Nucleic Acids Res 35: W71–W74, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang N, Symons JD, Zhang H, Jia Z, Gonzalez FJ, Yang T. Distinct functions of vascular endothelial and smooth muscle PPARgamma in regulation of blood pressure and vascular tone. Toxicol Pathol 37: 21–27, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular PPARg controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab 8: 1–10, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol 587: 3911–3920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]