Abstract
Hypertension alters cerebrovascular regulation and increases the brain's susceptibility to stroke and dementia. We investigated the temporal relationships between the arterial pressure (AP) elevation induced by “slow pressor” angiotensin II (ANG II) infusion, which recapitulates key features of human hypertension, and the resulting cerebrovascular dysfunction. Minipumps delivering saline or ANG II for 14 days were implanted subcutaneously in C57BL/6 mice (n = 5/group). Cerebral blood flow was assessed by laser-Doppler flowmetry in anesthetized mice equipped with a cranial window. With ANG II (600 ng·kg−1·min−1), AP started to rise after 9 days (P < 0.05 vs. saline), remained elevated at 11–17 days, and returned to baseline at 21 days (P > 0.05). ANG II attenuated the cerebral blood flow increase induced by neural activity (whisker stimulation) or endothelium-dependent vasodilators, an effect observed before the AP elevation (7 days), as well as after the hypertension subsided (21 days). Nonpressor doses of ANG II (200 ng·kg−1·min−1) induced cerebrovascular dysfunction and oxidative stress without elevating AP (P > 0.05 vs. saline), whereas phenylephrine elevated AP without inducing cerebrovascular effects. ANG II (600 ng·kg−1·min−1) augmented neocortical reactive oxygen species (ROS) with a time course similar to that of the cerebrovascular dysfunction. Neocortical application of the ROS scavenger manganic(I-II)meso-tetrakis(4-benzoic acid)porphyrin or the NADPH oxidase peptide inhibitor gp91ds-tat attenuated ROS and cerebrovascular dysfunction. We conclude that the alterations in neurovascular regulation induced by slow pressor ANG II develop before hypertension and persist beyond AP normalization but are not permanent. The findings unveil a striking susceptibility of cerebrovascular function to the deleterious effects of ANG II and raise the possibility that cerebrovascular dysregulation precedes the elevation in AP also in patients with ANG II-dependent hypertension.
Keywords: reduced nicotinamide adenine dinucleotide phosphate oxidase, oxidative stress, functional hyperemia, acetylcholine, field potentials
the brain is a major target of the end-organ damage associated with elevated blood pressure, and brain diseases, mainly stroke and dementia, contribute substantially to the public heath burden of hypertension (15, 24). Hypertension is responsible for 62% of cerebrovascular diseases (23), and midlife hypertension is a risk factor for Alzheimer's diseases, the most common cause of cognitive impairment in the elderly (9). Although it is well known that cerebral blood vessels are a major target of the effects of hypertension on the brain (16), the mechanisms leading to brain dysfunction and increased risk for stroke and dementia have not been elucidated.
The brain is critically dependent on a continuous and well-regulated blood supply to support its dynamic needs for oxygen and glucose and to remove metabolic by-products of brain activity (17, 26). Complex regulatory mechanisms ensure that the brain receives sufficient cerebral blood flow (CBF) to maintain the homeostasis of the cerebral microenvironment. Thus active neurons evoke powerful increases in CBF to match substrate delivery with the metabolic requirements of activation (functional hyperemia), whereas endothelial cells release potent vasoactive substances that regulate the distribution of microvascular flow (1, 17). Hypertension disrupts these control mechanisms and increases the susceptibility of the brain to vascular insufficiency (16). However, the temporal relationships between the cerebrovascular dysfunction and the development of hypertension remain to be defined. This issue is of interest because evidence of cerebrovascular impairment can be present in patients who, based on the magnitude of blood pressure elevation, do not yet meet the criteria for hypertension (prehypertension) (5, 31, 36). Therefore, it is important for preventive purposes to determine whether the cerebrovascular alterations precede or follow the elevation in blood pressure.
Angiotensin II (ANG II) has emerged as a critical factor in essential hypertension, and its administration in experimental animals reproduces key features of hypertension, including the cerebrovascular dysfunction (16). Systemic administration of subpressor doses of ANG II for 2 wk, the so-called “slow pressor” model of hypertension, elicits a delayed increase in blood pressure (40). Because of the time lag between ANG II infusion and the onset of hypertension, this model offers the opportunity to examine the temporal relationships between cerebrovascular dysfunction and blood pressure elevation. Therefore, we used this model to investigate the temporal profile of the increase in blood pressure and cerebrovascular dysfunction. We found that the cerebrovascular dysfunction precedes the onset of hypertension and persists beyond the normalization of blood pressure at the end of the 2-wk infusion. Neocortical reactive oxygen species (ROS) increased in parallel with the cerebrovascular alterations and contributed to the impairment. The findings provide evidence for a remarkable susceptibility of the cerebral circulation to the deleterious effects of ANG II and raise the possibility that cerebrovascular dysfunction is an early event also in human hypertension.
METHODS
Methods for surgical preparation of mice, topical application of drugs, recording field potentials, blood pressure measurement, and monitoring CBF using laser-Doppler flowmetry have been described in detail in previous publications (3, 12, 19, 21, 22, 29) and are briefly summarized here.
General Surgical Procedures
All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. All studies were conducted in male C57BL/6J mice (age, 2 to 3 mo; and 20–30 g body wt), obtained from Jackson Laboratories (Bar Harbor, ME).
Minipumps implantation.
Osmotic minipumps containing vehicle (saline), ANG II, or phenylephrine (PE) were implanted subcutaneously in mice (n = 5/group) under isoflurane anesthesia. Concentrations and delivery rates of ANG II (600 ng·kg−1·min−1) and PE (3 μg·kg−1·min−1) were adjusted to produce comparable levels of blood pressure elevation. In some experiments, a concentration of ANG II that does not increase blood pressure (200 ng·kg−1·min−1) was used. Systolic blood pressure was monitored daily in awake mice using tail-cuff plethysmography, as previously described (4, 21, 22). At different times after pump implantation (3, 7, 14, 16, 18, 21, and 28 days), the mice were anesthetized and instrumented for assessment of cerebrovascular reactivity by laser-Doppler flowmetry as described in Monitoring CBF.
Radiotelemetry.
In some mice, arterial pressure was monitored by radiotelemetry (Data Sciences International) using a catheter implanted in the thoracic aorta via the left common carotid artery (3). Because of the carotid cannulation, these mice were not used in experiments involving CBF monitoring. These experiments with telemetry were used to select the time points at which to assess CBF reactivity. The changes in blood pressure in mice in which CBF was measured were confirmed by tail-cuff plethysmography and by the measurement of mean arterial pressure (MAP) through a femoral catheter during the CBF experiment.
Surgery for CBF experiments.
Mice were anesthetized with isoflurane in a mixture of N2 and O2 (induction, 5%; and maintenance, 2%). The trachea was intubated and the mice were artificially ventilated with an oxygen-nitrogen mixture. The O2 concentration in the mixture was adjusted to provide an arterial Po2 of 120–130 mmHg (4) (see supplemental Table S1; note: supplemental material may be found posted with the online version of this article). One of the femoral arteries was cannulated for recording MAP and collecting blood samples. Rectal temperature was maintained at 37°C using a thermostatically controlled rectal probe connected to a heating pad. End-tidal CO2, monitored by a CO2 analyzer (Capstar-100, CWE), was maintained at 2.6–2.7% to provide a Pco2 of 33–36 mmHg (4) (see supplemental Table S1). After surgery, isoflurane was discontinued and anesthesia was maintained with urethane (750 mg/kg ip) and chloralose (50 mg/kg ip). Throughout the experiment, the level of anesthesia was monitored by testing corneal reflexes and motor responses to tail pinch. To minimize the confounding effects of anesthesia on vascular reactivity, the time interval between the administration of urethane-chloralose and the testing of CBF responses was kept consistent among the different groups of mice studied.
Monitoring CBF
The parietal region was exposed through a small craniotomy (2 × 2 mm), the dura was removed, and the site was superfused with a modified Ringer solution (37°C; pH 7.3–7.4). CBF was continuously monitored at the site of superfusion with a laser-Doppler probe (Perimed) positioned stereotaxically on the cortical surface. The outputs of the flowmeter and blood pressure transducer were connected to a data acquisition system (PowerLab) and saved on a computer for off-line analysis. CBF values were expressed as percent increases relative to the resting level. Zero values for CBF were obtained after the heart was stopped by an overdose of isoflurane at the end of the experiment. Although laser-Doppler flowmetry is not quantitative, it monitors relative changes in CBF quite accurately (see Ref. 14 for a review).
ROS Detection and Immunocytochemistry
ROS production was assessed by dihydroethidium (DHE) microfluorography as previously described (4, 11, 12). Although there are many methods to assess ROS, each with advantages and disadvantages, DHE fluoromicrography is particularly well suited to in situ ROS detection with cellular resolution (4, 11, 13, 29, 37). DHE (2 μmol/l; Molecular Probes) was topically superfused on the somatosensory cortex for a total of 60 min (see Experimental Protocols). The brain was removed and frozen, and coronal sections (thickness, 20 μm) were cut through the cortex underlying the cranial window using a cryostat. Sections were analyzed using a Nikon Eclipse E800 fluorescence microscope equipped with a custom filter set for the detection of DHE oxidation products (4, 11, 12, 29). Images were acquired with a digital camera (Coolsnap, Roper Scientific) and analyzed in a blinded manner using the IPLab software (Scanalytics), as described (4). Fluorescent intensities of all sections (20 per animal) were added, divided by the total number of pixels analyzed, and expressed in relative fluorescence units. In experiments in which the cellular site of the ROS signal was established, DHE (10 mg/kg) was injected into the jugular vein and, 60 min later, the mice (n = 4–6/group) were perfused transcardially with PBS followed by 4% paraformaldehyde in PBS. We used this approach because it does not require a craniotomy and results in better preservation and fixation of the somatosensory cortex. A potential drawback, however, is that some of the fluorescence might be lost during the tissue processing for immunocytochemistry. To minimize the confounding effects of the loss in fluorescence, brain sections from saline- and ANG II-treated mice were processed in parallel and under identical conditions. Coronal brain sections (thickness, 20 μm) were cut through the somatosensory cortex using a cryostat. Sections were incubated with primary antibodies (glial fibrillary acidic protein, 1:200, Sigma-Aldrich; neuronal nuclei, 1:200, Chemicon International; and CD31, 1:200, BD Biosciences). Sections were then incubated with a Cy5-conjugated secondary antibody (1:200; Jackson ImmunoResearch), mounted on slides and examined using a Leica confocal microscope (4). Identical confocal settings were used for the acquisition of all images. To obtain a semiquantitative assessment of the increase in ROS induced by ANG II infusion in neurons, astrocytes, and endothelial cells, fluorescence was quantified in the cells immunopositive for the different markers using ImageJ. Data were expressed in arbitrary fluorescence units.
Experimental Protocols
Effect of ANG II or PE on CBF responses to whisker stimulation, endothelium-dependent vasodilators, or adenosine.
Mice were surgically prepared for CBF measurement at different time points after implantation of osmotic minipumps containing ANG II, PE, or vehicle (see Surgery for CBF experiments). After stabilization of MAP and blood gases (see supplementary Table S1), the whisker-barrel region of the somatosensory cortex was activated for 60 s by stroking the contralateral facial whiskers (4), and the evoked changes in CBF were recorded. CBF responses to acetylcholine (ACh; 10 μM), bradykinin (50 μM), the Ca2+ ionophore A-23187 (3 μM), and adenosine (400 μM) were also tested (4). These agents were selected because they produce vasodilation through different mechanisms. ACh induces CBF increases mediated by endothelial nitric oxide (NO) and muscarinic receptors (38), whereas bradykinin and A-23187 act via endothelial cyclooxygenase-1 products via receptor-dependent and -independent mechanisms, respectively (27). Adenosine is a smooth muscle relaxant whose action is independent of the endothelium (30). Agents were applied at concentrations previously determined not to be supramaximal (19). In experiments in which the ROS scavenger manganic(I-II)meso-tetrakis(4-benzoic acid)porphyrin (MnTBAP; 100 μM), the NADPH oxidase peptide inhibitor gp91ds-tat (1 μM), or a scrambled control peptide (1 μM) was used (33), the responses were tested before and after topical superfusion of these agents for 30 min (11, 12). We have previously determined that the concentrations of MnTBAP and gp91ds-tat used are effective in blocking ANG II-induced ROS production (11, 12).
Effect of ANG II on somatosensory field potentials evoked by whisker stimulation.
Mice were anesthetized and surgically prepared as described in Surgery for CBF experiments. The electrocorticogram was recorded using bipolar recording electrodes consisting of Teflon-coated silver wires (ID 0.005 inches; Stoelting). Electrodes were positioned stereotaxically in the left somatosensory cortex (3 mm lateral and 1.5 mm caudal to bregma; depth of 0.6 mm) (22, 29). A metal screw inserted into the occipital bone served as a reference electrode. The electrocorticogram was recorded for five epochs each lasting 5 min and separated by a 20-min interval. To avoid the confounding effects of anesthesia on cortical electrical activity, the timing of the recordings relative to the administration of the anesthetic was identical for all animals. The signals were amplified, digitized, and stored on a computer for off-line analysis (PowerLab, AD Instruments). Spectral analysis of the electrocorticogram was performed using a software module embedded in PowerLab. Field potentials were recorded using an electrode placed in the somatosensory cortex contralateral to the activated whiskers. The somatosensory cortex was activated by electrical stimulation of the whisker pad (2 V; 0.5 Hz; and pulse duration, 1 ms). Ten stimulation trials were averaged using a data acquisition system and stored on a hard drive for off-line analysis (22, 29).
ROS measurement.
The protocol for these experiments was identical to that of the CBF studies. After stabilization of MAP and blood gases, DHE was superfused on the cranial window or administered intravenously. The brain was removed 60 min later, and ROS were determined as described in ROS Detection and Immunocytochemistry.
Data Analysis
Data in the text and figures are expressed as means ± SE. Two-group comparisons were analyzed by the two-tailed Student's t-test. Multiple comparisons were evaluated by the analysis of variance and Tukey's test. Probability values of <0.05 were considered statistically significant.
RESULTS
Slow Pressor ANG II Infusion Elevates Blood Pressure and Induces Cerebrovascular Dysfunction
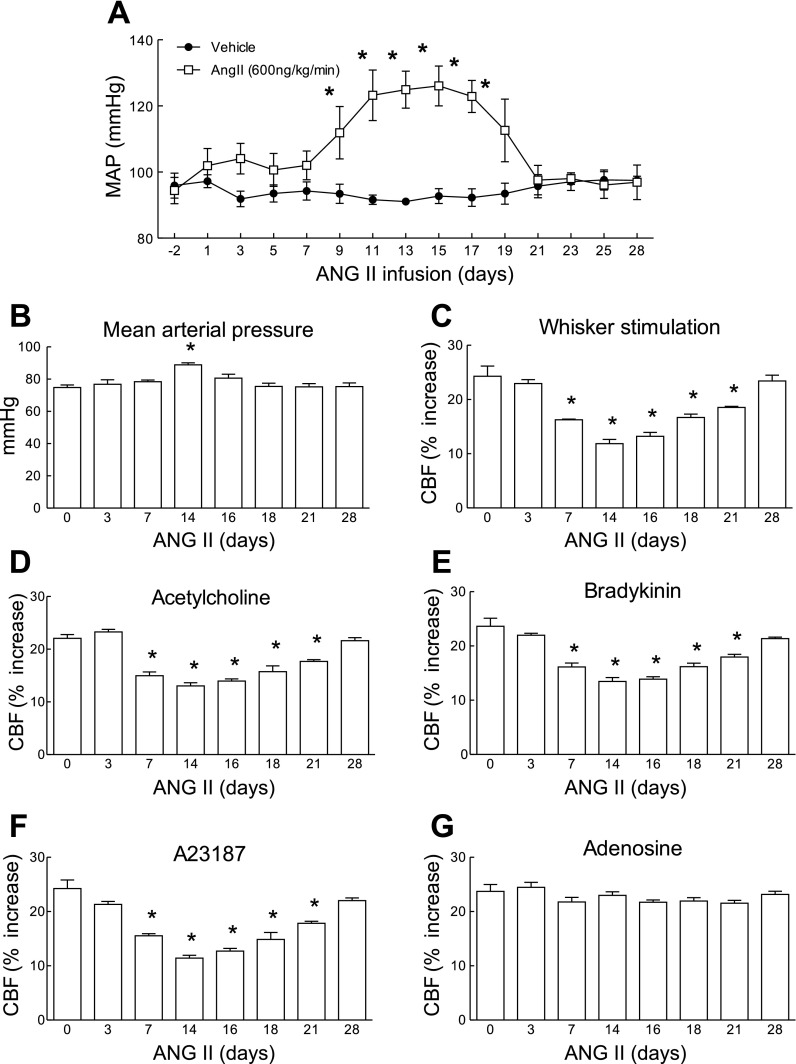
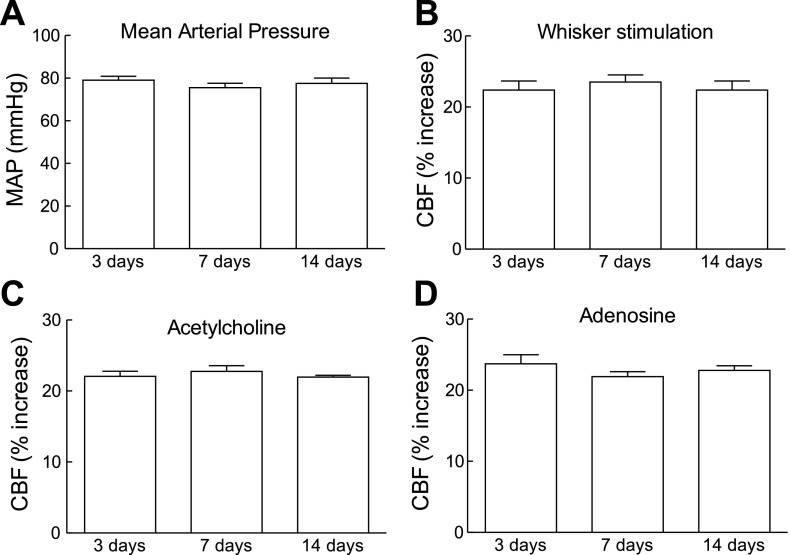
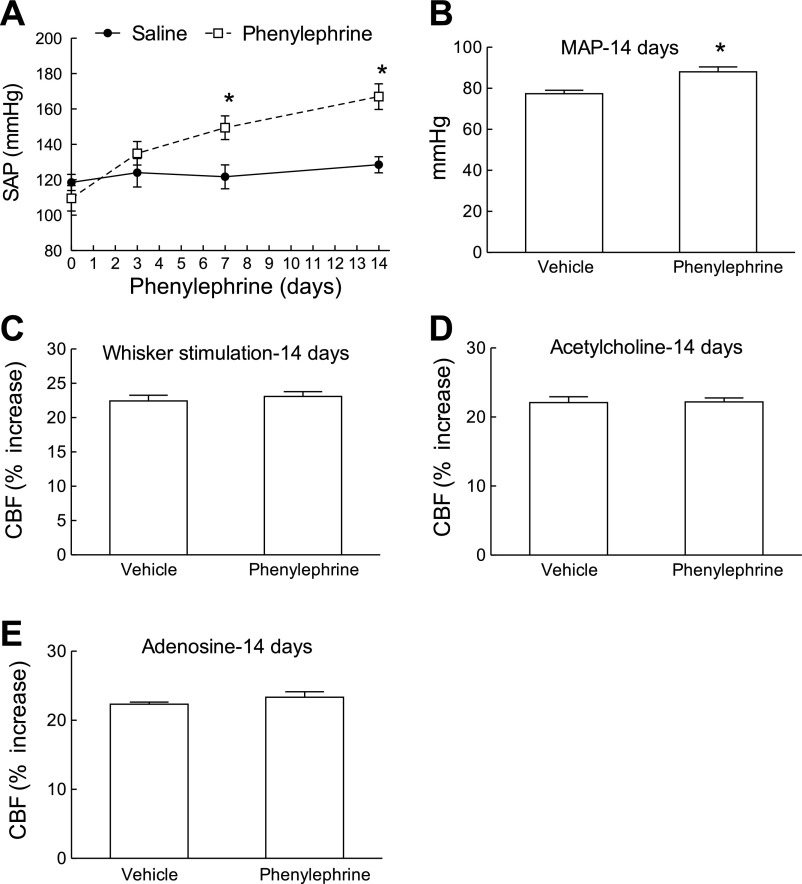
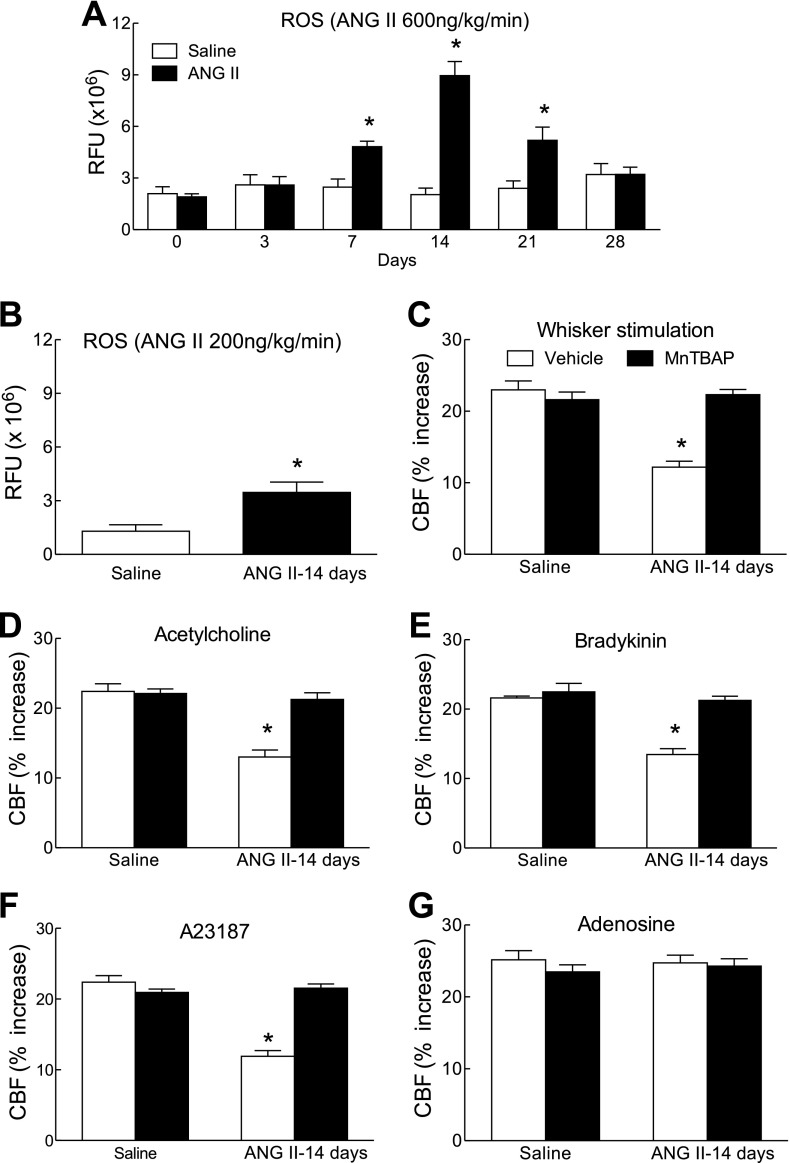
Using telemetric blood pressure measurements, we found that slow pressor ANG II infusion (600 ng·kg−1·min−1) for 14 days induces a delayed increase in MAP that started at day 9 and reached a plateau between days 11 and 17 (Fig. 1A). As the osmotic minipumps emptied, MAP started to decline and returned to normal by day 21, where it remained until the end of the study (day 28) (Fig. 1A). In separate mice, in which cerebrovascular reactivity was studied, ANG II infusion elevated MAP (Fig. 1B) and attenuated the increase in CBF induced by whisker stimulation and by the endothelium-dependent vasodilators ACh, bradykinin, and A-23187 (Fig. 1, C–F). In contrast, the increase in CBF induced by the smooth muscle relaxant adenosine was not affected (Fig. 1G). The cerebrovascular dysfunction was first observed at 7 days before the increase in MAP occurred and persisted after MAP returned to baseline at 21 days (Fig. 1, C–F). The stability of the increases in CBF in mice implanted with minipumps delivering saline was tested at 3, 7, and 14 days, and responses were stable in time (Fig. 2, A–D). To determine whether the sustained raise in blood pressure was by itself sufficient to induce cerebrovascular dysfunction, we elevated MAP for 14 days using PE. PE increased MAP to a level similar to that induced by ANG II (Fig. 3, A and B) but did not alter functional hyperemia, endothelium-dependent responses, or the increase in CBF produced by adenosine (Fig. 3, C–E).
Fig. 1.
Time course of the effect of ANG II infusion (600 ng·kg−1·min−1) on mean arterial pressure (MAP) and cerebral blood flow (CBF) responses. A: recordings of MAP by radiotelemetry in mice implanted with osmotic minipumps set to deliver ANG II or vehicle for 14 days. MAP increases significantly at 9 days, reaches a plateau at 11 days, and starts to decline at 19 days. MAP returns to baseline at 21 days. *P < 0.05 from baseline and vehicle (ANOVA and Tukey's test; n = 5/group). B: increases in MAP induced by ANG II infusion in anesthetized mice in which CBF was monitored. MAP was measured through an indwelling femoral artery catheter. A significant increase is observed only at 14 days. C–G: increases in CBF induced by whisker stimulation, ACh (10 μM), bradykinin (50 μM), A-23187 (3 μM), or adenosine (400 μM) in mice receiving ANG II. Responses to whisker stimulation, ACh, bradykinin, and A-23187, but not adenosine, are attenuated at 7 days when MAP is not yet increased. Responses remain reduced up to 21 days and return to baseline at 28 days. *P < 0.05 (ANOVA and Tukey's test; n = 5/group).
Fig. 2.
Stability of MAP and increases in CBF over time in mice implanted with osmotic minipumps loaded with saline. A: MAP measured through a femoral catheter during the CBF experiments. B–D: increases in CBF induced by whisker stimulation or topical application of ACh or adenosine. P > 0.05 (ANOVA and Tukey's test; n = 5/group).
Fig. 3.
Elevation of MAP with phenylephrine (PE) does not attenuate the CBF responses. A: time course of the systolic blood pressure elevation by tail-cuff plethysmography induced by infusion of PE (3 μg·kg−1·min−1) for 14 days. SAP, systolic arterial pressure. *P < 0.05 from saline; n = 5/group. B: increases in MAP induced by PE in anesthetized mice in which CBF was monitored. C–E: despite the MAP increase, PE does not attenuate the increase in CBF induced by whisker stimulation, ACh, or adenosine. P > 0.05 from vehicle; n = 5/group.
Nonpressor Doses of ANG II Induce Cerebrovascular Dysfunction
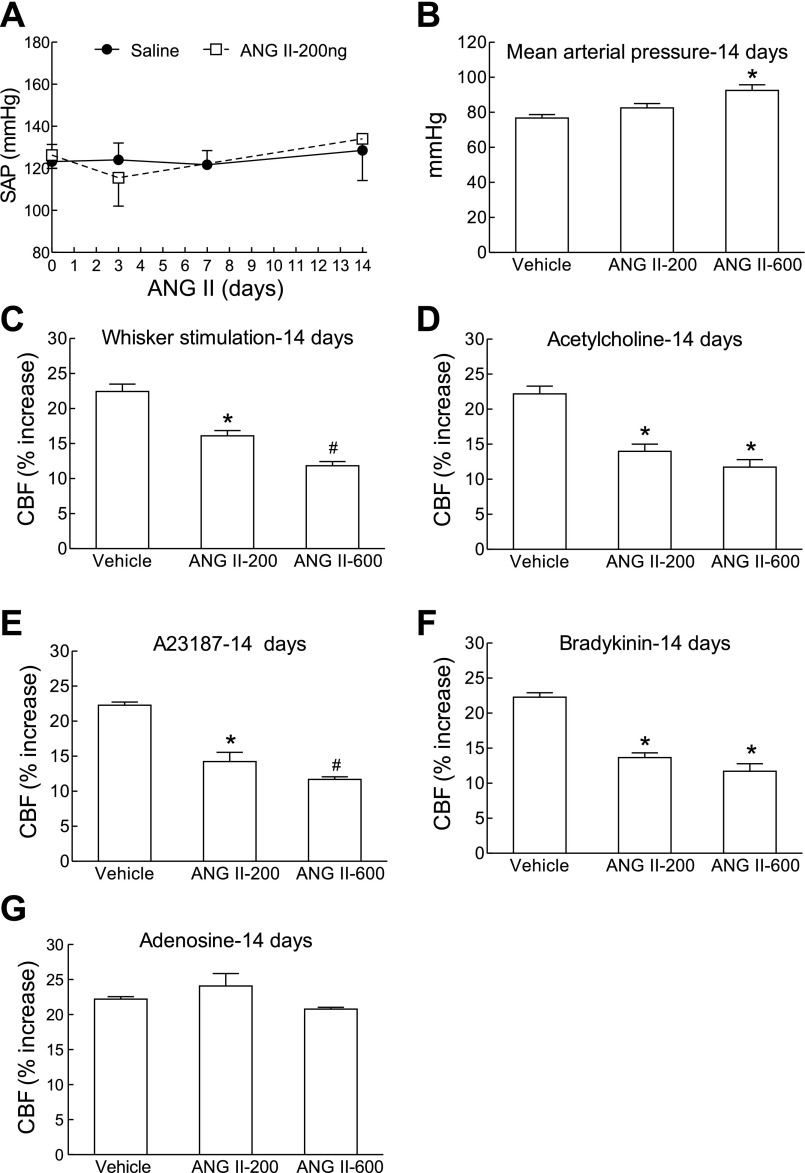
The data presented in the previous section suggest that ANG II may induce cerebrovascular dysfunction independently of the elevation in MAP. Therefore, we examined whether a dose of ANG II that does not elevate MAP is able to induce cerebrovascular dysfunction. Nonpressor doses of ANG II (200 ng·kg−1·min−1) did not elevate MAP (Fig. 4, A and B) but induced a cerebrovascular dysfunction that was comparable with that produced by slow pressor ANG II doses (600 ng·kg−1·min−1) (Fig. 4, C–G).
Fig. 4.
Nonpressor doses of ANG II attenuate CBF responses. A: systolic blood pressure measured by tail-cuff plethysmography during infusion of nonpressor doses of ANG II (200 ng·kg−1·min−1; n = 5/group). B: MAP measured through a femoral catheter during the CBF experiments in mice infused with ANG II (200 or 600 ng·kg−1·min−1) for 14 days. C–G: ANG II infusion (200 and 600 ng·kg−1·min−1) attenuate CBF responses to whisker stimulation, ACh, bradykinin, A-23197, but not adenosine. *P < 0.05 from vehicle; #P < 0.05 from ANG II-200 and vehicle (ANOVA and Tukey's test; n = 5/group).
Spontaneous or Evoked Neocortical Neural Activity Is Not Altered During Slow Pressor ANG II Hypertension
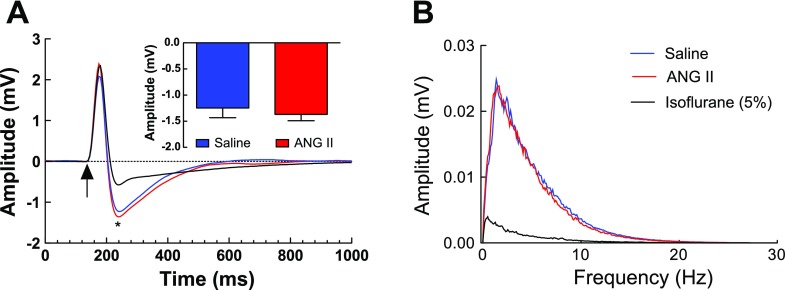
Neural activity is a powerful determinant of cerebrovascular reactivity (7). To determine whether the alterations in cerebrovascular function were associated with alterations in neural activity, we examined the effect of slow pressor doses of ANG II on the electrocorticogram and on the field potentials induced in the somatosensory cortex by whisker stimulation. As illustrated in Fig. 5, ANG II infusion for 14 days elevated MAP (vehicle, 79 ± 2; and ANG II, 88 ± 1 mmHg; P < 0.05; n = 4/group) but did not affect the amplitude of the field potentials or the frequency distribution of the electrocorticogram. In contrast, the anesthetic isoflurane attenuated the field potentials and the electrocorticogram at all frequencies (Fig. 5, A and B), providing a positive control for the sensitivity of the monitoring system. Therefore, the cerebrovascular dysfunction induced by ANG II is not associated with alterations in spontaneous or evoked neural activity in the neocortex in which CBF was measured.
Fig. 5.
Effect of a 14-day infusion of ANG II (600 ng·kg−1·min−1) on spontaneous and evoked neural activity in the somatosensory cortex (n = 4/group). A: ANG II does not affect the field potentials evoked by activation of the whiskers (2 V; 0.5 Hz; 1-ms pulse). In contrast, the anesthetic isoflurane (5%) markedly attenuates the response. Arrow marks the application of the stimulus. *Negative wave of the field potential the amplitude of which (in mV) is shown in inset. B: ANG II does not affect the frequency distribution of the electrocorticogram, whereas isoflurane potently attenuates signal amplitude at all frequencies.
ANG II Induces Oxidative Stress in Somatosensory Cortex
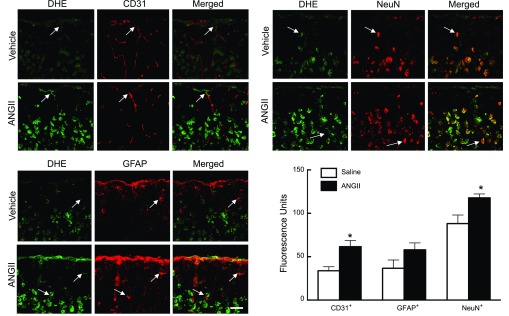
ANG II is well known to induce ROS production (10). However, it is not known whether slow pressor ANG II infusion increases ROS in the cerebral cortex and, if so, whether the increase parallels the elevation in blood pressure. With the administration of the slow pressor dose of ANG II (600 ng·kg−1·min−1), ROS started to increase at 7 days (P < 0.05), reached a maximum at 14 days (P < 0.05), were still increased at 21 days (P < 0.05), and returned to baseline at 28 days (Fig. 6A). A nonpressor dose of ANG II (200 ng·kg−1·min−1) also elevated ROS at 14 days, but the increase was smaller than that observed with the higher ANG II dose (Fig. 6B). In experiments with acute ANG II administration, we observed ROS increases predominantly in cerebral blood vessels (11). To determine whether this was also the case with sustained ANG II administration, we combined DHE microfluorography with immunocytochemistry for selected cell markers (11, 29). By visual inspection, ANG II administration for 14 days increased the ROS signal not only in cells expressing the endothelial marker CD31 but also in cells expressing the neuronal marker neuronal nuclei or the astrocytic marker glial fibrillary acidic protein (Fig. 7). Quantification of the ROS signal in neurons, astrocytes, and endothelial cells indicated increases in ROS signal in endothelial cells and neurons (Fig. 7; P < 0.05). A tendency to increase was seen also in astrocytes, but the change did not reach statistical significance (Fig. 7; P > 0.05).
Fig. 6.
ANG II increases reactive oxygen species (ROS) in the somatosensory cortex, and the ROS scavenger manganic(I-II)meso-tetrakis(4-benzoic acid)porphyrin (MnTBAP) reverses the cerebrovascular dysfunction. A: slow pressor dose of ANG II (600 ng·kg−1·min−1) increases ROS production at 7, 14, and 21 days. ROS return to baseline at 28 days. B: nonpressor dose of ANG II (200 ng·kg−1·min−1) also increases ROS at 14 days but to a lesser extent. *P < 0.05 from saline (ANOVA and Tukey's test; n = 5/group). C–F: topical neocortical application of the ROS scavenger MnTBAP (100 μM) reverses the effect of ANG II on CBF responses to whisker stimulation, ACh, bradykinin, and A-23187. G: CBF response to adenosine is not affected. *P < 0.05 from saline and MnTBAP; n = 5/group.
Fig. 7.
ANG II infusion for 14 days increases ROS-dependent fluorescence in endothelial cells (top, left), neurons (top, right), and astrocytes (bottom, left) compared with vehicle infusion. Microphotographs depict the somatosensory cortex from the pial surface to the deeper cortical layers. ROS were detected by the dihydroethidium (DHE) method (intravenous administration), and CD31, neuronal nuclei (NeuN), or glial fibrillary acidic protein (GFAP) immunoreactivity was used as endothelial, neuronal, and astrocytic markers, respectively. Arrows indicate colocalization of the ROS signal (DHE) with the cell markers. Bar size = 50 μm. Quantification of the fluorescent signal (bottom, right) demonstrates significant ROS increases in endothelial cells and neurons. The increase in ROS in astrocytes did not reach statistical significance. *P < 0.05; n = 4–6/group.
ROS Scavenger MnTBAP or the NADPH Oxidase Peptide Inhibitor gp91ds-tat Reverses the Cerebrovascular Dysfunction Induced by ANG II
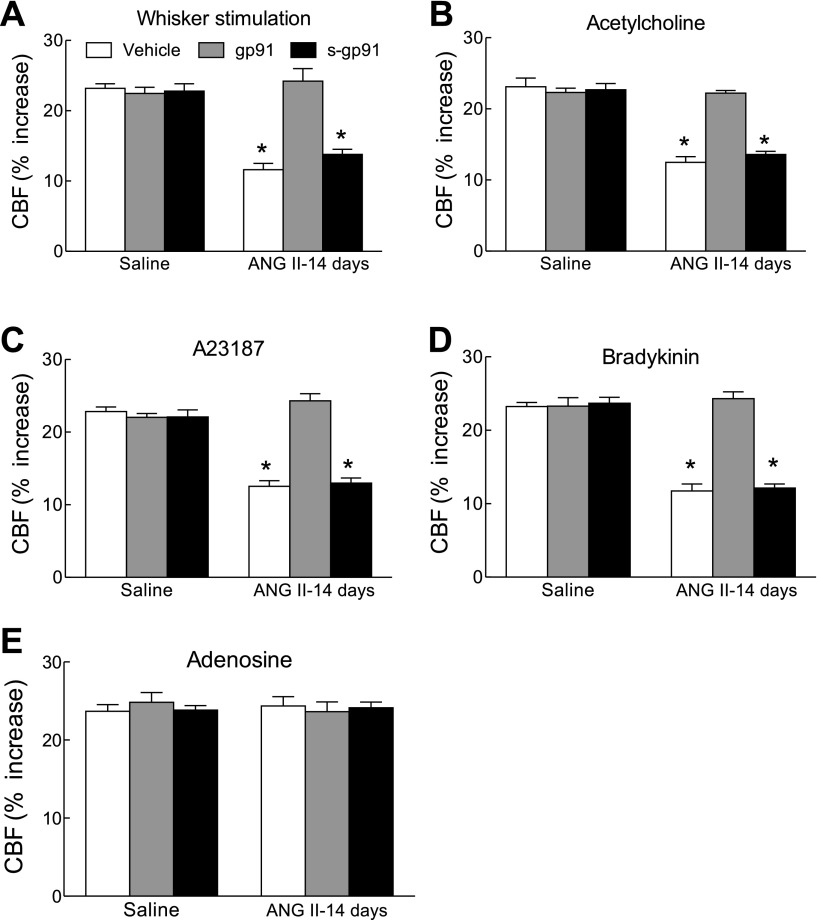
To determine whether the increase in ROS production produced by ANG II contributes to the cerebrovascular dysfunction, we used the ROS scavenger MnTBAP (4). Superfusion of the somatosensory cortex with MnTBAP (100 μM) reversed the cerebrovascular dysfunction induced by the administration of ANG II for 14 days (Fig. 6, B–E). As anticipated, MnTBAP did not affect the response to adenosine (Fig. 6F). Because the enzyme NADPH oxidase is a major source of ROS produced by ANG II (10), we examined the effect of the peptide inhibitor gp91ds-tat on the cerebrovascular dysfunction (11, 12, 33). Neocortical superfusion of the peptide (1 μM), but not its scrambled control, reversed the cerebrovascular dysfunction (Fig. 8, A–D) without affecting the response to adenosine (Fig. 8E). These findings implicate NADPH oxidase-derived ROS in the cerebrovascular dysfunction induced by slow pressor ANG II.
Fig. 8.
A peptide inhibitor of NADPH oxidase counteracts the cerebrovascular dysfunction induced by ANG II. A–D: gp91ds-tat (gp91; 1 μM), but not its scrambled control (s-gp91; 1 μM), reverses the effect of ANG II on CBF responses to whisker stimulation, A-23187, and bradykinin. E: CBF response to adenosine is not affected. *P < 0.05 from saline and gp91; n = 5/group.
DISCUSSION
We have demonstrated that slow pressor doses of ANG II induce a marked disruption in major cerebrovascular regulatory mechanisms, leading to the attenuation of the increases in CBF induced by neural activity and by endothelium-dependent vasodilators. The cerebrovascular dysfunction preceded the MAP elevation induced by ANG II and was still present after MAP returned to baseline at the end of the infusion. We also found that nonpressor doses of ANG II produce cerebrovascular alterations comparable with those observed with slow pressor doses, whereas the elevation of MAP with PE fails to alter cerebrovascular reactivity. These observations indicate that the elevation of MAP is not necessary or sufficient to alter cerebrovascular regulation. The cerebrovascular dysfunction was paralleled by an increase in ROS, which also preceded the onset of slow pressor hypertension and persisted beyond the normalization of MAP. Interestingly, immunolabel with cell markers suggested that the increase in ROS occurred not only in cerebral vessels but also in neocortical neurons and, possibly, astroglia, consistent with the extravascular effects of circulating ANG II. Collectively, these data demonstrate that slow pressor ANG II infusion has profound effects on the regulation of the cerebral circulation, which precede the onset of hypertension, persist after the hypertension subsides, and are mediated by oxidative stress.
The findings of the present study cannot be attributed to alterations in the physiological parameters of the mice, because MAP was monitored and body temperature and blood gases were carefully controlled. Furthermore, the dysfunction cannot be attributed to hypertension-induced structural or functional alterations in the vascular smooth muscle preventing vascular relaxation, because ANG II did not affect the increase in CBF induced by the smooth muscle relaxant adenosine. Although neural activity can have profound effects on cerebral blood vessels, the observation that ANG II does not affect the frequency profile of the electrocorticogram or the magnitude of somatosensory-evoked potentials indicates that the attenuation in functional hyperemia is not due to the suppression of neural activity. Furthermore, the fact that ANG II attenuates also nonneurally mediated responses, e.g., endothelium-dependent vasodilation, argues against a role of neural activity in the mechanisms of the effect. One limitation of the present study is that because of the cranial window, the CBF experiments had to be performed under anesthesia, which can have profound effects on MAP and CBF reactivity (18, 20, 35). However, this concern is mitigated by the fact that the time course of the MAP increase established by telemetry in awake mice was comparable with that of anesthetized mice, i.e., the increases in MAP at 14 but not 7 days of ANG II infusion. As for the CBF responses, the same conditions of anesthesia were used in saline- and ANG II-treated mice, minimizing the possibility that the observed differences in CBF responses were attributable to the anesthesia.
A new finding of the present study is that the cerebrovascular dysfunction and cerebral oxidative stress induced by ANG II precede the development of hypertension and persist even after the elevation in MAP has subsided. This observation suggests that cerebrovascular function is highly susceptible to the effects of circulating ANG II. Thus alterations in the regulation of CBF may be one of the early manifestations of the neurohumoral dysregulation that mediates the peripheral vascular effects of ANG II. This conclusion is also suggested by a study in which nonpressor doses of ANG II attenuated the response of the isolated basilar artery to ACh (6). Our finding that doses of ANG II not sufficient to elevate blood pressure induce marked cerebrovascular disruption also underlines the sensitivity of the cerebral circulation to ANG II-induced dysregulation. Because we did not assess vascular function in other arterial territories, we could not establish whether the regulation of cerebral vessels is more susceptible to the effects of ANG II than that of systemic vessels. However, cerebral blood vessels have a greater capacity to produce NADPH oxidase-derived ROS than systemic vessels (25), which could explain their increased vulnerability. Therefore, assuming that systemic resistance vessels contribute to the genesis of ANG II hypertension, the findings suggest that cerebral vessels are affected before the expression of the neurovascular and humoral changes driving the hypertension.
We also found that the infusion of slow-pressor doses of ANG II induces oxidative stress not only in cerebral blood vessels but also in neurons and, possibly, astrocytes. This is in contrast to the effects of an acute infusion of pressor doses of ANG II, which induce oxidative stress predominantly in cerebral blood vessels (11, 12). In addition to the data with DHE, further evidence of an involvement of ROS in the effects of ANG II is provided by our observations that ROS scavenger MnTBAP and the NADPH peptide inhibitors gp91ds-tat abrogate the cerebrovascular dysfunction. Although our finding with gp91ds-tat suggests that NADPH oxidase is involved in the ROS generation, the mechanisms by which circulating ANG II activates this enzyme in neurons and astrocytes remain to be defined. Circulating ANG II does not cross the blood-brain barrier, and its effects on vascular ROS production are thought to be mediated by the activation of vascular ANG II receptors linked to NADPH oxidase (21). One possibility is that ANG II, when infused chronically, acts at brain sites located outside the blood-brain barrier, i.e., the circumventricular organs. The subfornical organ (SFO), one of the circumventricular organs, is critically involved in the mechanisms of slow pressor ANG II hypertension (40), and it is conceivable that the increase in cortical ROS is mediated by neurohumoral mechanisms triggered by the SFO. In particular, the SFO projects heavily to the hypothalamic paraventricular nucleus, which can release vasoactive hormones, e.g., vasopressin, as well as activate neural projection that could reach the cerebral cortex multisynaptically (2, 34). Another possibility is that circulating ANG II, possibly through the SFO, induces endogenous ANG II production in the brain, resulting in a global increase in ROS. These issues need to be addressed in additional experiments in which the role of the SFO in the cerebrovascular dysfunction induced by ANG II is investigated.
ANG II administration attenuated the increase in CBF produced by neural activity, ACh, bradykinin, and A-23187, responses mediated by different mediators. The increase in CBF evoked by neural activity is mediated, in part, by NO derived from the neuronal NO synthase, whereas endothelial NOS-derived NO mediates the response to ACh (12, 38, 39). On the other hand, in the mouse microcirculation the CBF responses to bradykinin and A-23187 are mediated by cyclooxygenase-1 pathway (27). Therefore, alterations involving a single vasodilatatory mechanism cannot explain the diversity of the cerebrovascular effects of ANG II. Studies on acute ANG II administration have suggested that the cerebrovascular effects of ANG II are mediated by peroxynitrite, which can alter vascular responses through the nitration of proteins critical for vascular function (8, 12, 28). It remains to be determined whether a similar mechanism applies for the effects of chronic ANG II administration.
The vascular and neuronal oxidative stress observed with ANG II infusion has important implications for the central nervous system effects of ANG II-induced hypertension and, possibly, essential hypertension. Hypertension increases the susceptibility of the brain to ischemia and promotes cognitive dysfunction (15). Although the cerebrovascular alterations and related reduction in vascular reserves could play a role, neuronal and glial oxidative stress could also be involved. Inasmuch as the ANG II slow-pressor model recapitulates some of the features of essential hypertension (32), our data support the notion that preventive treatments should be instituted also in prehypertensive states (31), conditions in which the MAP elevation is below the threshold for hypertension (5). Indeed, dietary modifications and exercise may be beneficial for the cognitive effects observed in prehypertension (36), attesting to the reversibility of the brain dysfunction.
In conclusion, we have demonstrated that slow pressor ANG II infusion induces a delayed elevation in MAP and an alteration of key regulatory mechanisms of the cerebral circulation. The cerebrovascular dysfunction, which is also observed with ANG II doses that do not elevate MAP, precedes the onset of hypertension and persists beyond the return of MAP to baseline but is not permanent. In contrast, an elevation of MAP with PE fails to alter cerebrovascular regulation. The cerebrovascular dysfunction induced by slow pressor ANG II is mediated by oxidative stress, which involves not only cerebral microvessels but also neurons and, possibly, astroglia. The findings indicate that slow pressor ANG II infusion targets cerebrovascular function before inducing hypertension, highlighting the vulnerability of cerebral blood vessels to the damaging effects of ANG II. Although the relevance of these experimental data to human hypertension remains uncertain, the data support early preventive interventions in patients with prehypertension, especially in high-risk individuals with other cardiovascular risk factors.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-96571.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol 100:318–327, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res 15:254–263, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5:89–97, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Capone C, Faraco G, Anrather J, Zhou P, Iadecola C. Cyclooxygenase 1-derived prostaglandin E2 and EP1 receptors are required for the cerebrovascular dysfunction induced by angiotensin II. Hypertension 55:911–917, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, Jones D, Materson B, Oparil S, Wright J, Roccella E. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289:2560–2572, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Chrissobolis S, Faraci FM. Sex differences in protection against angiotensin II-induced endothelial dysfunction by manganese superoxide dismutase in the cerebral circulation. Hypertension 55:905–910, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donegan JH, Traystman RJ, Koehler RC, Jones MD, Jr, Rogers MC. Cerebrovascular hypoxic and autoregulatory responses during reduced brain metabolism. Am J Physiol Heart Circ Physiol 249:H421–H429, 1985. [DOI] [PubMed] [Google Scholar]
- 8. Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol 100:739–743, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol 5:649–658, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol 302:148–158, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol 26:826–832, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol 27:303–309, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iadecola C. Principles and methods for measurement of cerebral blood flow: experimental methods. In: Primer on Cerebrovascular Diseases, edited by Welsh KM, Caplan LR, Reis DJ, Sïesjo BK, Weir B. San Diego, CA: Academic Press, 1997, p. 34–37. [Google Scholar]
- 15. Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120:287–296, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab 7:476–484, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 10:1369–1376, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Iadecola C, Springston ME, Reis DJ. Dissociation by chloralose of the cerebrovascular and cardiovascular responses evoked from the cerebellar fastigial nucleus. J Cereb Blood Flow Metab 10:375–382, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 2:157–161, 1999. [DOI] [PubMed] [Google Scholar]
- 20. Iadecola C, Zhang F, Xu X. Cerebrovasodilation elicited by fastigial stimulation is preserved under deep halothane anesthesia. Am J Physiol Regul Integr Comp Physiol 265:R187–R194, 1993. [DOI] [PubMed] [Google Scholar]
- 21. Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH-oxidase-derived radicals. Circ Res 95:1019–1026, 2004. [DOI] [PubMed] [Google Scholar]
- 22. Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol 285:H1890–H1899, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. Part II: estimates of attributable burden. J Hypertens 24:423–430, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 371:1513–1518, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res 97:1055–1062, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 67:181–198, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res 88:600–608, 2001. [DOI] [PubMed] [Google Scholar]
- 28. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA 105:1347–1352, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillis JW. Adenosine in the control of the cerebral circulation. Cerebrovasc Brain Metab Rev 1:26–54, 1989. [PubMed] [Google Scholar]
- 31. Pimenta E, Oparil S, Medscape Prehypertension: epidemiology, consequences and treatment. Nat Rev Nephrol 6:21–30, 2010. [DOI] [PubMed] [Google Scholar]
- 32. Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 284:R893–R912, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89:408–414, 2001. [DOI] [PubMed] [Google Scholar]
- 34. Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol 28:50–60, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sloan TB. Anesthetics and the brain. Anesthesiol Clin North America 20:265–292, 2002. [DOI] [PubMed] [Google Scholar]
- 36. Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 55:1331–1338, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu CC, Liu YB, Lu LS, Lin CW. Imaging reactive oxygen species dynamics in living cells and tissues. Front Biosci (Schol Ed) 1:39–44, 2009. [DOI] [PubMed] [Google Scholar]
- 38. Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA 98:14096–14101, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang G, Iadecola C. Obligatory role of NO in glutamate-dependent hyperemia evoked from cerebellar parallel fibers. Am J Physiol Regul Integr Comp Physiol 272:R1155–R1161, 1997. [DOI] [PubMed] [Google Scholar]
- 40. Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95:210–216, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.