Abstract
Urocortins are members of the hypothalamic corticotropin-releasing factor (CRF) peptide family. Urocortin1 (UCN1) mRNA has been reported to be expressed in the brainstem neurons. The present investigation was carried out to test the hypothesis that microinjections of UCN1 into the nucleus ambiguus (nAmb) may elicit cardiac effects. Urethane-anesthetized, artificially ventilated, adult male Wistar rats, weighing between 300–350 g, were used. nAmb was identified by microinjections of l-glutamate (5 mM, 30 nl). Microinjections (30 nl) of different concentrations (0.062, 0.125, 0.25, and 0.5 mM) of UCN1 into the nAmb elicited bradycardic responses (26.5 ± 1, 30.1 ± 1.7, 46.9 ± 1.7, and 40.3 ± 2.6 beats/min, respectively). These heart rate responses were not accompanied by significant changes in mean arterial pressure. The bradycardic responses to maximally effective concentration of UCN1 (0.25 mM) were significantly (P < 0.05) attenuated by prior microinjections of a selective antagonist (NBI 27914, 1.5 mM) for CRF type 1 receptor (CRF1R). Prior microinjections of ionotropic glutamate receptor (iGLUR) antagonists [d-(−)-2-amino-7-phosphono-heptanoic acid and 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-(f)quinoxaline-7-sulfonamide disodium] also attenuated the bradycardia elicited by UCN1 microinjections into the nAmb. Microinjections of NBI 27914 (1.5 mM) into the nAmb did not alter baroreflex responses. Bilateral vagotomy abolished the bradycardic responses to microinjections of UCN1 into the nAmb. These results indicated that 1) microinjections of UCN1 into the nAmb elicited bradycardia, 2) the bradycardia was vagally mediated, 3) activation of CRF1Rs in the nAmb was responsible for the actions of UCN1, and 4) activation of iGLURs in the nAmb also participated in the bradycardia elicited by UCN1.
Keywords: corticotropin-releasing factor receptors, vagus
urocortins are members of the hypothalamic corticotropin-releasing factor (CRF) peptide family. These peptides include urocortin1 (UCN1, formerly known as urocortin), urocortin2 (UCN2, also known as stresscopin-related peptide), and urocortin3 (UCN3, also known as stresscopin) (11, 15, 18, 32). Urocortins mediate their actions via CRF receptors (CRFRs). There are two major subtypes of CRF receptors, CRF1Rs and CRF2Rs (8, 12, 14). These receptors are seven-transmembrane-domain Gs protein-coupled receptors and stimulate adenylate cyclase activity.
Information regarding the neurocircuitry of UCN1 has been reviewed recently (17, 21, 34) and can be summarized as follows. Initial reports indicated that UCN1-containing cells were primarily located in the Edinger-Westphal nucleus (EWN), which is involved in ocular function. Subsequently it was demonstrated that UCN1-containing neurons were not identical with cholinergic neurons of the EWN and formed a separate group of cells that has been named as nonpreganglionic EWN (33). In the hypothalamus, UCN1 is present in the supraoptic nucleus and lateral hypothalamus (23). UCN1-containing cells have also been identified in other areas of the brainstem including the lateral superior olive and the facial and hypoglossal nuclei (4, 40). UCN1-positive axons and terminals have been reported to be distributed diffusely throughout many brain regions and along the length of the spinal cord including lamina VII, which contains the sympathetic preganglionic neurons of the intermediolateral cell column (39).
Peripheral administration of urocortins elicits decrease in systemic blood pressure (BP) and reduction of plasma concentrations of vasoconstrictor hormones such as angiotensin II, arginine vasopressin, and endothelin-1 (10, 40, 44). Application of UCN2 to isolated adult rabbit ventricular cardiomyocytes exerts direct positive inotropic as well as lusitropic effects via activation of CRF2Rs (46). In healthy humans, UCN2 infusion has been reported to increase cardiac output and decrease mean and diastolic BP and systemic vascular resistance (9). There is increasing evidence that urocortins can be useful in the management of cardiovascular diseases such as heart failure, ischemic heart disease, and hypertension (41). For example, in sheep with pacing-induced heart failure, administration of UCN1, UCN2, and UCN3 increased cardiac output and decreased peripheral resistance and left atrial pressure (29, 30, 31). In the periphery, the positive inotropic and vasodilator effects of urocortins are mediated via activation of cardiac and vascular CRF2Rs, respectively.
CRFRs are distributed widely in the brain (8, 38). The information available regarding the cardiovascular effects of activation of central CRFRs is limited. Because urocortins can reach the central nervous system via a unique transport mechanism (28), these peptides are expected to affect cardiovascular function. Indeed direct microinjections of urocortins into different brain regions elicit different responses. For example, microinjections of urocortins into the medial subnucleus of the nucleus tractus solitarius (mNTS) decrease BP and heart rate (HR) and sympathetic nerve activity (24, 25, 45), whereas similar microinjections into the hypothalamic paraventricular nucleus elicit opposite responses (20). The presence of UCN1 and CRF1Rs, but not UCN2, UCN3, or CRF2Rs, has been reported in the nucleus ambiguus (nAmb) of the rat (4, 18, 19, 32, 38, 40). UCN1-immunoreactive fibers have also been identified in the ventral medulla, but the sources of these projections remain to be determined (4). On the basis of these reports, it was hypothesized that activation of CRF1Rs in the nAmb by UCN1 may elicit cardiac effects. This hypothesis was tested in the present study by direct microinjections of UCN1 into the nAmb and area surrounding its compact portion, which is the predominant brain area providing parasympathetic innervation of the heart in the rat (2, 6, 26–27, 37).
MATERIALS AND METHODS
General procedures.
Experiments were carried out in adult male Wistar rats (n = 50) (Charles River Laboratories, Wilmington, MA) weighing 300–350 g. All animals were housed under controlled conditions with a 12-h:12-h light/dark cycle. Food and water were available to the animals ad libitum. The experiments were performed according to the NIH Guide for the Care and Use of Laboratory Animals (7th Edition, 1996) and with the approval of the Institutional Animal Care and Use Committee of University of Medicine and Dentistry of New Jersey.
The general procedures have been described in detail elsewhere (7). Briefly, the rats were anesthetized with inhalation of isoflurane (2–3% in 100% oxygen), the trachea was cannulated, and the rats were artificially ventilated to avoid cardiovascular effects secondary to respiratory changes, if any, induced by microinjections of UCN1 into the nAmb. The tidal volume and frequency on the ventilator were adjusted to maintain the end-tidal CO2 at 3.5–4.5%. The end-tidal CO2 was measured continuously in the expired gas using an infrared CO2 analyzer for small animals. One of the femoral veins was cannulated, and urethane (1.2–1.4 g/kg) was injected intravenously in 12–15 aliquots at 2-min intervals. Isoflurane inhalation was terminated after the administration of five to six aliquots of urethane. Absence of withdrawal of the limb in response to pinching of a hind paw indicated that the rats were properly anesthetized. Rectal temperature was maintained at 36.5 ± 0.5°C. One of the femoral arteries was cannulated, and BP was recorded using a polygraph. The BP signals were digitized, and mean arterial pressure (MAP) and HR signals were derived from them using 1401 A/D converter and Spike 2 software (Cambridge Electronic Design, Cambridge, UK). All of the tracings were stored on a computer hard drive.
Vagotomy.
Vagotomy was necessary in experiments designed to investigate the role of parasympathetic innervation to the heart in mediating the UCN1-induced HR responses. In these experiments, silk sutures were placed loosely around the vagus nerves bilaterally for subsequent identification and sectioning.
Baroreflex function test.
To measure the parasympathetic component of baroreflex, phenylephrine (PE) was infused intravenously and baroreflex function curves were obtained by plotting reflex decreases in HR in response to increases in MAP. The following protocol was used (7). Approximately linear increase in MAP was induced by infusing PE (125 μg/ml) with a 5-ml syringe connected to the femoral venous cannula and mounted on an infusion pump (model no. 341; Sage Instruments, Cambridge, MA). PE was administered continuously every 30 s by ramped infusion at a rate of 0.12, 0.17, and 0.25 ml/min until the MAP increased by 70 mmHg. The protocol was repeated after bilateral microinjections of artificial cerebrospinal fluid (aCSF) and CRF1R antagonist (NBI 27914; 1.5 mM) into the nAmb. In each experiment, PE infusions were made before any microinjections into the nAmb (control), after microinjection of aCSF, and after microinjection of CRF1R antagonist; the interval between PE infusions was at least 20 min.
Microinjections.
The details of this technique have been described elsewhere (7). Briefly, the rats were placed in a prone position in a stereotaxic instrument with bite bar 18 mm below the interaural line. The microinjections were made using a dorsal approach. Four-barreled glass micropipettes (tip size 20–40 μm) were mounted on a micromanipulator, and each barrel was connected to one of the channels on a picospritzer. One of the barrels contained l-glutamate (l-GLU), whereas the contents of other barrels varied according to the requirements of the experiment. The following coordinates were used for the identification of the nAmb: 0.3 caudal to 1.1 mm rostral and 1.8–2.0 mm lateral to the calamus scriptorius and 2.0–2.4 mm deep from the dorsal medullary surface. The sites eliciting bradycardia in the nAmb were identified by microinjections of l-GLU (5 mM). The volume of all microinjections into the nAmb was 30 nl; the selection of this volume was based on our preliminary studies in which 30-nl volume of l-GLU (5 mM) elicited maximum bradycardic responses. The volumes were pressure ejected (30–35 psi) and visually confirmed by the displacement of fluid meniscus in the barrel containing the solution. The duration of microinjection was 5–10 s. Microinjections (30 nl) of aCSF (pH 7.4) were used as controls.
Decerebration.
Midcollicular decerebration was performed in isoflurane-anesthetized rats as described elsewhere (7). After the decerebration, isoflurane administration was discontinued and a stabilization period of 60 min was allowed.
Histology.
At the end of the experiment, the sites of microinjections into the nAmb were marked by diluted India ink and the brain tissue was processed for histology as described elsewhere (7).
Drugs and chemicals.
The following drugs and chemicals were used: d-(−)-2-amino-7-phosphono-heptanoic acid [d-AP7; N-methyl-d-aspartate (NMDA) receptor antagonist], 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-(f)quinoxaline-7-sulfonamide disodium (NBQX disodium salt; non-NMDA receptor antagonist), 5-chloro-N-(cyclopropylmethyl)-2-methyl-N-propyl-N′-(2,4,6-trichlorophenyl)-4,6-pyrimidinediamine hydrochloride (NBI 27914; selective antagonist for CRF1R) (5), UCN1, l-GLU, PE, isoflurane, and urethane. All of the solutions for the microinjections were freshly prepared in aCSF (pH 7.4). The concentration of drugs refers to their salts where applicable. The sources of different drugs and chemicals were as follows: UCN1 (American Peptide, Sunnyvale, CA), NBI 27914 (Tocris Cookson, Ellisville, MO), and isoflurane (Baxter Pharmaceutical Products, Deerfield, IL). All other drugs and chemicals were obtained from Sigma Chemicals (St. Louis, MO).
Statistical analyses.
The mean and SE were calculated for the maximum decreases in HR in response to microinjections of UCN1 or l-GLU into the nAmb. In the concentration-response studies, comparisons of the maximum decreases in HR in different groups of rats were made by using a one-way analysis of variance (ANOVA) followed by Tukey-Kramer's multiple comparison. In experiments testing for tachyphylaxis and effect of CRFR antagonists, comparisons of the maximum decreases in HR elicited by microinjection of UCN1 into the nAmb were made by using repeated-measures ANOVA followed by Tukey-Kramer's multiple comparison. Student's paired t-test was used for comparisons of maximum decreases in HR elicited by the following: 1) microinjection of UCN1 into the nAmb before and after the bilateral vagotomy and 2) microinjection of l-GLU into the nAmb before and after the microinjections of CRFR antagonist. Unpaired t-test was used for comparisons of the maximum decreases in HR elicited by microinjection of UCN1 into the nAmb in anesthetized and decerebrate rats. In all cases, the differences were considered significant at P < 0.05.
RESULTS
Baseline values for MAP and HR in urethane-anesthetized rats were 100.0 ± 8.3 mmHg and 404 ± 11.4 beats/min, respectively (n = 45). The values for baseline MAP and HR in the decerebrate rats (n = 5) were 92.1 ± 2 mmHg and 411 ± 34 beats/min, respectively. There were no significant differences (P > 0.05) between the baseline values of MAP and HR in urethane-anesthetized and decerebrate rats.
Concentration response of UCN1.
Bradycardic responses without significant changes in MAP elicited by microinjections (30 nl each) of different concentrations of UCN1 into the nAmb are shown in Table 1. The nAmb was previously identified by microinjections of l-GLU (5 mM), which elicited a decrease in HR (80 ± 7.3 beats/min) without significant changes in MAP (n = 10). Maximum bradycardic responses were elicited by the 0.25 mM concentration of UCN1. This concentration was selected for further studies in other experiments.
Table 1.
Concentration response of microinjections of UCN1 into the nAmb (n = 10)
| UCN1, mM | Decrease in HR, beats/min | Decrease in MAP, mmHg |
|---|---|---|
| 0.062 | 26.5 ± 1.0 | 1.2 ± 0.8 |
| 0.125 | 30.1 ± 1.7 | 1.3 ± 1.5 |
| 0.250 | 46.9 ± 1.7* | 2.0 ± 1.6 |
| 0.500 | 40.3 ± 2.6 | 2.5 ± 1.7 |
Applicable values are means ± SE. UCN1, urocortin1; nAmb, nucleus ambiguus; HR, heart rate; MAP, mean arterial pressure. The bradycardic response was significantly greater than the responses elicited by other concentrations;
P < 0.001.
Reproducibility of UCN1 responses.
The concentration of UCN1 that elicited maximal bradycardic responses (0.25 mM) was microinjected into the nAmb at least three times, at 40-min intervals (n = 5). The decreases in HR induced by three consecutive microinjections of UCN1 were 38 ± 5, 45.7 ± 3, and 46 ± 5 beasts/min, respectively (P > 0.05). Thus no tachyphylaxis of bradycardic responses was observed with repeated microinjections of UCN1 when the interval between injections was 40 min.
Site specificity of UCN1 responses.
The concentrations of UCN1 (0.25 mM, 30 nl) that elicited maximal bradycardic responses when microinjected into the nAmb (n = 5) did not elicit a response when injected intravenously. Microinjections of UCN1 (0.25 mM) into areas adjacent to the nAmb (e.g., ventral region of the dorsal medullary reticular nucleus; 0.6 mm rostral and 2.3 mm lateral to the calamus scriptorius and 2.6 mm deep from the dorsal medullary surface) elicited no HR responses.
Effect of UCN1 in decerebrate rats.
The nAmb was identified by microinjection of l-GLU (5 mM) in a group of decerebrate rats (n = 5); a decrease in HR (137 ± 23 beats/min) was observed. Five minutes later, microinjections of UCN1 (0.25 mM) at the same site elicited bradycardia (49 ± 2 beats/min), which was not statistically different (P > 0.05) when compared with bradycardia elicited by the microinjection of the same dose of UCN1 into the nAmb of urethane-anesthetized rats (45.7 ± 2.7 beats/min).
Effect of bilateral vagotomy.
The nAmb was identified by microinjection of l-GLU (5 mM); a decrease in HR (66.7 ± 10.5 beats/min ) was observed. Five minutes later, microinjections of UCN1 (0.25 mM) at the same site elicited a bradycardia (42.8 ± 2.1 beats/min). Forty minutes later, bilateral vagotomy was performed, and a 10-min period of stabilization was allowed. At this time, microinjection of UCN1 into the nAmb failed to elicit a bradycardic response (Table 2). Five minutes later, l-GLU was microinjected into the nAmb; the bradycardic response to l-GLU was also abolished.
Table 2.
Effect of bilateral vagotomy or different antagonists on changes in HR and MAP elicited by UCN1 (0.25 mM)
| UCN1-Induced Decreases in HR, beats/min |
UCN1-Induced Decreases in MAP, mmHg |
||||
|---|---|---|---|---|---|
| n | Procedure or Antagonist | Before | After | Before | After |
| 5 | Bilateral vagotomy | 42.8 ± 2.1 | * | 2.8 ± 2.1 | 2.5 ± 2.0 |
| 5 | NBI 27914 (1.0 mM) | 32.l ± 3 | 20 ± 3.1† | 2.1 ± 1.5 | 2.0 ± 1.6 |
| 5 | NBI 27914 (1.5 mM) | 42.1 ± 4.5 | 7.6 ± 1.0‡ | 3.2 ± 0.9 | 3.0 ± 1.6 |
| 5 | NBQX (2 mM) + d-AP7 (5 mM) | 30 ± 2.6 | 13.5 ± 1.5‡ | 2.0 ± 1.8 | 1.9 ± 1.8 |
Values are means ± SE. The antagonists and UCN1 were microinjected into the nAmb.
Abolition of the bradycardic response.
Decreases in HR before and after the antagonist were not significantly different (P > 0.05).
Decreases in HR after the antagonist were significantly smaller compared to the corresponding values before the antagonist (P < 0.05). NBQX, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-(f)quinoxaline-7-sulfonamide disodium; d-AP7, d-(−)-2-amino-7-phosphono-heptanoic acid.
Effect of selective CRF1R antagonist on UCN1 responses.
Microinjections of l-GLU (5 mM) into the nAmb elicited decreases in HR (80 ± 13 beats/min) without concomitant changes in MAP (n = 5). Five minutes later, microinjection of the UCN1 (0.25 mM) into the nAmb elicited a decrease in HR without significant changes in MAP (Table 2). Forty minutes later, microinjections of NBI 27914 (1 mM) did not attenuate the bradycardic responses to microinjections of UCN1 (0.25 mM) (Table 2). In another group of rats (n = 5), a higher concentration of CRF1R antagonist (1.5 mM) was microinjected into nAmb, which was followed by another microinjection of UCN1 (0.25 mM). The bradycardic responses to microinjections UCN1 (0.25 mM) were significantly attenuated (Table 2). Within 40–50 min, the bradycardic responses to microinjection of UCN1 into the nAmb (31.1 ± 3.2 beats/min) recovered. A tracing of these results is shown in Fig. 1.
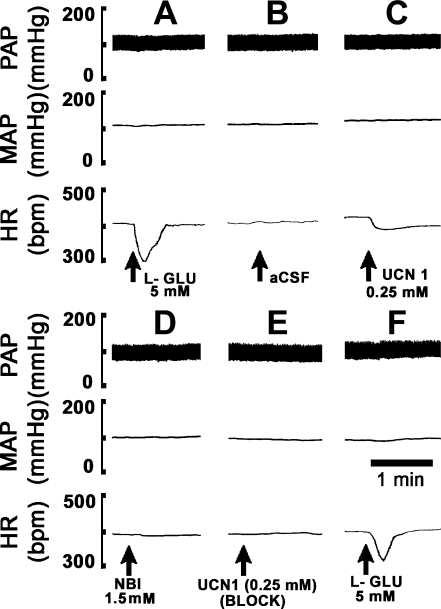
Fig. 1.
A typical tracing showing blockade of urocortin1 (UCN1)-induced bradycardia. Top: pulsatile arterial pressure (PAP, mmHg). Middle: mean arterial pressure (MAP, mmHg). Bottom: heart rate (HR, beats/min). A: microinjections of l-glutamate (l-GLU) (5 mM) into the nucleus ambiguus (nAmb) elicited a decrease in HR with no significant changes in MAP. B: after 5 min, microinjection of artificial cerebrospinal fluid (aCSF) at the same site did not elicit any changes in HR. C: after an interval of 5 min, UCN1 (0.25 mM) microinjection into the nAmb elicited bradycardia but no significant changes in MAP. D: corticotropin-releasing factor type 1 receptor (CRF1R) antagonist [NBI 27914 (NBI); 1.5 mM] was microinjected at the same site after 40 min. No significant changes in MAP and HR were observed. E: 2 min after the microinjection of the antagonist, UCN1 (0.25 mM) was microinjected again. The bradycardia induced by UCN1 was blocked. F: after 5 min, microinjection of l-GLU (5 mM) into the nAmb elicited usual bradycardia, indicating that the CRF1R antagonist did not alter the responses to an unrelated agonist.
Effect of iGLUR antagonists on UCN1-induced response.
The nAmb was identified by microinjections of l-GLU (5 mM) as in other experiments (n = 5). After an interval of 5 min, microinjections of UCN1 (0.25 mM) at the same site elicited bradycardia with no significant changes in MAP. Forty minutes later, NBQX (2 mM) and d-AP7 (5 mM) were microinjected sequentially (within 2 min) at the same site. The concentrations of NBQX and d-AP7 were selected from our previous report (24). The microinjection of UCN1 (0.25 mM) was repeated at the same site within 8–10 min. UCN1-induced decrease in HR was significantly attenuated by ionotropic glutamate receptor (iGLUR) antagonists (Table 2).
Effect of CRF1R antagonist on baroreflex.
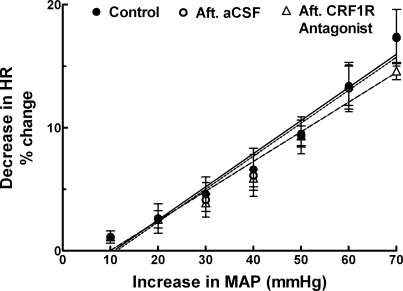
The nAmb was identified bilaterally by microinjections of l-GLU (5 mM); the decrease in HR was 80 ± 7.3 beats/min (n = 5). The following three protocols were used to test the baroreceptor function (Fig. 2). First, control baroreflex responses were obtained before any microinjections into the nAmb; reflex decreases in HR (change from basal value) were 1.1 ± 0.6, 2.6 ± 1.2, 4.6 ± 1.4, 6.6 ± 1.7, 9.5 ± 1.1, 13.4 ± 1.9, and 17.4 ± 2.2% in response to 10, 20, 30, 40, 50, 60, and 70 mmHg increases in MAP, respectively. Twenty minutes later, the same protocol was repeated after bilateral microinjections of aCSF into the nAmb; reflex decreases in HR were 1.1 ± 0.5, 2.6 ± 1.2, 4.1 ± 1.4, 6.1 ± 1.7, 9.4 ± 1.5, 13.2 ± 1.9, and 17.3 ± 2.3%, respectively. After an interval of 20 min, the same protocol was repeated after bilateral microinjections of CRF1R antagonist (NBI 27914, 1.5 mM) into the nAmb; reflex decreases in HR were 1.1 ± 0.3, 2.6 ± 0.7, 3.9 ± 0.7, 5.9 ± 0.7, 9.3 ± 0.8, 13.4 ± 1.6, and 14.6 ± 0.7%, respectively. No significant differences (P > 0.05) in the reflex HR decreases in response to different increases in MAP were observed after the microinjections of CRF1R antagonist into the nAmb. Microinjections of aCSF into the nAmb also did not alter reflex HR decreases. No significant changes in basal HR were elicited by bilateral microinjections of either the CRF1R antagonist or aCSF. The concentration of CRF1R antagonist (1.5 mM) used in these experiments was previously shown to completely block the effects of the maximally effective concentration of UCN1 (0.25 mM) in the nAmb.
Fig. 2.
Effect of UCN1 on baroreflex-induced bradycardia. Linear regression curves showing the control baroreflex responses before any microinjections into the nAmb (● and solid line), after (Aft) bilateral microinjections of aCSF (30 nl) into the nAmb (○ and dotted line), and after bilateral microinjections of a selective CRF1R antagonist (NBI 27914, 1.5 mM) into the nAmb (▵ and dashed line). The r2 values for the regression curves of the bradycardic reflex were 0.96, 0.95, 0.96 for control, aCSF, and CRF1R antagonist (NBI 27914) groups, respectively.
Histology.
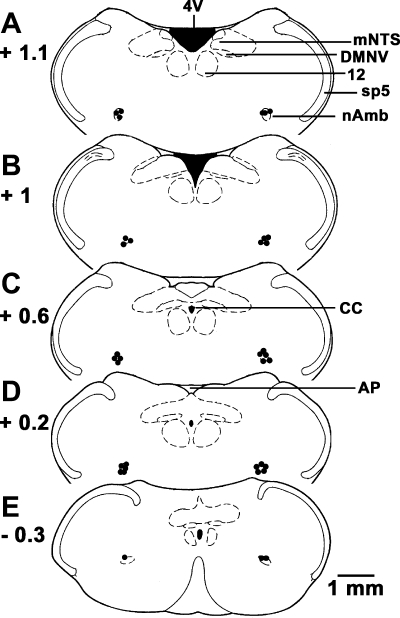
The nAmb sites, where microinjections of l-GLU and UCN1 elicited bradycardia, were marked with India ink in 35 rats. Composite diagrams of the microinjection sites in the nAmb are presented in Fig. 3.
Fig. 3.
Histological identification of microinjection sites. A–E: drawings of coronal sections at levels 1.1 mm rostral to 0.3 mm caudal to the calamus scriptorius. Microinjection sites are shown as dark spots (n = 35); each spot represents a site in one animal. AP, area postrema; CC, central canal; DMNV, dorsal motor nucleus of vagus; mNTS, medial nucleus tractus solitarius; Sp5, spinal trigeminal tract; 4V, fourth ventricle; 12, hypoglossal nucleus.
DISCUSSION
The main observation in this study was that microinjections of UCN1 into the nAmb elicited bradycardic responses. There are no reports in the literature regarding the cardiovascular effects of UCN1 in the nAmb for comparison of our results. Local distortion of brain tissue or any nonspecific effects were not responsible for the bradycardic effects elicited by microinjections of UCN1 into the nAmb because microinjections of aCSF at the same site did not elicit any response. Concentrations of UCN1 microinjections (0.25 mM, 30 nl) into the nAmb that elicited maximum decreases in HR did not elicit a response when injected intravenously, indicating that leakage of UCN1, if any, from the microinjection site into the peripheral circulation was not responsible for the observed responses. The UCN1-induced bradycardic responses elicited from the nAmb were site specific because similar microinjections into the adjacent areas elicited no responses.
The mechanism of the bradycardic responses elicited by UCN1 microinjections into the nAmb can be explained as follows on the basis of our present knowledge regarding the medullary control of cardiac function (22, 35–36). UCN1 microinjections may have excited the neurons located in the region surrounding the compact formation of the nAmb, increasing the activity of vagal input to the heart and causing bradycardia. The bradycardic responses to microinjections of UCN1 into the nAmb were blocked by prior microinjections of NBI 27914 at the same site, suggesting that these responses were mediated via CRF1Rs in this brain area. NBI 27914 has been reported to be a selective antagonist for CRF1Rs (1). Furthermore, this antagonist did not alter responses to microinjections of an unrelated agonist, l-GLU, into the nAmb. Because unilateral or bilateral microinjections of the antagonist alone into the nAmb area did not elicit significant changes in basal MAP and HR, it was concluded that under normal physiological conditions CRF1Rs in the nAmb were not under tonic control of endogenous UCN1.
The bradycardic effects elicited by microinjections of UCN1 into the nAmb were lesser in magnitude than those elicited by microinjections of l-GLU. Weaker responses to UCN1 may be due to lesser density of CRFRs in the nAmb (8, 38).
Microinjections of UCN1 into the nAmb showed a bell-shaped concentration response. A similar type of concentration response has been reported for microinjections of urocortins into the mNTS (25). This type of concentration response has been explained by homotropic allostery in which the agonist at higher concentrations binds to a modulator site, which is different from the primary binding site, and thereby affects the function of the receptor, resulting in attenuated responses (3). Another possibility is that, at higher concentrations, UCN1 may activate inhibitory pathways located in the nAmb. For example, the presence of inhibitory glycinergic and GABAergic pathways has been reported in the nAmb (13). Activation of these pathways by UCN1 at higher concentrations is expected to reduce the vagal input to the heart and increase HR, causing a reduction in UCN1-induced bradycardic responses. The role of inhibitory mechanisms in the nAmb in reducing the cardiac responses at higher concentrations of UCN1 remains to be tested.
In midcollicular decerebrate rats, microinjections of UCN1 into the nAmb elicited bradycardic responses, which were not statistically different from those elicited in urethane-anesthetized rats. These results suggested that urethane anesthesia did not alter the responses to UCN1 qualitatively or quantitatively. Moreover, the presence of neural structures located rostral to the brainstem (e.g., hypothalamus) was not necessary for UCN1-induced effects in the nAmb.
The decreases in HR induced by microinjections of UCN1 into the nAmb were partly mediated via iGLURs. This conclusion was based on our observation that microinjections of iGLUR antagonists into the nAmb attenuated the UCN1-induced decreases in HR. We have previously reported similar actions of urocortins in the mNTS in which the specificity of the iGLUR antagonists used was also established (24). It may be pointed out that most neurons derive their on-going activity from glutamatergic synaptic inputs and that iGLUR antagonists may attenuate the effect of exogenously applied excitatory substances by virtue of the fact that the neurons in the brain area under investigation may be hyperpolarized. Therefore, results using iGLUR antagonists need to be interpreted with caution. However, we have previously demonstrated that the doses of NBQX and d-AP7 used in this study did not attenuate the effect of exogenously applied excitatory substances indiscriminately because the effects of an unrelated agonist, carbachol, remained unaltered (16, 24). The presence of glutamatergic inputs to the cardiac vagal neurons in the nAmb region has been reported (43). UCN1 microinjections may have activated CRFRs on the terminals of glutamatergic inputs in the nAmb and released glutamate, which, in turn, elicited decreases in HR. Consistent with this conclusion is the report that activation of CRFRs released glutamate in other brain areas (e.g., the ventral tegmental area) (42).
As mentioned in the introduction, there is very little information available regarding the central cardiovascular actions of urocortins. We have published studies on the actions of UCN1 and UCN3 in the NTS (24–25). In the present manuscript, we have focused on the action of UCN1 in the nAmb because neurons and fibers immunoreactive for this peptide and CRF1Rs have been reported to be present in this brain area (4, 18, 19, 38, 40). UCN2 and UCN3 were not included in the present article because there is no evidence for the presence of these peptides or CRF2Rs in the nAmb (4, 18, 19, 32, 38, 40). In this context, it may be noted that UCN1 mediates its actions via both CRF1Rs and CRF2Rs, whereas UCN2 and UCN3 mediate their actions primarily via CRF2Rs (14).
Several pharmacological studies have shown that urocortins are involved in the regulation of stress, anxiety states, food consumption, social behaviors, and drug self-administration (17, 28, 33–34). Pharmacological studies conducted in our and other laboratories indicate that urocortins may play a role in cardiovascular regulation also (9, 10, 24–25, 29–31, 40, 41, 44, 46). The presence of UCN1-positive neurons, fibers, and CRF1Rs in the nAmb and adjacent areas (4, 38, 40) is of interest in the context of our results. It is possible that UCN1-containing neurons are activated in the nAmb in some pathological situations, releasing UCN1 in this nucleus and causing bradycardia. However, further studies are warranted to establish the cardiovascular and other functions of endogenous urocortins.
The blockade of CRF1Rs in the nAmb did not significantly alter baroreflex-induced bradycardia. This observation rules out the possibility that UCN1 may be a neurotransmitter released in the nAmb in response to baroreflex activation. In this context, it may be noted that published reports suggest that glutamate may be the neurotransmitter in the nAmb that is mediating reflex bradycardic responses (43). The facts that microinjections of the CRF1R antagonist alone into the nAmb did not elicit significant HR responses and did not alter baroreflex-induced bradycardia indicate that UCN1 in the nAmb does not have a tonic influence on HR control under normal states. However, our results showed that activation of CRF1Rs by UCN1 elicits vagally mediated bradycardia. This observation prompts a speculation that UCN1 may be released in the nAmb during some pathological states (e.g., heart failure) to counterbalance disease-induced tachycardia.
The potential clinical significance of our results regarding centrally mediated bradycardic effect of UCN1 can be speculated to be as follows. There is a general consensus that, irrespective of the etiology, the first manifestation of congestive heart failure is usually tachycardia. Bradycardic responses elicited by UCN1 via its central action on the nAmb neurons could be beneficial in this situation. This possibility becomes relevant in view of the reports that peripherally administered urocortins can cross blood-brain barrier by a unique transport system (28). However, it should be noted that positive chronotropic and inotropic effects exerted by the peripheral administration of UCN1 may have an adverse impact on myocardial oxygen demand and may offset the potential beneficial effects of this peptide in heart failure (29, 30). The potential beneficial and adverse actions of UCN1 remain to be carefully assessed.
In summary, microinjections of UCN1 into the nAmb elicited bradycardic responses, which were mediated via CRF1Rs. iGLURs in the nAmb also participated in the UCN1-induced bradycardia. It was concluded that activation of neurons in the nAmb region caused bradycardia via increase in vagal activity because bilateral vagotomy abolished the bradycardic effect of UCN1.
GRANTS
This work was supported in part by NIH grants HL024347 and HL076248 awarded to Dr. H. N. Sapru.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Baram TZ, Chalmers DT, Chen C, Koutsoukos Y, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res 770: 89–95, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol 262: 546–562, 1987 [DOI] [PubMed] [Google Scholar]
- 3. Bindslev N. A homotropic two-state model and auto-antagonism. BMC Pharmacol 4: 11–22, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bittencourt JC, Vaughan J, Arias C, Riesman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol 415: 285–312, 1999 [PubMed] [Google Scholar]
- 5. Chen C, Dagnino R, Jr, De Souza EB, Grigoriadis DE, Huang CQ, Kim KI, Liu Z, Moran T, Webb TR, Whitten JP, Xie YF, McCarthy JR. Design and synthesis of a series of non-peptide high-affinity human corticotropin-releasing factor1 receptor antagonists. Med Chem 39: 4358–4360, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Cheng SB, Hayakawa T, Kuchiiwa S, Maeda S, Ito H, Seki M, Nakagawa S. Evidence for the collateral innervation of the esophagus and the heart from neurons in the compact formation of the nucleus ambiguus of the rat. Brain Res 832: 171–174, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Chitravanshi VC, Bhatt S, Sapru HN. Microinjections of alpha-melanocyte stimulating hormone into the nucleus ambiguus of the rat elicit vagally mediated bradycardia. Am J Physiol Regul Integr Comp Physiol 296: R1402–R1411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci 23: 71–77, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Davis ME, Pemberton CJ, Yandle TG, Fisher SF, Lainchbury JG, Frampton CM, Rademaker MT, Richards AM. Urocortin 2 infusion in healthy humans: hemodynamic, neurohormonal, and renal responses. J Am Coll Cardiol 49: 461–471, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Dieterle T, Meili-Butz S, Buhler K, Morandi C, John D, Buser PT, Rivier J, Vale WW, Peterson KL, Brink M. Immediate and sustained blood pressure lowering by urocortin 2: a novel approach to antihypertensive therapy? Hypertension 53: 739–744, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, Vaughan JM, Vale WW. Cloning and characterization of human urocortin. Endocrinology 137: 2167–2170, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Drolet G, Rivest S. Corticotropin-releasing hormone and its receptors; an evaluation at the transcription level in vivo. Peptides 22: 761–767, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Griffioen KJ, Venkatesan P, Huang ZG, Wang X, Bouairi E, Evans C, Gold A, Mendelowitz D. Fentanyl inhibits GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res 1007: 109–115, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotrophin releasing factor and their ligands. Pharmacol Rev 55: 21–26, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med 7: 605–611, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Kasamatsu K, Chitravanshi VC, Sapru HN. Depressor and bradycardic responses to microinjections of endomorphin-2 into the nucleus tractus solitarius are mediated via ionotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol 287: R715–R728, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol 391: 1–10, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci 22: 991–1001, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, Fan M, Shen L, Cao Y, Zhu D, Hong Z. Excitatory responses of cardiovascular activities to urocortin3 administration into the PVN of the rat. Auton Neurosci 154: 108–111, 2010 [DOI] [PubMed] [Google Scholar]
- 21. McAllen RM, Spyer KM. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J Physiol 282: 353–364, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 14: 155–161, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Morin SM, Ling N, Liu XJ, Kahl SD, Gehlert DR. Differential distribution of urocortin- and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience 92: 281–291, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Nakamura T, Sapru HN. Cardiovascular responses to microinjections of urocortins into the NTS: role of ionotropic glutamate receptors. Am J Physiol Heart Circ Physiol 296: H2022–H2029, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura T, Kawabe K, Sapru HN. Cardiovascular responses to microinjections of urocortin 3 into the nucleus tractus solitarius of the rat. Am J Physiol Heart Circ Physiol 296: H325–H332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nosaka S, Yamamoto T, Yasunaga K. Localization of vagal cardioinhibitory preganglionic neurons within rat brain stem. J Comp Neurol 186:79–92, 1979 [DOI] [PubMed] [Google Scholar]
- 27. Nosaka S, Yasunaga K, Tamai S. Vagal cardiac preganglionic neurons: distribution, cell types, and reflex discharges. Am J Physiol Regul Integr Comp Physiol 243: R92–R98, 1982 [DOI] [PubMed] [Google Scholar]
- 28. Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol 84: 148–156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rademaker MT, Cameron VA, Charles CJ, Richards AM. Integrated hemodynamic, hormonal, and renal actions of urocortin 2 in normal and paced sheep: beneficial effects in heart failure. Circulation 112: 3624–3632, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Rademaker MT, Charles CJ, Espiner EA, Frampton CM, Lainchbury JG, Richards AM. Four-day urocortin-I administration has sustained beneficial haemodynamic, hormonal, and renal effects in experimental heart failure. Eur Heart J 26: 2055–2062, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Rademaker MT, Cameron VA, Charles CJ, Richards AM. Urocortin 3: haemodynamic, hormonal, and renal effects in experimental heart failure. Eur Heart J 27: 2088–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 98: 2843–2848, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience 134:1317–1323, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev 52: 368–380, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol 29: 491–496, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Takanaga A, Hayakawa T, Tanaka K, Kawabata K, Maeda S, Seki M. Immunohistochemical characterization of cardiac vagal preganglionic neurons in the rat. Auton Neurosci 106: 132–137, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Vasconcelos LA, Donaldson C, Sita LV, Casatti CA, Lotfi CF, Wang L, Cadinouche MZ, Frigo L, Elias CF, Lovejoy DA, Bittencourt JC. Urocortin in the central nervous system of a primate (Cebus apella): sequencing, immunohistochemical, and hybridization histochemical characterization. J Comp Neurol 463: 157–175, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378: 287–292, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Venkatasubramanian S, Newby DE, Lang NN. Urocortins in heart failure. Biochem Pharmacol 80: 289–296, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 193: 283–294, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann NY Acad Sci 940: 237–246, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Wiley KE, Davenport AP. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Br J Pharmacol 143: 508–514, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamazaki T, Waki H, Kohsaka A, Nakamura T, Cui H, Yukawa K, Maeda M. Microinjection of urocortin into the rat nucleus tractus solitarii decreases arterial blood pressure. Auton Neurosci 142: 51–54, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Yang LZ, Kockskamper J, Heinzel FR, Hauber M, Walther S, Spiess J, Pieske B. Urocortin II enhances contractility in rabbit ventricular myocytes via CRF2 receptor-mediated stimulation of protein kinase A. Cardiovasc Res 69: 402–411, 2006 [DOI] [PubMed] [Google Scholar]