Abstract
Telmisartan, an angiotensin receptor blocker, may have unique benefits as it possesses partial peroxisome proliferator-activated receptor (PPAR)-γ agonist activity in addition to antihypertensive effects. In this study, we test whether treatment with telmisartan ameliorates cardiovascular abnormalities to a greater extent than olmesartan, which has little PPAR-γ activity. The hypertensive rodent model of tissue renin-angiotensin system activation, transgenic (mRen2)27 (Ren2) rats and their littermate Sprague-Dawley controls were used. Rats were treated with telmisartan (2 mg·kg−1·day−1), olmesartan (2.5 mg·kg−1·day−1), or vehicle via drinking water for 3 wk; these doses achieved similar blood pressure control, as measured by telemetry. Ren2 rats displayed impaired diastolic and systolic function using left ventricular (LV) pressure-volume (P-V) analysis. Load-independent diastolic indexes, including the time constant of isovolumic relaxation and the slope of the end-diastolic P-V relationship, as well as systolic indexes, including preload recruitable stroke work, the dP/dtmax-end-diastolic volume (EDV) relationship, and the P-V area-EDV relationship, were elevated in Ren2 rats compared with Sprague-Dawley controls (P < 0.05). The Ren2 myocardium exhibited parallel increases in the oxidant markers NADPH oxidase and 3-nitrotyrosine. The increase in the prohypertrophic protein Jak2 in Ren2 rats was associated with cardiac structural abnormalities using light microscopic and ultrastructural analysis, which included interstitial fibrosis, cardiomyocyte and LV hypertrophy, and mitochondrial derangements. Both angiotensin receptor blockers attenuate these abnormalities to a similar extent. Our data suggest that the beneficial effect of telmisartan and olmesartan on cardiac structure and function may be predominantly pressor-related or angiotensin type 1 receptor dependent in this model of renin-angiotensin system activation.
Keywords: oxidative stress, left ventricular hypertrophy, peroxisome proliferator-activated receptor-γ, Janus-activated kinase 2, AMP-activated protein kinase
numerous reports have demonstrated a seminal role for ANG II and the associated generation of ROS via NADPH oxidase in the development of myocardial remodeling and that angiotensin type 1 receptor (AT1R) blockade improves myocardial remodeling, hypertrophy, and ventricular compliance (4, 5, 31). A molecular modeling study (4) demonstrated a structural similarity between the AT1R blocker telmisartan and the peroxisome proliferator-activated receptor (PPAR)-γ agonists pioglitazone and rosiglitazone. Telmisartan, to a greater extent than other angiotensin receptor blockers (ARB), acts as a partial agonist of PPAR-γ independently of AT1R-blocking effects (4, 27). PPAR-γ agonism improves insulin sensitivity and inhibits vascular oxidative stress and inflammation (11). In this regard, telmisartan reduces glucose, insulin, and triglyceride levels in a rat model of high-fat diet-induced insulin resistance (4).
Some evidence suggests that PPAR-γ agonists improve myocardial contractile dysfunction after ischemia-reperfusion injury and inhibit the ventricular hypertrophic response to pressure overload (1). Moreover, PPAR-γ agonists promote glucose uptake in cardiac muscle through activation of AMP-activated protein kinase (AMPK) signaling (33). Hypothetically, telmisartan could provide additional protection against myocardial hypertrophy and dysfunction. This additional protection may occur through activation of AMPK to increase myocardial perfusion and substrate utilization as well as attenuate cardiomyocyte hypertrophy (24, 30). This beneficial metabolic signaling effect could be additive to attenuation of the conventional AT1R-mediated inflammatory signaling (e.g., via AT1R/NADPH oxidase/ROS or AT1R/Jak2 pathways).
We have previously reported that AT1R blockade attenuates myocardial NADPH oxidase-induced oxidative stress in conjunction with improvements in myocardial remodeling and hypertrophy and insulin metabolic signaling in the transgenic (mRen2)27 (Ren2) rat, a rodent model of tissue renin-angiotensin system (RAS) activation that manifests hypertension (19), dyslipidemia (17), and insulin resistance (31). Here, we extend our previous studies to test the hypothesis that in vivo treatment with telmisartan, a dual AT1R antagonist-PPAR-γ agonist, ameliorates myocardial oxidative stress and inflammation as well as cardiac dysfunction and hypertrophy to a greater extent than the AT1R antagonist olmesartan, which has little PPAR-γ activity.
MATERIALS AND METHODS
Animal Care
All animal procedures were approved in advance by the Harry S. Truman Veterans Memorial Hospital Subcommittee for Animal Safety as well as by the Institutional Animal Care and Use Committee of the University of Missouri. Animals were cared for in accordance with National Institutes of Health guidelines. Five-week-old male heterozygous transgenic Ren2 (n = 34) and Sprague-Dawley littermate (n = 30) rats were housed under standard laboratory conditions, where the room temperature was 21–22°C and light and dark cycles were 12 h each. Rats were randomly distributed into the following groups: control Sprague-Dawley group (SDC; n = 11), telmisartan-treated Sprague-Dawley group (SDT; n = 9), olmesartan-treated Sprague-Dawley group (SDO; n = 10), control Ren2 group (R2C; n = 8), telmisartan-treated Ren2 group (R2T; n = 15), and olmesartan-treated Ren2 group (R2O; n = 11). Beginning at 7 wk of age, rats received daily telmisartan (2 mg/kg), olmesartan (2.5 mg/kg), or vehicle solution in drinking water for a total of 21 days. These doses were based on preliminary telemetric experiments in Ren2 rats that established that these doses of telmisartan and olmesartan resulted in similar blood pressure reductions for the purpose of minimizing differences of blood pressure-dependent effects between the two ARBs.
Blood Pressure Monitoring Via Telemetry
Systolic blood pressures (SBPs) were obtained on a subset of rats, including five SDC, four SDT, five SDO, five R2C, seven R2T, and six R2O rats. At 6 wk of age, rats were anesthetized and instrumented with a radio telemetric transmitter (TA11PA-C40, Data Sciences, St. Paul, MN) for blood pressure monitoring. Rats recovered a minimum of 7 days before data collection. Recordings of SBP were collected at 0, 1, 2, and 3 wk posttreatment initiation. For each week, data were recorded continuously for 5 min at 15-min intervals (sampling rate: 1,000 Hz) for three light and three dark 12-h cycles.
Cardiac Catheterization and Cardiac Function Testing
After treatment, rats were anesthetized with isoflurane and ventilated with gas containing 1.75% isoflurane and 40% oxygen with the aid of a positive pressure ventilator set at 54–56 breaths/min. A 1.9-Fr Scisense rat pressure-volume (P-V) catheter (FTS-1912B-8018) was inserted into the right carotid artery and passed retrograde through the aortic valves into the left ventricle (LV) to monitor pressure and volume as well as admittance magnitude and phase angle signals in the LV. Data were acquired using a laptop computer and Labscribe 2.2 software, which applies a variety of algorithms to calculate as many as 30 hemodynamic parameters (see below). Signals were monitored continuously at a sampling rate of 1,000 Hz using an Advantage PV system (model FY897B, Scisense). The steps involved in the setup and use of the Advantage system, i.e., catheter calibration and measures of myocardial conductivity and permittivity, were performed and have been described in detail elsewhere (7). Before experiments, independent estimates of LV stroke volume (SV) were obtained using cine-MRI on 9- to 10-wk-old male Sprague-Dawley and Ren2 rats.
Baseline hemodynamics.
Initially, LV baseline hemodynamic variables, including heart rate (HR), end-systolic pressure (ESP), end-diastolic pressure (EDP), the maximal slope of the systolic pressure increment (dP/dtmax), the diastolic pressure decrement (dP/dtmin), maximum volume, minimum volume, end-systolic volume, end-diastolic volume (EDV), SV, cardiac output (CO), ejection fraction (EF), stroke work (SW), total peripheral resistance, arterial elastance (Ea), and P-V area were recorded in real time using Labscribe 2.215 software (iWorx Systems, Dover, NH). During this time period, the time constant of isovolumic relaxation (τ), which is a load-independent index of the active phase of early diastolic relaxation, was calculated by the method of Glantz (25). All hemodynamic data were collected with the ventilator off and while rats were apneic.
Assessment of diastolic function.
Diastolic dysfunction may involve abnormalities in active relaxation related to Ca2+ handling (see the description of τ above) and/or passive properties that alter the compliance of the ventricular wall (e.g., fibrosis). After the initial isovolumic or active phase of ventricular relaxation, the mitral valve opens, and blood from the left atrium begins to flow into the LV. The duration of this phase of diastole, the volume of blood residing in the LV at the end of diastole, and the EDP depend on the passive properties of the ventricle wall, i.e., those that affect chamber compliance or “stiffness.” After preload occlusion, Labscribe software was used to generate the EDP-volume relationship (EDPVR), which describes the passive filling phase of diastole. The slope of the curve provides an index of ventricular stiffness (6).
Assessment of systolic function.
P-V loops were generated during occlusion of the inferior vena cava to vary preload. Labscribe calculates several indexes of LV systolic function that are relatively insensitive to loading conditions, cardiac mass, and HR. Thus, unlike load-dependent systolic indexes (e.g., EF, CO, or dP/dtmax), differences in load-independent indexes reflect real differences in ventricular contractility rather than differences due to altered loading conditions [such as would occur in the hypertensive Ren2 rat with LV hypertrophy (LVH) and increased HR]. The first is the ESP-volume relation (ESPVR), which describes maximal LV pressure as a function of LV volume (using a quadratic equation). The slope of ESPVR represents the end-systolic elastance (Ees), and because Ees is relatively load independent, it is widely considered to be a superior index of myocardial contractility than load-sensitive parameters (8). Next is preload recruitable SW (PRSW), which is the slope of the relationship between SW and EDV. PRSW is similar to the Frank-Starling cardiac function curve except that EDP is replaced with EDV, yielding a highly linear relation (14). Another frequently reported systolic index is the slope of relationship between dP/dtmax and EDV (20). Unlike the other load-independent indexes, dP/dtmax-EDV accounts for the speed of contraction. Finally, P-V area, i.e., the area between ESPVR and EDPVR, measured as a function of EDV, is also used as an index of overall pump function (18). This index, like PRSW, integrates both systolic and diastolic properties and indicates the total mechanical work of the LV as a function of EDV.
Quantitation of Jak2 Protein
SDS-PAGE was performed on cytosolic fractions of LV homogenates. Samples (40 μg/lane) were separated and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories). Blots were incubated 3 h at 4°C with primary antibodies against Jak2 and phospho-Jak2 (Tyr1007/1008, 1:1,000 dilution; Cell Signaling Technology). After a rinse, blots were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5,000 dilution of each antibody) for 1 h at room temperature. Binding of the antibodies was detected by chemiluminescence, and images were recorded using a Bio-Rad ChemiDoc XRS image-analysis system. Quantitation of phosphorylated protein band density, normalized to the density of total protein for each sample, was performed using Quantity One software (Bio-Rad). Data are reported as the normalized protein band density in arbitrary units.
Measurement of Myocardial Tissue Oxidative Stress
NADPH oxidase activity in myocardial plasma membrane extracts.
As previously described (31), NADPH activity was determined in aliquots of plasma membrane fractions by measuring the conversion of Radical Detector (Cayman Chemical) in the absence and presence of the NADPH inhibitor diphenylene iodonium sulfate (500 μM) using spectrophotometric (450 nm) techniques. Initially data were calculated in millioptical density units per minute per milligram of protein. Because data were collected from several cohorts of rats and baseline NADPH oxidase activity in SDC rats varied between the cohorts, all data are expressed as percentages of the SDC value within each cohort.
Lucigenin-enhanced chemiluminescence.
Superoxide anions were measured via the lucigenin-enhanced chemiluminescence method as previously described (9). Briefly, the assay was performed in duplicate on homogenates of fresh LV samples (∼200 mg wet wt each). Superoxide production was calculated as counts [in relative light units (RLU)] per second per milligram of fresh tissue protein for each sample after subtraction of the background activity. Like the NADPH oxidase activity assay, all ROS data are expressed as percentages of the SDC value within a daily cohort or rats.
3-Nitrotyrosine.
To assess the level of oxidative damage to proteins, samples of the LV were harvested, fixed, embedded in paraplast, sectioned, and evaluated for 3-nitrotyrosine residue using immunofluorescence microscopy as previously described (15). Grayscale intensity was measured within a fixed sized region of interest rectangle as previously described.
Histomorphometric Analysis
Light microscopic analysis for myocardial interstitial fibrosis and cardiomyocyte hypertrophy.
Paraffin sections (5 μm thick) were mounted on glass slides and stained with Verhoeff van Gieson stain, which stains collagen fibers pink, to evaluate interstitial fibrosis, as previously described (31). The relative amount of collagen within 10 representative region of interest rectangles was determined with the aid of MetaVue software (Boyce Scientific, Gray Summit, MO), and an average value was recorded for each LV sample and expressed in arbitrary units. Samples from five rats from each of the six treatment groups were analyzed. To evaluate cardiomyoctye hypertrophy, digital images were captured on cardiomyocytes in cross section, and the cross-sectional area was determined with the aid of image-analysis software. To quantify cardiomyocyte size, ∼20 cells from 2 separate sites were measured per sample, and only cardiomyocytes with a well-defined cellular membrane and visible nucleus were measured. The average size of all measured cardiomyocytes within a sample was determined and expressed in units of cross-sectional area (in μm2).
Ultrastructural analysis with transmission electron microscopy.
Details of tissue fixation, embedding, sectioning, and staining have been previously described (31).
Statistical Analysis
Differences in outcomes among vehicle-, telmisartan-, and olmesartan-treated Sprague-Dawley and Ren-2 rats were determined using two-way ANOVA, and post hoc paired comparisons were made using the Tukey test and considered significant when P < 0.05 (Sigma Stat 3.1, Systat Software). Relationships between NADPH oxidase activity and LV mass or cardiomyocyte cross-sectional area were examined using linear regression analysis.
RESULTS
Experimental Parameters
Body and ventricular weights.
Pre- and posttreatment body weights and percent body weight increases (expressed as the percent increase relative to the initial body weight) are shown in Table 1. Ren2 rats gained slightly less weight than Sprague-Dawley rats (34 ± 5% vs. 44 ± 7%, P < 0.05). Neither telmisartan nor olmesartan affected weight gain in either rat strain. At the end of the study, R2C rats exhibited LVH, and treatment with telmisartan and olmesartan resulted in an almost complete attenuation of LVH (P < 0.05; Table 1).
Table 1.
Age, pre- and posttreatment body weights, and LV and RV weights at autopsy in SDC, SDT, SDO, R2C, R2T, and R2O rats
| Parameter | SDC Rats | SDT Rats | SDO Rats | R2C Rats | R2T Rats | R2O Rats | Significant Main Effect(s) (P < 0.05) |
|---|---|---|---|---|---|---|---|
| n | 6 | 6 | 9 | 6 | 9 | 9 | |
| Age, wk | 10.2 ± 0.2 | 10.4 ± 0.1 | 10.7 ± 0.2 | 10.6 ± 0.2 | 10.7 ± 0.2 | 10.8 ± 0.1 | ND |
| Pretreatment body weight, g | 249 ± 17 | 255 ± 9 | 262 ± 12 | 235 ± 17 | 267 ± 8 | 263 ± 9 | ND |
| Posttreatment body weight, g | 354 ± 10 | 355 ± 13 | 390 ± 12 | 312 ± 19 | 363 ± 12† | 355 ± 12 | Strain, treatment |
| Percent weight change | 44 ± 7 | 40 ± 6 | 51 ± 5 | 34 ± 5 | 36 ± 2 | 36 ± 2 | Strain |
| LV weight, mg | 769 ± 21* | 705 ± 27* | 776 ± 21* | 1,044 ± 66 | 871 ± 31† | 906 ± 40‡ | Strain, treatment |
| RV weight, mg | 210 ± 13 | 205 ± 7 | 218 ± 8 | 234 ± 17 | 243 ± 11 | 238 ± 11 | Strain |
| LV weight/body weight, mg/g | 2.18 ± 0.03* | 1.99 ± 0.05* | 1.99 ± 0.04* | 3.34 ± 0.09 | 2.41 ± 0.07† | 2.54 ± 0.08‡ | Strain, treatment, interaction |
| RV weight/body weight, mg/g | 0.59 ± 0.05* | 0.58 ± 0.01* | 0.56 ± 0.01* | 0.75 ± 0.04 | 0.67 ± 0.03 | 0.66 ± 0.01 | Strain, treatment |
Values are means ± SE; n, no. of rats/group. Rats were divided into the following groups: control Sprague-Dawley (SDC), telmisartin-treated Sprague-Dawley (SDT), olmesartan-treated Sprague-Dawley (SDO), control transgenic (mRen2)27 (Ren2) (R2C), telmistartan-treated Ren2 (R2T), and olmestartan-treated Ren2 (R2O). LV, left ventricular; RV, right ventricular; ND, no main effect difference. The LV weight includes the weight of the LV plus the septum.
P < 0.05 Sprague-Dawley vs. R2C rats;
P < 0.05, R2C vs. R2T rats;
P < 0.05, R2C vs. R2O rats (via the Tukey post hoc test).
Arterial blood pressure.
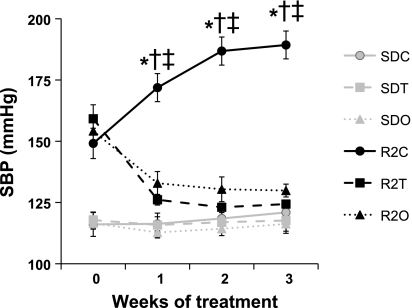
The time course for hypertension in Ren2 rats between 7 and 11 wk of age as recorded by telemetric monitoring is shown in Fig. 1. At 7 wk of age and before the initiation of ARB treatment, the average SBP of all Ren2 rats (n = 18) was 154 ± 1 compared 117 ± 1 mmHg (P < 0.05) for age-matched Sprague-Dawley rats. SBP in R2C rats was 189 ± 6 mmHg at the end of the 3-wk study period, whereas SBP in the SDC group was unchanged. Ren2 rats treated with either telmisartan or olmesartan experienced a steady decline in SBP to 124 ± 1 and 130 ± 3 mmHg, respectively, which did not differ from SBP in SDC rats (121 ± 4 mmHg, P > 0.05). The patterns for diastolic, mean arterial, and pulse pressure between 7 and 10 wk of age (not shown) were similar. No differences were detected via Tukey post hoc test in any blood pressure parameters between R2T and R2O rats.
Fig. 1.
Time course of hypertension in the control transgenic (mRen2)27 (Ren2) group (R2C) relative to the control Sprague-Dawley (SDC), telmisartan-treated Sprague-Dawley (SDT), olmesartan-treated Sprague-Dawley (SDO), telmisartan-treated Ren2 (R2T), and olmesartan-treated Ren2 (R2O) groups. Symbols represent mean (±SE) systolic blood pressures (SBPs) and are plotted versus the time course (in wk). The data shown were collected during the dark cycle. *P < 0.05, R2C vs. SDC rats; †P < 0.05, R2C vs. R2T rats; ‡P < 0.05, R2C vs. R2O rats.
P-V Loop Analysis of LV Function
Steady-state hemodynamic data.
LV hemodynamic parameters of anesthetized rats obtained during the acquisition of steady-state P-V loops can be found in the Supplemental Material (Supplemental Table 1).1 R2C rats exhibited elevated dP/dtmin, total peripheral resistance index, and Ea compared with SDC rats (P < 0.05). There was a trend toward increased dP/dtmax in R2C rats compared with SDC rats.
These findings support the notion that in 10- to 11-wk-old male Ren2 rats a compensatory increase in LV contractility occurs in response to increased afterload (>Ea). There were no significant differences among treatment groups in load-dependent diastolic (EDP and EDV) or systolic (SV, EF, CO, cardiac index, and dP/dtmax) parameters.
Load-independent indexes of LV function.
All load-independent indexes of cardiac function are shown in Table 2. With the exception of τ, these indexes were generated after occlusion of the inferior vena cava to vary preload.
Table 2.
Load-independent systolic and diastolic indexes in SDC, SDT, SDO, R2C, R2T, and R2O rats
| Parameter | SDC (5) | SDT (7) | SDO (9) | R2C (6) | R2T (9) | R2O (8) | Significant Main Effect(s) (P < 0.05) |
|---|---|---|---|---|---|---|---|
| n | 5 | 7 | 9 | 6 | 9 | 8 | |
| Systolic indexes | |||||||
| Ees, mmHg/μl | 1.23 ± 0.35 | 1.19 ± 0.24 | 1.13 ± 0.12 | 1.59 ± 0.20 | 1.23 ± 0.20 | 1.16 ± 0.11 | ND |
| PRSW, mmHg | 73 ± 10* | 77 ± 3* | 76 ± 4* | 106 ± 8 | 90 ± 8 | 76 ± 6‡ | Strain |
| Slope of dP/dtmax-EDV, mmHg·s−1·μl−1 | 9.4 ± 1.9* | 7.0 ± 1.4* | 11.7 ± 1.7 | 18.2 ± 4.1 | 9.9 ± 2.1† | 5.6 ± 0.9‡ | Treatment, interaction |
| Slope of P-V area-EDV, mmHg·μl−1·μl−1 | 82 ± 10* | 87 ± 4* | 94 ± 5* | 145 ± 14 | 102 ± 9† | 90 ± 7‡ | Strain, treatment, interaction |
| Diastolic indexes | |||||||
| Slope of EDPVR, mmHg/μl | 0.0024 ± 0.0005* | 0.0034 ± 0.0008* | 0.0024 ± 0.0003* | 0.0100 ± 0.0018 | 0.0033 ± 0.0006† | 0.0023 ± 0.0003‡ | Strain, treatment, interaction |
| Time constant of isovolumic relaxation (Glantz), ms | 13.9 ± 0.4* | 12.2 ± 0.6* | 12.8 ± 0.8* | 18.0 ± 1.4 | 12.4 ± 0.9† | 14.2 ± 0.6‡ | Strain, treatment |
Values are means ± SE; n, no. of rats/group. Ees, end-systolic elastance; PRSW, preload recruitable stroke work; EDV, end-diastolic volume; P-V, pressure-volume; EDPVR, end-diastolic P-V relationship, ND, no differences were observed in any of the ANOVA main effects.
P < 0.05, Sprague-Dawley rats vs. R2C rats;
P < 0.05, R2C vs. R2T rats;
P < 0.05, R2C vs. R2O rats (via the Tukey post hoc test).
SYSTOLIC INDEXES.
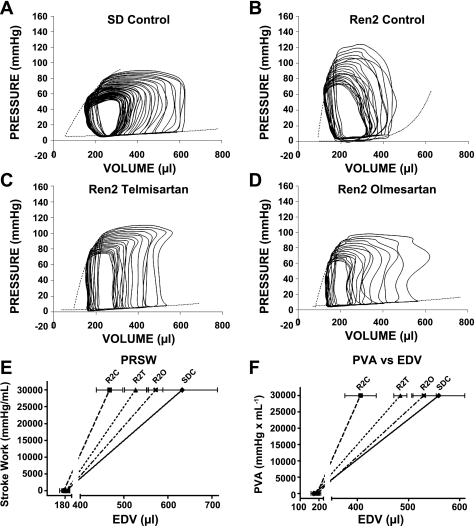
No differences were detected in Ees, i.e., the slope of the ESPVR (Table 2). On the other hand, the three other systolic indexes were different among the treatment groups. PRSW was elevated in R2C rats compared with SDC rats (P < 0.05; Table 2) and was normalized by olmesartan (P < 0.05 vs. R2C rats), whereas telmisartan failed to reduce PRWS (P = 0.21 vs. R2C rats; Table 2). Although systolic indexes tended to be lower in R2O rats compared with R2T rats, no differences were detected via Tukey post hoc tests. Graphical representation of PRSW showed a steeper slope in R2C rats and also showed that the volume axis intercepts were similar among groups (Fig. 2E). The volume axis intercept is also an indicator of the severity of contractile dysfunction. Two other indexes of LV contractility, the dP/dtmax-EDV relationship (Table 2) and P-V area-EDV relationship (Table 2 and Fig. 2F), were also elevated in R2C rats and normalized by treatment with ARBs (Table 2).
Fig. 2.
Representative left ventricular (LV) pressure-volume (P-V) loops, which were generated during preload reduction via occlusion of the inferior vena cava. Data are shown for SDC (A), R2C (B), R2T (C), and R2O (D) rats. SDT and SDO rats are not shown (but see Table 3). Dotted lines show the slopes of the end-systolic P-V relationship (Ees) and the end-diastolic P-V relationship (stiffness). E: preload recruitable stroke work (PRSW), which is the relationship between stroke work (SW) and end-diastolic volume (EDV), for SDC, R2C, R2T, and R2O rats. EDVs for each rat were generated using PRSW regression equations. Regression lines were generated using the average value for EDV when SW = 0 (x-axis intercept) and 30,000 mmHg/ml. SEs for EDV when SW = 30,000 mmHg/ml are shown as horizontal lines through the symbols. The steeper slope of the relationship seen in R2C rats indicates increased LV contractility compared with SDC rats and that olmesartan is effective at normalizing LV contractility. F: relationship between P-V area (PVA) and EDV for SDC, R2C, R2T, and R2O rats. Data were acquired as described for E. In the Ren2 LV, both telmisartan and olmesartan reduce the increase in LV contractility.
DIASTOLIC INDEXES.
τ, a load-independent index of LV active relaxation, was elevated in R2C rats compared with SDC rats (18.0 ± 1.4 vs. 13.9 ± 0.4 ms, P < 0.05) and was normalized by both telmisartan and olmesartan (12.4 ± 0.9 and 14.2 ± 0.6 ms, respectively, P < 0.05 vs. R2C rats). To evaluate LV wall compliance, we calculated the LV chamber stiffness constant, which is the slope of EDPVR during variable preload conditions. Representative P-V loops are shown in Fig. 2, A–D. The LV chamber stiffness constant was elevated in R2C rats compared with SDC rats (P < 0.05; Table 2) and was normalized by both telmisartan and olmesartan (P < 0.05 vs. R2C rats).
P-V area analysis of LV energetics.
Analysis of PVA normalized to the LV plus septum weight detected no significant strain, treatment, or interaction effects, suggesting that the myocardial O2 consumption per unit mass did not vary among treatment groups (P > 0.05; Supplemental Table 1).
Jak2 Phosphorylation
The binding of ANG II to the AT1R induces the activation of Jak2, an inflammatory marker that contributes to hypertrophy via the autophosphorylation of Tyr1007/1008 residues and the subsequent activation of STAT3 (2, 13). Therefore, we tested whether ARBs could prevent Tyr1007/1008 phosphorylation of Jak2 in the Ren2 LV. Compared with SDC rats, myocardial Tyr1007/1008 phosphorylation of Jak2, normalized to total Jak2, was elevated (activation) in R2C rats (P < 0.05; Fig. 3). Both ARBs significantly reduced the activation of Jak2.
Fig. 3.
Immunoblot analysis of myocardial Jak2 expression. Representative bands are shown for phosphorylated (p) and total (t) proteins. Bar graph shows the ratios of phosphorylated to total protein levels (n = 4–5 rats/group). *P < 0.05, R2C vs. SDC rats; †P < 0.05, R2C vs. R2T rats; ‡P < 0.05, R2C vs. R2O rats.
NADPH Oxidase Generation of Oxidative Stress
NADPH oxidase.
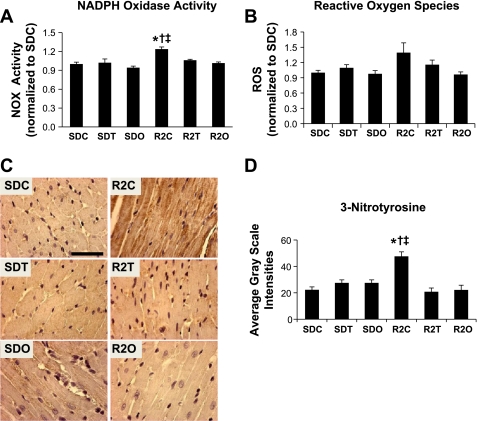
NADPH oxidase activity was higher in LV plasma membrane extracts of R2C rats compared with SDC rats (124 ± 4% vs. 100 ± 3% in SDC rats, P < 0.001; Fig. 4A), and treatment attenuated the increased enzyme activity in R2T and R2O rats (102 ± 2% and 106 ± 2%, respectively, P < 0.05 vs. SDC rats). Compared with SDC rats, there were no differences in NADPH oxidase activity in SDT or SDO rats (94 ± 2% and 102 ± 6%, respectively, P > 0.05).
Fig. 4.
Myocardial markers of oxidative stress. A and B: bar graph showing mean (±SE) NADPH oxidase (NOX) activity (in milli-optical density units·min−1·mg protein−1, n = 8–15 rats/group; A) and ROS levels (counts in relative light units·s−1·mg protein−1, n = 8–12 rats/group; B). Both parameters were normalized to values for SDC rats. C: representative micrographs of 3-nitrotyrosine (3-NTY)-stained sections of the LV myocardium. D: bar graph showing the quantitative analysis of 3-NTY immunostaining (n = 4–5 rats/group). *P < 0.05, SDC vs. R2C rats; †P < 0.05, R2C vs. R2T rats; ‡P < 0.05, R2C vs. R2O rats.
Myocardial superoxide levels.
There were nearly significant strain (P = 0.06) and treatment effects (P = 0.07), which suggests that LV superoxide tended to be higher in R2C rats compared with SDC rats (Fig. 4B). Olmesartan, but not telmisartan, tended to normalize superoxide in R2O rats compared with R2C rats. LV 3-nitrotyrosine content was higher in R2C rats compared with SDC rats (48 ± 3 vs. 22 ± 2, P < 0.001) and normalized in R2T and R2O rats (22 ± 3 vs. 21 ± 4, P < 0.001 vs. R2C rats; Fig. 4, C and D).
Myocardial Remodeling
Cardiomyocyte hypertrophy.
Cardiomyocyte cross-sectional area was greater in R2C rats than in SDC rats (737 ± 58 vs. 287 ± 18 μm2, P < 0.05; Fig. 5A). Both telmisartan and olmesartan normalized cardiomyoctye area (403 ± 23 and 357 ± 41 μm2, respectively, P < 0.05).
Fig. 5.
Myocyte hypertrophy and interstitial fibrosis. A: micrographs showing cardiomyocytes in cross section (top) and bar graph showing the average (±SE) cardiomyoctye size expressed as cross-sectional area (bottom). B: micrographs showing collagen fiber staining (in pink) upon Verhoeff-van Gieson staining (top) and bar graph showing the percent area of interstitial fibrosis (bottom). *P < 0.05, R2C vs. SDC rats; †P < 0.05, R2C vs. R2T rats; ‡P < 0.05, R2C vs. R2O rats.
LV fibrosis.
The relative amount of interstitial collagen staining was greater in R2C rats than in SDC rats (10.0 ± 1.2 vs. 2.5 ± 0.5 arbitrary units, P < 0.05; Fig. 5B). Treatment of Ren2 rats with telmisartan and olmesartan resulted in an attenuation of fibrosis (3.1 ± 0.3 and 3.9 ± 0.6, respectively, P < 0.001).
Intermyofibrillar mitochondria.
The normal ultrastructural arrangement in the SDC myocardium consists of a row of sarcomeres alternating with a row of intermyofibrillar mitochondria (Fig. 6A). This normal ultrastructural arrangement was disrupted in R2C rats (Fig. 6B). In this study, we also observed an increase in the number of intermyofibrillar mitochondria in R2C rats, which was associated with disruption of the normal cardiomyocyte sarcomere arrangement. In R2C rats, the intermyofibrillar mitochondria morphology was markedly deranged, and there were numerous breaks and loss of mitochondrial cristae associated with loss of the mitochondrial matrix (Fig. 6B, inset). The derangement in the ultrastructural morphology of intermyofibrillar mitochondria in R2C rats was improved by both telmisartan and olmesartan (Fig. 6, C and D, respectively).
Fig. 6.
Abnormalities in mitochondrial ultrastructure. Representative transmission electron micrographs (×1,000) demonstrating that, compared with SDC rats (A), there were increased numbers of intermyofibrillar mitochondria and disrupted sarcomeric structure in R2C rats (B). The insets show higher-magnification (×2,500) images. The inset in B shows the disrupted mitochondrial structure with numerous breaks and loss of cristae structure and loss of matrix in the R2C myocardium. Mitochondrial numbers and structure appeared more normal in R2T (C) and R2O (D) rats. Arrows point to intermyofibrillar mitochondria. Scale bar = 2 μm.
LVH.
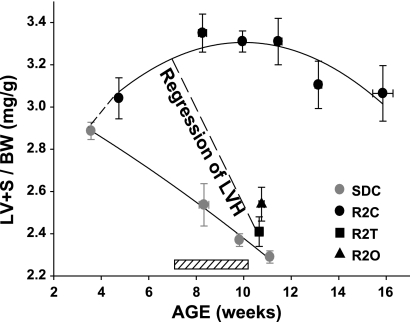
To determine the time course for the development of LVH in heterozygous male Ren2 rats, we normalized the LV plus septum weight to body weight and plotted the values as a function of the age group (i.e., 3–4.9, 7–8.9, 9–10.9, 11–12.9, 13–14.9, and 15–16.9 wk of age) for 50 Ren2 rats and 50 SD rats ranging in age between 4 and 16 wk old (Fig. 7). Additional age-matched rats were added to the cohort of control Sprague-Dawley and Ren2 rats used for this study to generate the relationships shown in Fig. 7. It is clear that Ren2 rats had established LVH at the start of the treatment period when they were between 7 and 8 wk of age. At autopsy, R2C rats exhibited LVH that was almost completely attenuated by treatment with telmisartan and olmesartan (P < 0.05; Fig. 7 and Table 1).
Fig. 7.
Angiotensin receptor blockers reverse established LV hypertrophy (LVH). The scatterplots shows LV + septum weight, adjusted for body weight, as a function of age in SDC and R2C rats. The sample sizes were 50 and 50 rats, respectively. The best-fit lines are second-order polynomials. The hatched box above the x-axis shows the timing of the 21-day treatment period. The dashed line indicates that telmisartan and olmesartan treatment regressed existing LVH in the Ren2 rat.
DISCUSSION
This is the first study to compare the beneficial effects of telmisartan, an ARB with partial PPAR-γ activity, to an ARB with little PPAR-γ activity (olmesartan) on abnormalities in cardiac structure and function in a rodent model of hypertension, insulin resistance, and dyslipidemia. Importantly, the doses of telmisartan and olmesartan tested achieved similar blood pressure reduction. A previous study (4) suggested that ARBs with selective PPAR-γ modulating activity may have insulin-sensitizing properties that are especially beneficial in insulin-resistant states. Our observations that the LV of hypertensive 10- to 11-wk-old male transgenic Ren2 rats display impairments in diastolic relaxation and increases in systolic contractility associated with myocardial oxidative stress, hypertrophy, fibrosis, and intramyofibrillar mitochondrial ultrastructural derangement suggest that these abnormalities occur in a state of compensated early heart failure. Furthermore, treatment with telmisartan or olmesartan comparably reduced LV fibrosis, hypertrophy, and oxidative stress while improving ultrastructure and cardiac function. The similar improvements in cardiac structure and function observed in telmisartan- and olmesartan-treated Ren2 rats suggest that the benefits of telmisartan may be predominantly pressor related or AT1R dependent in this model of RAS activation.
Our blood pressure measurements are consistent with prior measurements, derived by the tail-cuff method, demonstrating elevated SBPs beginning at 5 wk of age, which progress to a maximum of ∼240 mmHg between 11 and 14 wk of age (19). Unfortunately, detailed time courses for the parallel development of LVH, myocyte hypertrophy, and periarterial and interstitial fibrosis are not available, despite the likely contribution of these factors to the onset and progression of diastolic and systolic dysfunction. LVH is a significant predictor of heart failure (16), and antihypertensive therapy, especially targeting the RAS, leads to a regression of LVH (21). Our current observations suggest that LVH coincides with the onset of hypertension at ∼5 wk of age (Fig. 7). Periarterial fibrosis has been reported in these transgenic rats as young as 8–9 wk of age (28, 31), and extensive interstitial fibrosis and scarring have been reported in 13- to 14-wk-old Ren2 rats (23). The myocardium of 10- to 11-wk-old Ren2 male rats studied here exhibited modest interstitial fibrosis. Based on this study and previous reports, we conclude that fibrosis initially envelopes the vasculature in early stages of LVH and later progresses into the interstitium. Thus, at the onset of treatment in this study, the LV exhibited myocyte hypertrophy and periarterial fibrosis but not interstitial fibrosis. Thus, it appears that the establishment of diastolic dysfunction, as indicated by increases in τ and LV stiffness (slope of EDPVR), occurs in concert with the initial advancement of the extracellular matrix into the myocardial interstitium. The ARB therapies used in this study using the hypertensive Ren2 model at an early stage of heart disease were effective at reversing established LVH and the associated abnormalities in cardiac function and structure.
Our observations that the redox-sensitive Ser kinase phospho-Jak2 is elevated in the Ren2 myocardium and that both telmisartan and olmesartan reduce phospho-Jak is consistent with the notion that ANG II promotes LV hypertrophy via the Jak/STAT pathway (2, 22). After activation by Jak2/STAT proteins in myocardial tissue, this signaling pathway can induce an increase in angiotensinogen expression that establishes a positive feedback loop sustaining local cardiac RAS activation (22).
It is thought that myocardial remodeling in this transgenic rat model is due mostly to the consequences of expression of the mouse renin transgene in the myocardium, suggesting a autocrine/paracrine role for the RAS leading to high levels of ANG II (12, 34) and the generation of NADPH oxidase-derived superoxide (31, 32). The limited endogenous antioxidant potential of the heart increases myocardial susceptibility to oxidant damage (10). Indeed, mounting evidence suggests that the induction of ROS is necessary for cardiomyocyte hypertrophy (26), and the data reported here are consistent with this hypothesis (Figs. 3 and 6). The hypertrophic LV of the Ren2 rat exhibits enhanced oxidative stress as indicated by elevations in myocardial superoxide levels, 3-nitrotyrosine immunostaining, NADPH oxidase activity, and Rac1 and p47phox immunostaining, all of which are normalized by treatment with ARB or renin inhibition (31, 32). In this study, the elevated intensities of myocardial p47phox and Rac1 immunostaining were similarly decreased by treatment with telmisartan and olmesartan (P < 0.05, data not shown), suggesting that the increase in NADPH oxidase activity in this model is mediated via signaling through the AT1R.
Despite the ultrastructural derangements observed in the intermyofibrillar mitochondria (Fig. 6), there is little functional evidence of impaired myocardial energetics in 10- to 11-wk-old Ren2 rats. For instance, the P-V area, an estimate of myocardial O2 consumption (29), did not differ among the groups investigated (Supplemental Table 1). The P-V area is indicative of the total mechanical energy generated in a single ventricular contraction. The P-V area is a surrogate marker of myocardial O2 consumption per heart beat and is relatively insensitive to loading and contractile conditions (29). Additionally, the ratio between SW and P-V area, which provides a useful index of cardiac efficiency, did not vary among rat strains and treatment groups (Supplemental Table 1). Thus, we speculate that the increase in the number of intermyofibrillar mitochondria helps to maintain myocardial O2 consumption and efficiency despite the structural abnormalities observed in some mitochondria.
In this study, we were able to optimize the doses of telmisartan and olmesartan to achieve comparable blood pressure reduction. The doses of telmisartan and olmesartan used resulted in similar improvements in most measured hemodynamic, structural, and metabolic outcomes. Thus, one potential limitation of this study is that the potential additive benefits of PPAR-γ-dependent antioxidative and anti-inflammatory effects of telmisartan could have been masked by the influence of the AT1R-mediated maximal blood pressure reduction.
In conclusion, this investigation suggests that AT1R blockade attenuates the manifestations of early stage heart failure in the Ren2 rat. The benefits associated with the use of ARBs are likely related to reductions in NADPH oxidative-generated ROS and improvements in adverse myocardial remodeling and diastolic and systolic function. Although telmisartan and olmesartan were similarly beneficial in reducing cardiac dysfunction, the major therapeutic effects of telmisartan are primarily due to the blockade of AT1R-dependent signaling rather than AT1R-independent activation of PPAR-γ.
GRANTS
This research was supported, in part, by an investigator-initiated grant from Boehringer-Ingelheim (to J. R. Sowers and V. G. DeMarco), National Heart, Lung, and Blood Institute Grant R01-HL-73101-01A1 (to J. R. Sowers), Veterans Affairs Merit System Grant 0018 (to J. R. Sowers), Veterans Affairs CDA-2 (to A. T. Whaley-Connell), and the University of Missouri Research Board (to A. T. Whaley-Connell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical support of Rebecca I. Schneider, Mona Garro, Terry L. Carmack, Douglas R. Elliott, and Nathan T. Rehmer.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Asakawa M, Takano H, Nagai T, Uozumi H, Hasegawa H, Kubota N, Saito T, Masuda Y, Kadowaki T, Komuro I. Peroxisome proliferator-activated receptor γ plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation 105: 1240–1246, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Beckles DL, Mascareno E, Siddiqui MA. Inhibition of Jak2 phosphorylation attenuates pressure overload cardiac hypertrophy. Vascul Pharmacol 45: 350–357, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activity. Hypertension 43: 993–1002, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, Stas S, Kumar P, Sha Q, Ferrario CM, Sowers JR. Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab 288: E353–E359, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Clark JE, Kottam A, Motterlini R, Marber MS. Measuring left ventricular function in the normal, infarcted and CORM-3-preconditioned mouse heart using complex admittance-derived pressure volume loops. J Pharmacol Toxicol Methods 59: 94–99, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Connelly KA, Prior DL, Kelly DJ, Feneley MP, Krum H, Gilbert RE. Load-sensitive measures may overestimate global systolic function in the presence of left ventricular hypertrophy: a comparison with load-insensitive measures. Am J Physiol Heart Circ Physiol 290: H1699–H1705, 2006 [DOI] [PubMed] [Google Scholar]
- 9. DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Heart Circ Physiol 294: H2659–H2668, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Elmedal B, de Dam MY, Mulvany MJ, Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br J Pharmacol 141: 105–113, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45: 120–159, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Flesch M, Schiffer F, Zolk O, Pinto Y, Rosenkranz S, Hirth-Dietrich C, Arnold G, Paul M, Bohm M. Contractile systolic and diastolic dysfunction in renin-induced hypertensive cardiomyopathy. Hypertension 30: 383–391, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF, Wen X, Shi J, McGee R, Haley PJ, Shepard S, Rodgers JD, Yeleswaram S, Hollis G, Newton RC, Metcalf B, Friedman SM, Vaddi K. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 184: 5298–5307, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Jr, Rankin JS. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71: 994–1009, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Habibi J, Whaley-Connell A, Qazi MA, Hayden MR, Cooper SA, Tramontano A, Thyfault J, Stump C, Ferrario C, Muniyappa R, Sowers JR. Rosuvastatin, a HMG-CoA reductase inhibitor, decreases cardiac oxidative stress and remodeling in Ren2 transgenic rats. Endocrinology 148: 2181–2188, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med 287: 781–787, 1972 [DOI] [PubMed] [Google Scholar]
- 17. Kinnick TR, Youngblood EB, O'Keefe MP, Saengsirisuwan V, Teachey MK, Henriksen EJ. Modulation of insulin resistance and hypertension by voluntary exercise training in the TG(mREN2)27 rat. J Appl Physiol 93: 805–812, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension 47: 901–911, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Lee MA, Bohm M, Paul M, Bader M, Ganten U, Ganten D. Physiological characterization of the hypertensive transgenic rat TGR(mREN2)27. Am J Physiol Endocrinol Metab 270: E919–E929, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Little WC, Cheng CP, Mumma M, Igarashi Y, Vinten-Johansen J, Johnston WE. Comparison of measures of left ventricular contractile performance derived from pressure-volume loops in conscious dogs. Circulation 80: 1378–1387, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, Cifkova R, Clement D, Coca A, Dominiczak A, Erdine S, Fagard R, Farsang C, Grassi G, Haller H, Heagerty A, Kjeldsen SE, Kiowski W, Mallion JM, Manolis A, Narkiewicz K, Nilsson P, Olsen MH, Rahn KH, Redon J, Rodicio J, Ruilope L, Schmieder RE, Struijker-Boudier HA, Van Zwieten PA, Viigimaa M, Zanchetti A. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 27: 2121–2158, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci USA 95: 5590–5594, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinto YM, Buikema H, van Gilst WH, Scholtens E, van Geel PP, de Graeff PA, Wagner J, Paul M. Cardiovascular end-organ damage in Ren-2 transgenic rats compared to spontaneously hypertensive rats. J Mol Med 75: 371–377, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepinski JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun 393: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Raff GL, Glantz SA. Volume loading slows left ventricular isovolumic relaxation rate. Evidence of load-dependent relaxation in the intact dog heart. Circ Res 48: 813–824, 1981 [DOI] [PubMed] [Google Scholar]
- 26. Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol 34: 379–388, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation 109: 2054–2057, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Seccia TM, Belloni AS, Kreutz R, Paul M, Nussdorfer GG, Pessina AC, Rossi GP. Cardiac fibrosis occurs early and involves endothelin and AT-1 receptors in hypertension due to endogenous angiotensin II. J Am Coll Cardiol 41: 666–673, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Suga H, Yamada O, Goto Y, Igarashi Y. Oxygen consumption and pressure-volume area of abnormal contractions in canine heart. Am J Physiol Heart Circ Physiol 246: H154–H160, 1984 [DOI] [PubMed] [Google Scholar]
- 30. Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKα2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res 106: 1117–1128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump CS, Ferrario CM, Sowers JR. Angiotensin-II mediated oxidative stress promotes myocardial tissue remodeling in the transgenic TG (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Whaley-Connell A, Habibi J, Cooper SA, DeMarco VG, Hayden MR, Stump CS, Link D, Ferrario C, Sowers JR. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab 295: E103–E109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao X, Su G, Brown SN, Chen L, Ren J, Zhao P. Peroxisome proliferator-activated receptors γ and α agonists stimulate cardiac glucose uptake via activation of AMP-activated protein kinase. J Nutr Biochem 21: 621–626, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Zolk O, Flesch M, Nickenig G, Schnabel P, Bohm M. Alteration of intracellular Ca2+-handling and receptor regulation in hypertensive cardiac hypertrophy: insights from Ren2-transgenic rats. Cardiovasc Res 39: 242–256, 1998 [DOI] [PubMed] [Google Scholar]