Abstract
Cigarette smoking is a major independent risk factor for cardiovascular disease. While the association between chronic smoking and cardiovascular disease is well established, the underlying mechanisms are incompletely understood, partly due to the lack of adequate in vivo animal models. Here, we report a mouse model of chronic smoking-induced cardiovascular pathology. Male C57BL/6J mice were exposed to whole body mainstream cigarette smoke (CS) using a SCIREQ “InExpose” smoking system (48 min/day, 5 days/wk) for 16 or 32 wk. Age-matched, air-exposed mice served as nonsmoking controls. Blood pressure was measured, and cardiac MRI was performed. In vitro vascular ring and isolated heart experiments were performed to measure vascular reactivity and cardiac function. Blood from control and smoking mice was studied for the nitric oxide (NO) decay rate and reactive oxygen species (ROS) generation. With 32 wk of CS exposure, mice had significantly less body weight gain and markedly higher blood pressure. At 32 wk of CS exposure, ACh-induced vasorelaxation was significantly shifted to the right and downward, left ventricular mass was significantly larger along with an increased heart-to-body weight ratio, in vitro cardiac function tended to be impaired with high afterload, white blood cells had significantly higher ROS generation, and the blood NO decay rate was significantly faster. Thus, smoking led to blunted weight gain, hypertension, endothelial dysfunction, leukocyte activation with ROS generation, decreased NO bioavailability, and mild cardiac hypertrophy in mice that were not otherwise predisposed to disease. This mouse model is a useful tool to enable further elucidation of the molecular and cellular mechanisms of smoking-induced cardiovascular diseases.
Keywords: chronic cigarette smoke exposure, blood pressure, reactive oxygen species, nitric oxide, cardiovascular function
cigarette smoking is a major preventable cause of morbidity and mortality worldwide (10, 17, 52). It is estimated that >5 million people die worldwide from tobacco smoke-related illness each year (54). In the United States, 20.5% of all adults are current smokers, and smoking causes 1 in 5 deaths annually, or ∼443,000 deaths/yr (7, 52, 54). The outlook for the future is grim, as this number is expected to increase 10-fold during the 21st century (52). Since the number of smokers is continuing to increase (54), it is expected that there will be a further increase in the number of smoking-related deaths. Smoking is a major independent risk factor for cardiovascular disease (CVD), including atherosclerotic vascular disease, hypertension, myocardial infarction, unstable angina, sudden cardiac death, and stroke (1, 14, 25, 26, 36, 49, 52, 53). An epidemiological study (13) reported that >1 in 10 cardiovascular deaths, which make up 54% of all deaths worldwide, are attributable to smoking. While the deleterious effects of cigarette smoke (CS) on cardiovascular morbidity and mortality are well established, the onset and/or temporal progression of CS-induced pathological processes and manifestations of physiological disorders are poorly understood. Fundamental questions also remain regarding the underlying mechanisms by which smoking induces CVD.
Animal models have proven to be valuable tools for the investigation of CS-induced lung diseases (9, 56) and extrapulmonary manifestations in chronic obstructive pulmonary disease (COPD) (18). Chronic smoking predisposes the individual to several different clinical syndromes, and it has been reported that CS exposure can increase systemic oxidative stress (4, 11), alter nitric oxide (NO) bioavailability (59), cause endothelial dysfunction (ED) (41), and influence the levels of other major risk factors, such as blood pressure (BP) (35, 37). Importantly, even low levels of CS and secondhand smoke significantly increase the risk of cardiovascular mortality (52). Because CVD is the single largest cause of death worldwide and smoking is a preventable global problem, knowing when, to what extent, and how CS per se is involved in the pathogenesis of various CVD disorders might help in limiting smoking-induced premature deaths. Moreover, the precise mechanism of temporal progression of smoking-induced cardiovascular disorders remains largely unknown, partly due to the lack of adequate in vivo animal models. While mice are widely used in many experimental models and provide a unique potential for genetic modification, only a few studies (14, 15, 21, 27, 44) have been reported on models of smoking-induced cardiovascular disorders in mice. Furthermore, there is no prior mouse model that demonstrates a composite picture of high BP, oxidative stress, and cardiac dysfunction/ED after chronic CS exposure. The development of a well-characterized mouse model of smoking-induced CVD in a widely available background mouse strain would greatly facilitate the delineation of the mechanisms of disease pathogenesis allowing for the use of specific knockout or overexpressed genetic models.
Therefore, the present study was designed to generate and characterize an in vivo mouse model of chronic CS-induced CVD for morphological, physiological, cellular, biochemical, oxidative, and molecular end points.
METHODS
This study was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University and conformed with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996).
Mice and chronic smoking.
Male C57BL/6J mice of 8–9 wk of age (Harlan Sprague Dawley) were randomly assigned to either CS-exposed (smoking) or air-exposed (control) groups. The C57Bl/6J mouse strain, which is widely used in a number of transgenic models, has been reported to be susceptible to CS exposure-induced lung disease, body weight (BW) changes, and oxidative stress end points (18, 20, 58). Therefore, in view of its wide use in cardiovascular studies and genetic models, we chose to use the C57Bl/6J mouse strain for our experiments. While in previous reports the dose, time, type of cigarette, or exposure machine have varied from laboratory to laboratory (14, 15, 18, 20, 21, 27, 44, 58), in general, standard cigarettes from the University of Kentucky are commonly used along with exposure protocols following the Federal Trade Commission (FTC)/International Standard Organization (ISO) standard of 35-ml puffs of 2-s duration, taken once a minute (ISO 1991). Considering the available previous data from the literature, we chose mainstream whole body CS exposure with University of Kentucky 3R4F research cigarettes according to the FTC/ISO protocol aiming for a moderate CS exposure that would be expected to induce disease with chronic exposure, as is the case with human smoking-related disease. Mice were exposed to whole body mainstream CS generated from 3R4F research cigarettes (9.4 mg tar/0.726 mg nicotine, University of Kentucky) by the SCIREQ “InExpose” smoking system (SCIREQ, Montreal, QB, Canada) using the following standard parameters (ISO 1991): one 35-ml puff of 2-s duration followed by 58 s of fresh air at a rate of 6 ml/s. The CS was directed in the exposure chamber (5-liter volume) at a smoke-to-air ratio of 1:10. Our CS exposure protocol gave a concentration of 200 mg/m3 of total particulate matter (TPM) of air, and this TPM level was within the reported range previously used in mice (15, 58). Mice were exposed to CS for 48 min/day, 5 days/wk, for 16 or 32 consecutive weeks and were killed 24 h after the last CS exposure. Air-exposed mice served as controls. Carboxyhemoglobin (CO-Hb) levels in whole blood were measured spectrophotometrically using an Agilent 8453 Diode Array UV/VIS spectrophotometer (Agilent Technologies). The CO-Hb level immediately after the exposure was ∼12% in CS-exposed mice and ∼1% in air-exposed mice. The BW of each mouse was determined before the exposure protocol started and after 16 or 32 wk of exposure. The terms “smoking” and “CS exposed” are used interchangeably throughout this report.
BP measurements in conscious mice.
BP was measured in conscious animals using the tail-cuff method (CODA-2, Kent Scientific, Torrington, CT). Briefly, mice were trained for 7 days by measuring BP daily, after which BP recordings were made 3 days/wk. Each session consisted of 5 acclimatization cycles followed by 15 BP measurements cycles. On the data collection day, 2 sessions of 15 BP measurements were obtained; a set was accepted if the computer identified >50% successful readings. The average from one session was used for systolic BP (SBP), diastolic BP (DBP), and mean BP in each individual mouse.
Mouse cardiac cine MRI.
To measure in vivo left ventricular (LV) function and cardiac morphology, cine MRI was performed in mice under light isoflurane anesthesia. Cine MRI was performed on a Bruker 11.7-T 500-MHz NMR system using an external ECG-triggered fast gradient echo. Six to eight contiguous ventricular short-axis slices (1-mm thickness) were acquired to cover the entire heart for LV end-systolic volume, LV end-diastolic volume, heart rate (HR), LV mass, wall thickening, stroke volume (SV), and ejection fraction (EF). Images were converted to DICOM format and then analyzed using ARGUS image-analysis software (Siemens Medical Solutions).
Langendorff-perfused heart preparation for cardiac function.
After scheduled smoking exposures, hearts were isolated from CS-exposed mice as previously described (46) in parallel with hearts from air-exposed mice. Briefly, mice were anesthetized with pentobarbital (50 mg/kg ip), and hearts were excised, aorta cannulated, and perfused in the Langendorff mode at a constant pressure of 80 mmHg with modified Krebs-Henseleit buffer (KHB) equilibrated with 95% O2-5% CO2 at 37°C. The constituents of KHB were (in mM) 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 MgCl2, 2.5 CaCl2, 0.5 EDTA, and 16.7 glucose. A fluid-filled balloon was inserted into the LV across the mitral valve and connected to a pressure transducer permitting the continuous measurement of LV pressure (LVP). Hearts were immersed in a water-jacketed bath maintained at 37°C, and the LV balloon was filled with water to yield a LV diastolic pressure of 3–6 mmHg. Coronary flow was continuously monitored via a Doppler flow probe (T206, Transonic Systems, Ithaca, NY) placed in the aortic perfusion line. Aortic and LVP were recorded on a PowerLab/400 multichannel data-acquisition system (AD Instruments, Castle Hill, Australia) using ADI Chart software (version 4.2). The LVP signal was digitally processed to yield diastolic and systolic pressures, LV developed pressure (LVDP), HR, and the rate-pressure product (RPP = LVDP × HR). After 20 min of equilibration, a pressure-volume (P-V) curve was constructed for each heart by an incremental increase in the LV balloon volume (2 μl) at 30-s intervals until the maximal developed pressure.
Isolated mouse aortic ring preparation for vascular function.
Preparation of the isolated mouse aorta was similar to that previously described (47). After the heart was collected, the thoracic aorta was gently dissected out from the same mouse. After careful removal of fat and connective tissues, the aorta was cut transversely into two to three rings of 2–3 mm in length. The rings were mounted on a wire myograph (Multi Myograph System-610M, Danish Myo, Aarhus, Denmark) with extreme care taken not to damage the endothelium and then suspended in 5-ml organ baths containing modified KHB [containing (in mM) 118 NaCl, 24 NaHCO3, 4.6 KCl, 1.2 NaH2PO4, 1.2 CaCl2, 4.6 HEPES, and 18 glucose] and continuously gassed with 95% O2-5% CO2 (37°C, pH 7.4). Aortic rings were allowed to equilibrate for 90 min with an initial resting tension of 1 g (the length-tension relationship was determined separately), and the bathing solution was changed at 15-min intervals. Changes in isometric tension were recorded on a PowerLab/8sp multichannel data-acquisition system (AD Instruments) using ADI Chart software (version 5.3) for digital processing and data analysis.
After equilibration, the responsiveness and stability of each individual ring were checked by the successive administration of a submaximally effective concentration of l-phenylephrine hydrochloride (phenylephrine; 1 μM). The integrity of the vascular endothelium was assessed pharmacologically by ACh-induced relaxation of phenylephrine-precontracted rings. Preparations were then washed three times with drug-free buffer and allowed to relax fully for 30 min before the experimental protocol began. To determine the vasodilator responses to ACh, the aortic rings were precontracted with phenylephrine, and dose-response curves for aortic relaxation were obtained by the cumulative addition of ACh in the organ bath. The concentration of agonist in the organ bath was increased in steps of 1-log units. ACh was added to yield the next higher concentration only when the response to the lower dose reached a steady state. One dose-response curve for ACh was constructed for each ring. The vasodilator (relaxant) responses were expressed as percent decreases of phenylephrine-induced precontraction, where the contraction produced by 1 μM phenylephrine in each ring from its initial resting tension (1 g) was considered as 100%. The terms “vasodilation” and “vasorelaxation” are used interchangeably throughout this report.
Preparation of white blood cells and measurement of ROS.
Whole blood was obtained from the mice (CS or air exposed) through a cardiac puncture. Blood cells were separated from the plasma by centrifugation at 1,000 rpm for 2 min. The buffy coat was carefully aspirated from the remaining red blood cells (RBCs), and the sample was resuspended in HBSS (pH 7.4). ROS generation in white blood cells (WBCs) was determined using the redox-sensitive probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; for H2O2) or dihydroethidium (DHE; for superoxide) along with nuclear stain (Hoechst-33342 dye) as previously reported (22). H2DCF-DA can readily pass through cells and is deacetylated by cellular esterases to the nonfluorescent probe 2′,7′-dichlorohydrofluorescein (DCFH), which is then trapped inside the cell by its polar nature. DCFH is converted by intracellular oxidants, such as H2O2, to the green fluorescent molecule 2′,7′-dichlorofluorescein (DCF). Similarly, DHE enters the cell freely and is dehydrogenated to ethidium. H2DCF-DA and DHE at a concentration of 50 μM were added simultaneously with Hoechst-33342 dye (1 μM) to the cell suspensions and incubated for 30 min at 37°C. To investigate the effects of a known potent activator of leukocyte ROS generation (48), additional sets of WBCs were incubated with 50 nM 12-O-tetradecanoylphorbol 13-acetate (TPA) for 30 min. After an incubation, cells were washed three times and then mounted on a glass slide using mounting media. The fluorescence emissions for DCF and DHE were detected at excitation/emission wavelengths of 490 and 555 nm, respectively, and images were acquired by a Nikon Fluorescent microscope using a ×40 objective. Averaged individual intensities of WBCs were analyzed by Metamorph software (version 7.0).
Electrochemical measurements of the NO decay rate in blood.
Blood was collected into a heparinized tube from control and CS-exposed mice 24 h after the last exposure. Blood samples were centrifuged at 2,300 g for 10 min. The supernatant containing the plasma was removed, and the RBC and WBC pellet was washed three times with PBS [15 mM phosphate (potassium) plus 0.9% NaCl; pH 7.4]. The packed RBCs/WBCs were then resuspended in the same buffer and stored on ice for use. Measurements of NO concentration were performed in a four-port water-jacketed electrochemical chamber (NOCHM-4, WPI, Sarasota, FL) at 37°C with a carbon fiber NO electrode (ISO-NOPF200, WPI). The carbon fiber NO electrode was inserted (through the electrode port on the cap of the chamber) into the chamber. NO (1 μM) was added into the solution (through a hole on the cap of the chamber) with a Hamilton syringe by a bolus injection in the presence or absence of diluted (1:3,000) blood samples to measure the NO decay rate after hemoglobin of the RBCs was completely oxidized by previously added NO. The NO decay rate was determined in a manner similar to that previously described (29).
Data analysis.
All results are expressed as means ± SE. Data were analyzed by two-tailed Student's t-test for paired data from the same experiment and unpaired data from different experiments. Dose-response curves for multiple comparisons were analyzed by two-way repeated-measures ANOVA followed by a Bonferroni t-test using SigmaPlot software (version 11.0, Systat Software, Chicago, IL). P value of <0.05 were considered to be statistically significant.
RESULTS
Effects of CS exposure on BW and BP.
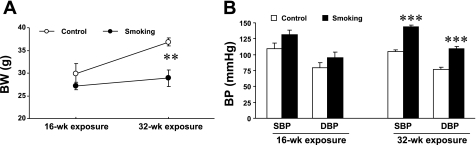
To determine the time-dependent effects of smoking on physiological parameters, BW and BP in conscious mice were recorded at 16 and 32 wk of CS exposure and compared with age-matched, air-exposed control mice. BW and BP were randomly measured in nonexposed mice (at the age of 8–9 wk) and were within the same range. BW, SBP, and DBP in nonexposed mice (n = 20) were 25 ± 0.5 g, 114 ± 1.4 mmHg, and 76 ± 2.4 mmHg, respectively. Figure 1A shows that BW increased with age in control mice; however, CS exposure blunted this increase in BW, and, at 32 wk of exposure, BW was significantly lower in CS-exposed mice compared with control mice. Interestingly, compared with control mice, BP showed a trend to increase at 16 wk of CS exposure, and it was significantly higher at 32 wk of CS exposure (Fig. 1B), with SBP of 143 ± 3.5 versus 105 ± 2.2 mmHg (P < 0.001) and DBP of 110 ± 3.5 versus 77 ± 3.1 mmHg (P < 0.001).
Fig. 1.
Effects of cigarette smoke (CS) exposure on body weight (BW) and blood pressure (BP) under normal physiological conditions. Mice were divided into air-exposed (control) and CS-exposed (smoking) groups. CS exposure for 16 wk had no significant effects, but 32 wk of CS exposure significantly blunted BW gain (A) and increased BP (B) compared with control mice. Values are means ± SE; n = 5–7 mice/group. **P < 0.01 and ***P < 0.001 vs. control.
Effects of CS exposure on in vitro vascular function.
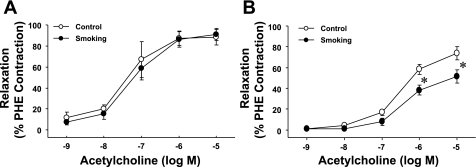
To determine the time-dependent effects of smoking on vascular endothelial function, isolated aortic rings from both control and CS-exposed mice were investigated for endothelium-dependent vasorelaxation. As shown in Fig. 2, 16 wk of CS exposure did not affect endothelium-dependent vasorelaxation induced by ACh; however, 32 wk of CS exposure resulted in a significant impairment of endothelium-dependent vasorelaxation, with rightward and downward shifts of the ACh-induced dose-response curve.
Fig. 2.
Effects of CS exposure on in vitro vascular endothelial function of the mouse aorta. CS exposure for 16 wk had no effects (A), but 32 wk of CS exposure significantly shifted ACh-induced aortic relaxation to the right and downward (B). PHE, phenylephrine. Values are means ± SE; n = 5 mice/group. *P < 0.05 vs. the respective control dose.
Effects of CS exposure on in vitro and in vivo cardiac parameters.
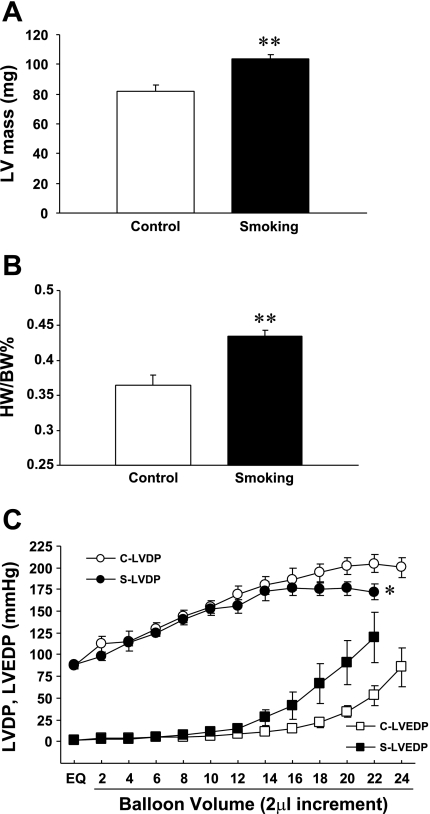
To determine if chronic CS exposure has any in vivo effect on the heart, cine MRI under light anesthesia was performed to compare cardiac morphology and LV function between 32-wk CS-exposed and control mice. Table 1 shows baseline cardiac parameters in anesthetized mice determined by MRI. The LV wall was thicker and end-diastolic volume was significantly smaller in CS-exposed mice compared with control mice. As a consequence, SV and cardiac output were markedly smaller in CS-exposed mice compared with control mice. EF, an index of cardiac contractile function, was, however, comparable between the two groups. Importantly, LV mass was significantly higher in CS-exposed mice compared with control mice (Fig. 3A).
Table 1.
MRI comparison of left ventricular volumes, thickness, and function between 32-wk control and CS-exposed anesthetized mice
| Parameters | Control Mice | CS-Exposed Mice | P Value |
|---|---|---|---|
| End-diastolic volume, mm3 | 59.4 ± 2.7 | 47.6 ± 3.2 | <0.05 |
| End-systolic volume, mm3 | 12.4 ± 1.8 | 8.3 ± 1.3 | 0.109 |
| Stroke volume, mm3 | 47 ± 1.9 | 39 ± 2.7 | 0.052 |
| Ejection fraction, % | 79 ± 2.6 | 83 ± 2.3 | 0.361 |
| Heart rate, beats/min | 365 ± 16 | 342 ± 23 | 0.413 |
| Cardiac output, ml/min | 16.8 ± 1.2 | 13.3 ± 0.85 | <0.05 |
| End-diastolic thickness, mm | 0.95 ± 0.06 | 1.13 ± 0.04 | <0.05 |
| End-systolic thickness, mm | 1.79 ± 0.08 | 2.08 ± 0.11 | 0.067 |
| Wall thickening, % | 91 ± 13 | 84 ± 8 | 0.674 |
Values are means ± SE; n = 5 mice/group. CS, cigarette smoke.
Fig. 3.
Effects of 32 wk of CS exposure on cardiac mass and contractile parameters. Left ventricular (LV) mass (A) and the heart weight-to-BW ratio (HW/BW; B) were significantly larger in CS-exposed mice compared with control mice. C: LV pressure-volume (P-V) relationship, which exhibited a trend toward LV systolic and diastolic dysfunction at high afterload in CS-exposed mice (S) compared with control mice (C). LVDP, LV developed pressure; LVEDP, LV end-diastolic pressure. Values are means ± SE; n = 5 mice/group. *P < 0.05 and **P < 0.01 vs. control.
To determine the intrinsic cardiac effects of chronic smoking, isolated hearts from both 32-wk control and CS-exposed mice were then investigated for baseline cardiac parameters and cardiac contractile performance. Table 2 shows baseline functional parameters in isolated hearts determined at the end of the 30-min equilibration period. Baseline coronary flow (normalized to the wet weight of each heart), HR, and LV contraction in isovolumetrically beating hearts were not different between control and CS-exposed mice; however, the heart weight (HW)-to-BW ratio was significantly larger in CS-exposed mice compared with control mice (Fig. 3B). Importantly, the LV P-V relationship was impaired in CS-exposed hearts at high afterload (Fig. 3C).
Table 2.
Effects of 32 wk of CS exposure on baseline cardiac parameters of isolated hearts
| Parameters | Control Group | CS-Exposed Group |
|---|---|---|
| Coronary flow, ml•min•g | 14.2 ± 0.9 | 14.2 ± 1 |
| Heart rate, beats/min | 282 ± 12 | 290 ± 14 |
| Left ventricular developed pressure, mmHg | 88 ± 5 | 89 ± 4 |
| Rate-pressure product, ×10−3 mmHg•beats•min−1 | 24.9 ± 2 | 25.7 ± 2 |
Values are means ± SE; n = 5 hearts/group.
Effects of CS exposure on systemic oxidative stress.
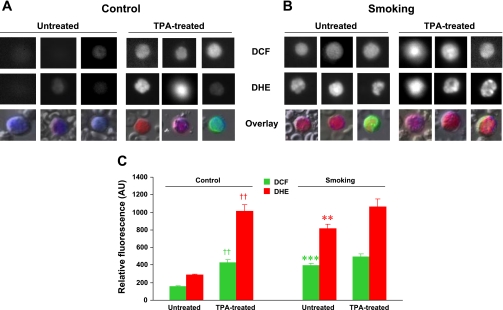
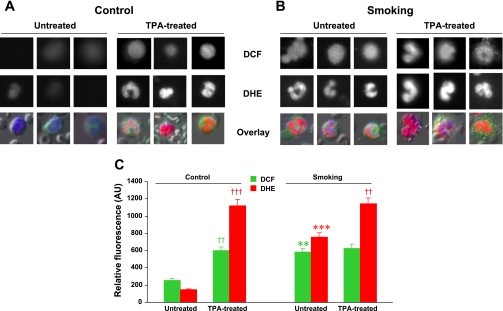
Blood cells are regularly subjected to high oxygen tension and are the first among body fluids exposed to oxidative substances either inhaled or ingested. Reactive oxygen radicals like superoxide and the hydroxyl radical are highly unstable species that initiate the oxidation of proteins, DNA, and lipids, which might cause a wide variety of cellular responses. To determine whether 32 wk of CS exposure induces leukocyte activation and oxidative stress, ROS generation was measured in WBCs of freshly collected whole blood from control and CS-exposed mice using the fluorescent probes DCF and DHE. Figures 4 and 5 show measurements of ROS in WBCs of CS-exposed mice compared with control mice. Interestingly, in CS-exposed mice, both mononuclear cells (Fig. 4) and polymorphonuclear cells (Fig. 5) produced more H2O2 and superoxide than comparable cells from control mice. Importantly, when WBCs were activated with TPA, markedly increased levels of ROS were seen in WBCs of control mice compared with nonactivated cells, and in WBCs from CS-exposed mice, ROS levels approached those seen in control TPA-treated cells. TPA treatment also further increased ROS generation in WBCs of CS-exposed mice. Thus, CS exposure by itself caused increased ROS generation in WBCs of smoking animals, but further activation was still possible.
Fig. 4.
Effects of 32 wk of CS exposure on ROS generation in mononuclear cells. A and B: representative mononuclear cells from control (A) and smoking (B) mice showing the fluorescence of 2′,7′-dichlorofluorescein (DCF) and dihydroethidium (DHE) as grayscale intensities at the top and middle rows, respectively. The bottom row shows the overlay with Hoechst (nuclei; blue), DCF (green), and DHE (red) fluorescences at the original magnification (×20). C: bar graphs of mean fluorescence intensities in untreated and 22-O-tetradecanoylporbol 13-acetate (TPA)-treated mononuclear cells from control and CS-exposed mice. In control cells, TPA induced activation with increased ROS generation (††P < 0.002, TPA-treated vs. respective untreated cells). For untreated cells, smoking induced clear activation with enhanced ROS generation (***P < 0.001 and **P < 0.002 vs. untreated cells, respectively, for DCF and DHE). For the smoking group, TPA induced only mild further increase in ROS, indicating that these cells are basally activated. AU, arbitrary units. Values are means ± SE; n = 8 samples/group.
Fig. 5.
Effects of 32 wk of CS exposure on ROS generation in polymorphonuclear cells. A and B: representative polymorphonuclear cells from control (A) and smoking (B) mice showing the fluorescence of DCF and DHE as grayscale intensities at the top and middle rows, respectively. The bottom row shows the overlay with Hoechst (nuclei; blue), DCF (green), and DHE (red) fluorescences at the original magnification (×20). C: bar graphs showing mean fluorescence intensities in untreated and TPA-treated polymorphonuclear cells. In control cells, TPA induced marked activation with increased ROS generation [††P < 0.002 (DCF) and †††P < 0.001 (DHE) for TPA-treated vs. respective untreated cells]. For untreated cells, smoking induced clear activation with enhanced ROS generation (**P < 0.002 and ***P < 0.001 vs. untreated cells, respectively, for DCF and DHE). For the smoking group, TPA induced further increases in ROS [††P < 0.002 (DHE)], indicating that these white blood cells are basally activated by CS exposure but still capable of further activation by TPA. Values are means ± SE; n = 8 samples/group.
Effects of CS exposure on NO bioavailability.
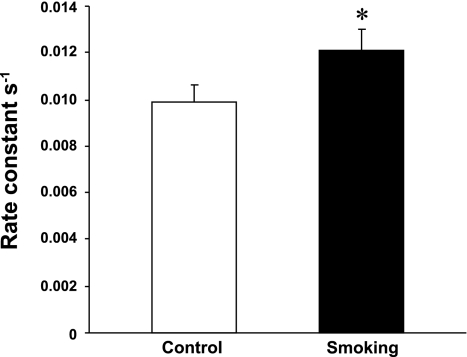
To determine whether 32 wk of CS exposure has an effect on NO bioavailability, the in vitro NO decay rate was measured in the absence or presence of RBCs/WBCs collected from control and CS-exposed mice. In these experiments, hemoglobin was previously oxidized by exogenous NO administrations to eliminate the effect of hemoglobin on NO consumption rates. As shown in Fig. 6, there was a significantly increased rate constant for the NO decay rate in RBCs/WBCs of CS-exposed mice compared with control mice. The increased NO decay was inhibited by an inhibitor of superoxide-generating enzymes, diphenyleneiodonium (30), thus indicating that the raised ROS levels may be responsible for the increased NO decay rate.
Fig. 6.
Effects of 32 wk of CS exposure on the nitric oxide (NO) decay rate in red blood cells/white blood cells of control and CS-exposed mice. NO decay rate constants in diluted (1:3,000) blood samples from mice in the CS-exposed and control groups were determined by carbon fiber electrodes. Normalized calculated values show that the rate constant of NO decay in CS-exposed mice was greater compared with control mice. Values are means ± SE; n = 8 samples/group. *P < 0.05 vs. the respective control.
DISCUSSION
The major goal of this study was to determine the time-dependent effects of CS exposure on the physiological and systemic parameters of mice in relation to CVD processes. We observed that 32 wk of CS exposure in mice resulted in 1) blunted BW gain, increased BP, and increased HW-to-BW ratio; 2) impaired vascular endothelial function; 3) mild cardiac remodeling; 4) increased leukocyte-specific ROS generation; and 5) faster NO decay rate. To our knowledge, this is the first study that demonstrates significant deleterious effects of CS exposure on the general physiology, cardiovascular hemodynamics, endothelial function, and systemic oxidative status in a mouse model leading to disease. Of note, in a pilot study (45) with earlier time points of similar 8 or 16 wk of CS exposure, no effects on BW, HW-to-BW ratio, BP, endothelial function, or cardiac contractile function of mice were observed.
Chronic smoking and BW.
It is well recognized that chronic smoking is associated with weight loss regardless of gender, culture, or socioeconomic status (50). Smokers, particularly those with COPD, often have significant weight loss (12), and almost all of the models with COPD have demonstrated a failure in weight gain for smoke-exposed animals (55). Consistent with previous findings in mice (18), we also observed that chronic CS exposure significantly blunted BW gain in CS-exposed mice compared with air-exposed mice (Fig. 1A). While the mechanisms of reduced BW gain with smoking are not completely understood, these could be different due to the amount and duration of smoking. Of note, a recent report (8) with chronic moderate CS exposure (12 wk) in mice demonstrated that CS exposure increases uncoupling protein expression, induces spontaneous hypophagia, and reduces weight gain.
Chronic smoking and BP.
Hypertension is a major risk factor for CVD, and the risk for cardiovascular events doubles with each 20-mmHg increase in SBP (∼10-mmHg DBP) (28). Several epidemiological studies including healthy subjects, hypertensive subjects, diabetic, and renal patients have clearly documented that smokers have higher BPs than nonsmokers (for a review, see Ref. 37). Importantly, the prevalence of hypertension was higher in former smokers than in never smokers (37), and the risk of hypertension was associated with the number of cigarettes smoked daily and the duration of smoking (37). Despite the strong association between smoking and BP in humans, there is a significant lack of animal studies for the cardiovascular effects of chronic CS exposure. In a rat model, CS exposure for 4 days mildly increased mean arterial BP in CS-smoked rats compared with sham control rats (24). Recently, in a mouse model, CS exposure for 6 or 16 wk has been shown to mildly increase mean arterial BP to a similar extent in both groups. Interestingly, switching to air exposure decreased BP in the 6-wk group but not in the 16-wk group (21), indicating an irreversible state in BP with long-term CS exposure.
In our study protocol, BP showed a trend to increase at 16 wk of CS exposure, and it was significantly different from that of control mice at 32 wk of CS exposure. The significantly elevated BP and reduced BW gain at 32 wk in CS-exposed mice compared with air-exposed mice indicate that a biologically active significant level of CS exposure was achieved in our model. Although the mechanism of elevated BP in our model was not investigated, it has been reported that the increase in BP with CS is mediated via direct stimulation of the sympathetic nervous system and subsequent increased plasma concentrations of norepinephrine and epinephrine (19). Of note, the main biologically active ingredients in CS that have been considered as casual constituents for CVD are nicotine, carbon monoxide, oxidant gases, and particulate matter (1, 4, 5, 11).
Chronic smoking and ED.
ED is an early event of atherosclerosis in chronic smokers as well as after acute cigarette smoking (for a review, see Ref. 41), and an important manifestation of ED is reduced endothelium-dependent vasodilation/vasorelaxation. Endothelium-dependent vasomotion is the most widely used clinical end point for the assessment of endothelial function, and it is often compared with an endothelium-independent dilator such as nitroglycerine. In vivo and in vitro studies have demonstrated that smoking impairs endothelium-dependent vascular responses in virtually every vascular bed (for a review, see Ref. 41); however, there is limited information regarding the temporal onset of vascular ED and its relationship with CS-induced alterations in physiological and biological parameters (41). Using a chronic CS exposure protocol in otherwise healthy mice, we observe that 16 wk of CS exposure did not, but 32 wk of CS exposure did, significantly impair endothelium-dependent vasorelaxation (Fig. 2). The CS exposure-related vascular ED support a causative role of smoking in hypertension and atherogenesis. Importantly, the impaired aortic endothelial function was concurrent with elevated BP (Fig. 1). ED with accompanying risk factors, such as high BP, may be a critical pathological state preceding the development of overt atherosclerosis with long-term or higher-level exposure. Of note, 8 wk of CS exposure decreased endothelium-dependent aortic relaxation in hypercholesterolemic rabbits (33). The precise mechanisms of CS-induced ED are not fully understood, but several potential mechanisms are likely to be involved. Oxidative damage seems to constitute the primary mechanism because CS contains a large number of oxidants and introduces ROS in the circulatory system (4, 11, 39). ROS generated by long-term smoking has been reported to directly damage endothelial cells and inactivate NO (11, 39). Decreased NO release, dysfunctional NO synthase, and increased consumption of NO have been reported with ED in healthy smokers (2, 11, 23, 39).
Chronic smoking and the heart.
In addition to the well-established effects of smoking on BP and endothelial function (2, 19, 23, 37, 39, 41), smoking also can impair cardiac performance independent of coronary atherosclerosis. A previous study (32) showed that smoking compromises cardiac function and leads to cardiac remodeling. Greater LV mass was reported in chronic smokers independent of any changes in their BP and LV fractional shortening (16), and a recent report (38) showed a positive association of smoking with exercise-induced LV growth in young men. Cardiac hypertrophy was determined by the crude HW-to-BW ratio, and this was complemented by in vivo cardiac MRI. We observed that 32 wk of CS exposure caused an increase in the LV wall thickness both at diastole and systole with a resultant decrease in SV and cardiac output (Table 1); however, global in vivo cardiac contractile function (EF) was unaffected. Of note, LV mass and the HW-to-BW ratio at 32 wk were significantly larger in smoking mice compared with control mice (Fig. 3), and this was concurrent with elevated BP (Fig. 1). Thus, it is possible that the adaptive cardiac hypertrophic changes might have contributed to the maintenance of cardiac contractile function under normal physiological conditions. In Wistar rats (6), CS exposure has been shown to increase BP and ventricular weight without altering BW. Moreover, in spontaneously hypertensive rats (SHRs), smoking significantly enhanced the progression of cardiac hypertrophy with an increased HW-to-BW ratio compared with sham SHRs (31). We did not observe any adverse effect on the in vitro baseline cardiac contractile function at the organ level (Table 2); however, the LV P-V relationship demonstrated a trend toward LV systolic and diastolic dysfunction at high afterload in isolated hearts of CS-exposed mice compared with control mice (Fig. 3C). This impairment in the LV P-V relationship suggests that under stress conditions, CS-exposed animals would be at a greater risk of cardiac contractile dysfunction. Importantly, in the Multi-Ethnic Study of Atherosclerosis, MRI demonstrated regional myocardial dysfunction in smokers compared with nonsmokers, despite the absence of clinical manifestation of disease (42).
Since hypertension and smoking are main risk factors for CVD, either of these individual risk factors can cause cardiac hypertrophy, and they may accelerate the progression of hypertrophy into overt cardiac remodeling when present together. Indeed, CS-induced cardiac remodeling has been reported in rats with potential mechanisms including neurohumoral activation, oxidative stress, and MAPK activation (32). Although the mechanisms underlying the clear association of CS exposure with cardiac hypertrophy and remodeling were not investigated in mice, the increased BP in our study provides strong evidence that exposure to CS results in the stimulation of intracellular signaling pathways of cardiac hypertrophy and remodeling.
Chronic smoking and systemic oxidative stress.
The association between CS and oxidative stress is widely recognized, and there is a general consensus that CS-induced increased oxidative stress plays a major role in the pathogenesis of different cardiovascular disorders (1, 4, 11). In the setting of cigarette smoking, free radicals could arise from the CS itself; circulating or in situ activated neutrophils, monocytes, and macrophages; and endogenous sources of ROS, such as uncoupled endothelial NO synthase, xanthine oxidase, and the mitochondrial electron transport chain (4, 11, 39, 57, 60). Blood is the first body fluid exposed to substances either inhaled or ingested, and reactive oxidants contained in CS may readily traverse into the blood and modify the function of blood cells.
We observed that chronic CS exposure increased ROS generation in both mononuclear and polymorphonuclear leukocytes compared with control mice (Figs. 4 and 5), and these findings are in agreement with increased systemic oxidative stress reported in humans (3, 40). CS has been reported to cause marked activation of leukocytes and platelets and contributes to oxidative vascular damage in smokers (1, 11). Interestingly, WBCs of the CS-exposed group exhibited markedly enhanced ROS generation with levels much above those of untreated WBCs of control mice, approaching the levels seen when control cells were treated with TPA. Thus, these data indicate that CS exposure alone caused increased oxidative stress in CS-exposed animals. While high-level ROS formation was seen in WBCs of CS-exposed mice, a further increase was seen after TPA treatment, indicating that further activation was possible. Importantly, along with ROS formation, myeloperoxidase release by activated neutrophils has been reported to contribute to CS-dependent vascular inflammation and atherosclerosis by reducing endothelial NO bioavailability (43). Lipid peroxidation and protein nitration by increased ROS/reactive nitrogen species might play a role in the inflammatory response. While the causes of CS-induced systemic oxidative stress and inflammation are multifaceted, our results provide clear evidence that free radicals and secondary oxidants are released endogenously from activated leukocytes of chronically CS-exposed mice, and this, in turn, can induce vascular dysfunction, leading to hypertension and atherogenesis (1, 3, 4, 11, 39, 40, 43).
Chronic smoking and NO bioavailability.
Several clinical and animal studies have clearly documented the critical role of NO in smoking-induced ED (for reviews, see Refs. 34, 38, and 41), and altered NO function with CS exposure may result from a break or alteration in the cascade of NO metabolism (2, 21, 23, 59). We observed that the NO decay rate was significantly accelerated with a shortened half-life in RBCs/WBCs of CS-exposed mice compared with RBCs/WBCs of control mice (Fig. 6). Of note, the increased NO decay was inhibited by the flavoprotein inhibitor diphenyleneiodonium, which is well known to inhibit leukocyte NADPH oxidase (30), suggesting that the increased NO decay rate is caused by the raised superoxide generation from leukocytes. We also observed increased oxidative stress in the blood of CS-exposed mice (Figs. 4 and 5). If ROS levels are raised, NO would be trapped by superoxide in the vascular wall to form the potent oxidant peroxynitrite (51) there, and this would decrease NO bioavailability, contributing to ED.
Perspectives and study limitations.
Our study was designed to investigate the effects of chronic CS exposure in a mouse model and provide substantial data regarding the pathogenic processes in this model. In particular, we sought to demonstrate whether the CS exposure-dependent alterations in physiological and cardiovascular parameters are concurrent with systemic oxidative stress. Although our findings provide convincing insights into a versatile mouse model of chronic smoking, the exposure time was moderate, and by design only one dose of low-level CS exposure was tested. Further studies with longer periods and different levels of CS exposure would be useful in further determining the relationship between duration and dose of exposure in the process of chronic cigarette smoking-induced CVD. Along with the future use of genetically altered mice, this mouse model of smoking-induced CVD should be a useful tool to determine the mechanisms of smoking-induced disease and approaches for early detection and prevention.
In conclusion, the present in vivo mouse model provides convincing evidence that chronic CS exposure induces significant alterations in vascular and cardiac function, structure, and physiology with cellular oxidative stress and decreased NO bioavailability. Further molecular and cellular studies in this model should enable the determination of important mechanistic insights in CS-induced diseases.
GRANTS
This work was supported by a gift from Lorillard Tobacco Company and by National Heart, Lung, and Blood Institute Grants HL-63744, HL-65608, HL-38324, EB-0890, and EB-4900.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Shouvik D. Mahamud for help with BP measurements, Craig Hemann for assistance with CO-hemoglobin assays, and Manzoor Wani for organizational support.
REFERENCES
- 1. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43: 1731–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation 104: 1905–1910, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bergmann S, Siekmeier R, Mix C, Jaross W. Even moderate cigarette smoking influences the pattern of circulating monocytes and the concentration of sICAM-1. Respir Physiol 114: 269–275, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Bernhard D, Wang XL. Smoking, oxidative stress and cardiovascular diseases–do anti-oxidative therapies fail? Curr Med Chem 14: 1703–1712, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens 3: 332–350, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Castardeli E, Duarte DR, Minicucci MF, Azevedo PS, Matsubara BB, Matsubara LS, Campana AO, Paiva SA, Zornoff LA. Exposure time and ventricular remodeling induced by tobacco smoke exposure in rats. Med Sci Monit 14: BR62–BR66, 2008 [PubMed] [Google Scholar]
- 7. Center for Disease Control and Prevention Smoking-attributable mortality, years of potential life lost, and productivity lossess-United States, 2000–2004. Morb Mortal Wkly Rep 57: 1226–1228, 2005 [PubMed] [Google Scholar]
- 8. Chen H, Hansen MJ, Jones JE, Vlahos R, Anderson GP, Morris MJ. Long-term cigarette smoke exposure increases uncoupling protein expression but reduces energy intake. Brain Res 1228: 81–88, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 294: L612–L631, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Cokkinides V, Bandi P, McMahon C, Jemal A, Glynn T, Ward E. Tobacco control in the United States–recent progress and opportunities. CA Cancer J Clin 59: 352–365, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci 14: 3128–3144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Decramer M, De Benedetto F, Del Ponte A, Marinari S. Systemic effects of COPD. Respir Med 99, Suppl B: S3–S10, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation 112: 489–497, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Gairola CG, Drawdy ML, Block AE, Daugherty A. Sidestream cigarette smoke accelerates atherogenesis in apolipoprotein E−/− mice. Atherosclerosis 156: 49–55, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Gandley RE, Jeyabalan A, Desai K, McGonigal S, Rohland J, DeLoia JA. Cigarette exposure induces changes in maternal vascular function in a pregnant mouse model. Am J Physiol Regul Integr Comp Physiol 298: R1249–R1256, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gidding SS, Xie X, Liu K, Manolio T, Flack JM, Gardin JM. Cardiac function in smokers and nonsmokers: the CARDIA study. The Coronary Artery Risk Development in Young Adults study. J Am Coll Cardiol 26: 211–216, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Giovino GA. The tobacco epidemic in the United States. Am J Prev Med 33, Suppl 6: S318–S326, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gosker HR, Langen RC, Bracke KR, Joos GF, Brusselle GG, Steele C, Ward KA, Wouters EF, Schols AM. Extrapulmonary manifestations of chronic obstructive pulmonary disease in a mouse model of chronic cigarette smoke exposure. Am J Respir Cell Mol Biol 40: 710–716, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, Del Bo A, Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 90: 248–253, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 170: 974–980, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Guo X, Oldham MJ, Kleinman MT, Phalen RF, Kassab GS. Effect of cigarette smoking on nitric oxide, structural, and mechanical properties of mouse arteries. Am J Physiol Heart Circ Physiol 291: H2354–H2361, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Han Z, Varadharaj S, Giedt RJ, Zweier JL, Szeto HH, Alevriadou BR. Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells. J Pharmacol Exp Ther 329: 94–101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Münzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 86: E36–E41, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Houdi AA, Dowell RT, Diana JN. Cardiovascular responses to cigarette smoke exposure in restrained conscious rats. J Pharmacol Exp Ther 275: 646–653, 1995 [PubMed] [Google Scholar]
- 25. Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) study. JAMA 279: 119–124, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Khalili P, Nilsson PM, Nilsson JA, Berglund G. Smoking as a modifier of the systolic blood pressure-induced risk of cardiovascular events and mortality: a population-based prospective study of middle-aged men. J Hypertens 20: 1759–1764, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kunitomo M, Yamaguchi Y, Kagota S, Yoshikawa N, Nakamura K, Shinozuka K. Biochemical evidence of atherosclerosis progression mediated by increased oxidative stress in apolipoprotein E-deficient spontaneously hyperlipidemic mice exposed to chronic cigarette smoke. J Pharmacol Sci 110: 354–361, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Liu X, Liu Q, Gupta E, Zorko N, Brownlee E, Zweier JL. Quantitative measurements of NO reaction kinetics with a Clark-type electrode. Nitric Oxide 13: 68–77, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Liu X, El-Sherbiny GA, Collard E, Huang X, Follmer D, El-Mahdy M, Zweier JL. Application of carbon fiber composite minielectrodes for measurement of kinetic constants of nitric oxide decay in solution. Nitric Oxide 23: 311–318, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meurrens K, Ruf S, Ross G, Schleef R, von Holt K, Schlüter KD. Smoking accelerates the progression of hypertension-induced myocardial hypertrophy to heart failure in spontaneously hypertensive rats. Cardiovasc Res 76: 311–322, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Minicucci MF, Azevedo PS, Paiva SA, Zornoff LA. Cardiovascular remodeling induced by passive smoking. Inflamm Allergy Drugs Targets 8: 334–339, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Mudaliar JH, Freischlag JA, Johnson D, Coe DA, Kelly H, Hanson L, Cambria RA, Seabrook GR, Towne JB. Combined exposure to cigarette smoke and hypercholesterolemia decreases vasorelaxation of the aorta. J Vasc Surg 25: 884–889, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Münzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med 40: 180–196, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Nakamura K, Barzi F, Lam TH, Huxley R, Feigin VL, Ueshima H, Woo J, Gu D, Ohkubo T, Lawes CM, Suh I, Woodward M, Asia Pacific Cohort Studies Collaboration Cigarette smoking, systolic blood pressure, and cardiovascular diseases in the Asia-Pacific region. Stroke 39: 1694–1702, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 96: 3243–3247, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Orth SR. Effects of smoking on systemic and intrarenal hemodynamics: influence on renal function. J Am Soc Nephrol 15, Suppl 1: S58–S63, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Payne JR, Eleftheriou KI, James LE, Hawe E, Mann J, Stronge A, Kotwinski P, World M, Humphries SE, Pennell DJ, Montgomery HE. Left ventricular growth response to exercise and cigarette smoking: data from LARGE Heart. Heart 92: 1784–1788, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Powell JT. Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med 3: 21–28, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol 5: 276–292, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Rosen BD, Saad MF, Shea S, Nasir K, Edvardsen T, Burke G, Jerosch-Herold M, Arnett DK, Lai S, Bluemke DA, Lima JA. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol 47: 1150–1158, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Rudolph TK, Rudolph V, Baldus S. Contribution of myeloperoxidase to smoking-dependent vascular inflammation. Proc Am Thorac Soc 5: 820–823, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Schroeter MR, Sawalich M, Humboldt T, Leifheit M, Meurrens K, Berges A, Xu H, Lebrun S, Wallerath T, Konstantinides S, Schleef R, Schaefer K. Cigarette smoke exposure promotes arterial thrombosis and vessel remodeling after vascular injury in apolipoprotein E-deficient mice. J Vasc Res 45: 480–492, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Talukder MA, Johnson W, Varadharaj S, Lian J, Kearns P, Druhan L, Liu X, Zweier J. Chronic cigarette smoke exposure causes hypertension, leukocyte-specific reactive oxygen species (ROS) generation, endothelial dysfunction, and cardiac hypertrophy in mice (Abstract). FASEB J 23: 1017.–39., 2009 [Google Scholar]
- 46. Talukder MA, Kalyanasundaram A, Zhao X, Zuo L, Bhupathy P, Babu GJ, Cardounel AJ, Periasamy M, Zweier JL. Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. Am J Physiol Heart Circ Physiol 293: H2418–H2428, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Talukder MA, Morrison RR, Mustafa SJ. Comparison of the vascular effects of adenosine in isolated mouse heart and aorta. Am J Physiol Heart Circ Physiol 282: H49–H57, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Tasat DR, Mancuso R, O'Connor S, Molinari B. Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging Cell 2: 159–164, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L, Zhang X, Yusuf S, INTERHEART Study Investigators Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet 368: 647–658, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Wack JT, Rodin J. Smoking and its effects on body weight and the systems of caloric regulation. Am J Clin Nutr 35: 366–380, 1982 [DOI] [PubMed] [Google Scholar]
- 51. Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. J Biol Chem 271: 29223–29230, 1996 [DOI] [PubMed] [Google Scholar]
- 52. White WB. Smoking-related morbidity and mortality in the cardiovascular setting. Prev Cardiol 10, Suppl 2: 1–4, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke. The Framingham study. JAMA 259: 1025–1029, 1988 [PubMed] [Google Scholar]
- 54. World Health Organization WHO Report on the Global Tobacco Epidemic, 2009: Implementing Smoke-Free Environments (online). www.who.int/tobacco/mpower [10 November 2010]
- 55. Wright JL, Churg A. Animal models of cigarette smoke-induced COPD. Chest 122, Suppl 6: 301S–306S, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 295: L1–L15, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273: 25804–25808, 1998 [DOI] [PubMed] [Google Scholar]
- 58. Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Zhang WZ, Venardos K, Chin-Dusting J, Kaye DM. Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertension 48: 278–285, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Zweier JL, Talukder MAH. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res 70: 181–190, 2006 [DOI] [PubMed] [Google Scholar]