Abstract
Resting intracellular Ca2+ can be raised, in neonatal rat cardiac myocytes, by exposure to very low concentration of thapsigargin (TG). Such a Ca2+ rise yields calcineurin (CN) activation demonstrated by increased expression of transfected luciferase cDNA under control of nuclear factor of activated T-cells (NFAT) promoter and increased translocation of NFAT to nuclei. We found that exposure of cardiac myocytes to TG is followed by increase of sarcroplasmic reticulum Ca2+ transport ATPase (SERCA2) expression, which is further increased when CN inactivation by CAMKII (calmodulin-dependent kinase) is prevented with KN93 (CAMKII inhibitor). On the other hand, SERCA2 expression is reduced by CN inhibition with cyclosporine. We have now induced calcineurin A (CNA) α- or β-subunit gene silencing with small interfering RNA (siRNA) and observed strong interference with expression of SERCA2, both in control myocytes and following exposure to TG. Such interference is also obtained following NFAT displacement from CN with 9,10-dihydro-9,10[1′,2′]-benzenoanthracene-1,4-dione (INCA-6). We have also observed analogous effects on expression of phospholamban (PLB) and Na+/Ca2+ exchanger (NCX). Pertinent to these findings, we have identified, by in-silico analysis, NFAT binding sites in SERCA2, PLB, and NCX1 promoters. Our experiments indicate that activation of the calcineurin-NFAT pathway by rise of resting cytosolic Ca2+ elevates transcription/expression of SERCA2, PLB, and NCX1, providing a homeostatic mechanism for long-term control of cytosolic Ca2+.
Keywords: sarcoplasmic reticulum Ca2+-ATPase; thapsigargin; calcineurin silencing; nuclear factor of activated T-cells displacement; 9,10-dihydro-9,10[1′,2′]-59 benzenoanthracene-1,4-dione
calcium signaling serves as a common mechanism to couple membrane excitation to intracellular functions in most biological tissues (6, 8). In cardiac muscle, variations of cytosolic Ca2+ are involved in several signaling functions including activation of transcription and contraction (4). In this regard, the Ca2+ transport ATPase (SERCA2) of cardiac sarcoplasmic reticulum (14) plays an important role as it fills intracellular stores with Ca2+ to be released for contractile activation, and in turn, sequesters cytosolic Ca2+ to allow relaxation. Severe alterations of Ca2+ signaling and contractile function have been demonstrated (16) following specific inhibition of SERCA2 transport activity with thapsigargin (TG), reduction of expression by a SERCA2 gene null mutation (24), and SERCA2 gene silencing with short interference RNA (siRNA) (30). In fact, a prominent feature of cardiac hypertrophy and failure is SERCA2 downregulation, leading to deficient Ca2+ signaling (3, 10, 13, 25).
The mechanism of SERCA2 trascriptional regulation for optimal control of Ca2+ homeostasis in cardiac muscle is a subject of interest. Previous studies identified regulatory regions (2) in the SERCA2 promoter, some of them corresponding to Sp1 sites (5, 33). Recent work has shown that the SERCA2 promoter can be activated by oversexpression of silent information regulator in cardiac myocytes (31), yielding corrections of SERCA2 expression in disease states. With the work reported here, we characterized the role of cytosolic Ca2+ concentration on transcription/expression of SERCA2, through intervention of the calcineurin (CN)-nuclear factor of activated T-cell (NFAT) pathway and with possible implications for long-term Ca2+ homeostasis in cardiac myocytes.
METHODS
Primary cell cultures.
Neonatal cardiac myocytes were obtained from neonatal (1 day old) rats. Harvesting of cardiac tissue was performed using protocols approved by the California Pacific Medical Center Research Institute animal care and use committee and using the method previously described in detail (26, 32). The dissociated myocytes were preplated in an uncoated P150 dish for 1 h at 37°C under 5% CO2, thereby eliminating nonmyocyte cells by adhesion to the plate. The unattached myocytes were then removed and plated on gelatin-coated dishes or laminin-coated glass surfaces and cultured under 5% CO2 in “plating medium” containing 0.1 mM bromodeoxyuridine.
Twenty four hours after plating was completed, the attached myocytes were washed with PBS. A 4:1 mixture of DMEM and Medium 199 contained 0.1 mM bromodeoxyuridine, 10 μg/ml ITS (insulin, transferrin, and selenium; Mediatech), 0.1% BSA, 0.1 mM vitamin C, and 2 μg/ml vitamin B12. The myocytes were then maintained at 37°C under 5% CO2.
The cultured myocytes were observed by phase contrast microscopy or following immunofluorescence staining. Myofibrillar structure was evidenced in cells grown on four-chambered slide wells, fixed (20 min) with 4% paraformaldehyde (Sigma), and permeabilized (15 min) with 0.1% Triton X-100 in PBS. The myocytes were then stained with Alexa Fluor 488 conjugated phalloidin (SKU no. 12379, Molecular probes) (1:100 in PBS) to visualize F-actin filaments and myofibrillar structure (1). The phalloidin-stained cells were viewed under a fluorescence microscope (×20 objective).
siRNA construct and adenoviral vectors.
DNA templates for endogenous transcription of silencing RNA were cloned into a pSilencer 1.0-U6 plasmid under the control of the U6 RNA Polymerase III promoter (−315 to +1) (Ambion). Coding sequences for targeting mRNAs were selected using the “siRNA Target Finder and Design tool” from Ambion. The potential siRNA target sequence was subjected to BLAST search (NCBI database) against EST libraries of rat to ensure that no other gene(s) was targeted.
The target sequence for rat calcineurin A β (CNA-β) was the following: 5′-AAGCTCCAATTACAGTGTGTG-3′.
To obtain transcription of a complementary sequence to the target, we designed the following sequence where the underlined segment indicates the loop. Sense template: 5′-GCTCCAATTACAGTGTGTGTTCAAGAGACACACACTGTAATTGGAGCTTTTTT-3′; antisense template: 5′-AATTAAAAAAGCTCCAATTACAGTGTGTGTCTCTTGAACACACACTGTAATTGGAGCGCCC-3′.
The target sequence for rat calcineurin A α (CNA-α) was the following: 5′-AACAAGATCCGAGCAATAGGC-3′. Sense template: 5′-CAAGATCCGAGCAATAGGCTTCAAGAGAGCCTATTGCTCGGATCTTGTTTTTT-3′; antisense template: 5′-AATTAAAAAACAAGATCCGAGCAATAGGCTCTCTTGAAGCCTATTGCTCGGATCTTGGGCC-3′.
The plasmid and the oligonucleotides were digested at the ApaI and EcoRI sites and then ligated together. The position of the DNA oligonucleotide was such that it was immediately preceded by the U6 promoter. The ligated DNA was transformed into competent DH5α cells, and the cells were selected for ampicillin resistance. Adenoviral vectors were constructed using a pAd-lox plasmid with an SV40 polyadenylation signal (21). The U6 promoter and siRNA construct were subcloned into the pAd-lox plasmid. The silencing construct, or separately the control construct with just the promoter, were cotransfected with purified ψ5 adenovirus genome into CRE8 cells (12). Infections of myocytes with adenovirus vectors, empty or containing cDNA templates, were performed using viral titers of 5 pfu/cell. The cells infected with Psi5 or silencing vector were kept for 1 h in half volume of the 4:1 mixture of DMEM and Medium 199, and then the remaining half volume of a medium was added to reach the final composition. The cells were examined and collected for RNA extract or luciferase essays 72 h thereafter.
Real-time quantitative RT-PCR.
Total RNA was isolated using the RNeasy mini kit (Qiagen Cat no. 74104) with on-column DNase digestion using an RNase-free DNase set (Qiagen Cat no. 79254) according to the manufacturer's instructions. Primers and probes were designed using Beacon Designer 4.0 software (BD) and are shown in Table 1.
Table 1.
List of real-time RT PCR primers
| Genes | Primers for Real-Time RT-PCR |
|---|---|
| Rat GAPDH (sense) | 5′ GTTCCAGAGACAGCCGCATC 3′ |
| Rat GAPDH (antisense) | 5′ CGTTCACACCGA CCTTCACC 3′ |
| Rat SERCA2 (sense) | 5′ CAGTTCATCCGCTACCTCATCTCC 3′ |
| Rat SERCA2 (antisense) | 5′ CGCAGTGGCAGG CAGACC 3′ |
| Rat PLB (sense) | 5′ CATGCCAACGCAGTTACAACCT 3′ |
| Rat PLB (antisense) | 5′ TCGTGACCCTTCACGACGAT 3′ |
| Rat NCX1 (sense) | 5′ CGAAATGGATGGGAAAGTAGTCAAC 3′ |
| Rat NCX1 (antisense) | 5′ TCTTTGTCGGGATGCTTCTGC 3′ |
| Rat CNA-β (sense) | 5′ TGAACACCGCACATACCACTG 3′ |
| Rat CNA-β (antisense) | 5′ GCCCTCAAGCCTCCATCCG 3′ |
| Rat CNA-α (sense) | 5′ CAGACAGCAACGGCAGTAATAGC 3′ |
| Rat CNA-α (antisense) | 5′ CGTCAGAGGCAAGAACATCCAAC 3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SERCA2, sacroplasmic reticulum Ca2+ transport ATPase; PLB, phospholamban; NCX1, Na+/Ca2+ exchanger 1; CNA, calcineurin A. See methods for more information.
RT-PCR was performed by the SYBR Green method using an Applied Biosystems 7500 Fast Real-Time PCR System. The procedure was as follows: 1.0 μg total RNA was used to synthesize cDNA by reverse transcription using the iScript cDNA Synthesis kit (Bio-Rad) in a 20-μl volume. PCR amplification was performed in a total volume of 20 μl, containing 5 ng of the cDNA derived from reverse transcription, 25 pmol of each primer, and 10 μl iQ SYBR Green Supermix. Each reaction was incubated for 2 min at 50°C, 10 min at 95°C, and then subjected to 40 cycles involving denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. The threshold cycle (CT) for fluorescence development was measured. All samples were run in triplicate.
The ratios of the transcript levels of genes of interest in experimental and control samples were compared with the ratios of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript levels in corresponding samples. GAPDH is widely considered a stable reference in this methodology.
RT-PCR was also performed by the TaqMan gene expression assays (Applied biosystem) using an Applied Biosystems 7500 Fast Real-Time PCR System. Primers and probes [GAPDH: Rn99999916_S1 Gapdh; SERCA2: Rn00568762_A1 Atp2a2; Na+/Ca2+ exchanger-1 (NCX-1): Rn00570527_A1 Slc8a1] for TaqMan assays were obtained from Applied Biosystems. Primers listed in Table 1 were used only for the SYBR Green method.
Western blot analysis and immunofluorescence.
Total protein was measured by the BCA assay kit (Pierce) after sonication of the harvested cells. Various protein components were separated in 7.5%, 12%, or 15% polyacrylamide gels (18), transferred onto nitrocellulose paper, and stained with primary and secondary antibodies. Reactive bands were visualized by the Supersignal ECL Western blotting detection kit (Pierce), and densitometry was obtained in a NucleoVision workstation (Nucleotech) with Gel Expert software. Primary monoclonal antibodies for Western blots and immunostaining of whole cells were NB100-237A (1:2,000) (Novus Biologicals) for SERCA2, MA3-922 (1:2,000) (Affinity Bioreagents) for phospholamban, and sc-8321 (1:50) (Santa Cruz biotechnology) for NFATc3, MF-20 (Developmental Studies Hybridoma Bank, University of Iowa) for myosin. The primary antibody for actin (1:5,000) was obtained from Sigma (A2066). For immunostaining the cultured myocytes were grown on four-chambered slide wells, fixed (20 min) with 4% paraformaldehyde (Sigma), and permeabilized (15 min) with 0.1% Triton X-100 in PBS. The myocytes were blocked with 10% horse serum in PBS for 1 h at room temperature. The myocytes were then incubated with primary antibodies (NFATc3) (diluted in 10% horse serum in PBS, 1:50) for overnight at 4°C followed by three times washing with 1% horse serum in PBS for 10 min. After being washed, the myocytes were incubated with secondary antibody (catalog no. 11034, Alexa Fluor 488 goat anti-Rabbit IgG, Molecular Probes) (1:100, diluted in 10% horse serum in PBS) for 2 h. Cells were washed three times with 1% horse serum in PBS for 10 min. For nuclear staining, myocytes were incubated with propidium iodide (10 μg/ml) for 10 min followed by two times wash with PBS. Fluoromount G (Electron Microscopy Science, catalog no. 17984-25) was used to mount the coverslip on the glass slide. The stained cells were viewed under a confocal microscope (×40 objective).
ATP-dependent Ca2+ transport in cell homogenates.
After being rinsed with PBS, the cultured cells were harvested by scraping in a resuspension medium (10 ml/100 mm dish) containing 50 mM MOPS, pH 7.0, 10 mM NaF, 1 mM EDTA, 0.3 M sucrose, and protease inhibitors [0.4 mM Pefabloc SC (Roche), 0.5 mM dithiothreitol, 10 μg aprotinin/ml, 2 μg leupeptin/ml, 1 μg pepstatin A/ml]. Suspensions were centrifuged for 5 min at 2,000 g, and the cell pellets were frozen and stored at −70°C.
ATP-dependent (45Ca) Ca2+ transport was assayed using homogenates of cultured cells. The reaction conditions were as previously described (32). Transport by residual mitochondrial fragments was inhibited with 1 μM ruthenium red and 5 mM NaN3 in the reaction medium. Control assays in the presence of 1 μM TG were performed to ensure that no additional activity remained after specific inhibition of SERCA2.
Cytosolic Ca2+ transients.
Cytosolic Ca2+ transients were measured in cells grown on special culture dishes with laminin-coated glass coverslips. Cells were loaded for 30 min at 22°C, with a membrane permeant form of Fura-2 (1 μM) (Molecular Probes) and Pluronic F-127 (0.2%) (Molecular Probes) in pH 7.4 Ringer solution containing 10 mM HEPES, 135 mM NaCl, 4.0 mM KCl, 1 mM Mg Cl2, 1.8 mM CaCl2, and 10 mM glucose. The cells were then washed with dye-free Ringer solution. Coverslips loaded with cells were placed in a special chamber mounted on an Olympus 1 ×70 inverted microscope and connected to a circulating bath with Ringer solution held at 30 ± 2°C. Measurements were performed using the Ion Wizard high-speed fluorescence imaging system with a MYO100 Myocam (Ion Optix; Milton, MA). Fluorescence emission from single cells and dye calibration were obtained using 380 or 340 nm excitation (7). The cells were subjected to field stimulation of 10 V and 20 ms duration delivered most commonly at 1 Hz frequency. Cytoplasmic free Ca2+ was calculated (11) from background-corrected fluorescence ratios (R = F340/F380) using the equation [Ca2+] = Kd [(R − Rmin)/(Rmax − R)]× Q. Rmax was obtained at the end of each experiment in the presence of 1 mM Ca2+, and Rmin was estimated in the presence of 1 mM EGTA with no Ca2+. Q was the ratio Rmin/Rmax at 380 nm. Data are shown as means ± SD where n > 15. The threshold for statistical significance was set as P < 0.05 following a Student's two-tailed t-test.
NFAT-dependent transcriptional activity.
NFAT transcriptional activity of myocytes in culture was assessed by transfection with pNFAT-luciferase (cDNA encoding luciferase under control of NFAT-dependent promoter), as recommended by the kit supplier [Stratagene, pCIS-CK plasmid (catalog no. 219088), pNFAT-LUC plasmid (catalog no. 219090), luciferase assay kit (catalog no. 219020)]. The myocytes were transfected 72 h following exposure to experimental variables [i.e., TG, KN-93, cyclosporin (CsA), or ionomycin] and collected 24 h after transfection for determination of luciferase expression by luminescence assay in a Luminometer. In parallel experiments with green fluorescence protein (GFP) we found that 8–10% of the cells were effectively transfected under these conditions, as also reported by Djurovic et al. (9).
Statistical evaluation.
Data are expressed as means ± SD. Statistical analyses were performed using a paired Student's t-test or one-way ANOVA. Student's t-test was used for the comparison of two means, and a P value of <0.05 was taken to be significant. ANOVA was used for the comparison of multiple means. Where appropriate, differences among treatments were determined by ANOVA. When ANOVA revealed significant differences, Tukey's post hoc test for multiple comparisons was performed. P values of <0.05 were considered significant.
RESULTS
Upregulation of SERCA2 expression and NFAT-dependent luciferase transcription by TG.
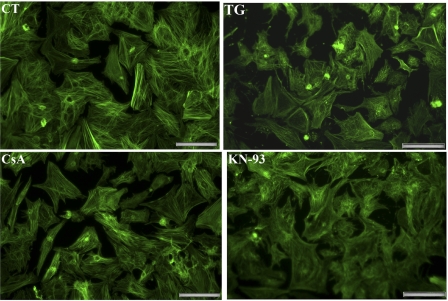
As previously reported (27), more than 95% of the cells in our primary cultures (Fig. 1) are identified with viable cardiac myocytes by immunostaining of specific proteins such as myosin and SERCA2. This constitutes a very advantageous system, permitting biochemical assays on samples collected from uniformly healthy cells. It is also important to note that agents used in our experiments (i.e., TG, CsA, or KN-93) do not produce obvious morphological changes (Fig. 1) at the concentrations used, although higher TG concentrations (50 nM) reduce cell survival significantly (27). Exposure of the myocytes to very low (10 nM) concentrations of TG yields strong inhibition of ATP-dependent Ca2+ transport by SERCA2 in cell homogenates (Fig. 2A) and alteration of Ca2+ signaling in whole myocytes (Fig. 2B), whereas no change in size of the myocytes or reduction in cell survival are noted. In fact, after 7 days exposure of myocytes to increasing concentrations of TG, we found that full inhibition of Ca2+ transport was produced by TG within the nanomolar range (29), whereas cell death was only produced by much higher (μM range) concentrations of TG (see supplemental Fig. 1 at the AJP-Cell Physiology website).
Fig. 1.
Fluorescence microscopy of cultured neonatal rat cardiac myocytes. Cardiac myocytes were prepared as described in methods and maintained in serum-free medium. As indicated, the culture medium was supplemented with 10 nM thapsigargin (TG), 200 nM cyclosporin (CsA), or 5 μM KN-93 (added 24 h after seeding). The myocytes were then fixed after 3 days, permeabilized, stained with Alexa Fluor 488-phalloidin, and viewed with a fluorescence microscope (×20 magnification). The images shown are representative of numerous observations of independent preparations, and the magnification bar corresponds to 50 μm.
Fig. 2.
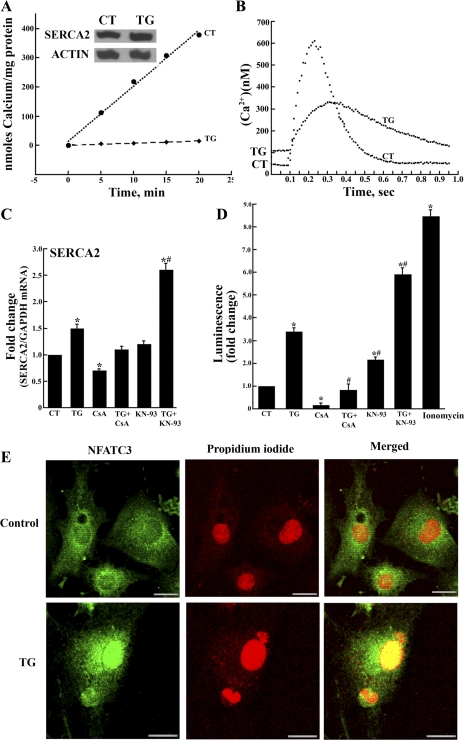
TG inhibits Ca2+ transport and Ca2+ signaling but increases sarcroplasmic reticulum Ca2+ transport ATPase (SERCA2) transcript and protein levels, expression of nuclear factor of activated T-cell (NFAT)-dependent luciferase, as well as NFATc3 nuclear translocation. A: ATP-dependent Ca2+ transport (measured as described in methods) and protein levels (SERCA2) in homogenates of cardiac myocytes (control or exposed to 10 nM TG for 7 days). Results are given as means ± SD from (n = 4). For calcium transport, the variations of TG-treated myocytes when compared with control myocytes were significant with P = 0.05 (Student's two-tailed t-test). Inset: SERCA2 protein levels were measured by Western blot analysis and compared with the levels of actin protein. B: Ca2+ signaling in cardiac myocytes. Cells (control or exposed to 10 nM TG) were loaded with Fura-2 and subjected to field stimulation. Fluorescence was measured at 340 and 380 nm excitation (see methods). Each trace represents the average of transients obtained from 25 cells per group over three separate experiments. Statistical significance was determined by Student's two-tailed t-test. The threshold for statistical significance was set as P < 0.05 following a Student's two-tailed t-test. C: SERCA2 transcript levels in control myocytes and myocytes exposed to TG (10 nM), CsA (0.2 μM), TG + CsA, KN-93 (5 μM), and TG + KN-93 added 24 h after seeding. Transcript levels were determined by RT-PCR. Data are means ± SD from n = 6 preparations. Statistical significance was determined by ANOVA. *Significance (P < 0.05) versus control and all other treatments; #significance (P < 0.05) versus TG. D: NFAT-dependent luciferase expression. Control myocytes and myocytes exposed to TG (10 nM), CsA (0.2 μM), TG + CsA, KN-93 (5 μM), TG + KN-93, or ionomycin (3.0 μM, in the absence of TG) added 24 h after seeding were transfected with cDNA encoding luciferase under the control of NFAT-dependent promoter. The cells were harvested 24 h later for determination of luciferase expression by luminescence assay. Data are means ± SD from n = 4 preparations. Statistical significance was determined by ANOVA. *Significance (P < 0.05) versus control; #significance (P < 0.05) versus TG or KN-93. E: immunostaining of myocytes with total NFATc3 reactive antibodies (shown in green) and with propidium iodide for nuclear stain (shown in red). Merging demonstrates nuclear localization (yellow color) of NFATc3 in myocytes exposed to TG. The magnification bar corresponds to 15 μm.
The Ca2+ signaling alterations produced by 10 nM TG include rise of the resting intracellular Ca2+ concentration, reduction of peak signal intensity, and prolongation of signal duration (Fig. 2B). These alterations are evidently due to inhibition of SERCA activity by TG and interference with sarcoplasmic reticulum (SR) function.
In parallel with alterations of Ca2+ signaling, a significant increase in SERCA2 transcript level (Fig. 2C) and protein expression (Fig. 2A, inset) is produced by 10 nM TG. It is noteworthy that exposure to 10 nM TG also increases three to four times expression of transfected luciferase cDNA under control of NFAT-dependent promoter (Fig. 2D), indicating involvement of the CN-NFAT pathway. Dephosphorylation and nuclear transfer of NFAT following CN activation by TG was also demonstrated by immunostaining (Fig. 2E). On the other hand, direct involvement of the cytosolic Ca2+ rise (even in the absence of TG) in activation of luciferase expression was demonstrated by permitting entry of extracellular Ca2+ by addition of ionomycin (3.0 μM), which was followed by a eight- to ninefold increase in luciferase expression (Fig. 2D).
In the absence as well as in the presence of 10 nM TG, both SERCA2 transcript levels and luciferase expression are reduced by CsA, a known CN inhibitor (Fig. 2, C and D). On the other hand, SERCA2 transcript levels and luciferase expression are increased following inhibition of calmodulin-dependent kinase (CAMKII) with KN-93 (Fig. 2, C and D), evidently due to interference with CN phosphorylation (and consequent inactivation) by CAMKII. These observations suggest that SERCA2 transcription, in analogy to transfected luciferase transcription, is influenced by the CN-NFAT pathway.
Effects of CNA subunit gene silencing or NFAT displacement from CN by INCA-6.
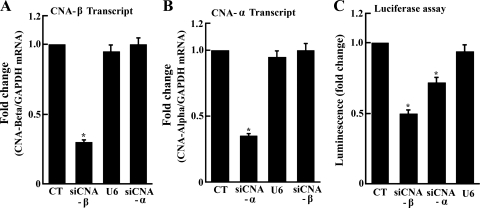
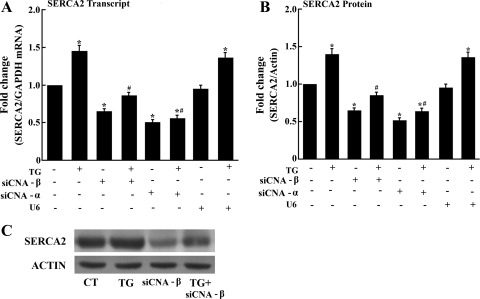
To demonstrate directly CN-NFAT pathway involvement, we have induced downregulation of CNA α- or β-subunits gene expression by means of endogenous transcription of siRNA encoded by exogenous cDNA templates. To this aim, we delivered cDNA templates by adenovirus vector, producing downregulation of related transcripts (Fig. 3, A and B). No effect of empty vector (U6) was observed in control experiments. As expected, a 35% and 50% reduction of (transfected) NFAT-dependent luciferase expression was produced by silencing CNA α- or β-subunit, respectively (Fig. 3C). We have then found that the SERCA2 transcript levels as well as protein expression are also reduced following CNA α- or β-subunits gene silencing (Fig. 4). Such a reduction by CN subunit A-α or A-β silencing is produced both in control myocytes and in myocytes exposed to TG (Fig. 4). Most importantly, no effect on SERCA2 transcription/expression is produced by the U6 control virus (i.e., no CN silencing). These experiments provide direct demonstration that CN is involved and rate limiting in SERCA2 transcription.
Fig. 3.
Downregulation of CNA-α and CNA-β subunits gene expression following endogenous transcription of small interfering RNA (siRNA) encoded by exogenous cDNA templates. cDNA templates were delivered efficiently by adenovirus vector to all cells in culture. In the figure, siCNA refers to silenced CN A subunit. Reduction of CNA β (A) and α (B) subunit transcript levels is produced by infection with adenovirus vectors carrying specific siRNA and not by control virus (U6). Luciferase expression (C) is reduced by either CNA α or β subunit but not by the control virus (U6). Data are means ± SD from n = 3 preparations. Statistical significance was determined by ANOVA. *Significance (P < 0.05) versus control.
Fig. 4.
Reduction of SERCA2 transcript levels (A) and protein expression (B and C) following CNA-α and CNA-β subunits gene silencing. Transcript levels were determined by RT-PCR. Data are means ± SD from n = 6 preparations. Statistical significance was determined by ANOVA. *Significance (P < 0.05) versus control; #significance (P < 0.05) versus TG. Representative Western blots obtained with cell homogenates are shown in C.
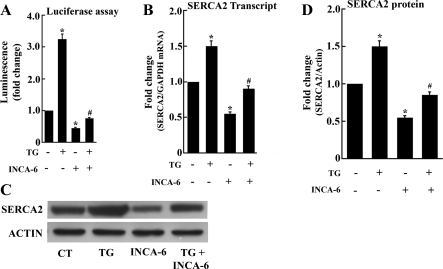
To distinguish whether the effect of CN silencing is related to a general reduction of the CN phosphatase activity or specifically due to lack of dephosphorylation and activation of NFAT(P), we then used 9,10-dihydro-9,10[1′,2′]-benzenoanthracene-1,4-dione (INCA-6). INCA-6 is a small organic molecule that specifically blocks targeting of NFAT(P) substrate to the CN phosphatase site and is an effective inhibitor of CN-NFAT signaling (28). We first documented the INCA-6 effect on cultured myocytes by demonstrating reduction of NFAT-dependent luciferase expression (Fig. 5A). We then found that INCA-6 reduces SERCA2 transcript levels as well as protein expression, in the absence or in the presence of TG (Fig. 5, B–D). It is important to note that INCA-6 interferes with NFAT(P) substrate recognition but does not block the catalytic site directly. Therefore, NFAT(P) dephosphorylation and NFAT nuclear import are inhibited independent of CN phosphatase activity on other possible substrates.
Fig. 5.
Reduction of NFAT-dependent luciferase expression (A), SERCA2 transcript levels (B), and protein expression (C and D) by exposure of myocytes to, 10-dihydro-9,10[1′,2′]-59 benzenoanthracene-1,4-dione (INCA-6). INCA-6 (5 μM) was added, in the absence or presence of TG (10 nM), 1 day after initial plating, and the myocytes were harvested 3 days thereafter. Luciferase expression was obtained by transfection with cDNA encoding luciferase under the control of NFAT-dependent promoter on the third day of treatment with INCA-6. Data are means ± SD from n = 3 preparations. Statistical significance was determined by ANOVA. *Significance (P < 0.05) versus control; #significance (P < 0.05) versus TG.
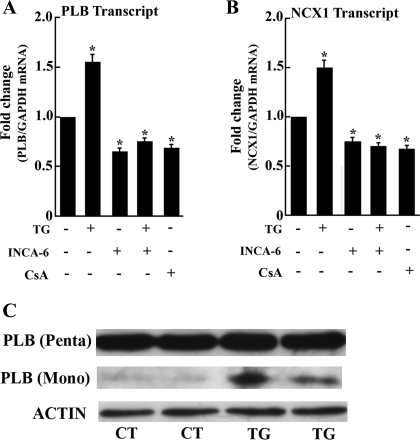
We also found that, in analogy to SERCA2, the phospholambdan (PLB) and NCX1 transcript levels are also increased following exposure of myocytes to TG (Fig. 6). Furthermore, reduction of the PLB and NCX1 transcript levels is produced by NFAT displacement from CN with INCA-6 (Fig. 6). Reduction is also observed following CN inhibition with CsA (Fig. 6). It is noteworty that the PLB protein of rat cardiac myocytes is mostly present as a pentameric oligomer (Fig. 6C), in agreement with previous reports demonstrating that the PLB monomer-to-pentamer ratio is much lower in murine than in human heart muscle (35). On the other hand, a small band corresponding to PLB monomer appears in TG-treated myocytes (Fig. 6C), suggesting that the newly formed PLB protein is transiently present as a monomer before aggregating into oligomers.
Fig. 6.
Phospholamban (PLB) (A) and Na+/Ca2+ exchanger 1 (NCX1) (B) transcript levels in control myocytes or following exposure to INCA-6 or CsA and PLB Western blot (C). INCA-6 (5 μM) was added, with or without TG (10 nM), 1 day after initial plating, and the myocytes were harvested 3 days thereafter. PLB and NCX1 transcripts levels were measured by real-time PCR as described above. Data are means ± SD from n = 3 preparations. Statistical significance was determined by ANOVA. *Significance (P < 0.05) versus control; #significance (P < 0.05) versus TG. C: Western blots showing PLB protein levels and state of oligomerization in control myocytes or following 7 days exposure to 10 nM TG.
DISCUSSION
Expression of transfected luciferase cDNA into cardiac myocytes under control of NFAT-dependent promoter indicates that the activity of endogenous CN is increased by a cytosolic Ca2+ rise produced by TG (Fig. 2D). CN activation is also demonstrated by nuclear transfer of NFAT (Fig. 2E). It is important to note that TG is not involved directly in these effects, since CN activation and luciferase expression are obtained through a cytosolic Ca2+ rise produced with ionomycin, even in the absence of TG (Fig. 2D).
Under condition of CN activation by TG, increased SERCA2 expression is also obtained, indicating that the cytosolic Ca2+ rise and consequent CN activation affects SERCA2 transcription. Furthermore, inhibition of CAMKII with KN-93 allows further increase in luciferase and SERCA2 expression, suggesting that if CN activation is not hindered by phosphorylation and inactivation by CAMKII, dephosphorylated NFAT is produced at higher rates, yielding further increase in expression and transcription of luciferase and SERCA2. In fact, constitutively active CAMKII (exogenous CAMKII overexpressed in the cytosol) was shown to downregulate CN-NFAT signaling by phosphorylation and subsequent inhibition of CN (22). CN and CAMKII are both activated by a rise of cytosolic Ca2+ and therefore, under physiological conditions, CAMKII may limit and possibly optimize the extent of CN activation. It should be pointed out that our observed changes in transcript levels are expressed with reference to GAPDH, and the changes in protein expression levels are expressed with reference to actin. Therefore, the observed effects of stimulation or inhibition of NFAT activation are not general but affect prevalently a selective set of genes including SERCA2.
The effects of CN subunits gene silencing, as well as NFAT substrate displacement from CN by INCA-6, demonstrate unambiguously that expression of SERCA2 is influenced by the CN-NFAT pathway. Expression of PLB and NCX1 appears to be affected in parallel with SERCA2. We then gained further insight on this subject by in-silico analysis of the SERCA2 promoter, searching for NFAT binding sites that may be involved in the mechanism of NFAT influence on SERCA2 transcription. The rat SERCA2 promoter sequence was obtained from the Transcriptional Regulatory Element Database (http://rulai.cshl.edu/cgi-bin/TRED/tred.cgi?process=home), as well as from NCBI (accession no. AY948198). Considering that NFAT consensus-binding site (17, 19) is [A/T]GGAAA[A/N][A/T/C]N, we searched using rvista analysis (http://rvista.dcode.org) and found three putative NFAT binding sites [−320bp (GGAAA), −1244bp (TTTCC), and −1424bp (GGAAA)] in the 1,500 bp upstream region (see supplemental Fig. 2). NFAT binding sites were also noted by Zarain-Herzberg (36) in consensus sequences of rabbit, human, rat, and mouse SERCA2 promoter. We have also identified putative NFAT binding sites in the rat PLB promoter sequence (accession no. AH002227.1) (15), i.e., five NFAT sites [−412 (GGAAA), −490 (TTTCC ), −802(GGAAA), −1120(GGAAA), −1418(GGAAA )] in the 1500 bp upstream region by in-silico analysis (see supplemental Fig. 3). Furthermore, three putative NFAT binding sites [−97bp (GGAAA), −961 (GGAAA), −1278 (TTTCC)] were identified in the 1500 bp upstream region (see supplemental Fig. 4) of the NCX1 promoter (Slc8a1) (accession no. NM_019268.2) (20).
In our experiments, the dependence of SERCA2, PLB, and NCX1 transcription on CN/NFAT is shown not only by upregulation in TG experiments but also by downregulation in the experiments with INCA-6 (NFAT-P displacement from CN). It is noteworthy that the CN-NFAT pathway may be a common point of regulation under some conditions but not in others. For instance, it was reported (23) that SERCA2 and PLB mRNA are both reduced in pressure overload hypertrophy, but SERCA2 is upregulated in hyperthyroid and downregulated in hypothyroid conditions. On the other hand, PLB is downregulated in hyperthyroid but unchanged in hypothyroid conditions. Therefore, SERCA2 and PLB regulation may be convergent or divergent in various cardiac remodeling models. We show here that they share CN-NFAT dependence and the related transcriptional pathway may be rate limiting. We also consider that functional regulation of SERCA2 by PLB, even under condition of overexpression, is likely to be limited due to its prevalent polymerization state in murine when compared with cardiac muscle (34).
The effects of CN silencing described above indicate that CN phosphatase activity is rate limiting for production of active (dephosphorylated) NFAT, which is then involved in transcription/expression of SERCA2 and also of PLB and NCX1. In turn, elevation of resting cytosolic Ca2+ plays an important role in activating CN, with consequent increase in transcription expression of Ca2+-handling proteins, such as SERCA2, PLB, and NCX1. In our experiments, TG was used as an experimental perturbation to obtain a rise in cyosolic Ca2+. This rise produced CN activation and increased transcription of exogenous genes such as luciferase and endogenous genes such as SERCA2, PLB, and NCX1. This rise was prevented by CN inactivation or silencing. Even though the expressed SERCA2 is inactivated by TG, the observed transcriptional response to cytosolic Ca2+ rise suggests that increased expression of SERCA, PLB, and NCX1 provides a homeostatic mechanism for long-term control of cytosolic Ca2+. Furthermore, our findings raise the possibility that competitive engagement of the CN-NFAT pathway by other genes that are dependent on NFAT binding promoters may influence SERCA2 expression in physiological or pathological conditions.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant RO1-69830.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors are grateful to Dr. Hailun Ma for reagents used in construction of adenoviral vectors.
REFERENCES
- 1. Aoki H, Sadoshima J, Izumo S. Myosin light chain kinase mediates sarcomere organization during cardiac hypertrophy in vitro. Nat Med 6: 183–188, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Aoyagi T, Yonekura K, Eto Y, Matsumoto A, Yokoyama I, Sugiura S, Momomura S, Hirata Y, Baker DL, Periasamy M. The sarcoplasmic reticulum Ca2+-ATPase (SERCA2) gene promoter activity is decreased in response to severe left ventricular pressure-overload hypertrophy in rat hearts. J Mol Cell Cardiol 31: 919–926, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Armoundas AA, Rose J, Aggarwal R, Stuyvers BD, O'rourke B, Kass DA, Marbán E, Shorofsky SR, Tomaselli GF, William Balke C. Cellular and molecular determinants of altered Ca2+ handling in the failing rabbit heart: primary defects in SR Ca2+ uptake and release mechanisms. Am J Physiol Heart Circ Physiol 292: H1607–H1618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Brady M, Koban MU, Dellow KA, Yacoub M, Boheler KR, Fuller SJ. Sp1 and Sp3 transcription factors are required for trans-activation of the human SERCA2 promoter in cardiomyocytes. Cardiovasc Res 60: 347–354, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA 99: 1115–1122, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavagna M, O'Donnell JM, Sumbilla C, Inesi G, Klein MG. Exogenous Ca2+-ATPase isoform effects on Ca2+ transients of embryonic chicken and neonatal rat cardiac myocytes. J Physiol 528: 53–63, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Djurovic S, Iversen N, Jeansson S, Hoover F, Christensen G. Comparison of nonviral transfection and adeno-associated viral transduction on cardiomyocytes. Mol Biotechnol 28: 21–32, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Gomez AM, Valdivia HH, Cheng H, Lederer M, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276: 800–806, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 12. Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol 71: 1842–1849, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34: 951–969, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Inesi G, Ebashi S, Watanabe S. Preparation of vesicular relaxing factor from bovine heart tissue. Am J Physiol 207: 1339–1344, 1964 [DOI] [PubMed] [Google Scholar]
- 15. Johns DC, Feldman AM. Identification of a highly conserved region at the 5′ flank of the phospholamban gene. Biochem Biophys Res Commun 188: 927–933 1992 [DOI] [PubMed] [Google Scholar]
- 16. Kirby MS, Sagara Y, Gaa S, Inesi G, Lederer WJ, Rogers TB. Thapsigargin inhibits contraction and Ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum Ca2+ pump. J Biol Chem 267: 12545–12551, 1992 [PubMed] [Google Scholar]
- 17. Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116: 3114–3126, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 19. Lin Z, Murtazaa I, Wanga K, Jiaoa J, Gaoa J, Lia PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA 106: 12103–12108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Low W, Kasir J, Rahamimoff H. cloning of the rat heart Na(+)-Ca2+ exchanger and its functional expression in HeLa cells. FEBS Lett 316: 63–67, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Ma H, Sumbilla CM, Farrance IK, Klein MG, Inesi G. Cell-specific expression of SERCA, the exogenous Ca2+ transport ATPase, in cardiac myocytes. Am J Physiol Cell Physiol 286: C556–C564, 2004 [DOI] [PubMed] [Google Scholar]
- 22. MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res 105: 316–325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagai R, Zarain-Herzberg A, Brandl CJ, Fujii J, Tada M, MacLennan DH, Alpert NR, Periasamy M. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci USA 86: 2966–2970, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, Nieman ML, Riddle T, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem 274: 2556–2562, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res 85: 38–46, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Prasad AM, Inesi G. Effects of thapsigargin and phenylephrine on calcineurin and protein kinase C signaling functions in cardiac myocytes. Am J Physiol Cell Physiol 296: C992–C1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prasad AM, Ma H, Sumbilla C, Lee DI, Klein MG, Inesi G. Phenylephrine hypertrophy, Ca2+-ATPase (SERCA2), and Ca2+- signaling in neonatal rat cardiac myocytes. Am J Physiol Cell Physiol 292: C2269–C2275, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Roehrl MHA, Kang S, Aramburu J, Wagner G, Rao A, Hogan PG. Selective inhibition of calcineurin-NFAT signaling by blocking protein-protein interaction with small organic molecules. Proc Natl Acad Sci USA 101: 7554–7559, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sagara Y, Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem 266: 13503–13506, 1991 [PubMed] [Google Scholar]
- 30. Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A, Soboloff J, Gill DL, Inesi G. Sarco(endo)plasmic reticulum Ca2+ATPase (SERCA) gene silencing and remodeling of the Ca2+signaling mechanism in cardiac myocytes. Proc Natl Acad Sci USA 101: 16683–16688, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 298: H833–H843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sumbilla C, Cavagna M, Zhong L, Ma H, Lewis D, Farrance I, Inesi G. Comparison of SERCA1 and SERCA2a expressed in COS-1 cells and cardiac myocytes. Am J Physiol Heart Circ Physiol 277: H2381–H2391, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Takizawa T, Arai M, Tomaru K, Koitabashi N, Baker DL, Periasamy M, Kurabayashi M. Transcription factor Sp1 regulates SERCA2 gene expression in pressure-overloaded hearts: a study using in vivo direct gene transfer into living myocardium. J Mol Cell Cardiol 35: 777–783, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Zhao W, Waggoner JR, Zhang ZG, Lam CK, Han P, Qian J, Schroder PM, Mitton B, Kontrogianni-Konstantopoulos A, Robia SL, Kranias EG. The anti-apoptotic protein HAX-1 is a regulator of cardiac function. Proc Natl Acad Sci USA 106: 20776–20781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao W, Yuan Q, Qian J, Waggoner JR, Pathak A, Chu G, Mitton B, Sun X, Jin J, Braz JC, Hahn HS, Marreez Y, Syed F, Pollesello P, Annila A, Wang HS, Schultz Jel J, Molkentin JD, Liggett SB, Dorn GW, 2nd, Kranias EG. The presence of Lys27 instead of Asn27 in human phospholamban promotes sarcoplasmic reticulum Ca2+-ATPase superinhibition, and cardiac remodeling. Circulation 113: 995–1004, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Zarain-Herzberg A. Regulation of the sarcoplasmic reticulum Ca2+. ATPase expression in the hypertrophic and failing heart. Can J Physiol Pharmacol 84: 309–521, 2006 [DOI] [PubMed] [Google Scholar]