Abstract
Treatment of aortic smooth muscle cells with PDGF induces the upregulation of protein tyrosine phosphatase 1B (PTP1B). PTP1B, in turn, decreases the function of several growth factor receptors, thus completing a negative feedback loop. Studies have reported that PDGF induces the downregulation of PKG as part of a repertoire of dedifferentiation of vascular smooth muscle cells. Other studies have reported that chronic nitric oxide (NO) treatment also induces the downregulation of PKG. In the present study, we tested the hypothesis that the downregulation of PKG by PDGF or NO in differentiated rat aortic smooth muscle cells can be attributed to the upregulation of PTP1B. We found that treatment with PDGF or NO induced an upregulation of PTP1B levels. Overexpression of PTP1B induced a marked downregulation of PKG mRNA and protein levels, whereas the expression of dominant negative PTP1B or short interfering RNA directed against PTP1B blocked the capacity of PDGF or NO to decrease PKG levels. We conclude that the upregulation of PTP1B by PDGF or NO is both necessary and sufficient to induce the downregulation of PKG via an effect on PKG mRNA levels.
Keywords: protein kinase G, platelet-derived growth factor, vascular smooth muscle
neointimal enlargement is of critical importance to the process of vascular remodeling that occurs in angioplasty-induced restenosis or in atherosclerosis (9, 22). The formation of the neointima is, in part, dependent on the migration of smooth muscle cells from the media to intima (24). PDGF is thought to play a pivotal role in inducing the movement of vascular smooth muscle cells from the media to neointima (12, 19, 34). In addition to motility, PDGF induces the dedifferentiation of cultured vascular smooth muscle cells from a contractile phenotype to a fibroblastic phenotype, together with an enhanced secretion of extracellular matrix proteins (8, 16).
We (6) have previously reported that treatment of differentiated cultured vascular smooth muscle cells with PDGF induces the upregulation of protein tyrosine phosphatase 1B (PTP1B), which is an enzyme that targets several receptor tyrosine kinases. We (5) have also reported that PTP1B levels are markedly upregulated in a model of rat carotid artery injury and that the enzyme plays an important counterregulatory role in attenuating vascular smooth muscle cell motility and neointima formation. It is also well established that the PDGF receptor β-isoform is important in transducing the effects of PDGF, both in cultured cells and in vivo (12, 34). We (37) have recently shown that PTP1B induces phosphotyrosyl dephosphorylation of PDGF receptor-β, a finding that supports the existence of a classical negative feedback loop involving the phosphatase.
Decreased force generation induced by nitric oxide (NO) in vascular smooth muscle cells is thought to depend, in a large measure, on the second messenger cGMP, via the activation of soluble or membrane-bound guanyl cyclases (23, 33). cGMP function, in turn, significantly depends on the activity of a serine kinase, cGMP-dependent protein kinase (PKG)I. PKGI induces the phosphorylation of several proteins involved in regulation of vascular tone and exists as two isoforms, α and β, derived from splice variants of a single gene (11). PKGI-α and PKGI-β express different amino acids in their NH2-terminal tails. PKGI-α is predominantly expressed in vascular smooth muscle cells, cerebellar neurons, and platelets, whereas PKGI-β is more prevalent in the central nervous system, uterine smooth muscle, and adrenal glands.
Cultured vascular smooth muscle cells treated with PDGF, or cells generated as part of the response to vascular injury, express reduced levels of PKGI-α (1, 30) together with reduced expression of proteins involved in vascular force generation, such as smooth muscle myosin heavy chain 2 and calponin. A study (4) has indicated that prolonged treatment of vascular smooth muscle cells with NO also decreases PKG levels. Moreover, considerable evidence supports the concept that PKG modulates the expression of proteins involved in mediating vascular smooth muscle cell contractility. In the present study, we report the novel finding that upregulation of PTP1B is both necessary and sufficient to induce the downregulation of PKGI induced by either PDGF or NO.
MATERIALS AND METHODS
Experiments were performed via a protocol approved by the Animal Care and Use Committee of the University of Tennessee Health Sciences Center in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 86-23, Revised 1996).
Materials.
Tissue culture plates were from Falcon/Becton-Dickinson (Oxnard, CA). DMEM and DMEM-Ham's F-12 (1:1) medium was from Cellgro Mediatech (Herndon, VA). Porcine pancreatic elastase and clostridium histolyticum collagenase were from Worthington Biochemical (Lakewood, NJ). Soybean trypsin inhibitor, FBS, and BSA (fraction V) were from Atlanta Biologicals (Lawrenceville, GA). Recombinant human PDGF-BB was from R&D Systems (Minneapolis, MN). Anti-PTP1B mouse monoclonal antibody for Western blot analysis, isotype control mouse IgG2a, crystallized porcine pancreatic elastase, and phosphatase inhibitor cocktail set I were from Calbiochem (La Jolla, CA). Clostridium histolyticum collagenase type I, soybean trypsin inhibitor, BSA (fraction V), protease inhibitor cocktail, and p-nitrophenyl phosphate were from Sigma (St. Louis, MO). Diethylenetriamine (DETA)-NONOate (NOC-18; DETA-NO) and S-nitroso-N-acetylpenicillamine (SNAP) were from Alexis Biochemicals (Carlsbad, CA). Protein G-Sepharose 4 Fast Flow was from GE Healthcare Bio-Sciences AB (Piscataway, NJ). The Transcriptor First Strand cDNA Synthesis Kit was from Roche (Indianapolis, IN). The Total RNA Mini Kit was from Bio-Rad (Hercules, CA). Purified mouse anti-PTP1B monoclonal antibody was from BD Biosciences (San Jose, CA). Adenoviral vectors expressing enhanced green fluorescent protein (EGFP), PTP1B, or C215S-PTP1B were prepared as described in a previous publication from our laboratory (29). Rabbit antiserum directed against PKG was obtained as previously described (36). Plasmids with a sequence expressing short interfering (si)RNA against rat PTP1B and plasmids with a sequence expressing a nontargeting siRNA control were from Dharmacon (Lafayette, CO). The nitrate/nitrite colorimetric assay kit was from Cayman (Ann Arbor, MI). All other reagents not mentioned specifically were obtained from Sigma.
Cell culture.
Lactating female rats of the Sprague-Dawley strain and their pups were purchased from Charles River Labs (Wilmington, MA). Smooth muscle cells were obtained from the thoracic aortas of newborn Sprague-Dawley rats (age: 6–9 days) as previously described (29). Cells were grown in DMEM-Ham's F-12 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS in 5% CO2 at 37°C. Cultures were maintained in mitogenic quiescence medium lacking FBS for 24 h before being harvested. All experiments in the present study were performed with the use of primary cultures; moreover, each individual experiment represents results from one such cell isolate, generally obtained from one newborn rat litter.
Measurement of PTP1B enzyme activity.
Cells were subjected to lysis using ice-cold HEPES buffer of following composition: 50 mM HEPES, 150 mM NaCl, 2.5 mM EDTA, and 1% Triton X-100 supplemented with protease inhibitor cocktail (0.5 mM PMSF, 0.4 μM aprotinin, 10.5μM leupeptin, 18 μM bestatin, 7.5 M pepstatin A, and 7 μM E-64) and serine/threonine phosphatase inhibitor cocktail (25 μM p-bromotetramisole oxalate, 5 μM cantharidin, and 5 nM microcystin). Tissue lysates equivalent to 500 μg protein were incubated with 1 μg PTP1B antibody at 4°C for 3 h followed by an incubation with 30 μl protein G-Sepharose beads (1:1 slurry) at 4°C for 2 h. After being washed two times with 500 μl buffer, beads were incubated with 10 mM p-nitrophenyl phosphate at 37°C for 30 min, and levels of the product, p-nitrophenol, were determined by absorption spectrophotometry.
Western blot analysis.
Cells were subjected to lysis using the following buffer: 188 mM Tris·HCl, 1 mM EDTA, 15% glycerol, and 3% SDS (pH 6.8) further supplemented with 1 mM sodium vanadate, 0.25 mM PMSF, 0.2 μM aprotinin, 5.25 μM leupeptin, 9 μM bestatin, 3.75 μM pepstatin A, and 3.5 μM E-64. Lysates were subjected to Western blot analysis, and band densities were measured using NIH software. Further details concerning the Western blot image analysis are available online at http://rsb.info.nih.gov/nih-image/manual/tech.html#analyze.
Measurement of PKG mRNA levels via semiquantitative real-time PCR.
Vascular smooth muscle cells were infected with adenovirus expressing EGFP, adenovirus expressing active PTP1B (wild-type PTP1B), or adenovirus expressing catalytically inactive PTP1B (C215S-PTP1B; dominant negative PTP1B) at multiplicity of infection values of 5–10 in the medium for 24 h. Cells were rinsed to remove residual virus, incubated for 24 h, and subjected to lysis using RIPA buffer followed by the isolation of total RNA via a commercial kit from Bio-Rad. mRNA was transcribed into cDNA followed by the performance of real-time PCR for PKG or cyclophilin D mRNA levels using a LC 480 Real-Time PCR instrument (Roche Diagnostics). Real-time PCR was performed using the following primers derived via Universal probe library software (universalprobelibrary.com, Roche Applied Science): PKG, forward 5′-GAACAGCGAACGTCATTGC-3′ and reverse 5′-CTCCAATTAAATGCTTGAAAGAGT-3′; and cyclophilin D, forward 5′-GGAGACTTCTCAAATCAGAATGG −3′ and reverse 5′-ACCCTCCCGATCATGCTT-3′. Results were normalized against the housekeeping gene cyclophilin D.
Knockdown of PTP1B levels via the use of siRNA.
Adenoviruses expressing siRNA against rat PTP1B or nontargeting siRNA were prepared as follows using the pSilencer 1.0 CMV kit from Ambion. Hairpin DNA inserts (from IDT or Dharmacon) were cloned into the adenovirus shuttle vector using XhoI/SpeI sites. After PacI restriction for both shuttle and backbone, both were transfected into human embryonic kidney (HEK)-293 cells. Sequences of siRNA against rat PTP1B were designed as follows: duplex 1, sense 5′-GAUCGAGGGUGCAAGUUCUU-3′ and antisense 5′-GAACUUUGCACCCUCGAUCUU-3′; and duplex 2, sense 5′-GAACAGGUACCGAGAUGUCUU-3′ and antisense: 5′-GACAUCUCGGUACCUGUUCUU-3′. Two adenovirus constructs, containing either duplex 1 or 2 against PTP1B, were premixed [1:1 (vol/vol)] before being used for cell infection. Sequences serving as controls were as follows: sense 5′-AGACUACCGUUGUUAUAGGUG-3′ and antisense 5′-GACCUAUAACAAUGGUAGUUU-3′.
Measurement of the nitrate/nitrite concentration in the cell culture medium.
Aortic smooth muscle cells were cultured in serum-free medium for 24 h followed by treatment with 20 ng/ml PDGF in serum-free medium in the presence or absence of the NO synthase (NOS) inhibitor N-monomethyl-l-arginine (l-NMMA) for 24 h. Nitrate/nitrite concentrations in cell culture media were measured with a nitrate/nitrite colorimetric assay kit (Cayman).
RESULTS
Overexpression of PTP1B mimics the capacity of PDGF to induce the downregulation of PKG.
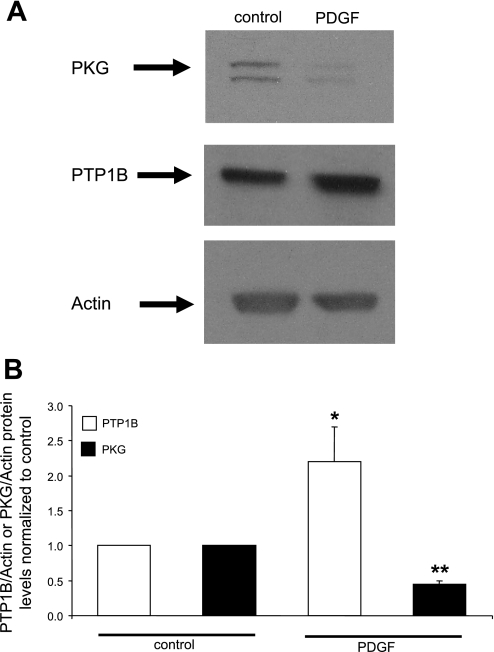
Our initial experiments were designed to verify the observation that PDGF induces the downregulation of PKGI-α and to test the hypothesis that the upregulation of PTP1B is sufficient to mediate this effect. As shown in Fig. 1, treatment of cells with PDGF induced a marked decrease of PKGI-α levels, confirming previous findings. Simultaneously, we confirmed the upregulation of PTP1B by PDGF (Fig. 1), in accordance with previous work from our laboratory (6).
Fig. 1.
PDGF induces a suppression of PKG levels together with an upregulation of protein tyrosine phosphatase 1B (PTP1B) levels. Aortic smooth muscle cells were cultured in serum-free medium for 24 h followed by treatment with 20 ng/ml PDGF in serum-free medium for 24 h. A: Western blots from a representative experiment. Duplicate bands in the PKG blot represent α- and β-isoforms. B: summary of results (mean ± SE) from 3 experiments, normalized to α-actin levels. *P < 0.05, PDGF + PTP1B compared with control; **P < 0.05, PDGF + PKG compared with control.
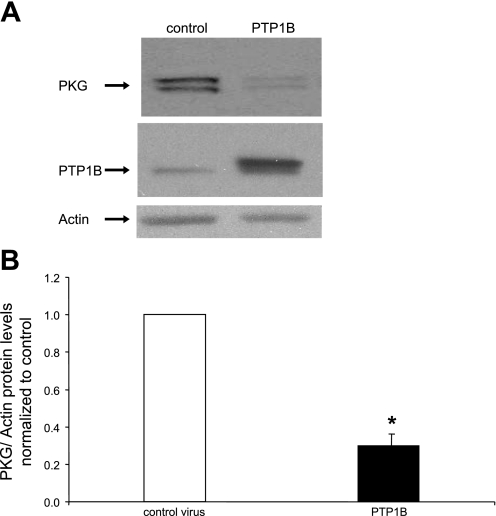
Next, we tested the hypothesis that the overexpression of PTP1B is sufficient to induce the downregulation of PKG protein levels. As shown in Fig. 2, overexpression of PTP1B, via an adenoviral vector, induced a marked decrease in the levels of PKGI-α. In view of the capacity of PDGF to increase PTP1B levels, this finding is consistent with the hypothesis that the upregulation of PTP1B is sufficient to mediate PDGF-induced suppression of PKGI-α levels in differentiated rat aortic smooth muscle cells.
Fig. 2.
Overexpression of PTP1B induces a suppression of PKG levels. Cells were cultured for 48 h in the presence of control adenovirus expressing enhanced green fluorescent protein (EGFP) or adenovirus expressing PTP1B at a multiplicity of infection of 5–10. A: Western blots from a representative experiment. B: summary of results (means ± SE) from 5 experiments, normalized to α-actin levels. *P < 0.05 compared with the control.
Treatment with dominant negative PTP1B or siRNA directed against PTP1B rescues cells from PDGF-induced downregulation of PKG.
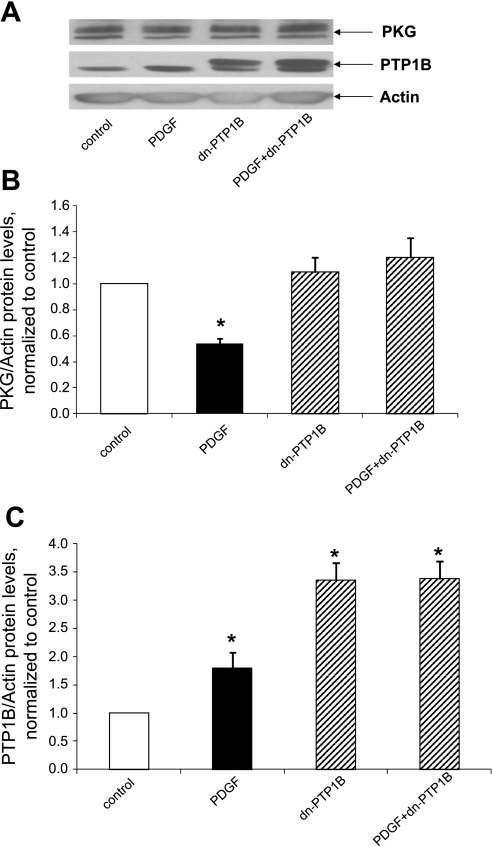
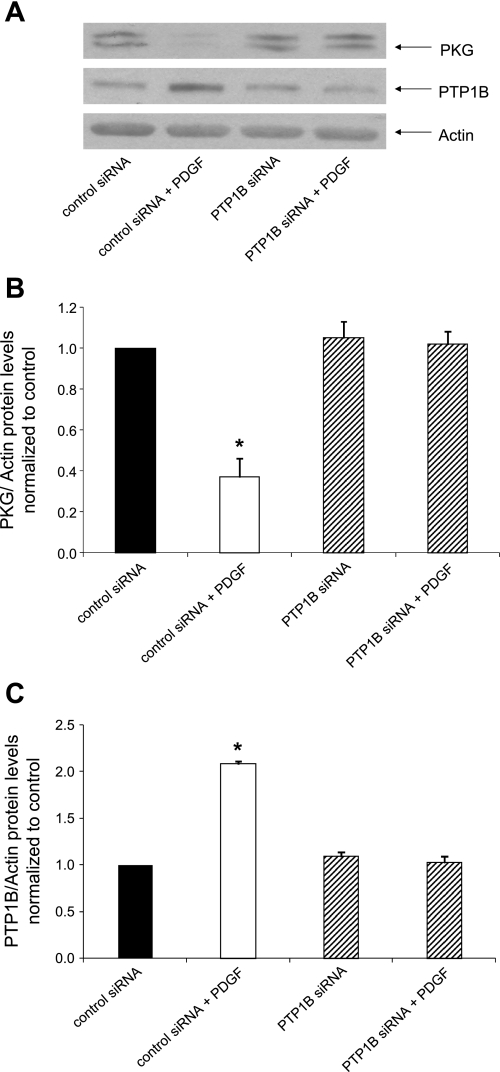
We next tested the hypothesis that the function of PTP1B is necessary for the capacity of PDGF to suppress PKG levels. Accordingly, we treated cells with dominant negative PTP1B using an approach that is known to interfere with PTP1B function, by the presumed sequestration of potential substrates (5). As shown in Fig. 3, the suppression of PKG induced by PDGF was abrogated in cells treated with adenovirus expressing dominant negative PTP1B but not in cells treated with control virus. To strengthen these findings, we determined the effect of treatment with siRNA directed against PTP1B on the capacity of PDGF to alter PKG levels. As shown in Fig. 4, adenovirus expressing siRNA directed against PTP1B, but not adenovirus expressing control siRNA, rescued cells from the PDGF-induced suppression of PKG. Taken together, these results are consistent with the hypothesis that PTP1B function is necessary for transduction of the capacity of PDGF to decrease PKG protein levels.
Fig. 3.
Expression of dominant negative (dn-)PTP1B rescues cells from the suppression of PKG induced by PDGF. Cells were cultured for 24 h in the presence of serum-free medium containing control adenovirus expressing EGFP or adenovirus expressing dn-PTP1B (C215S-PTP1B) at a multiplicity of infection of 5–10 followed by a 24-h incubation in serum-free medium and 24 h in serum-free medium supplemented with 20 ng/ml PDGF plus control virus or virus expressing dn-PTP1B. A: Western blots from a representative experiment. B and C: summary of results (means ± SE) from 5 experiments for PKG (B) or PTP1B (C), normalized to α-actin. *P < 0.05 compared with the control.
Fig. 4.
Expression of short interfering (si)RNA directed against PTP1B rescues cells from the suppression of PKG induced by PDGF. Cells were cultured for 24 h in the presence of serum-free medium containing adenovirus expressing control siRNA or adenovirus expressing siRNA directed against PTP1B at a multiplicity of infection of 5–10 followed by a 24-h incubation in serum-free medium and 24 h in serum-free medium supplemented with 20 ng/ml PDGF plus virus expressing control siRNA or virus expressing siRNA directed against PTP1B. A: Western blots from a representative experiment. B and C: summary of results (means ± SE) from 3 experiments for PKG (B) and PTP1B (C), normalized to α-actin. *P < 0.05 compared with the control.
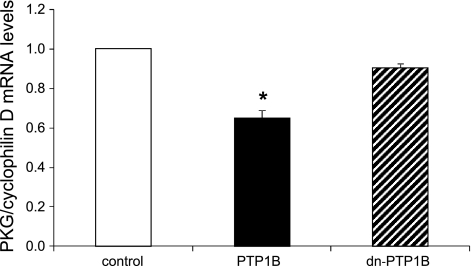
Overexpression of PTP1B, but not expression of dominant negative PTP1B, induces the suppression of PKG mRNA levels.
A previous study (30) reported that treatment of vascular smooth muscle cells with PDGF decreased the levels of PKG mRNA, thus providing a mechanism that explains the effect of PDGF on PKG protein levels. On the basis of this finding, we were prompted to test the hypothesis that PTP1B overexpression would similarly decrease the levels of PKG mRNA. As shown in Fig. 5, overexpression of PTP1B indeed significantly decreased the levels of PKG mRNA, whereas catalytically inactive dominant negative PTP1B had no significant effect.
Fig. 5.
Overexpression of PTP1B, but not expression of dn-PTP1B, induces the suppression of PKG mRNA levels. Cells were cultured for 24 h in the presence of adenovirus expressing EGFP (control), PTP1B, or dn-PTP1B (C215S-PTP1B) at a multiplicity of infection of 5–10 followed by 24 h in serum-free medium. Real-time PCR was performed as described in materials and methods, and results were normalized to mRNA levels of cyclophilin D. Results are means ± SE of 6 experiments. *P < 0.05 compared with the control.
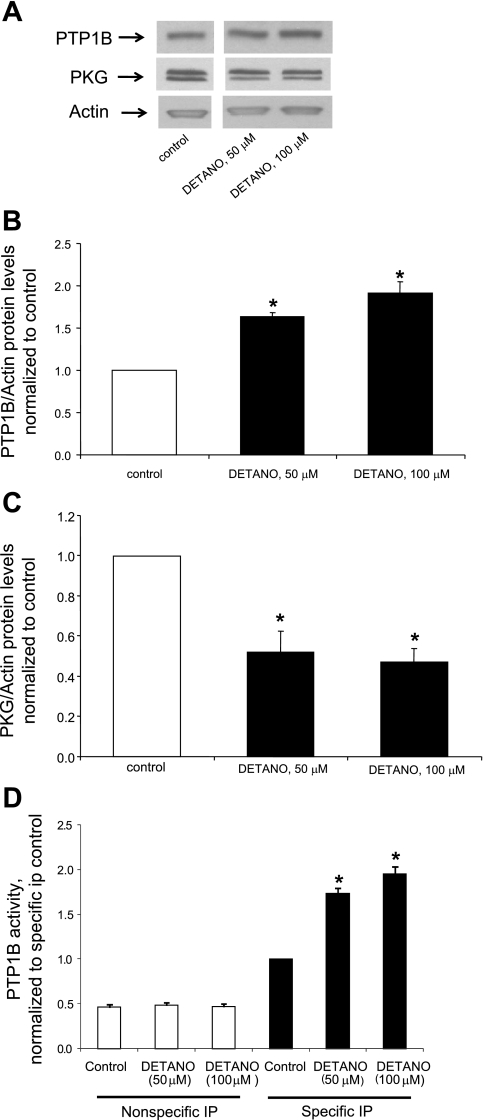
Chronic treatment with a NO donor decreases PKG levels together with the upregulation of PTP1B levels and activity.
Studies (4, 28) have reported that chronic exposure of cultured vascular smooth muscle cells to NO or cGMP or chronic activation of soluble guanyl cyclase induces the downregulation of PKGI-α. These findings prompted us to test the hypothesis that the downregulation of PKGIα by NO is mediated by an upregulation of PTP1B levels. As shown in Fig. 6, treatment with the NO donor DETA-NO increased both the levels and activity of PTP1B. Moreover, NO simultaneously decreased PKGI-α levels, consistent with the hypothesis that the suppression of PKGI-α is mediated by the upregulation of PTP1B. To verify the specificity of the effect of the NO donor DETANO, we also measured the levels of PKG and PTP1B in cells treated with a second NO donor, SNAP, and observed that treatment for 24 h with 50 μM SNAP decreased PKG levels to 45 ± 4% of control (n = 3 experiments, P < 0.05) while increasing PTP1B levels to 1.72 ± 0.14-fold above control (n = 3 experiments, P < 0.05).
Fig. 6.
Chronic treatment with a nitric oxide (NO) donor decreases PKG levels together with the upregulation of PTP1B levels and activity. Cells were cultured for 48 h in the presence of 50 or 100 μM diethylenetriamine NONOate (DETA-NO) followed by the measurement of protein levels via Western blot analysis. A: Western blots from a representative experiment. The gap between the lanes is because the control and DETA-NO lanes were not originally contiguous on the gel due to the splicing out of other data irrelevant to the presented results. B: summary of results (means ± SE) for PTP1B protein levels obtained from 3 experiments. *P < 0.05 compared with the control. C: summary of results (means ± SE) for PKG protein levels obtained from 3 experiments, normalized to α-actin levels. *P < 0.05 compared with the control. D: effect of DETA-NO on PTP1B activity. Results are means ± SE of 3 experiments, normalized to the control. *P < 0.05 compared with the control.
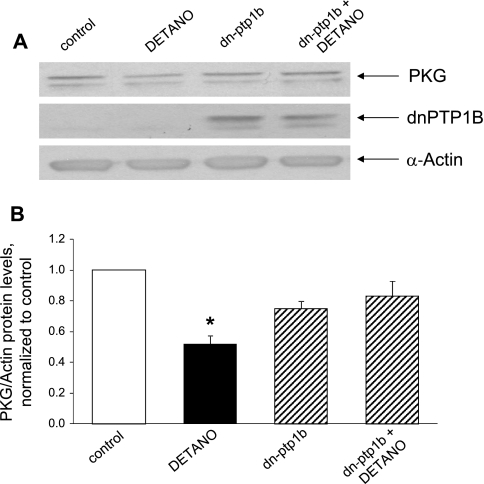
Treatment with dominant negative PTP1B rescues cells from NO-induced PKG downregulation.
The aforementioned findings raised the possibility that the upregulation of PTP1B is necessary for transduction of the suppressive effect of NO on PKG. To test this hypothesis, we treated cells with dominant negative PTP1B followed by treatment with NO. As shown in Fig. 7, dominant negative PTP1B rescued cells from NO-induced downregulation of PKG, consistent with the hypothesis that PTP1B function is necessary to mediate the effect of NO on PKG.
Fig. 7.
Expression of dn-PTP1B rescues cells from the suppression of PKG induced by a NO donor. Cells were cultured for 24 h in the presence of serum-free medium containing control adenovirus expressing EGFP or adenovirus expressing dn-PTP1B (C215S-PTP1B) at a multiplicity of infection of 5–10 followed by a 24-h incubation in serum-free medium and 24 h in serum-free medium supplemented with 100 μM DETA-NO plus control virus or virus expressing dn-PTP1B. A: Western blots from a representative experiment. B: summary of results (means ± SE) from 3 experiments, normalized to α-actin. *P < 0.05 compared with the control.
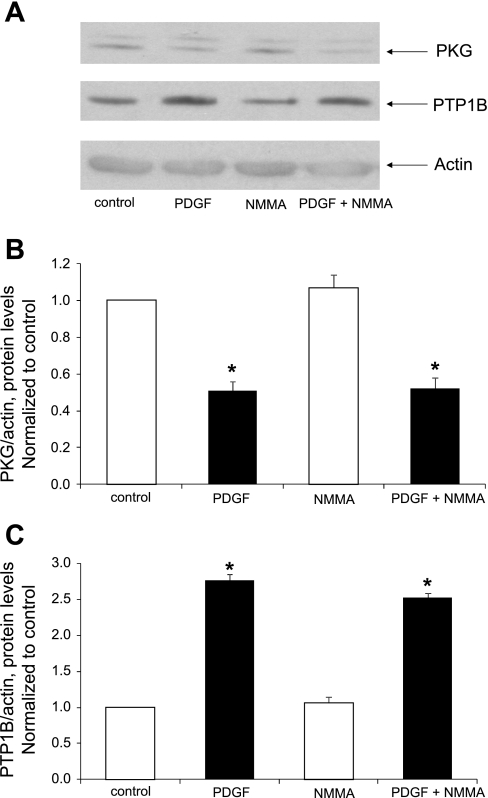
PDGF fails to increase NO levels, whereas inhibition of NOS fails to alter the capacity of PDGF to downregulate PKG protein levels.
PDGF has been reported to induce the upregulation of neuronal NOS and to increase the release of NO in dedifferentiated aortic smooth muscle cells (20). We were thus prompted to investigate a potential interaction of PDGF, NO, and the suppression of PKG in primary cultured cells. Treatment with PDGF (20 ng/ml) failed to induce an increase in nitrite levels found in culture media (data not shown). Moreover, treatment of cells with the general NOS inhibitor l-NMMA failed to alter the capacity of PDGF to suppress PKG levels or to increase PTP1B levels (Fig. 8).
Fig. 8.
Lack of effect of the NO synthase inhibitor N-monomethyl-l-arginine (l-NMMA) on PDGF-induced PKG suppression and PTP1B upregulation. Cells were cultured in serum-free medium for 24 h followed by treatment for 30 min without or with 0.5 mM l-NMMA and by treatment for 24 h without or with 20 ng/ml PDGF in the continued absence or presence of l-NMMA. A: Western blots from a representative experiment. B and C: summary of results (means ± SE) from 3 experiments for PKG (B) or PTP1B (C) protein levels, normalized to α-actin. *P < 0.05 compared with control.
DISCUSSION
Several studies (10, 30, 32) have reported that exposure of cultured vascular smooth muscle cells to PDGF induces phenotypic dedifferentiation, including a downregulation of PKG. Other studies have indicated that PKG is an important modulator of phenotypic differentiation. Thus, expression of PKG in subcultured cells manifesting low levels of expression recovers most of the original contractile phenotype (2, 3, 18), whereas inhibition of PKG activity induces the opposite effect (18). Chronic exposure to NO also induces the downregulation of PKG, presumably as part of a negative feedback mechanism (28). Mechanistically, the expression of PKG appears to be under complex regulatory control, involving several transcription factors (25, 26, 35).
An in vivo study (1) found that the induction of vascular injury, involving a prominent role of growth factors, was associated with reduced levels of PKG. Moreover, in human arteries, neointimal areas express lower levels of PKG (1). Similarly, treatment with NO donors in vivo is associated with reduced PKG levels (28). Furthermore, a long-standing clinical problem is related to the resistance to NO that develops with the chronic use of nitroglycerin or other nitrovasodilators (7, 21, 31).
PTP1B, originally discovered as a phosphatase that targets the insulin signaling pathway, is now known to target a variety of other proteins involved in intracellular signaling. We (6) have previously reported that treatment of cells with several growth factors, including PDGF, induces the upregulation of PTP1B as part of a negative feedback mechanism. In the present study, we tested the hypothesis that decreased expression of PKG in cells treated with PDGF or NO requires the upregulation of PTP1B. Overexpression of PTP1B alone induced a suppression of PKG expression, indicating that the upregulation of PTP1B is sufficient to elicit PKG downregulation. A previous study (30) reported that PDGF decreases the levels of PKG mRNA, an effect that accounts for lower protein expression. On the basis of this finding, we tested the hypothesis that overexpression of PTP1B would similarly decrease the levels of PKG mRNA, and we obtained data that support this hypothesis. Thus, the mechanism of the suppression of PKG can be attributed to decreased mRNA levels induced by PTP1B. This finding is similar to results from other studies (17, 27) indicating that PTP1B has the capacity to modulate mRNA levels of proteins involved in several signaling pathways.
We (6) have previously reported that PDGF induces an upregulation of PTP1B levels, and, in the present study, we found that treatment of cells with NO induces a similar upregulation of PTP1B. These findings raised the possibility that upregulation of PTP1B may be necessary in the transduction of PKG suppression induced by either PDGF or NO. To test this hypothesis, we treated cells with a dominant negative allele of PTP1B followed by treatment with PDGF or NO, and we found that dominant negative PTP1B blocked the capacity of both PDGF and NO to decrease the expression of PKG. This conclusion was further supported by the similar effect of siRNA directed against PTP1B.
A previous study (20) reported the upregulation of neuronal NOS by PDGF in presumably dedifferentiated subcultured cells, suggesting the regulation of PKG levels via a potential interaction of PDGF and NO. However, on the basis of the lack of effect of PDGF on NO levels in culture and the lack of effect of a NOS inhibitor on PKG levels, we could find no evidence for this occurrence in our differentiated cultured cells, perhaps indicating a divergence of signaling mechanisms related to the differentiation status of the two cell types.
Taken together, our results support the novel hypothesis that the expression of PKG is under the regulatory control of PTP1B and that the suppression of PKG by PDGF or NO is mediated by the upregulation of PTP1B. Moreover, because NO induces the activation of PTP1B via a PKG-dependent mechanism, as shown in a previous study (15), the present results support the existence of a negative feedback loop involving the NO-PTP1B-PKG axis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-63886 and HL-72902.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank D. Luedemann for editorial assistance.
REFERENCES
- 1. Anderson PG, Boerth NJ, Liu M, McNamara DB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase expression in coronary arterial smooth muscle in response to balloon catheter injury. Arterioscler Thromb Vasc Biol 20: 2192–2197, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Boerth NJ, Dey NB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res 34: 245–259, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Brophy CM, Woodrum DA, Pollock J, Dickinson M, Komalavilas P, Cornwell TL, Lincoln TM. cGMP-dependent protein kinase expression restores contractile function in cultured vascular smooth muscle cells. J Vasc Res 39: 95–103, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Browner NC, Sellak H, Lincoln TM. Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells. Am J Physiol Cell Physiol 287: C88–C96, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chang Y, Ceacareanu B, Zhuang D, Zhang C, Pu Q, Ceacareanu AC, Hassid A. Counter-regulatory function of protein tyrosine phosphatase 1B in platelet-derived growth factor- or fibroblast growth factor-induced motility and proliferation of cultured smooth muscle cells and in neointima formation. Arterioscler Thromb Vasc Biol 26: 501–507, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Chang Y, Zhuang D, Zhang C, Hassid A. Increase of PTP levels in vascular injury and in cultured aortic smooth muscle cells treated with specific growth factors. Am J Physiol Heart Circ Physiol 287: H2201–H2208, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Colditz GA, Halvorsen KT, Goldhaber SZ. Randomized clinical trials of transdermal nitroglycerin systems for the treatment of angina: a meta-analysis. Am Heart J 116: 174–180, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Dandre F, Owens GK. Platelet-derived growth factor-BB and Ets-1 transcription factor negatively regulate transcription of multiple smooth muscle cell differentiation marker genes. Am J Physiol Heart Circ Physiol 286: H2042–H2051, 2004 [DOI] [PubMed] [Google Scholar]
- 9. De Meyer GR, Bult H. Mechanisms of neointima formation–lessons from experimental models. Vasc Med 2: 179–189, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Fager G, Hansson GK, Gown AM, Larson DM, Skalli O, Bondjers G. Human arterial smooth muscle cells in culture: inverse relationship between proliferation and expression of contractile proteins. In Vitro Cell Dev Biol 25: 511–520, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res 93: 907–916, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science 253: 1129–1132, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Fishbein I, Waltenberger J, Banai S, Rabinovich L, Chorny M, Levitzki A, Gazit A, Huber R, Mayr U, Gertz SD, Golomb G. Local delivery of platelet-derived growth factor receptor-specific tyrphostin inhibits neointimal formation in rats. Arterioscler Thromb Vasc Biol 20: 667–676, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Giese NA, Marijianowski MM, McCook O, Hancock A, Ramakrishnan V, Fretto LJ, Chen C, Kelly AB, Koziol JA, Wilcox JN, Hanson SR. The role of α and β platelet-derived growth factor receptor in the vascular response to injury in nonhuman primates. Arterioscler Thromb Vasc Biol 19: 900–909, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Hassid A, Yao J, Huang S. NO alters cell shape and motility in aortic smooth muscle cells via protein tyrosine phosphatase 1B activation. Am J Physiol Heart Circ Physiol 277: H1014–H1026, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res 71: 1525–1532, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Kaszubska W, Falls H, Schaefer V, Haasch D, Frost L, Hessler P, Kroeger P, White D, Jirousek M, Trevillyan J. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol 195: 109–118, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci 11: 356–367, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Myllarniemi M, Calderon L, Lemstrom K, Buchdunger E, Hayry P. Inhibition of platelet-derived growth factor receptor tyrosine kinase inhibits vascular smooth muscle cell migration and proliferation. FASEB J 11: 1119–1126, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Nakata S, Tsutsui M, Shimokawa H, Tamura M, Tasaki H, Morishita T, Suda O, Ueno S, Toyohira Y, Nakashima Y, Yanagihara N. Vascular neuronal NO synthase is selectively upregulated by platelet-derived growth factor. Involvement of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway. Arterioscler Thromb Vasc Biol 13: 2502–2508, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Parker JO. Intermittent transdermal nitroglycerin therapy in the treatment of chronic stable angina. J Am Coll Cardiol 13: 794–795, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801–809, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, Kleppisch T, Ruth P, Hofmann F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res 87: 825–830, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Schwartz SM. Smooth muscle migration in vascular development and pathogenesis. Transpl Immunol 5: 255–260, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Sellak H, Choi C, Browner N, Lincoln TM. Upstream stimulatory factors (USF-1/USF-2) regulate human cGMP-dependent protein kinase I gene expression in vascular smooth muscle cells. J Biol Chem 280: 18425–18433, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Sellak H, Yang X, Cao X, Cornwell T, Soff GA, Lincoln T. Sp1 transcription factor as a molecular target for nitric oxide- and cyclic nucleotide-mediated suppression of cGMP-dependent protein kinase-Iα expression in vascular smooth muscle cells. Circ Res 90: 405–412, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Shimizu S, Ugi S, Maegawa H, Egawa K, Nishio Y, Yoshizaki T, Shi K, Nagai Y, Morino K, Nemoto K, Nakamura T, Bryer-Ash M, Kashiwagi A. Protein-tyrosine phosphatase 1B as new activator for hepatic lipogenesis via sterol regulatory element-binding protein-1 gene expression. J Biol Chem 278: 43095–43101, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Soff GA, Cornwell TL, Cundiff DL, Gately S, Lincoln TM. Smooth muscle cell expression of type I cyclic GMP-dependent protein kinase is suppressed by continuous exposure to nitrovasodilators, theophylline, cyclic GMP, and cyclic AMP. J Clin Invest 100: 2580–2587, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sreejayan N, Lin Y, Hassid A. NO attenuates insulin signaling and motility in aortic smooth muscle cells via protein tyrosine phosphatase 1B-mediated mechanism. Arterioscler Thromb Vasc Biol 22: 1086–1092, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Tamura N, Itoh H, Ogawa Y, Nakagawa O, Harada M, Chun TH, Suga S, Yoshimasa T, Nakao K. cDNA cloning and gene expression of human type Iα cGMP-dependent protein kinase. Hypertension 27: 552–557, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Thadani U, Fung HL, Darke AC, Parker JO. Oral isosorbide dinitrate in angina pectoris: comparison of duration of action an dose-response relation during acute and sustained therapy. Am J Cardiol 49: 411–419, 1982 [DOI] [PubMed] [Google Scholar]
- 32. Thyberg J, Palmberg L, Nilsson J, Ksiazek T, Sjolund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation 25: 156–167, 1983 [DOI] [PubMed] [Google Scholar]
- 33. Winquist RJ, Hintze TH. Mechanisms of atrial natriuretic factor-induced vasodilation. Pharmacol Ther 48: 417–426, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Yamasaki Y, Miyoshi K, Oda N, Watanabe M, Miyake H, Chan J, Wang X, Sun L, Tang C, McMahon G, Lipson KE. Weekly dosing with the platelet-derived growth factor receptor tyrosine kinase inhibitor SU9518 significantly inhibits arterial stenosis. Circ Res 88: 630–636, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Zeng Y, Zhuang S, Gloddek J, Tseng CC, Boss GR, Pilz RB. Regulation of cGMP-dependent protein kinase expression by Rho and Kruppel-like transcription factor-4. J Biol Chem 281: 16951–16961, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Zhuang D, Ceacareanu AC, Ceacareanu B, Hassid A. Essential role of protein kinase G and decreased cytoplasmic Ca2+ levels in NO-induced inhibition of rat aortic smooth muscle cell motility. Am J Physiol Heart Circ Physiol 288: H1859–H1866, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Zhuang D, Pu Q, Ceacareanu B, Chang Y, Dixit M, Hassid A. Chronic insulin treatment amplifies PDGF-induced motility in differentiated aortic smooth muscle cells by suppressing the expression and function of PTP1B. Am J Physiol Heart Circ Physiol 295: H163–H173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]