Abstract
The extracellular K+ concentration ([K+]o) has been proposed to link cardiac metabolism with coronary perfusion and arrhythmogenesis, particularly during ischemia. Several animal studies have also supported K+ as an EDHF that activates Na+-K+-ATPase and/or inwardly rectifying K+ (Kir) channels. Therefore, we examined the vascular reactivity of human coronary arterioles (HCAs) to small elevations in [K+]o, the influence of risk factors for coronary disease, and the role of K+ as an EDHF. Changes in the internal diameter of HCAs were recorded with videomicroscopy. Most vessels dilated to increases in [K+]o with a maximal dilation of 55 ± 6% primarily at 12.5–20.0 mM KCl (n = 38, average: 16 ± 1 mM). Ouabain, a Na+-K+-ATPase inhibitor, alone reduced the dilation, and the addition of Ba2+, a Kir channel blocker, abolished the remaining dilation, whereas neither endothelial denudation nor Ba2+ alone reduced the dilation. Multivariate analysis revealed that cigarette smoking was the only risk factor associated with impaired dilation to K+. Ouabain significantly reduced the vasodilation in HCAs from subjects without cigarette smoking but not in those with smoking. Cigarette smoking downregulated the expression of the Na+-K+-ATPase catalytic α1-subunit but not Kir2.1 in the vessels. Ouabain abolished the dilation in endothelium-denuded vessels to a same extent to that with the combination of ouabain and Ba2+ in endothelium-intact vessels, whereas neither ouabain nor ouabain plus Ba2+ reduced EDHF-mediated dilations to bradykinin and ADP. A rise in [K+]o dilates HCAs primarily via the activation of Na+-K+-ATPase in vascular smooth muscle cells with a considerable contribution of Kir channels in the endothelium, indicating that [K+]o may modify coronary microvascular resistance in humans. Na+-K+-ATPase activity is impaired in subjects who smoke, possibly contributing to dysregulation of the coronary microcirculation, excess ischemia, and arrhythmogenesis in those subjects. K+ does not likely serve as an EDHF in the human coronary arteriolar dilation to bradykinin and ADP.
Keywords: cardiovascular disease, ion channels
the extracellular k+ concentration ([K+]o) is estimated to increase to ∼15 mM in the interstitial space during hypoxia and a brief period of myocardial ischemia, implicating as a cause of electrophysiological alterations leading to arrhythmias (18, 41). Activation of endothelial cells (ECs) also induces K+ efflux into the myoendothelial space to a similar extent (10). Since small elevations in [K+]o can elicit hyperpolarization and relaxation of vascular smooth muscle cells (VSMCs), K+ has been proposed as a candidate for EDHF (10). However, studies (2, 38) from some animal models have suggested that K+ does not serve as an EDHF in all vasculatures.
Na+-K+-ATPase and inwardly rectifying K+ (Kir) channels, which are both sensitive to changes in [K+]o, maintain the cellular resting membrane potential and thus regulate microvascular resistance through hyperpolarization and vasodilation. Na+-K+-ATPase is composed of α-, β-, and γ-subunits (4). The Kir2.1 subunit of the Kir channel has been identified as responsible for Kir channel activity and for vasodilation in response to a low to moderate rise in [K+]o (49), although not all vessels express functional Kir channels (37).
Thus, a critical gap exists in our knowledge of the role played by K+-induced vasomotor responses in the human coronary microcirculation. This might be important in further understanding of the mechanism of coronary microvascular dilation in health and disease. Therefore, we examined the potential role of Kir channels and Na+-K+-ATPase as mediators of vascular responses of human coronary arterioles (HCA) to a small rise in [K+]o and analyzed the influence of coronary risk factors on the responses. We also studied the potential for K+ to serve as an EDHF in the human coronary microcirculation.
METHODS
Materials.
All chemicals were obtained from Sigma Chemical and were dissolved in distilled water except for indomethacin (Indo), which was dissolved in 20 mM Na2CO3 (30, 31). All concentrations represent the final molar concentrations (in mol/l) in the organ chambers.
General preparation.
Fresh specimens of the right atrial appendage were obtained from 73 patients undergoing cardiac surgery. All protocols and procedures were approved by the appropriate Institutional Review Boards. After surgical removal, the atrial appendage was placed in cold oxygenated HEPES buffer as previously described (30, 31) (see the Supplemental Material).1
Vascular response to a rise in [K+]o.
Vascular responses of HCAs to cumulative increases in [K+]o (6–30 mM) were studied in the presence or absence of BaCl2 [3 × 10−5 M, a Kir channel blocker (20)], ouabain [10−6 or 10−4 M, a Na+-K+-ATPase inhibitor (25)], a combination of BaCl2 and ouabain, or after mechanical EC denudation (30, 31). In some vessels, the effects of ouabain or the combination of ouabain and BaCl2 on dilations to aprikalim, an ATP-sensitive K+ channel opener (31), papaverine, bradykinin (BK), or ADP (29–31) were also tested in the presence or absence of Nω-nitro-l-arginine methyl ester (l-NAME, an nitric oxide synthase inhibitor, 10−4 M) and Indo (10−5 M, a cyclooxygenase inhibitor) to focus on the EDHF-mediated dilation. Separately, the role of osmolarity (15) in the response to the different KCl-containing solutions was studied by determining the response to isotonic high-K+ physiological salt solution (PSS) in which KCl was increased by isotonic replacement with NaCl.
Real-time PCR analysis.
Quantitative real-time PCR was performed as previously reported (see the Supplemental Material).
Statistical analysis.
Vascular responses to rises in [K+]o, aprikalim, BK, and ADP were expressed as percentages of dilation or constriction, with 100% representing the change in diameter to papaverine (10−4 M) (30, 31). Vasodilation to papaverine was expressed as the percent passive diameter obtained with Ca2+-free PSS containing papaverine (10−4 M) (30, 31). Student's paired t-test was used to compare maximal dilations and ED50 values (the molar concentration of dilator that produced a 50% of the maximal response). Responses to K+, aprikalim, papaverine, BK, and ADP under different conditions were compared using two-way repeated-measures ANOVA with a Bonferroni correction. Multivariate analysis assessed the influence of basal vascular tone, vessel size, age, gender, cigarette smoking, and underlying diseases (coronary artery disease, diabetes mellitus, hypertension, hypercholesterolemia, myocardial infarction, valvular disease, and congestive heart failure; Tables 1 and 2) on the responses. Regression models for all doses or time intervals isolated the confounding effects of the other diseases on the responses (30, 31). An analysis of covariance was performed to adjust for contributions of each factor to an impaired response (30, 31). SAS for Windows (version 8) was used for analyses. Significance was defined as P < 0.05. Data are expressed as means ± SE.
Table 1.
General patient demographics
| No. of Subjects | |
|---|---|
| Sex (men/women) | 54/19 |
| Age, yr | 63 ± 12 |
| Range, yr | 34∼82 |
| Surgical procedures | |
| CABG | 65 |
| Valve replacement | |
| Aortic | 14 |
| Mitral | 16 |
| Tricuspid | 2 |
| Congenital heart disease repair | 0 |
| Underlying diseases | |
| Coronary artery disease | 66 |
| Diabetes mellitus | 20 |
| Hypertension | 41 |
| Hypercholesterolemia | 28 |
| Myocardial infarction | 12 |
| Congestive heart failure | 10 |
| Cigarette smoking | 32 |
| None of the above | 1 |
Seventy-three subjects in total were studied.
CABG, coronary artery bypass graft.
Table 2.
Patient demographics by surgical procedures
| CABG |
Aortic Valve Replacement |
Mitral Valve Replacement |
Tricuspid Valve Replacement |
|||||
|---|---|---|---|---|---|---|---|---|
| No. of subjects | % | No. of subjects | % | No. of subjects | % | No. of subjects | % | |
| Total no. of subjects | 65 | 14 | 16 | 2 | ||||
| Coronary artery disease | 65 | 100 | 13 | 93 | 9 | 56 | 2 | 100 |
| Diabetes Mellitus | 19 | 29 | 3 | 21 | 2 | 13 | 1 | 50 |
| Hypertension | 40 | 62 | 10 | 71 | 4 | 25 | 1 | 50 |
| Hypercholesterolemia | 27 | 42 | 5 | 36 | 4 | 25 | 0 | 0 |
| Myocardial infarction | 12 | 18 | 0 | 0 | 1 | 6 | 0 | 0 |
| Congestive heart failure | 8 | 12 | 1 | 7 | 3 | 19 | 0 | 0 |
| Cigarette smoking | 28 | 43 | 3 | 21 | 7 | 44 | 2 | 100 |
| None of above | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 |
RESULTS
Seventy-three HCAs with an average passive internal diameter of 149 ± 7 μm (range: 58–271 μm) were dissected. Patient demographics are shown in Tables 1 and 2.
HCA responses to increases in [K+]o.
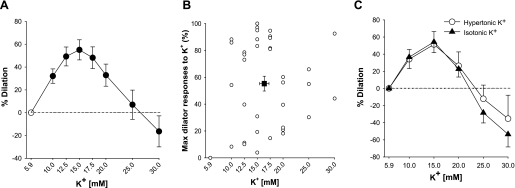
In HCAs, extraluminally added KCl induced a dose-dependent and sustained vasodilation between 10 and 25 mM [K+]o, as shown in Fig. 1A. Constriction was seen at higher doses. Although most vessels tested responded with vasodilation to increasing [K+]o with a maximal dilation of 55 ± 6%, maximal dilator responses of individual vessels markedly varied (range: 0% to 100%, n = 38; Fig. 1B) and were primarily observed between 12.5 and 20.0 mM (average: 16 ± 1 mM), concentrations comparable with elevations in [K+]o in myocardial interstitial and myoendothelial spaces during ischemia or EC activation (10, 18).
Fig. 1.
Vascular response to elevations in extracellular K+ concentration ([K+]o). A: increases in [K+]o produced graded vasodilation of human coronary arterioles (HCAs; n = 38). At concentrations higher than 25 mM, competing dose-dependent constriction was seen. B: maximal vasodilator responses to K+ and the concentrations that elicited the maximal response varied among the subjects tested (n = 38). C: vasodilation to isotonic [K+]o was identical to that to hypertonic [K+]o (n = 10). Values are means ± SE.
Since the addition of KCl increases the osmolarity of circulating PSS, which may independently result in vasodilation (15), the vascular response to isotonic high-K+ PSS, in which KCl was increased by isotonic replacement with NaCl, was also tested. When KCl was substituted for NaCl to maintain the osmolarity, dilator responses were similar to those in hypertonic buffer at the concentration of [K+]o lower than 20 mM with a tendency of greater constriction at higher concentrations (n = 10). Maximal responses to hypertonic and isotonic [K+]o were 54 ± 15% and 60 ± 12%, respectively [P = not significant (NS); Fig. 1C]. These results suggest that modest elevations in [K+]o, at least at concentrations lower than 20 mM, induce vasodilation in HCAs by mechanisms independent of osmolarity.
Roles of Na+-K+-ATPase and Kir channels in vasodilation to K+.
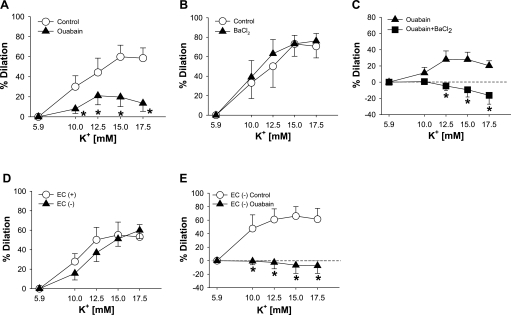
Since a role for Na+-K+-ATPase, Kir channels, or both in the vasodilation to K+ has been demonstrated in other species (8, 9, 20, 37, 49), we studied mechanisms for vasodilation to elevations in [K+]o in HCAs. K+-induced vasodilation was significantly inhibited by pretreatment with ouabain (10−4 M, maximum dilation: 29 ± 9% vs. 61 ± 8% in control, P < 0.05, n = 14; Fig. 2A). BaCl2 (3 × 10−5 M) alone failed to inhibit the vasodilation at a dose selective for Kir channels (20) (maximum dilation: 80 ± 6% vs. 81 ± 7% in control, P = NS, n = 5; Fig. 2B), but the addition of BaCl2 to ouabain further reduced the dilation (maximum dilation: 5 ± 3% vs. 35 ± 9% with ouabain alone, P < 0.05, n = 5; Fig. 2C). Ouabain alone or in combination with BaCl2 may nonspecifically reduce vasodilator responses due to excessive depolarization and tone. However, the combination of ouabain with BaCl2 had no inhibitory effect on papaverine-induced dilation [maximum dilation: 97 ± 1% vs. 97 ± 1% and −log(ED50): 5.7 ± 0.3 vs. 5.6 ± 0.1, P = NS, n = 5, respectively]. Similarly, hyperpolarization-mediated vasodilation to aprikalim (31) was not affected by the combination [maximum dilation: 95 ± 1 vs. 95 ± 2% and −log(ED50): 6.6 ± 0.5 vs. 6.8 ± 0.4, P = NS, n = 3, respectively]. Thus, HCA dilation to elevations in [K+]o was mediated largely by Na+-K+-ATPase, and the role of Kir channels was discernibly unmasked when Na+-K+-ATPase activity was blocked. We next determined whether Na+-K+-ATPase in either ECs, VSMCs, or both mediates K+-evoked dilation in HCAs. EC denudation did not change the response to K+ (maximum dilation: 68 ± 9% vs. 67 ± 12% with ECs, P = NS, n = 9; Fig. 2D). In EC-denuded vessels, ouabain alone (10−4 M) completely abolished the dilation to K+ (maximum dilation: 9 ± 9% vs. 73 ± 20% in control, P < 0.05, n = 5; Fig. 2E) to the same extent as that with the combination of ouabain and BaCl2 in EC-intact vessels (Fig. 2C). In contrast, BaCl2 alone (3 × 10−5 M) did not alter the dilation to K+ in EC-denuded vessels (maximum dilation: 57 ± 22% vs. 58 ± 16% in control, P = NS, n = 6). Therefore, elevations in [K+]o dilated HCAs mainly via activating Na+-K+-ATPase on VSMCs. Taken together, these findings suggest that Na+-K+-ATPase in VSMCs could be a primary target for K+ in the vasculature, whereas Kir channels in ECs may play a considerable role in dilation.
Fig. 2.
Role of vascular smooth muscle cell (VSMC) Na+-K+-ATPase in K+-induced vasodilation. A: vasodilation to increases in [K+]o was inhibited by treatment with ouabain (10−4 M, n = 14). B: BaCl2 [3 × 10−5 M, P = not significant (NS)] had no effect on the vasodilation to K+ (n = 5). C: BaCl2 reduced the vasodilation only in the case where Na+-K+-ATPase activity was inhibited (n = 5). D: endothelial denudation had no inhibitory effect on the vasodilation (n = 9). E: vasodilation to K+ was abolished by treatment with ouabain alone (10−4 M) in endothelial cell (EC)-denuded vessels (n = 5). Values are means ± SE. *P < 0.05 vs. control (A and E) or ouabain alone (C).
Na+-K+-ATPase is a physiological regulator of vascular tone.
We next determined the roles of Na+-K+-ATPase and Kir channels in the regulation of coronary microvascular tone, since the activities of these proteins have not been studied in the human coronary microcirculation. Isolated HCAs developed myogenic tone over wide range of values (14–60% of passive diameters, 27 ± 3%), and the degree of resting myogenic tones was not affected by any risk factors, as we have previously reported (27, 31, 42). Inhibition of Na+-K+-ATPase with ouabain produced a marked increase in vascular tone in a dose-dependent manner (−6 ± 2% at 10−6 M and −18 ± 6% at 10−4 M, P < 0.05 vs. resting tone, n = 12). In contrast, 3 × 10−5 M BaCl2, a dose selective for Kir channels (20), had no effect on vascular tone (0%, P = NS vs. resting tone, n = 10). These results indicate in vitro that Na+-K+-ATPase but not the Kir channel plays an important role in the regulation of basal vascular tone in HCAs.
Influence of clinical factors on vasodilation to elevations in [K+]o.
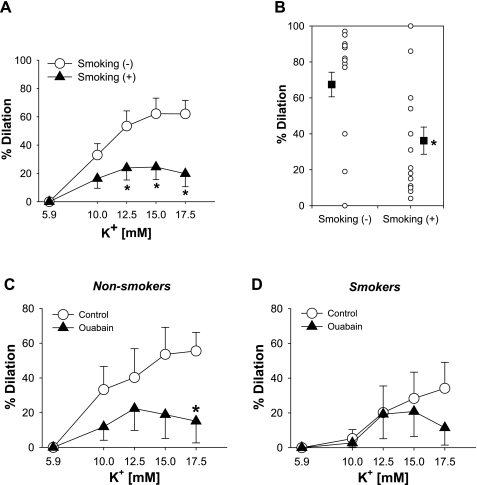
We evaluated the influence of basal vascular tone, vessel diameter, age, gender, cigarette smoking, and underlying diseases (coronary artery disease, diabetes mellitus, hypertension, hypercholesterolemia, myocardial infarction, valvular disease, and congestive heart failure) on the vasodilation to elevations in [K+]o and to papaverine (10−4 M) (30, 31). Multivariate analysis revealed that only the presence of cigarette smoking predicted impaired dilation to elevations in [K+]o (r2 = 0.21, coefficient: −34.5, P = 0.03), whereas no factor affected the dilation to papaverine. To confirm this result, analysis of covariance (30, 31) was used to adjust the dilation in cigarette smoking for the contribution of other factors to the impaired response. Cigarette smoking was still independently correlated with impaired dilation to K+. As shown in Fig. 3A, there was a significant reduction in K+-induced dilation in the presence of cigarette smoking compared with the absence of cigarette smoking (at 15 mM [K+]o: 25 ± 9% vs. 62 ± 11%, P < 0.05, and at 17.5 mM [K+]o: 20 ± 9% vs. 62 ± 10%, P < 0.05). The maximal dilation was decreased by 46% in vessels from subject who smoked (36 ± 8%, n = 15, vs. 67 ± 7%, n = 22, P < 0.05; Fig. 3B). These finding indicate that cigarette smoking specifically impairs the vasodilation of coronary microvessels to K+ in humans.
Fig. 3.
Influence of cigarette smoking on K+-induced vasodilation. A: the presence of tobacco use was associated with reduced vasodilation to K+ (n = 33). B: maximal dilations to K+ were also decreased in subjects who smoke cigarette (n = 18 nonsmokers and 15 smokers). C and D: the inhibitory effect of ouabain on vasodilation to K+ was substantial in HCAs from subjects without tobacco use (n = 7; C), whereas no inhibition was seen in those from subjects who smoke cigarette (n = 6; D). Values are means ± SE. *P < 0.05 vs. smoking(−) or control.
Since Na+-K+-ATPase largely contributes to vasodilation to K+ and cigarette smoking is an independent factor associated with impaired dilation, the influence of cigarette smoking on the Na+-K+-ATPase component of the dilation to K+ was determined. In HCAs from subjects who did not smoke, ouabain (10−4 M) significantly reduced the vasodilation to K+ (maximum dilation: 32 ± 12% vs. 76 ± 6% in control, P < 0.05, n = 7; Fig. 3C), whereas the vasodilation was relatively unchanged after ouabain in HCAs from subjects who smoked (maximum dilation: 23 ± 13% vs. 41 ± 14% in control, P = NS, n = 6; Fig. 3D). These results suggest that Na+-K+-ATPase activity is impaired in HCAs from subjects who smoke cigarettes.
Expression of Na+-K+-ATPase and Kir channels in HCAs.
The catalytic α1-subunit of Na+-K+-ATPase is ubiquitous and assumes a housekeeping function in all cells, and Na+-K+-ATPase isozymes formed with the α1-subunit are the most sensitive one to changes in [K+]o (4). In the present study, 10−4 M ouabain, which blocks all α-isoforms, markedly increased vascular tone in HCAs, whereas 10−6 M ouabain, which inhibits α-isoforms other than α1, induced minimal changes. The Kir2.1 subunit of Kir channels has also been reported to be responsible for K+-induced vasodilation in mouse cerebral arteries (49). Therefore, we determined the influence of cigarette smoking on the expression of α1- and Kir2.1 subunits in HCAs to assess whether altered expression of these proteins could participate in the mechanism of impaired dilation to elevations of [K+]o in subjects who smoke cigarettes. As shown in Table 3, the presence of cigarette smoking significantly reduced the expression of mRNA for the Na+-K+-ATPase α1-isoform by ∼37%, which was very compatible with the impairment in the maximal dilation to rises in [K+]o by cigarette smoking (Fig. 3B), whereas the transcript for Kir2.1 was unchanged. These findings suggest that cigarette smoking specifically downregulates the expression of the Na+-K+-ATPase α1-isoform in HCAs, implying its causal role in the impaired vasodilation to elevations of [K+]o in subjects who smoke cigarettes.
Table 3.
Influence of cigarette smoking on channel expression in the human coronary microcirculation
| Nonsmoking Group | Smoking Group | |
|---|---|---|
| No. of subjects | 8 | 9 |
| Sex (men/women) | 4/4 | 4/5 |
| Age, yr | 72 ± 10 | 62 ± 11 |
| Underlying diseases | ||
| Coronary artery disease | 8 | 9 |
| Diabetes mellitus | 2 | 3 |
| Hypertension | 5 | 5 |
| Hypercholesterolemia | 4 | 2 |
| Myocardial infarction | 2 | 3 |
| Congestive heart failure | 2 | 1 |
| Cigarette smoking | 0 | 9 |
| mRNA expression | ||
| GAPDH | ||
| Ct | 24.5 ± 2.0 | 24.0 ± 0.3 |
| Na+-K+-ATPase α1-subunit | ||
| ΔCt | −4.0 ± 2.0 | −3.4 ± 0.2* |
| ΔΔCt (2ΔΔCt) | −0.66 (≈0.63-fold) | |
| Kir2.1 subunit | ||
| ΔCt | 0.64 ± 0.21 | 0.66 ± 0.43 |
| ΔΔCt (2ΔΔCt) | −0.02 (≈0.99-fold) | |
Real-time PCR analysis of Na+-K+-ATPase α1-subunit and Kir2.1 expression in human coronary arterioles is shown.
Ct, cycle threshold. ΔCt = Ct,X − Ct,GAPDH; ΔΔCt = ΔCt, no smoking − ΔCt, smoking. 2ΔΔCt indicates the fold increase in expression compared with the nonsmoking group.
P < 0.05 vs. the nonsmoking group.
Is K+ an EDHF in the human coronary microcirculation?
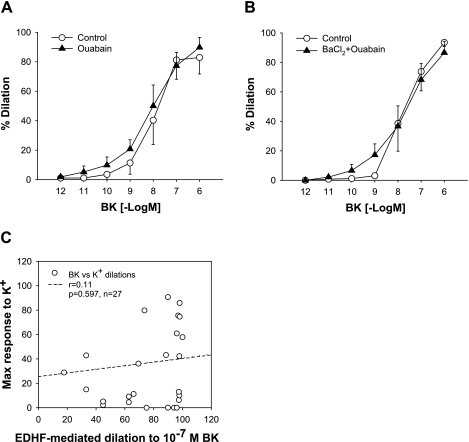
It has been reported in rat hepatic and mesenteric arteries that K+ released from ECs in response to EC-dependent vasodilators hyperpolarize the underlying VSMCs and thereby induce vasodilation through activations of Na+-K+-ATPase and/or Kir channels, supporting K+ as an EDHF (10). We (28, 29) previously reported that vasodilations of HCAs to BK and ADP are mediated by EDHF. Therefore, we tested the role of K+ in EDHF-mediated vasodilation in HCAs. We first studied whether EDHF-induced vasodilation to BK is mediated by Na+-K+-ATPase and/or Kir channels in HCAs. In the presence of l-NAME and Indo, ouabain (10−4 M) had no effect on the EDHF-mediated vasodilation to BK in HCAs [maximum dilation: 86 ± 11% vs. 90 ± 7% and −log(ED50): 8.1 ± 0.3 vs. 8.0 ± 0.2, P = NS, n = 5, respectively; Fig. 4A]. It is possible that EC-derived K+ stimulates Na+-K+-ATPase and/or Kir channels on ECs to release other vasorelaxing factors. However, vasodilation to BK in EC-intact vessels was unchanged after treatment with the combination of ouabain and BaCl2 [maximum dilation: 87 ± 8% vs. 94 ± 3% and −log(ED50): 8.0 ± 0.4 vs. 7.7 ± 0.3, P = NS, n = 5, respectively; Fig. 4B]. The EDHF-mediated dilation of HCAs to ADP was similarly unaffected by the combination [maximum dilation: 95 ± 2% vs. 97 ± 2% and −log(ED50): 6.1 ± 0.3 vs. 6.2 ± 0.1, P = NS, n = 5, respectively]. If K+ and the Na+-K+-ATPase axis involves EDHF-mediated dilations in HCAs, the efficacy of K+-induced dilation is supposed to correlate with that of EDHF-mediated dilations. As shown in Fig. 4C, the degree of maximal vasodilator responses to K+ was not correlated with the magnitude of EDHF-mediated vasodilation to a submaximal dose of BK (10−7 M, r2 = 0.01, P = 0.60, n = 27) (22, 28), and the maximal K+-induced vasodilation was significantly less than the EDHF-mediated vasodilation to BK (10−7 M, maximum dilation: 37 ± 7% vs. 75 ± 5%, P < 0.05, n = 27). The dilation to 10−8 or 10−6 M BK was also not correlated with the maximal response to K+ dilation (data not shown). No correlation between these vasodilator responses was also observed in either group of smokers or nonsmokers (data not shown). These findings suggest that K+, Na+-K+-ATPase, and/or Kir channels are unlikely to be responsible for or contribute to EDHF-mediated regulation of the human coronary microcirculation by BK and ADP.
Fig. 4.
No discernible role of Na+-K+-ATPase and inwardly rectifying K+ channels as targets for EDHF in vasodilations in the human coronary microcirculation. A and B: neither ouabain alone (10−4 M, n = 5; A) nor the combination of ouabain and BaCl2 (3 × 10−5 M, n = 5; B) had an inhibitory effect on EDHF-mediated response to bradykinin (BK; P = NS). C: there was no correlation between the magnitudes of maximal K+-induced vasodilation and EDHF-mediated vasodilation to 10−7 M BK (n = 27). Values are means ± SE.
DISCUSSION
This study is the first to describe mechanisms of vasodilation to elevations in [K+]o in the human coronary microcirculation. The major new findings are fivefold. First, in HCAs, elevations in [K+]o evoke a potent vasodilation primarily in an EC-independent manner at physiological and pathophysiological concentrations. Second, dilation to K+ is mediated largely by Na+-K+-ATPase in VSMCs with a considerable contribution of Kir channels in ECs. Third, in subjects who smoke cigarettes, vasodilation to K+ is impaired. Fourth, mRNA expression for the catalytic α1-isoform of Na+-K+-ATPase but not for the Kir2.1 subunit is suppressed in coronary microvessels from subjects who smoke cigarettes. Fifth, Na+-K+-ATPase and Kir channels play no role in the EDHF-mediated vasodilation to BK and ADP. These findings indicate that elevations of [K+]o may contribute to vasodilation of HCAs largely through the activation of Na+-K+-ATPase on VSMCs. The impaired activities of Na+-K+-ATPase may modify microvascular resistance in disease states in subjects who smoke cigarettes. However, K+ is unlikely to act as an EDHF in the human coronary arteriolar dilation to BK and ADP.
[K+]o in the heart.
Changes in interstitial [K+]o in the heart are closely linked to cardiac function and metabolism and to the regulation of the coronary circulation. Even under physiological conditions, an increase in heart rate rapidly induces a small rise of [K+]o (∼1 mM) in the myocardial interstitial space (18). During a brief period of myocardial ischemia, interstitial [K+]o increases to ∼15 mM and is associated with alterations in electrocardiographic metabolic parameters such as pH and Pco2 (18, 41). It is generally known that an increase in interstitial [K+]o in the heart causes changes in metabolism, excitability, refractoriness, and conduction, together with a shortening of the action potential, mainly due to an increase of Kir and delayed rectifying K+ conductance and, to a lesser extent, to a decrease of Na+ conductance, leading to the occurrence of reentry arrhythmias (5, 40). Importantly, the increase in interstitial [K+]o in the heart is regulated largely by myocardial uptake of K+ through Na+-K+-ATPase activity and by the coronary perfusion rate (18). These findings suggest the significant contribution of [K+]o and Na+-K+-ATPase activity to the regulations of coronary vasomotor tone and of cardiac function and metabolism under physiological and pathophysiological conditions.
Vasodilation to small elevations in [K+]o.
A number of studies (20, 21, 37) have demonstrated vasodilation to elevations in [K+]o in different vasculatures from several species. This is the first report of HCA responsiveness to elevations in [K+]o. The mechanism for K+-induced vasodilation varies with the vasculature and species studied. Knot et al. reported EC-independent vasodilation of rat coronary and cerebral arteries (20), whereas EC-dependent vasodilation was demonstrated in some vasculatures, such as rat mesenteric arteries (21). In the present study, we showed that vasodilation to K+ is primarily EC independent in HCAs.
The roles of Kir channels and Na+-K+-ATPase on the K+-induced vasodilator response also seems species and vascular bed dependent. In rat coronary and cerebral arteries, Ba2+ blocks vasodilation, but ouabain has no effect (20). In rabbit renal arteries, ouabain inhibits K+-induced dilation, but Ba2+ has no effect (37). In mice saphenous and mesenteric arteries, K+-induced vasodilations were partially reduced by either Ba2+ or ouabain and completely abolished by the combination of Ba2+ and ouabain (9), whereas K+-induced dilation was completely sensitive to Ba2+ in mice cerebral arteries (49). We showed in HCAs a novel mechanism of dilation involving an ouabain-sensitive VSMC component and a BaCl2-sensitive EC component that is only manifest in the presence of ouabain.
Kir channels and Na+-K+-ATPase in the human coronary microcirculation.
In the present study, ouabain significantly increased the basal tone of isolated HCAs, whereas BaCl2 at a dose selective for Kir channels had no effect. This is consistent with a human in vivo study (8) showing that an infusion of ouabain into brachial arteries reduced forearm blood flow, but Ba2+ (22 μM) had no effect. Other investigators (49), using an animal model, also showed that vascular tone is not altered by Ba2+ at the same doses used in our study. Genetic knockout of Kir channels elicits no change in the vascular tone of cerebral arteries in mice (49). These studies are consistent with our finding that Na+-K+-ATPase but not the Kir channel contributes to maintaining resting vascular tone in the human coronary microcirculation.
The expression and molecular characteristics of Kir channels and their functional role in the human coronary microcirculation are not fully understood. We detected mRNA for Kir2.1 in isolated HCAs. It is noteworthy that disruption of the Kir2.1 gene diminished Kir current in VSMCs from cerebral arteries of mice and abolished dilator responses to K+, whereas knockout of the Kir2.2 gene had no effect on vasodilation (49), suggesting a predominant role for Kir2.1 in Kir activity in the vasculature. However, electrophysiological examinations revealed no Kir currents in VSMCs from human coronary arteries (13), whereas Kir activity was detectable in both macro- and microvascular ECs from human coronary vessels (50). The contribution of Kir channels in ECs to K+-induced vasodilation has been reported in some animal arteries (21). Although neither BaCl2 alone nor EC denudation inhibited K+-induced vasodilation in this study, the addition of BaCl2 to ouabain further reduced the dilation in EC-intact vessels, and ouabain alone but not BaCl2 completely abolished the dilation in EC-denuded vessels. It is speculated that Na+-K+-ATPase activity in VSMCs might fully compensate for the contribution of Kir activity in ECs or that Kir channels in ECs may act as a substitute for the blocked Na+-K+-ATPase activity.
Four Na+/K+-ATPase catalytic α-isoforms have been reported, which determine the affinity of the enzyme for ouabain (4). The α1-isoform is the most prevalent one, being expressed ubiquitously in all cells. Human Na+-K+-ATPase formed with the α1-isoform is the most sensitive to change in [K+]o (4). In the present study, real-time PCR analysis detected abundant transcripts for the α1-isoform in isolated arterioles. Human umbilical vein ECs express α1- and α3-isoforms but not α2-isoforms (25). Rat mesenteric VSMCs express α1-, α2-, and α3-isoforms (45). A variety of cells express the low-affinity ouabain isoform (α1) as well as one or more of the independently regulated high-affinity isoforms (α2, α3, and/or α4) (25). In the present study, 10−4 M ouabain was used to block the activity of all α-isoforms. It is likely that the α1-isoform is the most predominant catalytic isoform in HCAs, since the higher dose of ouabain markedly increased vascular tone in HCAs and the α1-isoform is the most sensitive isoform to changes in [K+]o compared with other α-isoforms (4).
Smoking.
In the present study, we found that the dilation of HCA to rises in [K+]o is impaired only by cigarette smoking but not by other factors. The mechanism of impaired vasodilation to K+ with reduced expression of the Na+-K+-ATPase α1-isoform in subjects with cigarette smoking has not fully been determined. In a wide variety of cell systems, including VSMCs, elevations of intracellular Na+ concentration (46) and several hormones (34) increase Na+-K+-ATPase α1-isoform mRNA expression and activity to maintain a physiological intracellular Na+ concentration and the essential transmembrane Na+ gradient. Recent studies (14, 47) have proposed in VSMCs that the phosphotidyl inositol 3-kinase (PI3K)/Akt pathway is associated with the increase in the transcript and with protein translocation to the cell membrane. Importantly, chronic smoking causes impairment of Na+-K+-ATPase activity in human erythrocytes and platelets, coinciding with altered membrane properties such as membrane lipid peroxidation, an increase in the ratio of cholesterol and phospholipid, and an decrease in membrane fluidity (35, 36). An in vivo study (44) of canines subjected to chronic cigarette smoking also showed impaired Na+-K+-ATPase activity with similar alterations in the microenvironment of the VSMC membrane. More importantly, increasing evidence has indicated that smoking and cigarette smoke extract inhibit PI3K/Akt signaling in several cell types (24, 32). Thus, it is speculated that chronic cigarette smoking impairs Na+-K+-ATPase activity at least in part by reducing the number of Na+-K+-ATPase polypeptides at the cell surface as well as by decreasing the expression of its transcripts via inhibition of PI3K/Akt signaling in VSMCs.
Does K+ serve as an EDHF in the human coronary microcirculation?
We showed in HCAs that the inhibition of Na+-K+-ATPase and/or Kir channels blocked K+-induced dilation but not EDHF-mediated dilation to BK and ADP (28, 29). Other regional microcirculations in humans also exhibited no role of K+ in EDHF-mediated vasodilation (7). Some experiments have used extremely high doses of ouabain (e.g., 10−3 M) to support the role of K+ for EDHF (10). High doses of ouabain have the potential for profound effects on VSMCs, by nonspecifically blocking other mechanisms of vasodilation. In our study, ouabain, at a concentration lower than those reported, abolished vasodilation to K+ in EC-denuded arterioles, suggesting the specific role of VSMC Na+-K+-ATPase in K+-induced responses. The dose of ouabain had no effect on dilation to papaverine or aprikalim or on EDHF-mediated responses to BK and ADP, indicating 1) no detrimental effect on VSMC dilator responsiveness, 2) no discernible role of K+ as an EDHF, and 3) no role for Na+-K+-ATPase and Kir channels as targets for EDHF. In addition, we (28) have previously reported that aging is the only factor that impairs EDHF-mediated vasodilation to BK in HCAs, whereas smoking but not aging was the factor for impaired vasodilation to K+. Thus, it is unlikely that K+ plays a discernible role as an EDHF in human coronary arteriolar dilation to BK and ADP, although K+ might act as an EDHF for other endothelium-dependent vasodilators.
Potential problems.
In this study, we used BaCl2 at a dose of 3 × 10−5 M and ouabain at doses of 10−4 and 10−6 M. This dose of Ba2+ has been reported to be relatively specific to Kir channels (33). Higher doses of Ba2+ can exert nonspecific effects such as inhibition of ATP-sensitive K+ channels, Ca2+-activated K+ channels, and voltage-dependent K+ channels. The affinities of ouabain for α-isoforms also vary. The pharmacokinetics of native Na+-K+-ATPase activity is not fully understood due to the complex molecular heterogeneity that results from the expression and differential association of multiple isoforms (4). The role of each α-subunit of native Na+-K+-ATPase in the vasodilation of HCAs to K+ remains to be elucidated.
Transcripts for the Na+-K+-ATPase α1-isoform and Kir2.1 were examined in isolated HCAs. Protein expression and localization of these channels were not determined, because the small sample was insufficient for Western blot analysis. Further studies should examine the vascular cell types that express each isoform of the ATPase and channel.
We had no controls without any diseases, since all tissues were obtained as discarded specimens from patients undergoing cardiopulmonary bypass surgeries, including valve replacement and coronary artery bypass graft surgeries. Thus, we cannot comment on whether the dilation to small elevations of [K+]o in healthy subjects is different from that in diseased subjects. However, dilation may be the most important in the population we studied with ischemia (disease), where the largest levels of [K+]o elevation occurred (18, 41). We also cannot relate the degree of altered expression and dilation to the duration or frequency of tobacco use, since we did not have access to this personal information and no contact with the subject occurred. However, all subjects smoked regularly at the time before tissue acquisition.
We showed a considerable role of endothelial Kir channels in the vasodilation to elevations of [K+]o, whereas a significant contribution of endothelial Kir channels has been reported in some animal arteries (21). The cholesterol content of the cell membrane significantly affects the function of classical Kir channels, with the channels being targeted into cholesterol-rich lipid rafts without altering the channel expression (39), and this could be involved in some diseases (3, 12, 23, 26). Thus, we cannot exclude the possibility that the Kir current density has comparably been reduced in all HCAs tested.
We cannot determine the influence of treatments (prescription and diet/excise therapy) on findings in this study, since access to detailed patients' health information was not allowed. We assume that all patients were provided with treatments adequate to the underlying diseases diagnosed (Tables 1 and 2). However, it is not clear whether cigarette smokers and nonsmokers had the same treatments for the underlying diseases. It has been shown that treatments for cardiovascular diseases such as statins, β-blockers, and angiotensin-converting enzyme inhibitors ameliorate endothelial function not only in patients with the target diseases but also in chronic smokers (6, 43, 48). While we assume that all patients regardless of cigarette smoking had been treated with medications before surgery that could potentially restore microvascular function, we cannot rule out the possible influence of treatments.
Clinical implications.
Interstitial [K+]o in the myocardium rises slightly by increases in heart rate and markedly during a brief period of ischemia (18, 41), implying that [K+]o is positioned to play a role in the regulation of coronary microcirculation under both physiological and pathophysiological conditions, such as exercise and ischemia. The present study demonstrates the mechanism for coronary arteriolar dilation to small rises in [K+]o, showing a crucial role for Na+-K+-ATPase. We also demonstrated in subjects who smoke cigarettes that this mechanism of dilation is significantly impaired and that transcripts for the protein are downregulated. A genetic knockout study (16) revealed in mouse hearts that Na+-K+-ATPase α1-isoform heterozygous knockout mice are hypocontractile. Chronic cigarette smoking is also known to alter systolic and diastolic functions of right and left ventricles in healthy young subjects (11). These findings could contribute to the adverse effects of tobacco use on the coronary circulatory control, which include low exercise tolerance (1), increased O2 consumption (17), and reduced coronary flow reserve (19). More importantly, the increase in interstitial [K+]o in the heart is regulated largely by myocardial uptake of K+ through Na+-K+-ATPase activity and the coronary perfusion rate, associating with arrhythmogenesis during ischemia (18, 41). Thus, these findings could have important implications for the excess cardiovascular morbidity and mortality in subjects who smoke, since they may suffer impairment of an important mediator for regulating coronary microvascular and collateral perfusions and for buffering interstitial [K+]o levels during ischemia.
In summary, in HCAs, vasodilation to elevations in [K+]o is mediated largely by the activation of Na+-K+-ATPase in VSMCs with a considerable role of Kir channels in ECs. This vasodilation is impaired in subjects who smoke because of downregulation. EDHF-mediated dilations to BK and ADP are unlikely to be mediated by K+.
GRANTS
This work was supported by American Heart Association-Northland Affiliate Beginning Grant-In-Aid 0360035Z, an Advancing a Healthier Wisconsin grant from Medical College of Wisconsin, a Veterans Affairs Merit Review award, and National Heart, Lung, and Blood Institute Grants R01-HL-080173, HL-60592, P01-HL-68769, and P50-HL-65203.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank the Division of Cardiothoracic Surgery at the Medical College of Wisconsin, the Cardiovascular Surgery Associates of Milwaukee, the Cardiothoracic Surgery Group of Milwaukee, the Midwest Heart Surgery Institute, and the Wisconsin Heart Group for providing surgical specimens.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Abbott RD, Levy D, Kannel WB, Castelli WP, Wilson PW, Garrison RJ, Stokes J., 3rd Cardiovascular risk factors and graded treadmill exercise endurance in healthy adults: the Framingham Offspring study. Am J Cardiol 63: 342–346, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Andersson DA, Zygmunt PM, Movahed P, Andersson TL, Hogestatt ED. Effects of inhibitors of small- and intermediate-conductance calcium-activated potassium channels, inwardly-rectifying potassium channels and Na+/K+ ATPase on EDHF relaxations in the rat hepatic artery. Br J Pharmacol 129: 1490–1496, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res 73: 379–385, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 79: 917–1017, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Chalon S, Moreno H, Jr, Hoffman BB, Blaschke TF. Angiotensin-converting enzyme inhibition improves venous endothelial dysfunction in chronic smokers. Clin Pharmacol Ther 65: 295–303, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Coats P, Johnston F, MacDonald J, McMurray JJ, Hillier C. Endothelium-derived hyperpolarizing factor: identification and mechanisms of action in human subcutaneous resistance arteries. Circulation 103: 1702–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Dawes M, Sieniawska C, Delves T, Dwivedi R, Chowienczyk PJ, Ritter JM. Barium reduces resting blood flow and inhibits potassium-induced vasodilation in the human forearm. Circulation 105: 1323–1328, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Ding H, Kubes P, Triggle C. Potassium- and acetylcholine-induced vasorelaxation in mice lacking endothelial nitric oxide synthase. Br J Pharmacol 129: 1194–1200, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Eroglu E, Aydin S, Yalniz F, Kalkan AK, Bayrak F, Degertekin M. Chronic cigarette smoking affects left and right ventricular long-axis function in healthy young subjects: a Doppler myocardial imaging study. Echocardiography 26: 1019–1025, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Fang Y, Mohler ER, III, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circ Res 98: 1064–1071, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gollasch M, Ried C, Bychkov R, Luft FC, Haller H. K+ currents in human coronary artery vascular smooth muscle cells. Circ Res 78: 676–688, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Isenovic ER, Jacobs DB, Kedees MH, Sha Q, Milivojevic N, Kawakami K, Gick G, Sowers JR. Angiotensin II regulation of the Na+ pump involves the phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways in vascular smooth muscle cells. Endocrinology 145: 1151–1160, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Ishizaka H, Kuo L. Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity. Am J Physiol Heart Circ Physiol 273: H104–H112, 1997 [DOI] [PubMed] [Google Scholar]
- 16. James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB. Identification of a specific Role for the Na,K-ATPase α2 isoform as a regulator of calcium in the heart. Mol Cell 3: 555–563, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol 5: 541–552, 1985 [DOI] [PubMed] [Google Scholar]
- 18. Kleber AG. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res 52: 442–450, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Klein LW, Pichard AD, Holt J, Smith H, Gorlin R, Teichholz LE. Effects of chronic tobacco smoking on the coronary circulation. J Am Coll Cardiol 1: 421–426, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol 492: 419–430, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lacy PS, Pilkington G, Hanvesakul R, Fish HJ, Boyle JP, Thurston H. Evidence against potassium as an endothelium-derived hyperpolarizing factor in rat mesenteric small arteries. Br J Pharmacol 129: 605–611, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome P450 epoxygenases interaction between two endothelium-derived hyperpolarizing factors. Circ Res 102: 59–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marrelli SP, Johnson TD, Khorovets A, Childres WF, Bryan RM., Jr Altered function of inward rectifier potassium channels in cerebrovascular smooth muscle after ischemia/reperfusion. Stroke 29: 1469–1474, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Marti-Lliteras P, Regueiro V, Morey P, Hood DW, Saus C, Sauleda J, Agusti AG, Bengoechea JA, Garmendia J. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect Immun 77: 4232–4242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayol V, Dignat-George F, Gerbi A, Martin-Vasallo P, Lesaule G, Sampol J, Maixent JM. Evidence that human endothelial cells express different isoforms of Na,K-ATPase. J Hypertens 16: 145–150, 1998 [DOI] [PubMed] [Google Scholar]
- 26. McCarron JG, Halpern W. Impaired potassium-induced dilation in hypertensive rat cerebral arteries does not reflect altered Na+,K+-ATPase dilation. Circ Res 67: 1035–1039, 1990 [DOI] [PubMed] [Google Scholar]
- 27. Miller FJ, Jr, Dellsperger KC, Gutterman DD. Myogenic constriction of human coronary arterioles. Am J Physiol Heart Circ Physiol 273: H257–H264, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation 99: 3132–3138, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Miura H, Sato A, Saito T, Miura M, Gutterman DD. Evidence for multiple endothelium-derived hyperpolarizing factors in human coronary arterioles (Abstract). Circulation 104: II-39, 2001 [Google Scholar]
- 30. Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation 103: 1992–1998, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 92: 151–158, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Mortaz E, Lazar Z, Koenderman L, Kraneveld AD, Nijkamp FP, Folkerts G. Cigarette smoke attenuates the production of cytokines by human plasmacytoid dendritic cells and enhances the release of IL-8 in response to TLR-9 stimulation. Respir Res 10: 47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol 59: 171–191, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Oguchi A, Ikeda U, Kanbe T, Tsuruya Y, Yamamoto K, Kawakami K, Medford RM, Shimada K. Regulation of Na-K-ATPase gene expression by aldosterone in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 265: H1167–H1172, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Padmavathi P, Reddy VD, Kavitha G, Paramahamsa M, Varadacharyulu N. Chronic cigarette smoking alters erythrocyte membrane lipid composition and properties in male human volunteers. Nitric Oxide 23: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Padmavathi P, Reddy VD, Maturu P, Varadacharyulu N. Smoking-induced alterations in platelet membrane fluidity and Na+/K+-ATPase activity in chronic cigarette smokers. J Atheroscler Thromb 17: 619–627, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Prior HM, Webster N, Quinn K, Beech DJ, Yates MS. K+-induced dilation of a small renal artery: no role for inward rectifier K+ channels. Cardiovasc Res 37: 780–790, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Quignard JF, Feletou M, Thollon C, Vilaine JP, Duhault J, Vanhoutte PM. Potassium ions and endothelium-derived hyperpolarizing factor in guinea- pig carotid and porcine coronary arteries. Br J Pharmacol 127: 27–34, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romanenko VG, Fang Y, Byfield F, Travis AJ, Vandenberg CA, Rothblat GH, Levitan I. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys J 87: 3850–3861, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sagisaka K, Tamura M, Yamazaki I. The effect of K+ concentration on the energy metabolism in perfused rat heart. J Biochem 95: 1091–1103, 1984 [DOI] [PubMed] [Google Scholar]
- 41. Saito T, Miura H, Kimura Y, Watanabe H, Nakagomi A, Tamura Y, Hasegawa H, Kibira S, Miura M. Reduction of ST elevation in repeated coronary occlusion model depends on both altered metabolic response and conduction property. Int J Cardiol 92: 219–227, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Sato A, Terata K, Miura H, Toyama K, Loberiza FR, Jr, Hatoum OA, Saito T, Sakuma I, Gutterman DD. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Heart Circ Physiol 288: H1633–H1640, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Schmidt AC, Flick B, Jahn E, Bramlage P. Effects of the vasodilating beta-blocker nebivolol on smoking-induced endothelial dysfunction in young healthy volunteers. Vasc Health Risk Manag 4: 909–915, 2008 [PMC free article] [PubMed] [Google Scholar]
- 44. Tulenko TN, Rabinowitz JL, Cox RH, Santamore WP. Altered Na+-K+-ATPase, cell Na+ and lipid profiles in canine arterial wall with chronic cigarette smoking. Int J Biochem 20: 285–289, 1988 [DOI] [PubMed] [Google Scholar]
- 45. Weston AH, Richards GR, Burnham MP, Feletou M, Vanhoutte PM, Edwards G. K+-induced hyperpolarization in rat mesenteric artery: identification, localization and role of Na+/K+-ATPases. Br J Pharmacol 136: 918–926, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto K, Ikeda U, Okada K, Saito T, Kawakami K, Shimada K. Sodium ion mediated regulation of Na/K-ATPase gene expression in vascular smooth muscle cells. Cardiovasc Res 28: 957–962, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Ydemir-Koksoy A, Allen JC. Regulation of Na+ pump expression by vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 280: H1869–H1874, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Yoshida O, Kondo T, Kureishi-Bando Y, Sugiura T, Maeda K, Okumura K, Murohara T. Pitavastatin, an HMG-CoA reductase inhibitor, ameliorates endothelial function in chronic smokers. Circ J 74: 195–202, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res 87: 160–166, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Zunkler BJ, Henning B, Grafe M, Bass R, Hildebrandt AG, Fleck E. Electrophysiological properties of human coronary endothelial cells. Basic Res Cardiol 90: 435–442, 1995 [DOI] [PubMed] [Google Scholar]