Abstract
Long QT syndrome type 2 (LQT2) is caused by mutations in the human ether-a-go-go-related gene (hERG). Cryptic splice site activation in hERG has recently been identified as a novel pathogenic mechanism of LQT2. In this report, we characterize a hERG splice site mutation, 2592+1G>A, which occurs at the 5′ splice site of intron 10. Reverse transcription-PCR analyses using hERG minigenes transfected into human embryonic kidney-293 cells and HL-1 cardiomyocytes revealed that the 2592+1G>A mutation disrupted normal splicing and caused multiple splicing defects: the activation of cryptic splice sites within exon 10 and intron 10 and complete intron 10 retention. We performed functional and biochemical analyses of the major splice product, hERGΔ24, in which 24 amino acids within the cyclic nucleotide binding domain of the hERG channel COOH-terminus is deleted. Patch-clamp experiments revealed that the splice mutant did not generate hERG current. Western blot and immunostaining studies showed that mutant channels did not traffic to the cell surface. Coexpression of wild-type hERG and hERGΔ24 resulted in significant dominant-negative suppression of hERG current via the intracellular retention of the wild-type channels. Our results demonstrate that 2592+1G>A causes multiple splicing defects, consistent with the pathogenic mechanisms of long QT syndrome.
Keywords: ion channels, protein trafficking, RNA splicing
mutations in the human ether-a-go-go-related gene (hERG) cause the heritable form of long QT syndrome type 2 (LQT2) (8). The hallmark of LQT2 is the prolongation of the QT interval on the electrocardiogram, which is caused by delayed cardiac repolarization and can lead to ventricular arrhythmia, syncope, and sudden death (18). Congenital LQT2 is an autosomal dominant disorder caused by the loss of functional hERG channels. The protein encoded by hERG is a tetrameric K+ channel and a well-characterized component of the rapidly activating delayed rectifier current in the heart (16, 23, 28, 31).
Multiple pathogenic mechanisms induced by LQT2 mutations have been documented, including defects in hERG assembly and trafficking, abnormalities in channel gating and permeation, and the dominant-negative suppression of hERG current (2, 12, 22). Previous mechanistic studies of LQT2 mutations have predominantly focused on missense and frame-shift mutations that disrupt the coding sequence of hERG channels. Recent studies on the nonsense-mediated mRNA decay (NMD) of mutant hERG transcripts and on pathogenic cryptic splicing events induced by splice site mutations have underscored the importance of including RNA analysis in the characterization of LQT2 (7, 14). Over 20 LQT2 mutations are predicted to disrupt the splicing of hERG pre-mRNA, and, to date, only a few hERG splice site mutations have been characterized (7, 13, 29).
In normal eukaryotic pre-mRNA processing, the consensus sequence for the 5′ splice site is defined by a 9-bp region at the exon-intron boundary, in which the +1 and +2 positions are 100% conserved as a guanine and thymine, respectively. The LQT2 mutation 2592+1G>A disrupts the invariant +1 position of the 5′ splice site sequence of intron 10. To investigate the pathogenic mechanisms associated with 2592+1G>A, we performed mRNA analysis using wild-type (WT) and mutant minigenes to determine the specific splicing defects. Our results indicated that the 2592+1G>A mutation induces multiple splicing defects, including the activation of three cryptic 5′ splice sites and complete intron 10 retention. Three of the mutant splice products contained a premature termination codon (PTC), while the fourth transcript leads to an in-frame deletion of 24 amino acids from the highly structured, cyclic nucleotide binding domain in the COOH-terminus of the hERG channel. Biochemical and patch-clamp studies revealed trafficking and functional defects in the 2592+1G>A splice mutant channels. Importantly, mutant channels containing the large COOH-terminal deletion coassembled with WT channels, trapping them in the endoplasmic reticulum, which led to the dominant-negative suppression of hERG current. This study demonstrates that the 2592+1G>A mutation induces multiple splicing defects that can contribute to several pathogenic mechanisms associated with long QT syndrome.
MATERIALS AND METHODS
hERG minigenes and cDNA constructs.
Human genomic DNA was used as a template for PCR amplification of exons 8–12 from the hERG gene. PCR products were cloned into the pCRII vector using TA cloning (Invitrogen, Carlsbad, CA) and verified by DNA sequencing. The NH2-terminus of the hERG minigene was preceded by a Kozak sequence and translation start codon. The 2592+1G>A mutation was generated using the pAlter site-directed mutagenesis system (Promega, Madison, WI). A hERG cDNA construct with an in-frame deletion of 72 nt from exon 10 was made using overlap extension PCR. This construct, hERGΔ24, was designed to generate channels in which 24 amino acids from the cyclic nucleotide binding domain were deleted. For hemagglutinin (HA)-tagged hERG cDNA constructs, the HA epitope (YPYDVPDYA) was inserted in-frame at the COOH-terminus of hERG. The design of the Flag-tagged hERG cDNA construct has been previously described (12). The hERG minigenes and cDNA constructs were subcloned into the pcDNA3 vector (Invitrogen). Minigene and cDNA constructs were transiently and stably transfected into human embryonic kidney (HEK)-293 cells, as previously described (14, 30, 31). Green fluorescent protein cDNA (1 μg) was cotransfected with hERG cDNA (5 μg) to serve as an indicator in patch-clamp experiments. For the coexpression studies, a HEK-293 cell line stably expressing WT hERG was transiently transfected with hERGΔ24 or pcDNA3 empty vector (13, 21). HEK-293 cells were cultured in minimal essential medium supplemented with 10% fetal bovine serum at 37°C in 5% CO2. HL-1 murine cardiomyocytes (6) were also transiently transfected with the WT and mutant minigenes using Lipofectamine 2000 (Invitrogen). HL-1 cells were cultured in Claycomb medium (Sigma-Aldrich, St. Louis, MO), supplemented with 10% fetal bovine serum and 0.1 mM norepinephrine (Sigma-Aldrich) at 37°C in 5% CO2.
Reverse transcription-PCR analysis of mRNA splicing.
In minigene splicing assays, cytoplasmic mRNA was isolated from transfected HEK-293 and HL-1 cells using the Qiagen RNeasy kit (Qiagen, Valencia, CA). After reverse transcription (RT) using the SuperScript III First-Strand DNA Synthesis kit (Invitrogen), PCR was performed with primers in exon 9 (forward 5′-GTGCTGAAGGGCTTCCCTGAG-3′) and exon 11 (reverse 5′-CCGACTGAAGCCACCCTCTAAC-3′). The PCR products were analyzed by agarose gel electrophoresis and cloned into pCRII vector for sequence analysis.
Patch-clamp recordings.
Membrane currents were recorded in whole cell configuration using suction pipettes, as previously described (30). All patch-clamp experiments were performed at ∼22°C. The bath solution contained 137 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES (pH 7.4). The pipette solution contained 130 mM KCl, 1 mM MgCl2, 5 mM EGTA, 5 mM MgATP, and 10 mM HEPES (pH 7.2). An Axopatch-200B patch clamp amplifier was used to record membrane currents, and the computer software pCLAMP8 was used to analyze current signals. Data are presented as means ± SE and are analyzed by Student's t-test. P < 0.05 is considered statistically significant.
Immunofluorescence microscopy.
The characterization of hERG channels by immunofluorescence has been described previously (11, 30). To detect the presence of hERG at the cell surface, intact cells were blocked using a blocking buffer without Triton X-100 and then probed with an anti-hERG antibody specific to the extracellular loop between the S1 and S2 membrane-spanning domains of the channel (Alomone Laboratories, Jerusalem, Israel). Cells were fixed with 4% paraformaldehyde and reprobed with an Alexa Fluor 594-conjugated goat anti-rabbit IgG secondary antibody (Molecular Probes, Eugene, OR). To detect hERG in permeabilized HEK-293 cells, the cells were first fixed with 4% paraformaldehyde and blocked with PBS containing 5% goat serum and 0.2% Triton X-100. Cells were then incubated with the anti-hERG S1-S2 antibody for 1 h at room temperature and then probed with the Alexa Fluor 594-conjugated secondary antibody. Images were acquired with a Zeiss Axioskop 2 microscope.
Western blot and immunoprecipitation.
Western blot analysis and immunoprecipitation were performed, as previously described (30, 31). Briefly, proteins from whole cell lysates were subjected to SDS-PAGE, transferred onto nitrocellulose membranes, detected with anti-hERG, anti-HA, and anti-Flag antibody, and visualized with the Plus-ECL (PerkinElmer, Waltham, MA) detection kit. For proteinase K treatment, transfected cells were incubated with 2 ml of buffer containing 10 mM HEPES, 150 mM NaCl, and 2 mM CaCl2 (pH 7.4), with or without 200 μg/ml proteinase K (Sigma-Aldrich) for 30 min at 37°C. The proteinase K activity was stopped by adding ice-cold PBS containing 6 mM PMSF and 25 mM EDTA. In the immunoprecipitation experiments, cotransfected cells were lysed with immunoprecipitation buffer (10 mM Tris·HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mg/ml BSA, and protease inhibitors), and hERG channels were immunoprecipitated with anti-HA antibody. Proteins were detected by Western blot with anti-Flag and anti-HA antibody, as described above.
RESULTS
LQT2 2592+1G>A causes multiple splicing defects.
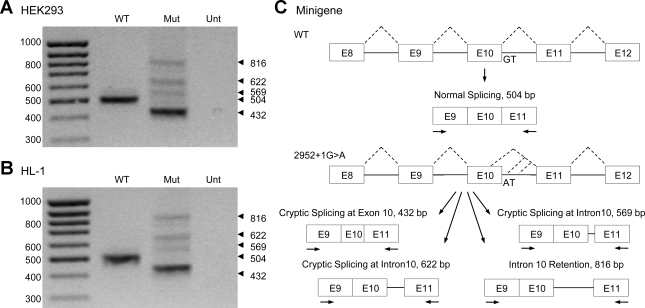
To determine the effect of the 2592+1G>A mutation on pre-mRNA splicing, we performed a minigene splicing assay. HEK-293 cells were transfected with WT and 2592+1G>A minigenes containing exon 8 to exon 12 of hERG genomic DNA (Fig. 1). Transcripts were analyzed by RT-PCR using primers in exons 9 and 11. The RNA isolated from the WT minigene yielded a single PCR product of 504 base pairs. In contrast, four unique splice products (432, 569, 622, and 816 bp) were generated in cells expressing the mutant minigene, and normal splicing was completely abolished (Fig. 1A). The PCR products were sequenced to determine the identity and origin of the aberrant splicing events observed with the mutant minigene (Supplemental Fig. 1; the online version of this article contains supplemental data). Sequence analyses confirmed that the WT PCR product was generated by normal splicing of intron 10. The major splice product from the mutant minigene was found to be generated by the activation of a 5′ cryptic splice site 72 nt upstream of the normal intron 10 donor site within exon 10. The three minor splice products arose from the activation of 5′ cryptic splice sites within intron 10, 65 nt and 118 nt downstream from the WT splice site, and another from the complete retention of intron 10. The inclusion of intron 10 sequence in these three transcripts results in the introduction of a PTC that is located >50–55 nucleotides upstream of the 3′-most exon-exon junction. According to the proposed rule, these transcripts are predicted to be degraded by NMD, precluding the generation of truncated hERG channels (15, 19). NMD has previously been shown to degrade several hERG nonsense mutations associated with long QT syndrome (5, 14). To determine whether the 2592+1G>A mutation also induced multiple splicing defects in the heart, the WT and mutant minigenes were transiently transfected into the HL-1 cardiomyocytes (Fig. 1B). RT-PCR analyses confirmed the same splicing pattern that was observed in the HEK-293 cell, suggesting that the mutation results in multiple splicing defects in the heart.
Fig. 1.
Analysis of the 2592+1G>A mutation (Mut) using minigenes transiently transfected into human embryonic kidney (HEK)-293 and HL-1 cells. Wild-type (WT) and 2592+1G>A minigenes were transfected into HEK-293 cells (A) and HL-1 cardiomyocytes (B). Isolated RNAs were amplified by RT-PCR. C: schematic of the WT and 2592+1G>A minigenes used in the transfection experiments. Arrows indicate primers used in RT-PCR experiments. The 2592+1G>A mutation activates cryptic splice sites in exon 10 and intron 10 and also can trigger complete intron 10 retention. Results shown are representative of three independent experiments. Unt, untransfected.
hERGΔ24 channels are nonfunctional.
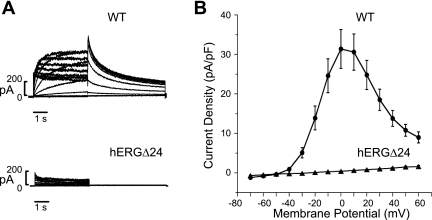
The major mutant splice transcript resulted from the activation of a cryptic splice site (GAG/gtgctg) within exon 10 and is predicted to delete 24 amino acids that normally encode the B and C α-helices of the cyclic nucleotide binding domain of hERG channels. To study the functional expression of this splice mutant, hERGΔ24, we performed patch-clamp experiments using HEK-293 cells transiently transfected with a cDNA construct lacking the corresponding 72 nt from the hERG cDNA (Fig. 2). The hERG channels were activated by 4-s depolarizing steps to voltages between −70 and 60 mV from a holding potential of −80 mV, and the hERG tail current was recorded on repolarization to −50 mV. The current-voltage plot of hERG current density, recorded at the end of each depolarizing step, indicated that WT channels exhibit typical hERG current with maximal outward current of 31.4 ± 4.9 pA/pF (n = 10) at 0 mV and a steep negative slope conductance at more positive voltages consistent with inward rectification due to voltage-dependent hERG inactivation (Fig. 2B) (23, 28, 31). In contrast, the 24-amino acid deletion within the cyclic nucleotide binding domain completely abolished hERG current.
Fig. 2.
Patch-clamp analysis of WT human ether-a-go-go-related gene (hERG) and hERGΔ24 channels expressed in HEK-293 cells. A: representative currents recorded from cells transiently transfected with WT hERG or hERGΔ24 cDNA. Currents were activated by depolarizing steps between −70 and 60 mV from a holding potential of −80 mV, and tail current was recorded on repolarization to −50 mV. B: current-voltage plot of WT hERG (circles, n = 10) and hERGΔ24 (triangles, n = 9) current densities, normalized to cell capacitance, recorded at the end of the depolarizing steps.
hERGΔ24 channels have trafficking defect.
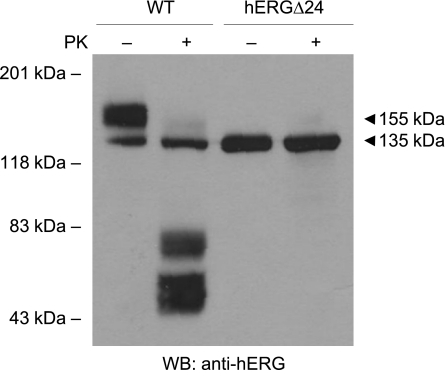
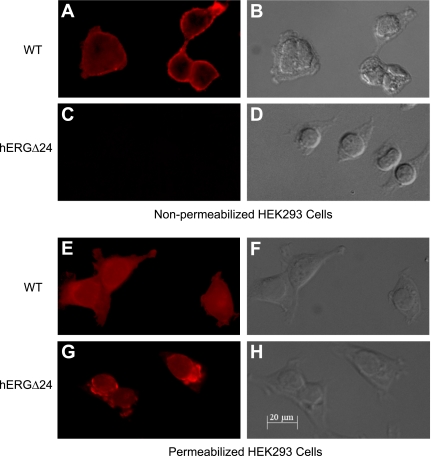
To investigate the mechanism underlying the absence of current from hERGΔ24 channels, we performed Western blot analysis of WT and mutant channels stably expressed in HEK-293 cells (Fig. 3). WT hERG was expressed as two bands at 135 and 155 kDa, which represent the core-glycosylated, immature channel and the mature, fully glycosylated channel expressed at the plasma membrane, respectively (31). In contrast, hERGΔ24 only expressed the immature form of the protein, indicating that the 24-amino acid deletion disrupted hERG trafficking. To further test trafficking, we treated HEK-293 cells stably expressing WT hERG or hERGΔ24 with a serine protease, proteinase K, which degrades the extracellular domains of proteins expressed at the cell surface. As shown in Fig. 3, the 155-kDa band of WT hERG was sensitive to proteinase K treatment, as digested fragments were observed as a 40- to 80-kDa smear. In contrast, the immature bands of both WT and hERGΔ24 channels were resistant to proteinase K treatment. We then further examined the cell surface expression of WT and mutant channels by immunofluorescence staining (Fig. 4). Permeabilized and nonpermeabilized transfected HEK-293 cells were treated with an antibody specific to the extracellular S1 and S2 domains of hERG. In nonpermeabilized cells, the immunofluorescent signal of WT hERG was detected predominantly along the plasma membrane, while no immunofluorescence was observed in cells transfected with hERGΔ24 (Fig. 4, A and C). In permeabilized cells, WT hERG exhibited a diffuse staining pattern visible throughout the cell, whereas hERGΔ24 distribution was restricted to perinuclear regions and was absent in the cell processes (Fig. 4, E and G). The combined results from our Western blot analysis, proteinase K digestion, and immunofluorescence strongly suggest that hERGΔ24 channels do not traffic to the cell surface.
Fig. 3.
Western blot (WB) analysis of WT hERG and hERGΔ24 channels. Intact HEK-293 cells were treated with (+) and without (−) proteinase K (PK). Proteins from whole cell lysates were subjected to SDS-PAGE and probed with anti-hERG antibody. Mature, fully glycosylated channels are 155 kDa; immature, core-glycosylated channels are 135 kDa. Results shown are representative of three independent experiments.
Fig. 4.
Immunofluorescence staining of WT hERG and hERGΔ24 channels. Nonpermeabilized (A–D) and permeabilized (E–H) HEK-293 cells transfected with WT hERG (A, B, E, and F) and hERGΔ24 (C, D, G, and H) were stained using an antibody specific to the S1-S2 extracellular domain of hERG. The phase-contrast image corresponding to the immunofluorescence image is shown for each transfection.
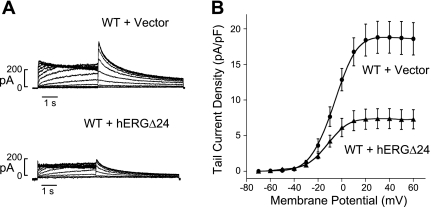
hERGΔ24 induced dominant-negative effects.
Patients with LQT2 mutations carry both WT and mutant alleles, which can result in dominant-negative effects. To determine whether the hERGΔ24 mutant can also suppresses WT hERG current in a dominant-negative manner, we performed patch-clamp studies in HEK-293 cells coexpressing both WT hERG and hERGΔ24 (Fig. 5). In these experiments, a HEK-293 cell line stably expressing WT hERG was transiently transfected with hERGΔ24 cDNA or control plasmid (pcDNA3 vector) (13, 21). We found a significant decrease in the hERG current when the WT hERG stable cell line was transfected with hERGΔ24 compared with the empty vector control (Fig. 5A). Current-voltage plots of the peak tail current densities measured at −50 mV following depolarizing voltages for WT hERG + vector and WT hERG + hERGΔ24 are shown in Fig. 5B. The average peak tail current density following test voltages of 30 mV of WT hERG + vector and WT hERG + hERGΔ24 were 18.8 ± 2.3 pA/pF (n = 6) and 7.4 ± 1.4 pA/pF (n = 8, P < 0.05), respectively, a decrease of ∼61% (Fig. 5B). These results suggest that hERGΔ24 is able to coassemble with WT channels to suppress hERG channel function.
Fig. 5.
Dominant-negative effects. A: representative currents from a WT hERG stable cell line transiently transfected with pcDNA3 vector or hERGΔ24 cDNA. hERG current was recorded using the protocol described in the Fig. 2 legend. B: current-voltage plot of hERG tail current amplitude measured at −50 mV following a depolarizing voltages from −70 to 60 mV for WT hERG + pcDNA3 vector (circles, n = 6) and WT hERG + hERGΔ24 (triangles, n = 8).
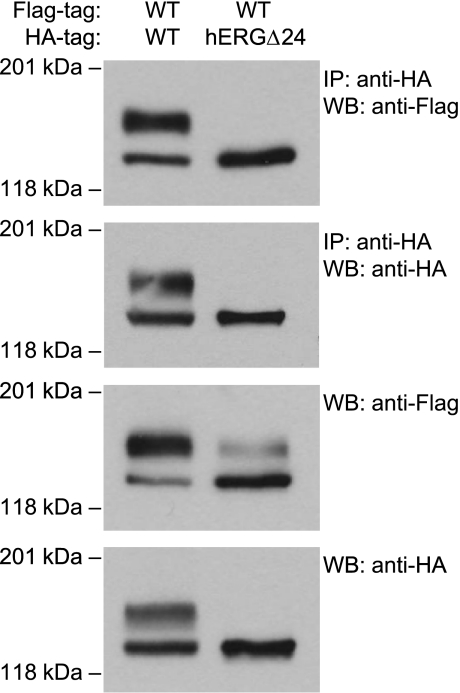
Heterotetrameric WT/hERGΔ24 channels are trapped within the cell.
To determine the mechanisms associated with the dominant-negative suppression of hERG current, we performed immunoprecipitation studies on HEK-293 cells coexpressing differentially tagged WT hERG and hERGΔ24 channels. We transiently transfected HEK-293 cells stably expressing Flag-tagged WT hERG with HA-tagged WT hERG or hERGΔ24. The coassembly of hERG channels was determined by immunoprecipitation with anti-HA antibody, followed by Western blot analysis with anti-Flag antibody (Fig. 6). WT-Flag hERG coimmunoprecipitated with WT-HA and hERGΔ24-HA channels. The association between WT-FL and WT-HA channels was observed in the immature and mature bands of the channel. The association between WT-FL and hERGΔ24-HA channels, however, was only observed in the immature band of the channel. This suggests that the WT hERG and hERGΔ24 channels were able to coassemble as core-glycosylated channels, but did not undergo complex glycosylation and trafficking to the cell surface. The membrane was also probed with anti-HA antibody, demonstrating the efficiency of immunoprecipitation of the HA-tagged channels. Western blot analysis with anti-Flag antibody without immunoprecipitation revealed that the coexpression of WT and hERGΔ24 led to the decrease in the expression of the mature band of WT-Flag and the concomitant increase in the expression of the immature band. These results suggest that the coassembly with hERGΔ24 causes a significant trafficking defect of WT hERG channels and thus contributes to the observed dominant-negative suppression of hERG current (Fig. 5).
Fig. 6.
Coassembly of WT and hERGΔ24 channels. HEK-293 cells stably expressing Flag-tagged WT hERG were transfected with hemagglutinin (HA)-tagged WT hERG or HA-tagged hERGΔ24 cDNA. Cell lysates were subjected to immunoprecipitation using anti-HA, followed by immunoblotting with anti-HA and anti-Flag antibody. Protein from the cell lysates was also subjected to Western blot using anti-Flag and anti-HA antibody. Results shown are representative of three independent experiments.
DISCUSSION
The hERG mutation 2592+1G>A was identified in a family in which two sudden deaths occurred at ages 9 and 20 yr, and one of the two living carriers underwent syncopes and a 2-day coma at age 4 yr (3). The present study demonstrates that this splice site mutation causes multiple defects that alter the function of hERG, including the dominant-negative suppression of WT hERG current, and may contribute to the observed severe phenotype. The hERG mutation 2592+1G>A disrupts the +1 position of the 5′ splice site of intron 10, GAT/gtgagt, a position that is 100% conserved in mammalian 5′ splice sites. RNA analysis using mutant minigenes transfected into HEK-293 and HL-1 cardiomyocytes revealed that 2592+1G>A abolished normal splicing of hERG and triggered multiple splicing defects, including complete intron 10 retention and the activation of 5′ cryptic splice sites in exon 10 (GAG/gtgctg) and intron 10 (GCT/gtgtga and ATG/gtatca). The consensus value scores compare the intrinsic strengths of splice sites relative to the eukaryotic 5′ consensus splice site sequence; most 5′ sites have scores >70 (4, 24). The 2592+1G>A exon 10 and intron 10 cryptic site scores, 64.5, 57.0, and 63.1, are significantly lower than the WT splice site score, 82.3. The intrinsically weak cryptic splice sites are inactive in the presence of the WT intron 10 splice site. The 2592+1G>A mutation completely abolishes normal splicing, allowing activation of the cryptic splice sites, which, despite having low splice scores, all contain the 100% conserved +1 and +2 nucleotides. Inefficient splicing from the weak, cryptic splice sites also results in the generation of unspliced transcripts containing the entire intron 10.
Over 20 hERG splice site mutations have been identified in patients with LQT2 (17, 20, 26, 27). Only three of the mutations, however, have been functionally characterized. The first, T1945+6T>C mutation, occurs at the last position of the 5′ splice site of intron 7, disrupts the base pairing between the splice site and the U1 snRNA, and results in complete intron 7 retention (29). The second, 2398+1G>C, activates an intronic 5′ cryptic splice, resulting in the insertion of 18 amino acids into the cyclic nucleotide binding domain between the β5- and β6-sheets (13). This large, in-frame insertion within hERG results in trafficking defective channels that are competent to coassemble with WT channels. The third, the IVS9–28A>G mutation, was recently found to disrupt the branch point and 3′ splice site of intron 9, which activates an upstream cryptic 3′ splice site in a hybrid minigene construct (7). The present characterization of the splice site mutation 2592+1G>A represents the first hERG mutation found to cause multiple splicing defects. We found that the three minor splice products result in transcripts that contain PTCs and are predicted to be degraded by the NMD mechanism. The major cryptic splice product, hERGΔ24, which lacks the last two α-helices of the cyclic nucleotide binding domain, is trafficking defective, as observed by Western blot analysis, by their expression as core-glycosylated, proteinase K-insensitive proteins and by their perinuclear, intracellular distribution in immunostaining studies. Although this deletion disrupts channel trafficking, it does not preclude teterameric assembly of mutant and WT channels. Furthermore, immunoprecipitation studies suggest that the dominant-negative suppression of hERG current by hERGΔ24 is due to the trapping of heterotetrameric channels within the cells.
It has been reported that LQT2 missense mutations located in highly structured regions, such as α-helices or β-sheets, correlate with a trafficking defective phenotype (2, 9, 10, 30). The cyclic nucleotide binding domain of hERG contains highly structured regions, and deletions of these regions have been shown to cause defective trafficking of the hERG channel (1). The present study on the LQT2 splice site mutation 2592+1G>A represents a relevant pathological example of large structural disruption of within the COOH-terminus of hERG. The mutant hERGΔ24 channel results in an in-frame deletion of the B and C α-helices of the COOH-terminal cyclic nucleotide binding domain. This mutant represents the largest deletion of highly structured domains caused by a disease-causing mutation in LQT2. A recent genotype-phenotype correlation study found that patients with mutations located in α-helical domains have significantly higher risk for arrhythmia-related cardiac events than do patients with mutations in either the β-sheet domains or other uncategorized locations (25). Our present finding that hERGΔ24 not only disrupts channel trafficking, but also leads to the dominant-negative suppression of hERG current, is consistent with the severe clinical phenotype observed in the patients carrying the 2592+1G>A mutation.
In summary, this report identifies multiple splicing defects induced by the 2592+1G>A mutation: the activation of a cryptic splice sites in exon 10 and in intron 10, and complete intron 10 retention. The activation of the cryptic splice site in exon 10 is the primary splicing defect and resulted in the in-frame deletion of 24 amino acids from the cyclic nucleotide binding domain. Functional and biochemical investigation of this splice mutant found that it gave rise to nonfunctional, trafficking defective channels, which coassembled with WT channels, trapped them in the endoplasmic reticulum, and resulted in the dominant-negative suppression of hERG current.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant HL-68854 (Z. Zhou). M. R. Stump is supported by an American Heart Association postdoctoral fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Akhavan A, Atanasiu R, Noguchi T, Han W, Holder N, Shrier A. Identification of the cyclic-nucleotide-binding domain as a conserved determinant of ion-channel cell-surface localization. J Cell Sci 118: 2803–2812, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113: 365–373, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Berthet M, Denjoy I, Donger C, Demay L, Hammoude H, Klug D, Schulze-Bahr E, Richard P, Funke H, Schwartz K, Coumel P, Hainque B, Guicheney P. C-terminal HERG mutations: the role of hypokalemia and a KCNQ1-associated mutation in cardiac event occurrence. Circulation 99: 1464–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bhasi A, Pandey RV, Utharasamy SP, Senapathy P. EuSplice: a unified resource for the analysis of splice signals and alternative splicing in eukaryotic genes. Bioinformatics 23: 1815–1823, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bhuiyan ZA, Momenah TS, Gong Q, Amin AS, Ghamdi SA, Carvalho JS, Homfray T, Mannens MM, Zhou Z, Wilde AA. Recurrent intrauterine fetal loss due to near absence of HERG: clinical and functional characterization of a homozygous nonsense HERG Q1070X mutation. Heart Rhythm 5: 553–561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A 95: 2979–2984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crotti L, Lewandowska MA, Schwartz PJ, Insolia R, Pedrazzini M, Bussani E, Dagradi F, George AL, Jr, Pagani F. A KCNH2 branch point mutation causing aberrant splicing contributes to an explanation of genotype-negative long QT syndrome. Heart Rhythm 6: 212–218, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80: 795–803, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J Biol Chem 277: 4989–4998, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Ficker E, Thomas D, Viswanathan PC, Dennis AT, Priori SG, Napolitano C, Memmi M, Wible BA, Kaufman ES, Iyengar S, Schwartz PJ, Rudy Y, Brown AM. Novel characteristics of a misprocessed mutant HERG channel linked to hereditary long QT syndrome. Am J Physiol Heart Circ Physiol 279: H1748–H1756, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Gong Q, Anderson CL, January CT, Zhou Z. Pharmacological rescue of trafficking defective HERG channels formed by coassembly of wild-type and long QT mutant N470D subunits. Am J Physiol Heart Circ Physiol 287: H652–H658, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Gong Q, Keeney DR, Robinson JC, Zhou Z. Defective assembly and trafficking of mutant HERG channels with C-terminal truncations in long QT syndrome. J Mol Cell Cardiol 37: 1225–1233, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Gong Q, Zhang L, Moss AJ, Vincent GM, Ackerman MJ, Robinson JC, Jones MA, Tester DJ, Zhou Z. A splice site mutation in hERG leads to cryptic splicing in human long QT syndrome. J Mol Cell Cardiol 44: 502–509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong Q, Zhang L, Vincent GM, Horne BD, Zhou Z. Nonsense mutations in hERG cause a decrease in mutant mRNA transcripts by nonsense-mediated mRNA decay in human long-QT syndrome. Circulation 116: 17–24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isken O, Maquat LE. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev 21: 1833–1856, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Jones EM, Roti Roti EC, Wang J, Delfosse SA, Robertson GA. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem 279: 44690–44694, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kapplinger JD, Tester DJ, Salisbury BA, Carr JL, Harris-Kerr C, Pollevick GD, Wilde AA, Ackerman MJ. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm 6: 1297–1303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104: 569–580, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Nagy E, Maquat LE. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci 23: 198–199, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, Nastoli J, Bottelli G, Cerrone M, Leonardi S. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. JAMA 294: 2975–2980, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Paulussen A, Raes A, Matthijs G, Snyders DJ, Cohen N, Aerssens J. A novel mutation (T65P) in the PAS domain of the human potassium channel HERG results in the long QT syndrome by trafficking deficiency. J Biol Chem 277: 48610–48616, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. Proc Natl Acad Sci U S A 93: 2208–2212, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Shapiro MB, Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res 15: 7155–7174, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimizu W, Moss AJ, Wilde AA, Towbin JA, Ackerman MJ, January CT, Tester DJ, Zareba W, Robinson JL, Qi M, Vincent GM, Kaufman ES, Hofman N, Noda T, Kamakura S, Miyamoto Y, Shah S, Amin V, Goldenberg I, Andrews ML, McNitt S. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol 54: 2052–2062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102: 1178–1185, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2: 507–517, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269: 92–95, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Vincent GM, Baralle M, Baralle FE, Anson BD, Benson DW, Whiting B, Timothy KW, Carlquist J, January CT, Keating MT, Splawski I. An intronic mutation causes long QT syndrome. J Am Coll Cardiol 44: 1283–1291, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem 273: 21061–21066, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J 74: 230–241, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.