Abstract
The major public health concern worldwide is coronary heart disease, with dyslipidemia as a major risk factor. Statin drugs are recommended by several guidelines for both primary and secondary prevention. Rosuvastatin has been widely accepted because of its efficacy, potency, and superior safety profile. Inflammation is involved in all phases of atherosclerosis, with the process beginning in early youth and advancing relentlessly for decades throughout life. C-reactive protein (CRP) is a well-studied, nonspecific marker of inflammation which may reflect general health risk. Considerable evidence suggests CRP is an independent predictor of future cardiovascular events, but direct involvement in atherosclerosis remains controversial. Rosuvastatin is a synthetic, hydrophilic statin with unique stereochemistry. A large proportion of patients achieve evidence-based lipid targets while using the drug, and it slows progression and induces regression of atherosclerotic coronary lesions. Rosuvastatin lowers CRP levels significantly. The Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial was designed after the observation that when both low density lipoprotein and CRP were reduced, patients fared better than when only LDL was lowered. Advocates and critics alike acknowledge that the benefits of rosuvastatin in JUPITER were real. After a review, the US Food and Drug Administration extended the indications for rosuvastatin to include asymptomatic JUPITER-eligible individuals with one additional risk factor. The American Heart Association and Centers of Disease Control and Prevention had previously recognized the use of CRP in persons with “intermediate risk” as defined by global risk scores. The Canadian Cardiovascular Society guidelines went further and recommended use of statins in persons with low LDL and high CRP levels at intermediate risk. The JUPITER study focused attention on ostensibly healthy individuals with “normal” lipid profiles and high CRP values who benefited from statin therapy. The backdrop to JUPITER during this period was an increasing awareness of a rising cardiovascular risk burden and imperfect methods of risk evaluation, so that a significant number of individuals were being denied beneficial therapies. Other concerns have been a high level of residual risk in those who are treated, poor patient adherence, a need to follow guidelines more closely, a dual global epidemic of obesity and diabetes, and a progressively deteriorating level of physical activity in the population. Calls for new and more effective means of reducing risk for coronary heart disease are intensifying. In view of compelling evidence supporting earlier and aggressive therapy in people with high risk burdens, JUPITER simply offers another choice for stratification and earlier risk reduction in primary prevention patients. When indicated, and in individuals unwilling or unable to change their diet and lifestyles sufficiently, the benefits of statins greatly exceed the risks. Two side effects of interest are myotoxicity and an increase in the incidence of diabetes.
Keywords: rosuvastatin, JUPITER study, statin drugs, C-reactive protein, dyslipidemia, cholesterol, primary prevention, cardiovascular risk, coronary heart disease, inflammation, low-density lipoprotein, high-density lipoprotein, diabetes, metabolic syndrome, Framingham risk score, Reynolds risk score, coronary artery calcification, carotid intima-media thickness, hypertension, obesity, HMG CoA reductase, mevalonate, prenylation, statin myopathy, pleiotropic, dolichol
Introduction
Cardiovascular disease (CVD) has been the leading cause of death for the past century in the United States (US), except for 1918, the year of an influenza pandemic. Even though deaths from coronary heart disease (CHD) have fallen during the past 2 decades, the mortality rate from this disease remains the top cause of death in the US, and, by 2020, will attain that status globally as well. An analysis of the fall in CHD death rates from 1980 to 2000 using the IMPACT model of analyzing data from the US National Center for Health Statistics1,2 revealed that half of this decline was due to improvements in risk factors; 79% was attributable to primary prevention and 21% to secondary prevention.3 Cholesterol reduction accounted for 42.7% of the death rate reduction in asymptomatic individuals, and for 34% in those with CHD. Use of statin drugs accounted for <20% of the improvement in death rate during those 20 years. Reductions in systolic blood pressure accounted for 38.8% of the fall in asymptomatic individuals and 52.8% in patients with CHD. Decreased use of tobacco accounted for 18.4% of the fall in deaths in asymptomatic individuals, and 12.9% in those with CHD. While the contribution of statins, 3-hydroxy-3- methyl-glutaryl-coenzyme A reductase (HMGR) inhibitors, was surprisingly small, these results illustrate the power of primary prevention in the management of CHD. Given the lower usage of less potent statins in many of the years surveyed, the data simultaneously suggest that using statin drugs earlier in the disease, for longer periods of time during pathogenesis, and titrated to lower targets, has substantial unrealized potential.
The cardiovascular risk burden in the populations of nearly all countries continues to rise at an alarming rate, and recent analyses show that gains in reducing the death rate from CHD are now being offset by the pressure of increases in reversible factors of obesity,4 metabolic syndrome, and diabetes, along with the progressive aging of the population.5–8 Control of risk factors, while impressive thus far, have also fallen short of guideline targets and public health goals. Hence, there is ongoing interest in the best use of all elements of primary prevention in order to improve national heart health. Statin drugs are uniquely effective and prominently recommended in current guidelines. The potency and favorable benefit to risk ratio of rosuvastatin account for its increasing usage in primary and secondary prevention.
Significant and voluminous research concerning the biochemistry, physiology, and clinical potential of measuring C-reactive protein (CRP), has intensified discussions concerning the role CRP monitoring will have in current guidelines for primary prevention. The Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, first reported in October 2008, presented evidence that a plasma CRP ≥2 mg/L reflects higher cardiovascular risk in asymptomatic individuals with low-density lipoprotein levels (LDL) <130 mg/dL, and that they would benefit from rosuvastatin therapy.9 Following the JUPITER report, there was lively debate about the data, clinical significance, and how the new information should be incorporated into clinical practice. Many fundamental questions concerning primary prevention have since been revisited, including the early beginnings of CHD and its long incubation period; methods of evaluating risk in the population; how populationbased versus individual risk-based approaches may best be employed; role and refinement of global risk factor scores; choice and merits of nontraditional risk factors; assessment of multiple biomarker panels; role of imaging techniques in evaluation and ongoing therapy; value of advanced lipid testing; weights assigned to traditional risk factors, cutoff values, and treatment targets, particularly LDL goals in guidelines; use of statins in primary prevention; reasons for low patient adherence with evidence-based therapies; causes of “clinical inertia” and lack of physician compliance with guidelines; and the etiologies, extent, and minimization of residual risk.
C-reactive protein
The fundamental role of inflammation in the pathogenesis of atherothrombotic disease is based upon strong evidence and is well-accepted.11–61 CRP protein is an acute phase reactant discovered by Tillett and Francis at Rockefeller University in 1930 in the blood of patients with pneumonia,62 was crystallized in 1947, and today, over 60 years later, there is still controversy about its physiology and applications in biomedicine. CRP protein is a pentraxin comprised of 5 subunits (Figure 1) which is primarily synthesized in the liver, and plays an active part in regulating the innate immune system. Since CRP mRNA levels rise in adipose tissue as CRP expression is enhanced in vitro by interleukin-6 (IL-6), adipose cells also have some ability to synthesize CRP. Innate immunity and adaptive immunity significantly modulate atherosclerosis,13,63,64 with both pro- and anti-atherogenic potential.64–66
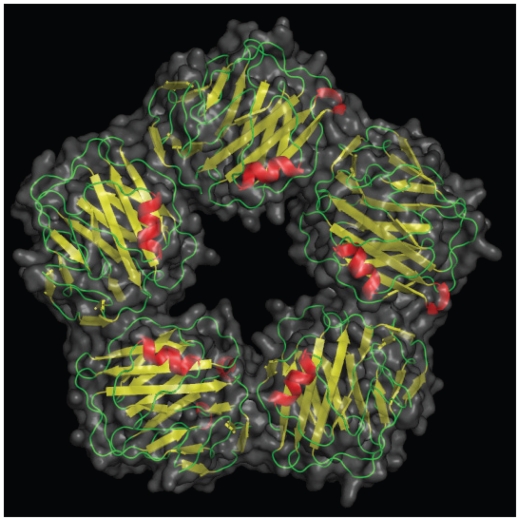
Figure 1.
A picture of C-reactive protein (CRP) from 1B09.pdb made using pymol.10 CRP is a pentameric molecule containing a recognition face that binds phosphocholine and calcium ions, and on the opposite side, an effector face that contains a C1q-binding site. Function depends upon Ca2+-dependent ligand binding. See text for details. Reproduced by permission of Skolstoe through Wikipedia commons.
CRP binding offers a glimpse into teleological function. CRP binds to phosphocholine, commonly found in cell membranes as well as in bacterial and fungal polysaccharides. Phosphocholine is used by the placenta and nematodes to cloak them from their host immune system. Binding to complement C1q complex and factor H, CRP assists in human complement activity to promote antigen presentation and phagocytosis,67 essentially functioning as an innate opsonin. In addition, CRP binds directly with phagocyte Fcγ receptors,68 literally becoming the interface between innate immunity, C1q on complement, and inflammation, Fcγ on macrophages. Details of CRP binding to its ligands and their implications have been recently reviewed.53 As an acute phase reactant during infections or diffuse tissue destruction, CRP concentrations may increase 50,000-fold quickly.69 A constant potent stimulus may sustain high circulating CRP concentrations, depending upon synthetic capacity; the half-life of CRP in the circulation is about 19 hours.70 Hepatic synthesis is driven at the transcriptional level by IL-6, largely expressed by macrophages, T cells, and adipocytes.11,71 Several upstream cytokines also regulate hepatic CRP release. In part because there is overlap in signaling, plasma CRP values are related to other inflammatory markers. However, despite a plethora of research, the precise role(s) of CRP remain unclear.
Variation and significance of CRP levels
Baseline values of CRP are influenced by genetics.72,73 Even after correction for age, CRP polymorphisms, and smoking, ancestry affects CRP levels, the highest values being found in blacks, with an average of 2.6 mg/L, followed by Hispanics, 2.51 mg/L, South Asians, 2.34 mg/L, whites, 2.03 mg/L, and East Asians with the lowest level at 1.01 mg/L.74 CRP levels also vary with the environment, eg, pollutant burdens;75 fiber intake,76–79 coffee ingestion,80 and other dietary factors;26,29,81–84 smoking;85 body mass index;86,87 alcohol consumption;88,89 drugs, including contraceptives90 and hormone replacement therapy;91 chronic infections,92,93 and noninfectious inflammatory conditions, such as the autoimmune diseases,94–97 cancer,98 and polycystic ovary syndrome;99–102 as well as multiple risk factors for atherosclerosis, including obesity, diabetes, metabolic syndrome, and hypertension. Triage of patients according to CRP levels generally control, correct for, and consider these variations.
Since CRP binds to LDL and can be identified within atherosclerotic plaque,103,104 is involved with inflammatory atherogenic processes,105–116 is elevated in patients with acute (ACS) and chronic coronary syndromes117–120 with unfavorable plaque compositions in these syndromes,55 and is associated with complications of heart failure121 a causal role in atherothrombotic disease has long been suspected. At this time, causality remains unproven.107,122,123 One intriguing property of CRP is the associated inhibition of endothelial nitric oxide synthase in vitro124 and the relationship to impaired vasoreactivity and hypertension in such models.125 Overall, the evidence for a role of CRP in endothelial cell dysfunction and monocyte activation in metabolic syndrome is impressive and difficult to ignore.126 The prevalence of this syndrome is approaching 53% in some populations (excluding those with diabetes)127 and the importance of endothelial dysfunction in pathogenesis is now unassailable.128
It is generally appreciated that a high CRP level (here-after meaning hs-CRP [hs, highly sensitive] measurements) usually portends a poorer prognosis in patients with CHD, diabetes, hypertension, pre- or postoperative surgery, and vascular comorbidities. A high prevalence of elevated circulating CRP levels in the general population has been known for some time. At the 53rd Annual Scientific Session of the American College of Cardiology, data were presented from the 1999–2000 National Health and Nutrition Examination Survey (NHANES) indicating that 47.1% of respondents aged 20 years or over had high CRP values.129,130 Levels were higher in females, the obese, and in the elderly, aged 55 to 74. About one-third of the population has CRP levels above 3 mg/L.131 However, smaller elevations in CRP are even more common, and over half of the population has a CRP ≥ 2 mg/L.132 Such “minor” elevations in CRP also imply a poorer prognosis compared with lower values, both in the ill and the apparently healthy.133 Raised values of CRP strongly suggest clinical attention is necessary. Common associations of CRP elevations may reflect not only inflammation but widespread tissue distress, degeneration, and/or destruction, much of which may be age-related. At the other end of the continuum, a low CRP has been considered a measure of wellness,134 and as lifestyle becomes progressively unhealthy in a population, CRP rises. As shown in the Monitoring Trends and Determinants in Cardiovascular Disease (MONICA) Augsburg Study, negative behaviors and factors, such as a poor quality “Western” diet with low intake of vegetables, fruits, and fiber, high consumption of saturated fats, poor physical fitness, and overfeeding and obesity, are all associated with higher CRP levels.135 In a global sense, when no overt disease is evident, the CRP level roughly follows the level of self-inflicted abuse. In these instances CRP elevations reflect poor lifestyle choices that lead to deranged metabolic signaling, inflammation, and the appearance and worsening of traditional risk factors during the early incubation period of atherosclerosis. As such, elevations tend to cluster with conventional risk factors, and teasing apart the significance has been difficult,136 but studies confirm that the properties of CRP and significance of changes are different. Lifestyle has a greater influence on CRP than does genetics.137 Conversely, sustained improvement in lifestyle, particularly weight loss with exercise, and measures that relieve the risk burden, including statins, all reduce plasma CRP levels.
In a review of hospital records of 22,962 patients in a large urban population, an analysis of change in CRP status – normal (≤ 3 mg/L) to elevated (>3 mg/L) and vice versa – and mortality, revealed a significant graded association between CRP levels and mortality.138 The patients were not healthy or chosen at random, but rather a cohort selected by physicians who believed their patients needed a CRP level performed. A change from normal to elevated CRP levels within a year was associated with a 6.7-fold hazard ratio (HR) for all-cause mortality during the following 4 years, compared to subjects whose CRP remained normal. When CRP levels changed from elevated to normal, the HR halved to 3.5. In the hospital setting as well, CRP levels reflect risk and subsequent survival, with a strong relationship to all-cause mortality, particularly in vascular diseases and cancer.139
In the elderly, high CRP values are associated with greater risk of all-cause mortality, especially in apolipoprotein E4 carriers, as well as with cognitive decline.140 The Rancho Bernardo Study reported that the lower the circulating CRP level in elderly men, the greater their longevity, and plasma concentrations of IL-6 were inversely related to survival time in elderly women who did not use estrogen.141 Finally, the classic paper that showed CRP was a stronger predictor of cardiovascular events than LDL, and measuring both together provided better prognostic information than screening for either alone, firmly established the potential clinical significance of following circulating CRP concentrations.142 This study also demonstrated the inverse relationship between CRP levels and the probability of event-free survival.
The work cited above supports the significance of circulating CRP levels in “ordinary” individuals as a sensitive but nonspecific index of quality of lifestyle prior to the diagnosis of disease, as a screen for risk refinement beyond traditional risk factors, and as an index that reflects the intensity of either the amount of inflammation or volume of cell distress.133
When CRP was compared to other inflammatory markers as a predictor of relative risk of hard coronary events (postmenopausal women, highest versus lowest quartile) relative risk scores were CRP, 4.4, serum amyloid A, 3.0, soluble intercellular adhesion molecule type 1, 2.6, IL-6, 2.2, total cholesterol, 2.4, LDL, 2.4, apolipoprotein B-100, 3.4, high-density lipoprotein (HDL), 0.3, and for the ratio total cholesterol/HDL, 3.4.35 The predictive power of CRP remained significant for LDL values <130 mg/dL. Of all nontraditional risk factors, CRP is the most studied, with over 34,000 general PubMed results, and 12,500 in connection with CVD, authored by investigators around the world over an extended period of time. CRP is involved in basic pathophysiology, and/or reflects additional risk and/or has predictive power in a number of CVDs, equivalents, or comorbidities, which include hypertension,143–151 hypertension and blood pressure variability,152 percutaneous coronary intervention,153,154 re-endothelialization,110 stent implantation,155 coronary artery bypass surgery,156–158 heart failure,159–167 peripheral arterial disease,168,169 carotid artery disease,170 sudden cardiac death (SCD),171–173 atrial fibrillation,174–176 ventricular tachycardia after myocardial infarction (MI) and implantable cardioverter-defibrillator implantation,177 calcific aortic valve disease,178 venous thromboembolism,179,180 pulmonary arterial hypertension,181 diabetes,116,182–186 metabolic syndrome,87,93,168,180,185,187–199 rheumatic mitral stenosis,200 chronic lung disease and asthma,201–203 chronic kidney disease,154,204–206 obstructive sleep apnea,207–208 air pollution vis-à-vis inflammation and cardiac risk,75,209,210 obesity,137,197,211–223 eclampsia,224 blood concentrations of reactive oxygen species,225 depression in the obese,211 depression associated with coronary artery disease,226 and HIV disease progression.225 Statins have been found useful in many of these clinical situations. The preponderance of the evidence, from many diverse sources, indicates that CRP measurement has significant broad-based value and is also uniquely related to vascular disease.
CRP and genetic studies
CRP genetic (pentraxin-related) polymorphisms exist, which, together with diet and other lifestyle factors, contribute to significant interindividual variation in plasma CRP levels, as well as differences in responses to therapeutic agents.227 Such variants in the CRP locus may account for 30% to 50% of the phenotypic variation in CRP levels,228 and may also explain some ethnic differences.229 CRP concentrations are 16% lower, for instance, in Asians, but 26% higher in black people than in whites.230 In addition, other genetic loci that encode for upstream cytokines (IL-6, IL-1, tumor necrosis factor α) that affect CRP synthesis influence CRP levels, as well as genes related to obesity and insulin resistance.187
Mendelian randomization, the random assortment of genes that occurs during reproduction, provides a method for assessing cause and effect that is not influenced by reverse causation.231–233 By comparing natural variation in known genes and phenotype, strengths of environmental influences may be inferred.234 A large genome-wide association (n = 17,967) and replication study (n = 13,615) examined the association of genetic loci with plasma CRP concentrations and risk of CHD.235 The authors found that genetic variants expected to lower the CRP expression by about 20% did not reduce CHD risk by the predicted amount of 6%. The discordance between anticipated versus actual CRP phenotype argued that, in genetic variants, high CRP levels throughout life do not cause increased risk for ischemic heart disease. Several limitations to the study, and in the use of Mendelian randomization generally, were identified.236 Another Mendelian randomization study of 47,000 individuals from the general population revealed a comparable situation elsewhere: high plasma CRP values were robustly associated with higher risk of atrial fibrillation, but not with genetically elevated CRP levels.176
Causation versus predictive utility
The Emerging Risk Factors Collaboration (ERFC) reported a meta-analysis of records of 160,309 people with medical histories of vascular disease, associating logeCRP concentrations with conventional risk factors and inflammatory markers.237 Their data confirmed that downstream markers of inflammation, especially fibrinogen levels, but also the leukocyte count, plasma albumin concentration, and erythrocyte sedimentation rate, were associated with CRP levels. As the investigators adjusted for conventional risk factors, the associations of CRP levels with risk of CHD weakened, but the most significant adjustment was for fibrinogen perturbations during inflammation. Hepatic synthesis of this protein is also regulated by IL-6. Hence, the ERFC concluded that most of the association of CRP with coronary artery disease depends upon conventional risk factors and fibrinogen levels.18,238–240 Their views are consistent with findings using Mendelian randomization discussed above, noting a disparity between CRP-related phenotypes, coronary risk, and subsequent coronary events.
Interestingly, after adjusting for conventional risk factors, the relative risks for CHD in this meta-analysis were CRP, 1.37, non-HDL cholesterol, 1.28, and systolic blood pressure, 1.35. Therefore, despite the conclusion, their data actually confirmed that CRP concentrations have a significant predictive association with risk of CHD, stroke, and vascular and nonvascular mortality.
While the ERFC appears to argue against a causative role of CRP in atherosclerosis, the issue is far from settled, since there is much convincing basic science that requires specific refutation.126 In addition, a significant putative role for the monomeric form of CRP (mCRP) has been proposed.241 Mediated by activated platelets, formation of mCRP,242 a conformationally distinct isoform which is prothrombotic243 and inflammatory,244 might confound conclusions if monomeric conversion of CRP from the pentameric moiety (pCRP) – commonly measured as hs-CRP – were not considered. Indeed, it has been shown that mCRP localizes monocyte-mediated inflammation in the atherosclerotic plaque, and may be deposited in plaques but not in healthy vessels, whereas pCRP is not found in either healthy or diseased arteries.244 Moreover, mCRP and pCRP have differing actions on neutrophil migration and thrombus evolution. The various metabolic roles of pCRP and mCRP are expertly reviewed in an editorial accompanying the aforementioned paper.115 There is further evidence that mCRP inserts into lipid raft microdomains within endothelial cell membranes, rather than binds to surface proteins, as a complex function of membrane cholesterol content. The result of this incorporation is endothelial cell activation, as part of an early inflammatory step in the atherosclerotic process.245 These data are but a small part of the burgeoning scientific literature that underscores the importance of CRP in atherothrombosis, and the need for further research.
A randomized trial using a drug that targets CRP will be necessary to decide whether or not CRP is causally involved in atherogenesis. However, even if proof of CRP participation in the atherothrombotic process is incomplete, separate evidence indicates that measurement of CRP levels is useful in predicting and monitoring particular groups of patients. For instance, AFCAPS-TexCAPS found that patients with higher CRP may anticipate a better response to statins,246 and selection of patients with high CRP levels in different circumstances adds to absolute risk assessment.142,247,248 As mentioned above, the ERFC, a large meta-analysis, reported a consistent 1.6-fold rise in vascular risk for each 1-SD rise in CRP levels.237
After de Beer reported that elevations in CRP levels predicted a poor outcome after MI,249 baseline levels were found to predict future cardiac events in stable and unstable angina.250,251 These observations were extended to people without heart disease in whom baseline CRP measurements also correlated with future cardiac prognosis.135,252 Lowering CRP concentrations in patients without elevations in LDL following statin administration improved prognosis.248,253 Reporting bias, with multiple ways of comparing CRP values, together with publication bias in CRP studies, may hamper full evaluation of prognostic capability.254 Several studies confirm that patients with higher CRP levels are at greater risk than those with lower values.118,255 These data support the view that measurement of CRP improves prediction of risk.118,256 Another approach, as suggested by the American Heart Association, the CDC, and the Canadian Cardiovascular Society, is that patients with heart disease falling in the “intermediate risk” category – with a Framingham Risk Score (FRS) of 10% to 20% – have increased CHD risk if CRP levels are elevated, and may be considered for statin therapy. The Canadian Cardiovascular Society recommends prophylactic statins for men >50 years and women >60 years with a CRP level >2 mg/L, and who are otherwise considered “intermediate risk”.257 Prior to the JUPITER study,9 considerable data showed that CRP levels ≥ 2 mg/L significantly raised risk, but currently laboratory reports continue to mention that CRP levels 2 to 3 mg/L reflect an average risk, and levels >3 mg/L reflect greater than average risk.258 Large prospective cohort studies reported age-adjusted relative risks for coronary artery disease from about 2.0 to 3.0 for the highest versus the lowest levels of CRP, again documenting a consistent association with heart disease that has predictive power.12,86,259
Some studies report that CRP does not discriminate sufficiently,260,261 adds little to traditional risk factors,86 in particular the FRS, and hence may lack cost-effectiveness. On the other hand, investigators have reported remarkable discrimination in a number of clinical settings,255 have found that when CRP is added to existing risk evaluation systems predictive power is enhanced,256,262–268 and reported that cost-effectiveness is indeed significant.269–271 All things considered, CRP is admittedly imperfect, as are all risk factors. However, it is much better than other nontraditional risk factors when used to refine risk, particularly when used along with traditional risk factors. In conclusion, CRP is an acknowledged sensitive marker of systemic inflammation,11 and in primary prevention, over 40 reports from prospective studes and 2 meta-analyses report a consistent association of CRP with subsequent CHD events.86,272,273 One evolving view is that traditional risk factors and scores should be used initially, and CRP measured in patients at intermediate risk or in complex patients. If elevated, a more aggressive therapeutic stance may be warranted.
Although lifestyle measures and some pharmacological agents do reduce CRP levels, statins are most frequently employed, and lower CRP levels about 15% to 35%.274,275 Rosuvastatin and atorvastatin in higher doses have the most significant CRP-lowering properties.274
Rosuvastatin
The word cholesterol derives from the Greek chole- (bile), stereos (solid), and –ol, the chemical suffix for alcohol. First identified in gallstones in 1769, the modern name was begun by chemist Eugène Chevreul in 1815. By 1971 Japanese biochemist Akira Endo began seeking compounds to lower cholesterol, and in 1987 Merck began marketing lovastatin isolated from Aspergillus terreus.
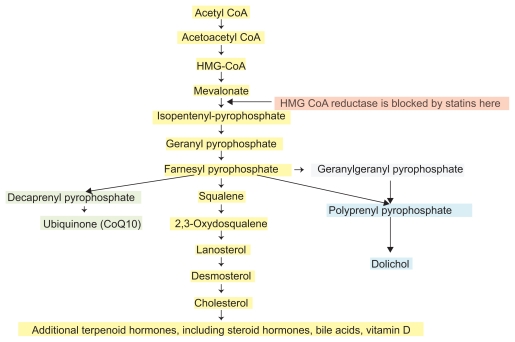
The endogenous biosynthesis of cholesterol occurs through the mevalonate pathway (Figure 1). HMGR is a highly regulated enzyme which catalyzes the rate-limiting step in cholesterol synthesis. Although HMGR is located within membranes of the endoplasmic reticulum, its catalytic domain remains active after it is cleaved from the transmembrane portion of the enzyme. The reduction of HMG to mevalonic acid involves the transfer of 4 electrons, using 2 molecules of nicotinamide adenine dinucleotide (NADPH) as a reductant in 2 steps. The carboxyl moiety of hydroxymethlyglutarate esterified to the thiol of CoA is first reduced to an aldehyde, and then to an alcohol. Statin drugs are highly efficient competitive inhibitors of HMGR.
Clinical pharmacology
Rosuvastatin (Figure 3) is a sulfur-containing, hydrophilic statin with multiple sites that form a strong interaction with HMGR and therefore provide more potent enzyme inhibition than other statins. Stereochemical details of the molecular binding between rosuvastatin and HMGR have been detailed elsewhere.276,277 Rosuvastatin, like other statins, is a competitive antagonist of HMGR, competing directly with the endogenous substrate for the active site cavity of the enzyme. Rosuvastatin employs a modified hydroxyglutaric acid moiety that mimics the 3-hydroxyglutaryl unit of the substrate, HMG CoA, and the mevaldyl CoA transition state intermediate. The pyrimidine ring in rosuvastatin binds tightly to the HMGR enzyme in the same area that normally binds the CoA component of the endogenous substrate HMG CoA. As a synthetic, “type 2”, or second-generation statin, rosuvastatin contains a fluorophenyl group which provides additional polar interactions that strengthen binding to HMGR. All told, rosuvastatin has an affinity for the HMGR active site that is >104-fold higher than that of HMG CoA.
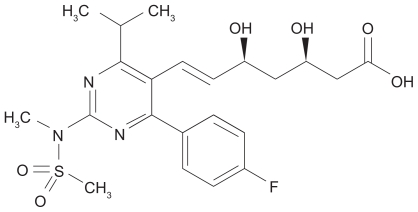
Figure 3.
The structure of rosuvastatin, uniquely containing sulfur, a fluorophenyl group, and a modified hydroxyglutaric acid moiety. The IUPAC name of rosuvastatin is (E,3R,5R)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan- 2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid.
Rosuvastatin is administered in its active acid form, meaning that the pharmacophore binding to the HMGR needs no transformation for activity compared with simvastatin or lovastatin, which are administered as lactones, a portion of which is hydrolyzed to the active acid form. Rosuvastatin is a hydrophilic statin, with relatively greater hepatoselectivity due to a unidirectional carrier-mediated active transport system. Hydrophilic statins tend to be retained in the liver, in part because they cannot passively diffuse out, whereas lipophilic statins concentrate in nonhepatic tissues. This property of hydrophilic statins is believed to account for fewer adverse events.278,279 As a result, rosuvastatin joins fluvastatin and pravastatin among statins least likely to produce myotoxicity.
Peak plasma concentration (Cmax) is reached 3 to 5 hours after oral administration,280 and both Cmax and the area under the plasma concentration-time curve (AUC) increase almost linearly with the dose. Absolute bioavailability is about 20%. Rosuvastatin is 88% bound to plasma proteins, chiefly albumin. Mean volume of distribution under steady-state conditions is ≈134 L. The half-life of rosuvastatin is 19 hours, the longest of the available statins. While pharmacokinetics do not differ meaningfully among white, Hispanic, or black individuals, Asians have about double the median exposure (Cmax and AUC) than whites. As a result, lower starting doses are advised in Asian-Americans.
Elimination is primarily in the liver, with ≈10% through the action of cytochrome CYP2C9, producing a metabolite, N-desmethyl rosuvastatin, having up to half the potency of rosuvastatin. After oral administration, 90% of rosuvastatin and its metabolites are excreted in the feces. Following an intravenous dose, about 72% of total body clearance is via the hepatic route, and 28% through the kidneys. Lipophilic statins must be metabolized to water-soluble forms for renal excretion, reactions that depend upon CYP450 isoenzymes. Compared to atorvastatin and some other statins, rosuvastatin has an advantage, since it is not metabolized predominantly through CYP3A4, eliminating many potential drug and food interactions. There are, however, other variables affecting pharmacokinetics and potential dose-related toxicity. P-glycoprotein (P-gp, also called ABCB1), an intestinal ATP-dependent drug efflux pump located in epithelial plasma membranes, prevents cellular uptake of xenobiotic foreign substances. P-gp also transports a number of endogenous biochemicals and drugs across cell membranes, noteably digoxin, and is inhibited by several statins. Available data do not indicate that rosuvastatin inhibits P-gp significantly, and therefore interactions with P-gp substrates and inhibitors, many of which are also CYP3A4 interactants, are not clinically relevant. Another potential interaction involves a hepatic uptake transporter, organic anion transporting polypeptide 1B1 (OATP1B1).281 Cyclosporine and gemfibrozil are OATP1B1 inhibitors, which may explain coadministration interactions.282 (See further discussion below under Side effects).
For each 39 mg/dL (1 mmol/L) fall in LDL, there is a 21% reduction in major cardiovascular events.283 Atorvastatin lowers LDL up to 60% at a maximal dose of 80 mg. Rosuvastatin lowers LDL by 45% to 63% (5 mg, 38%; 10 mg, 43%; 20 mg, 48%; 40 mg, 53%; 80 mg, 58%), reduces triglyceride levels by 10% to 35%, and raises HDL by 8% to 14%.179,284 The rise in HDL is believed to be due to a combination of a lower apolipoprotein A-1 (apoA-1) catabolism, raised hepatic apoA-1 synthesis, peroxisome proliferator-activated receptor-α activation and inhibition of cholesteryl ester transfer protein.280 Of all the statins, rosuvastatin has the lowest half maximum inhibitory concentration for cholesterol synthesis in the liver. The milligram-equivalent reductions in LDL and elevations in HDL are superior to those of other statins,179 thereby achieving evidence-based LDL targets in a higher portion of patients and greater success during intensive LDL reduction (Table 1). Rosuvastatin also lowers small, dense LDL concentrations. Although the reductions in LDL are dose-related, increasing the dose beyond 10 mg generally produces progressively smaller absolute reductions in LDL levels per unit.
Table 1.
Rough equivalent doses of rosuvastatin
| % Reduction in LDL | Rosuvastatin | Atorvastatin | Simvastatin | Fluvastatin | Lovastatin | Pravastatin |
|---|---|---|---|---|---|---|
| 30–40 | 5 mg | 10 mg | 20 mg | 80 mg | 40 mg | 40 mg |
| 40–45 | 5–10 mg | 20 mg | 40 mg | N/A | 80 mg | 80 mg |
| 46–50 | 10–20 mg | 40 mg | 80 mg | N/A | N/A | N/A |
| 50–55 | 20 mg | 80 mg | N/A | N/A | N/A | N/A |
| 56–60 | 40 mg | N/A | N/A | N/A | N/A | N/A |
Abbreviation: LDL, low-density lipoprotein.
Clinical studies with rosuvastatin
The Galaxy Program285 is a long-term, multi-trial research initiative sponsored by AstraZeneca, a planned portfolio of studies to investigate and support use of rosuvastatin to improve cardiovascular events and outcomes. The JUPITER study is one component, among others such as ASTEROID, METEOR, and CORONA. JUPITER sought to determine whether 20 mg of rosuvastatin could lower the number of major cardiovascular events in patients with acceptable LDL (under current guidelines) and elevated CRP levels, using a combined end point of death, MI, stroke, unstable angina or revascularization, and is further described below.
The Measuring Effects on Intima-Media Thickness: an Evaluation of Rosuvastatin (METEOR) trial randomized 984 asymptomatic participants with a mean age of 57 years, FRS < 10%, modest carotid intima-media thickness (CIMT) thickening (1.2 to <3.5), and elevated LDL (mean, 154 mg/dL) to either rosuvastatin 40 mg or placebo.286,287 The primary end point was the annualized rate of change in maximum CIMT at 12 sites during the 2-year study period. The rate of progression of maximum CIMT was significantly reduced by rosuvastatin therapy, but actual lesion regression was not achieved.
The Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) was conducted from 2003 to 2005 in 371 sites in 21 countries.288 The study randomized 5011 patients ≥ 60 years of age with chronic New York Heart Association (NYHA) class II, III, or IV ischemic systolic heart failure and an ejection fraction less than 40% to either rosuvastatin 10 mg or placebo. Median follow up was 32.8 months. There was a sharp fall in mean LDL (137 mg/dL) and CRP (3.1 mg/dL) at baseline to 3-month values of 76 and 2.1 respectively. The primary end point of the study was a composite of cardiovascular-related death, nonfatal MI, and nonfatal stroke. Secondary outcomes were total and cardiovascular mortality, time to first coronary event, and number of hospitalizations. By the end of the follow-up period, almost one-third of the participants sustained one end point event. There was no significant effect of rosuvastatin on primary or secondary end points, but a subsequent analysis suggested that those with CRP ≥ 2.0 had a 13% relative risk reduction and better outcomes.289 The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico Heart Failure (GISSI-HF) trial, which took place at 357 medical facilities in Italy, also prospectively randomized 4574 patients with NYHA class II-IV heart failure to rosuvastatin 10 mg or placebo, without an improvement in the primary end point.290 In GISSI-HF the event rate was similarly high, with over half the patients sustaining a cardiovascular death or hospitalization, reporting a 29% all-cause mortality during a follow-up of over 4 years. Although no explanation was forthcoming, it is apparent that these patients were quite ill with well advanced structural changes and pathophysiology. A recent commentary hypothesized that progression beyond a critical threshold in the pathogenesis of heart failure may have precluded any beneficial effect, citing the need for additional trials.291
Likewise, in A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events (AURORA), 2776 patients with end-stage chronic renal disease (ESRD) at 284 dialysis facilities in 25 countries were randomized to rosuvastatin 10 mg or placebo, and there was no significant difference between groups in a primary end point of time to a major cardiovascular event, even though LDL and CRP levels were substantially reduced in the treatment group.292 In this population the event rate of MI, stroke and cardiovascular death was >35%, again reflecting complex, advanced disease and extremely high absolute risk. One author proposed that in both ESRD and calcific aortic stenosis, once disease has progressed sufficiently, particularly with anatomical changes, statins may become unable to help achieve the end points chosen.293 When begun earlier during aortic sclerosis or mild stenosis, improvement is more likely than when stenosis is advanced. In ESRD, since cardiovascular mortality rises sharply by the time a patient requires dialysis, the threshold for benefit may have been passed prior to enrolling in the study.
The results of the A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID) study offer insight into the effects of rosuvastatin on the atherosclerotic process using two different, complementary techniques. First, monotherapy with 40 mg rosuvastatin for 24 months, which lowered LDL and raised HDL by about 53% and 15% respectively, significantly reduced atheroma volume in major coronary arteries that were angiographically normal or were stenosed, as assessed with intravascular ultrasound.294 Second, using quantitative coronary angiography (QCA), rosuvastatin decreased percentage diameter of coronary stenoses and improved minimum lumen diameter.295 Intravascular ultrasound images the coronary artery wall in proximal areas with minimal lumen stenosis, and QCA examines luminal narrowing in different segments of the coronary tree. Concordance of the findings strongly suggests rosuvastatin causes both slowing of progression of atherosclerosis and lesion regression in concert with LDL levels ≤ 70 mg/L (1.81 mmol/L). Reduction of luminal diameter, as detected by QCA, correlates with lower risk of future coronary events296 and subsequent mortality.297 Interestingly, changes in atheroma volume, when stratified above or below a median change of −37.1% in LDL and −21.4% in CRP levels, was greatest in those with reductions in both LDL and CRP (−2 mm3), less when CRP fell but not LDL (−1 mm3), progressed when LDL fell but CRP was high (+2 mm3), and progressed the most when both LDL and CRP were high (+8 mm3).294
The JUPITER study
Based upon (a) the ability of CRP to predict future vascular events,142,299–301 and (b) of statins to lower CRP,301 together with (c) additional evidence that the benefits of statins were greater when both LDL and CRP levels were lowered,249,253 and (d) a post-hoc analysis of the AFCAPS/TExCAPS study that indicated that healthy individuals with elevated CRP levels and normal LDL values could benefit from statin therapy,246 the global, multicenter JUPITER study was designed.9,302,303 After screening some 90,000 prospects, JUPITER enrolled 17,802 men (≥ 50 years) and women (≥ 60 years). The study population was not diagnosed with either CHD or diabetes, and had “normal” LDL levels (LDL < 130 mg/dL), but high hs-CRP levels (≥ 2.0 mg/L, median, 4.2 mg/L).
Participants were randomized to either treatment with rosuvastatin 20 mg daily or placebo. Participants’ median age was 66 years, and they comprised 62% men, 38% women, 71% Caucasians, 12.5% blacks, and 12.7% Hispanics (Table 2). Although free from clinical illness, the JUPITER population was not healthy, with 41% qualifying for the metabolic syndrome cluster of risk factors, 16% using tobacco, and 11.5% with a family history of premature heart disease. The cohort was fairly representative of the general population, with as-yet undetected, but substantial risk burden. This is an important point, since sometimes the term “healthy” may automatically be interpreted to mean “no treatment is necessary”.
Table 2.
Baseline clinical data in the JUPITER trial
| Characteristic | Treated group | Placebo group |
|---|---|---|
| Age, years (%) | 66 (median) | 66 |
| Female sex, number (%) | 3426 (38.5) | 3375 (37.9) |
| Ethnicity | ||
| White, number (%) | 6358 (71.4) | 6325 (71.1) |
| Black, number (%) | 1100 (12.4) | 1124 (12.6) |
| Hispanic, number (%) | 1121 (12.6) | 1140 (12.8) |
| Body mass index, kg/m2 | 28.3 (median) | 28.4 |
| Family history, number (%) | 997 (11.2) | 1048 (11.8) |
| Tobacco use number (%) | 1400 (15.7) | 1420 (16.0) |
| Systolic blood pressure, mm Hg | 134 (median) | 134 |
| Diastolic blood pressure, mm Hg | 80 | 80 |
| CRP | ||
| mg/L | 4.2 (2.8–7.1)a | 4.3 (2.8–7.2) |
| nmol/L | 40.0 (median) | 40.9 |
| Triglycerides | ||
| mg/dL | 118 (median) | 118 |
| mmol/L | 1.33 | 1.33 |
| Total cholesterol | ||
| mg/dL | 186 | 185 |
| mmol/L | 4.82 | 4.79 |
| LDL cholesterol | ||
| mg/dL | 108 | 108 |
| mmol/L | 2.8 | 2.8 |
| HDL cholesterol | ||
| mg/dL | 49 | 49 |
| mol/L | 1.27 | 1.27 |
| Glucose | ||
| mg/dL | 94 | 94 |
| mol/L | 5.22 | 5.22 |
| HbA1c % | 5.7 (5.4–5.9) | 5.7 (5.5–5.9) |
| Metabolic syndrome (%)b | 3652 (41.0) | 3723 (41.8) |
Notes: Interquartile range;
Metabolic syndrome was defined according to the National Heart, Lung, and Blood Institute definition or American Heart Association consensus criteria.
Abbreviations: HbA1c, glycated hemoglobin; LDL, low-density lipoprotein.
The primary end point composite included a nonfatal MI, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from a cardiovascular cause. The secondary end points included the individual components of the primary end point and all-cause mortality.
Although planned as a 5-year study, when patients in the rosuvastatin group had significantly fewer events and deaths than the controls, an independent data and safety monitoring board performed an interim efficacy analysis, and the trial was stopped after a median follow-up time of 1.9 years. About 75% of the treated participants were still taking rosuvastatin when the trial was ended. At that time there were 142 first major cardiovascular events in the rosuvastatin group, and 251 events in the placebo group, for a 44% reduction in first major cardiovascular events (HR 0.56, 95% confidence interval [CI] 0.46 to 0.69; P < 0.00001). There was also a 54% drop in nonfatal MI, a 48% reduction in nonfatal stroke, and a 47% fall in the cumulative incidence of hard end points of MI, stroke, or cardiovascular death in the rosuvastatin group (Table 3). In addition, there was a significant reduction in all-cause mortality of 20% in the treatment group, comparatively greater than in previous statin trials considering the duration. Rosuvastatin lowered CRP levels by 37%, with a 12 month mean of 2.2 mg/L, and lowered LDL by 50%, with a mean treated value of 55 mg/dL. Without a control group with normal CRP levels, whether CRP reduction was responsible for the observed benefits could not be answered.
Table 3.
JUPITER trial: comparison of outcomes between treated and nontreated patients
| End point | Patients in subgroup rosuvastatin (n = 8901) | Patients in subgroup placebo (n = 8901) | Hazard ratio (95% CI) |
|---|---|---|---|
| Primary end pointa | 142 | 251 | 0.56 (0.46–0.69) |
| Any MI | 31 | 68 | 0.46 (0.30–0.70) |
| Nonfatal MI | 22 | 62 | 0.35 (0.22–0.58) |
| Any stroke | 33 | 64 | 0.52 (0.34–0.79) |
| Nonfatal stroke | 30 | 58 | 0.52 (0.33–0.80) |
| Revascularization | 71 | 131 | 0.54 (0.41–0.72) |
| Hospitalization for unstable angina | 16 | 27 | 0.59 (0.32–1.10) |
| Revascularization or hospitalization for unstable angina | 76 | 143 | 0.53 (0.40–0.70) |
| MI, stroke, or death from cardiovascular causes | 83 | 157 | 0.53 (0.40–0.69) |
| Any death | 198 | 247 | 0.80 (0.67–0.97) |
Notes: Primary end point: composite of nonfatal MI, nonfatal stroke, hospitalization for unstable angina, revascularization, and death from cardiovascular causes.
Abbreviations: CI, confidence interval; MI, myocardial infarction.
The number needed to treat to prevent a first major primary end point event in 2 years was 95, falling to 31 in 4 years, and 25 for 5 years of treatment with rosuvastatin. The cardiovascular event rate in the rosuvastatin group was about 1 in 100, compared to approximately 2 in 100 in the placebo group, an absolute risk reduction of ≈1.2% for the 1.9-month study. Hence 25 individuals would require treatment for 5 years to prevent a single event, an absolute risk reduction of about 4%. Note that in primary prevention the baseline risk and event rate is low, so there is relatively less benefit in absolute risk anticipated. It is also instructive to compare the improvement in absolute risk achieved using the Mediterranean diet as the intervention, which may exceed 5% in some situations.304,305
JUPITER recorded 270 new cases of diabetes in the treatment group compared to 216 in the control group, and a rise in median glycated hemoglobin (HbA1c) levels, also noted in prior statin studies, amounting to a 25% increase in physician-reported incidence of diabetes over a 1.9-year period. There were 10 cases of myopathy in the rosuvastatin group versus 9 in the control group, with only 1 instance of rhabdomyolysis associated with rosuvastatin, numbers that are consistent with previous rosuvastatin trials.
Given prior data that CRP values improved prediction of risk, JUPITER suggested that a seemingly healthy patient population at high risk, previously ineligible for statin therapy, may benefit from rosuvastatin treatment, guided by the CRP level.306 Attention was focused on the large body of research that points to inflammation as a common denominator in atherothrombosis, ie, that inflammation matters at a clinical level.57 JUPITER, called a landmark study immediately after presentation, has since been the subject of many editorials, discussions, and intense debate. An online poll conducted by the New England Journal of Medicine followed publication, with 2553 votes cast over 18 days.307 Half of the respondents believed JUPITER data should change screening in the general population, with the remainder voting against any change. Similarly, the votes for and against a change in the therapeutic use of statins based upon JUPITER results were about evenly divided.
Subsequent analyses pertaining to JUPITER
Using data from 1999–2004 NHANES and applying NCEP ATP III 2004 Update (ATP III) criteria, Spatz et al308 calculated that 57.9% of older adults (men ≥ 60 years, women ≥ 50 years) are either taking a statin (24.4%) or eligible for a statin (33.5%). If one includes individuals satisfying strict JUPITER criteria – no history of cardiovascular disease, LDL <130 mg/dL, and CRP ≥ 2 mg/L – an additional 13.9% of the age group mentioned above, or nearly 8 million people, would become eligible for rosuvastatin therapy. If expanded JUPITER criteria were used – LDL between 130 and 160 mg/dL and CRP ≥ 2 mg/L – another 5.3%, or 3 million individuals, would become statin-eligible. Hence a grand total of just under 80% of the older population would have an indication for statin therapy. Compared to the remaining 20%, the “JUPITER group” was composed of more females, was older with a higher body mass index, and tended to have hypertension and metabolic syndrome. They also shared many characteristics with ATP III subjects, particularly the use of tobacco, visceral obesity, and hypertension. Interestingly, the investigators308 noted that in the Women’s Health Study, about 20% of the women with low and intermediate FRS were reclassified by using the Reynolds risk score, which incorporates CRP values.35,256,263 These findings support the growing realization that risk is greater than generally believed, and the validity of CRP-guided risk reduction.
Michos and Blumenthal309 also estimated the prevalence of JUPITER-eligible individuals in the US population using NHANES 1999–2004 data. They found about 6.5 million individuals would be added if strict JUPITER criteria were followed. Based upon the number needed to treat (NNT) of 25 at 5 years, they estimated about 260,000 cardiovascular events could be prevented with rosuvastatin therapy. A subsequent recalculation, after adding the number of venous thromboses that would be avoided, raised the number to about 500,000 per annum.
Ridker310 clarified aspects of the JUPITER study in mid- 2009, observing that all prespecified subgroups benefited by rosuvastatin therapy, including those participants considered at low risk. The decision to terminate the study early was based on rigorous pre-agreed principles. It was noted that the NNT to prevent 1 event for 5 years was comparable to that reported in AFCAPS/TexCAPS, and less than those accepted for the use of diuretics or beta-blockers in treating hypertension for primary prevention. Another point emphasized was that 80% of individuals who developed diabetes occurred among those participants who had impaired glucose tolerance at baseline, but those patients also benefited from treatment in terms of end points. Last, reduction of both LDL and CRP levels was especially successful, and dual targeting may simplify decisions to treat. Another detailed analysis of absolute risk reductions and consequent NNT within the JUPITER trial, including alternative statin regimens, concluded that NNT values are acceptable.311
Yang et al,312 using a JUPITER-eligible cohort from the Atherosclerosis Risk in Communities (ARIC) Study (men ≥ 50, women ≥ 60, CRP ≥ 2 mg/L, LDL <130 mg/dL), sought to determine if the absolute event rates and risk reduction seen in JUPITER would persist for a period longer than the 1.9 year follow-up. Of their JUPITER-eligible participants, there was an absolute CVD risk of ≈10.9% over a mean follow-up of 6.9 years, or 1.57% per year. Applying JUPITER HRs to this group generated an NNT of 38 over 5 years and 26 over 6.9 years. They concluded that the use of age and CRP level was a convenient method of identifying higher risk individuals.
Vaccarino and coworkers313 noted the absolute risk reduction of 0.59% per year for the primary end point required 169 persons to be treated for 1 year to prevent a single combination of events. To prevent a major coronary event, such as an MI, 500 patients needed to be treated for 1 year for a single event. An estimate of a drug cost for rosuvastatin of US$638,750 per year was made to prevent 1 event, and for a generic statin, US$24,000 per year. In their view, adding CRP screening costs of US$62,500, and then for additional liver function, glucose, and HbA1c monitoring, a total of over US$137,000 was reached per year for each event prevented. These authors suggested that the funds instead be invested in effective, proven population-based strategies to lower risk, as discussed elsewhere.314–316 In general, the costs per life year for lifetime treatment using simvastatin vary from US$2500 to US$10,990, depending on age and risk.317 Accad and Fred318 also opined that, according to their calculations, treatment of 95 individuals for 2 years to avoid 1 event is not a sufficient reward for the person with a high CRP level, but may be acceptable on a population level. On the other hand, Slejko et al269 maintained that statin therapy in JUPITER patients is cost-effective, at a cost of US$40,457 per quality adjusted life-year, below the customary threshold of US$50,000. MacDonald271 estimated that treating JUPITER-eligible individuals with rosuvastatin is highly cost-effective, but is a function of the initial FRS.
Kappagoda and Amsterdam319 reviewed treatment in the JUPITER study according to traditional risk factors and guidelines, and their calculations showed the following. Baseline characteristics of the participants revealed 2225 in each group with systolic blood pressures over 145 mm Hg. Treating these elevations would have lowered the number of strokes by 12 over the duration of the study. Similarly, by treating those individuals with LDL >119 mg/dL and HDL <40 mg/dL (about 25% of the subjects), and over-weight individuals (>50%) with weight loss, and eliminating tobacco use in the 16% who smoked, along with administering evidence-based aspirin prophylaxis in men for CHD prevention, and in women for stroke prevention, meaningful benefits would have changed outcomes. Aspirin alone could potentially have prevented 72 of the 99 MIs that occurred during the course of the JUPITER trial. Finally, since the baseline median HbA1c was 5.7% (reference range 4.8% to 5.9%), 2225 individuals in each group had levels >5.9%, suggesting that close to 25% of the entire JUPITER population met this criterion for diabetes. These authors suggested that because guidelines for care were not strictly followed, a spuriously high event rate may have caused an appearance of higher benefit from statin therapy. The event rate, however, was similar in the ARIC profile cohort that was JUPITER-eligible,312 although smaller, which would argue against this view.
A most significant dual target analysis of the JUPITER study evaluated the effects of rosuvastatin 20 mg versus placebo on the prespecified end points according to on-treatment values of LDL (<70 or ≥ 70 mg/dL) and CRP (<2 mg/L, or <1 mg/L).320 Compared to placebo, those in the treatment group who achieved an LDL < 70 mg/dL had a 55% reduction in vascular events per 100-person years (Table 4). Those who achieved CRP < 2 mg/L had a 62% reduction in events. Among those who reached dual reductions, there was a 65% reduction in events compared to a 33% reduction in those who achieved one or neither target. Those who reached an LDL < 70 mg/dL and a CRP < 1 mg/L enjoyed a 79% reduction in events (Table 5). Treatment CRP levels predicted the event rate no matter what lipid end point was used, including the ratio of aopB/aopA-1. Therefore, regardless of the lipid profile, the lower the CRP, the better was the prognosis. An editorial concluded that JUPITER provided “key experimental data” that inflammation mediated the benefits of rosuvastatin, but mused at the prospect of comparing the absolute risk reduction of statins with the effects of weight loss and regular exercise, remarking that such a study is unlikely,321 both for lack of funding and interest. Many of the pleiotropic effects of rosuvastatin are anti-inflammatory, and a catalog of those actions – significant improvement in endothelial function, immune responses, plaque stabilization, vascular remodeling, and oxidative stress – and antithrombotic actions322 further underpins the observation that targeting CRP is worthwhile and clinically rewarding.
Table 4.
Cardiovascular events fell based on LDL cholesterol and on hs-CRP levels <2 mg/L
| LDL cholesterol (mg/dL) and hs-CRP (mg/L) values | Event rate | Hazard ratio (95% CI) |
|---|---|---|
| ≥70 and ≥2 | 1.11 | 1.06 (0.72–1.55) |
| ≥70 and <2 | 0.54 | 0.42 (0.18–0.94) |
| <70 and ≥2 | 0.62 | 0.53 (0.38–0.74) |
| <70 and <2 | 0.38 | 0.35 (0.23–0.54) |
| ≥70 or ≥2 | 0.38 | 0.64 (0.49–0.84) |
Abbreviations: CI, confidence interval; LDL, low-density lipoprotein; hs-CRP, high CRP.
Table 5.
Cardiovascular events fell based on LDL cholesterol and on hs-CRP levels <1 mg/L
| LDL cholesterol (mg/dL) and hs-CRP (mg/L) values | Event rate | Hazard ratio (95% CI) |
|---|---|---|
| ≥70 and ≥1 | 0.95 | 0.89 (0.62–1.28) |
| ≥70 and <1 | 0.64 | 0.46 (0.11–1.85) |
| <70 and ≥1 | 0.56 | 0.49 (0.37–0.66) |
| <70 and <1 | 0.24 | 0.21 (0.09–0.51) |
| ≥70 or ≥1 | 0.67 | 0.59 (0.46–0.75) |
Abbreviations: CI, confidence interval; LDL, low-density lipoprotein; hs-CRP, high CRP.
The use of statins in women, particularly for primary prevention, has been heavily debated.323–325 Mora and coworkers326 performed a specific gender-specific analysis from JUPITER using rosuvastatin 20 mg or placebo employing the criteria mentioned above. Focusing only on 6801 female participants in JUPITER, treatment significantly lowered the relative risk of the primary end point, composite of MI, stroke, revascularization, hospitalization for unstable angina, and death from cardiovascular causes, by 46%. The greatest benefit was seen for revascularization, associated with a treatment-related reduction of 76% compared to placebo. A meta-analysis of 5 studies reporting sex-specific outcomes was also undertaken. Absolute CVD rates per 100 person-years in JUPITER women were lower than for men, with similar relative risk reductions between the sexes. A total of 13,154 women were included from primary prevention trials, and a significant reduction in primary CVD events with statins by one-third was found (relative risk 0.63; 95% CI 0.49 to 0.82; P < 0.001). These results were similar to prior results in men, and also to findings for secondary prevention in women. It was postulated that the greater numbers enrolled allowed JUPITER to demonstrate the benefit.327 CRP was noted as a tighter predictor of events in women than in men, along with the correlation between the degrees of CRP lowering and associated clinical benefit. These authors concluded that while CRP level as an independent predictor of events remains controversial, CRP is accepted as a marker which may be involved in the atherogenic process.237
The FDA approved rosuvastatin for primary prevention on February 9, 2010, following a 12-4 vote in a panel 2 months before, which only seemed to flare the ongoing controversy about its use in “healthy” individuals.328 The new indication was for men ≥ 50 years and women ≥ 60 years with fasting LDL ≤ 130 mg/dL, CRP ≥ 2.0 mg/L, triglycerides ≤ 500 mg/dL, no diagnosed CVD, and at least 1 additional CVD risk factor.
Summary of articles in the Archives of Internal Medicine
Kaul and collaborators329 challenged 3 aspects of the JUPITER study, asking (a) does CRP predict risk and treatment response? (b) did early stoppage of JUPITER unduly raise the benefit? and (c) what about guideline recommendations? First, they believed that the predictive value of 1.35%, although positive in JUPITER (241 events in 17,802 patients), was weak, and basically that treatment decisions should not be stratified using CRP values because there was no low-CRP, low-LDL control arm for comparison. This arm was excluded in the design of JUPITER because of the prior post-hoc analysis of AFCAPS/TexCAPS showing no benefit in a group with low CRP.246,330 The JUPITER authors hypothesized, with considerable supporting evidence from other investigators and studies outlined above, that high CRP levels reflected increased risk, and treatment with rosuvastatin addressed that component over and above any LDL lowering.310 A fair consensus from the many commentaries and blogs would be that a high level of CRP does have clinical utility, portends an otherwise poorer prognosis, but a normal CRP value does not assure low risk.
Second, the early termination of JUPITER may have led to a somewhat higher benefit than would have been observed from a longer study period. Compared to other statin studies in primary prevention, JUPITER reported unexpectedly larger reductions in ischemic end points including mortality. Studies terminated prematurely in other trials are known to exaggerate benefits, which regresses to the mean when follow-up is longer.331–334 A large systematic review reported that for interventions showing a 20% improvement in completed trials, stopping them early might double the apparent benefit.332 It is true that other studies used less potent statins, but a rapid mortality benefit within this period is exceptional. Details provided subsequent to JUPITER concerning the circumstances of the early termination did not reflect any intent to exaggerate the positive results. Kaul agreed that rosuvastatin benefits did occur in JUPITER, although perhaps were increased by the early stoppage of the study.330 The pathology in the CORONA and AURORA populations was sufficiently different from JUPITER to make a comparison of results difficult.
Third, Kaul et al329 question the conclusion that high CRP levels predict preferential improved response in JUPITER, based on an FDA analysis showing, essentially, that treatment effect of statins was not proportional to the degree of elevation in CRP.335 This conclusion was based on a stratification of FRS risk scores according to the CRP level. In addition, however, data presented in Tables 4 and 5 show a clear relationship between low CRP values and good prognosis.321 Kaul et al concluded that 44% risk reductions in JUPITER-eligible patients in the population will not be typically attained if CRP-guided rosuvastatin therapy is used. Their second conclusion, with which everyone agrees, is that control of risk factors with lifestyle modification trumps pharmacologic intervention in primary prevention. The third conclusion advises that if patients do not change their lifestyle, then statins may be used. In practice, since ≈95% of individuals will fall into this category, ultimately most patients will receive statins, simply because there are no comparable alternatives.
De Lorgeril et al336,337 maintained that a) there was a discrepancy between the reduction in mortality between end point subgroups; b) cardiovascular mortality was 5% to 18% compared with total mortality and the expected rate is about 40%; c) the case-fatality rate of MI was low compared to an expected figure of 50%; the case mortality rate in the placebo group was 8.8% compared to 29% in the rosuvastatin group; d) no sudden cardiac death was reported in JUPITER; and e) bias may have been introduced because of conflicts of interest and commercial sponsorship. These analysts believed that there was no significant difference in cardiovascular mortality between the two groups in JUPITER. Last, they lamented the secondary end point and subgroup analyses published concerning benefit in preventing venous thromboembolic disease,338 efficacy in women,326 lower stages of chronic renal disease,339 and in the elderly340 because they shared the limitations of the original study.
Several of the earnest concerns summarized in the papers by Kaul329 and de Lorgeril336 have been addressed by investigators other than JUPITER, and have also been answered in part by subanalyses and refinements presented by the JUPITER authors during the past 2 years. For instance, the association of high levels of CRP with vascular risk has considerable supportive evidence from multiple sources, and the recent meta-analysis of 54 prospective studies by the Emerging Risk Factors Collaboration confirmed this relationship.237 Specifically, the adjusted risk associated with a 1-standard deviation increase in CRP (HR 1.4; 95% CI 1.3 to 1.5) compared quite favorably with that associated with a similar increase in cholesterol (HR 1.2; 95% CI 1.1 to 1.3).
A closer evaluation of the relationship between base-line CRP measurements and cardiovascular risk is also available.268 Treating CRP as a continuous variable, an ordinal variable and as a threshold variable, relative risk reduction after rosuvastatin therapy was similar maintained across the entry levels of CRP. Absolute risk reductions therefore were greatest in the patients with the highest entry levels of CRP. Independent corroboration of these data is found in the ARIC study312 in which groups with similar FRS and LDL levels had higher vascular risk when the CRP was elevated, compared to counterparts with low CRP values.
JUPITER findings incorporated into the Canadian Cardiovascular Society guidelines
The Canadian Cardiovascular Society acknowledged JUPITER findings in their guidelines and recommended measurement of CRP in JUPITER-eligible individuals at “intermediate risk” as a step toward prophylactic statin treatment.257 The Society extended recommendations from the CDC in which CRP levels were considered an adjunct to risk assessment in healthy persons with an FRS between 5%–20%, ie, at intermediate risk.341 In JUPITER, the event rate in the placebo group was greater than in AFCAPS/TexCAPS study, and most individuals had an FRS between 5% and 20%. Analysis of JUPITER trial data stratified according to both the Framingham and Reynolds 10-year risk scores confirmed that treatment of JUPITER-eligible men and women in the “intermediate” categories (5% to 10% and 10% to 20% 10-year risk) significantly reduced cardiovascular events.342 In the 5% to 10% FRS category who were treated, HR was 0.55 (95% CI 0.36 to 0.84; 5-year NNT = 40, P = 0.005) (Table 6). In the 11% to 20% risk category, HR was 0.51 (95% CI 0.39 to 0.68, 5-year NNT = 18; P < 0.0001).
Table 6.
Event rates and hazard ratios for the primary end point correlated with estimated 10-year Framingham and Reynolds risk scores at baseline
| Risk level | Event rate/100-person years, rosuvastatin 20 mg group | Event rate/100-person years, placebo group | Hazard ratio (95% CI) |
|---|---|---|---|
| Framingham 10-year risk score | |||
| <5% (n = 2791, 173 men, 2618 women) | 0.22 | 0.34 | 0.64 (0.23–1.81) |
| 5%–10% (n = 6091, 2525 women, 3566 men) | 0.50 | 0.92 | 0.55 (0.36–0.84) 5-year NNT = 40a |
| 11%–20% (n = 7340,1404 women, 5936 men) | 0.95 | 1.84 | 0.51 (0.39–0.68) 5-year NNT = 18b |
| >20% (n = 1555, 242 women, 1313 men) | 1.72 | 2.41 | 0.70 (0.43–1.14) |
| Reynolds 10-year risk score | |||
| <5% (n = 3583, 944 men, 2639 women) | 0.26 | 0.41 | 0.62 (0.27–1.43) |
| 5%–10% (n = 6436, 2651 women, 3785 men) | 0.44 | 1.00 | 0.45 (0.29–0.68) |
| 11%–20% (n = 5040, 1151 women, 3889 men) | 1.07 | 1.65 | 0.65 (0.47–0.90) |
| >20% (n = 2651, 327 women, 2324 men) | 1.55 | 2.84 | 0.55 (0.38–0.80) |
Notes: Corresponds to estimated absolute risk difference between treated and placebo groups at 5 year of ≈2.5 events/100 person-years;
Corresponds to estimated absolute risk difference between treated and placebo groups at 5 year of ≈5.7 events/100 person-years.
Abbreviations: CI, confidence interval; NNT, number needed to treat.
Using the FRS, no significant benefit occurred in the groups with either low or very high risk. The Reynolds risk score reclassified more individuals into these two categories, low (<5%) and high (>20%), and rosuvastatin did produce a benefit in those at highest risk. Since the FRS underestimates risk in women, and frequently women do not have FRS > 10%, they may not be considered for treatment under current guidelines. Using CRP ≥ 2 mg/L as part of JUPITER criteria would include almost 7000 of such women who could potentially benefit from rosuvastatin therapy. This number becomes considerable in view of the growing awareness of serious gender disparities in CHD treatment.343
Summary of response of JUPITER authors
Ridker and Glynn330,344 and Ridker345 responded to the four major issues raised by De Lorgeril et al,336 namely a potential deficiency in trial design and execution; early termination of the study was inappropriate and/or favored to exaggerate benefit; inconsistencies existed concerning mortality, statin treatment and CRP; and pharmaceutical company sponsorship played a significant part in the positive outcomes, as summarized below.
JUPITER study design
The study was logically designed, participant follow-up was careful and full, end point adjudication was rigorous, and followed a pre-specified analysis plan.
Early termination of the trial
The details concerning the charter between the investigators and members of the independent Monitoring Board that decided to end the trial early were described. The decision to do so followed that agreement and was just and proper. The investigators reiterated that any overestimate of relative risk reduction involved would have been small, estimated by an FDA statistical analysis at ≤ 1%.346 Since the benefit was highly significant at 44% reduction in relative risk for the primary end point, there would be no clinically significant change in outcome interpretation. Simulation studies indicated that stopping the study early under similar circumstances produced valid treatment results.347,348
The benefits of rosuvastatin therapy in JUPITER were much larger than those reported in earlier statin trials. However, the relative risk lowering in JUPITER was similar to the analysis of a JUPITER-eligible cohort in the AFCAPS/TexCAPS study previously reported.246 In addition, earlier statin trials showed that a 1% fall in LDL level corresponded to a 1% reduction in relative risk. This was approximately the same in JUPITER, since rosuvastatin lowered LDL by 50%, and the resulting fall in relative risk was about the same magnitude.
A sequential review of the benefit of rosuvastatin treatment throughout JUPITER demonstrates that the differences between treatment and placebo groups increased steadily as the study progressed, rather than decreased.344
Inconsistencies in case-fatality rate
Instances of events and deaths required proof to be classified as cardiovascular, and many out-of-hospital deaths were termed “noncardiovascular” in the absence of such proof. There were 35 deaths from CVD in the treated group and 43 in the placebo group. The death rates for SCD were 16 in the rosuvastatin group versus 25 in the placebo group, suggesting a larger beneficial effect in the SCD component than was reported for total and cardiovascular mortality (Table 7).
Table 7.
Components of the primary end point reached in the JUPITER study
| End point | Rosuvastatin group | Placebo group | Hazard ratio | 95% CI | P value |
|---|---|---|---|---|---|
| Primary end point | 142 | 251 | 0.56 | 0.46–0.69 | <0.00001 |
| Nonfatal MI | 22 | 62 | 0.35 | 0.22–0.58 | <0.00001 |
| Any MI | 31 | 68 | 0.46 | 0.30–0.70 | <0.0002 |
| Nonfatal stroke | 30 | 58 | 0.52 | 0.33–0.80 | 0.003 |
| Any stroke | 33 | 64 | 0.52 | 0.34–0.79 | 0.002 |
| Revascularization or Unstable angina | 76 | 143 | 0.53 | 0.40–0.70 | <0.00001 |
| MI, stroke, CV death | 83 | 157 | 0.53 | 0.40–0.69 | <0.00001 |
| Total mortality | 198 | 247 | 0.80 | 0.67–0.97 | 0.02 |
| CV death (verified) | 35 | 43 | 0.82 | 0.52–1.27 | 0.37 |
| Sudden death | 16 | 25 | 0.64 | 0.34–1.20 | 0.16 |
Abbreviations: CI, confidence interval; CV, cardiovascular; MI, myocardial infarction.
Influence of the sponsor-affected outcomes
The sponsor, AstraZeneca, had no access to the data or any role in the analyses or manuscript writing.
Side effects
Pharmaceuticals nearly always have side effects, but most do not rise to clinical significance. Adverse events associated with statins have been exceptionally well studied. As the most potent member of its class, rosuvastatin is also associated with unwanted events. Molecular mechanisms have yet to be fully characterized. No one study imposes use of drugs on any patient – use is weighed on the ratio of potential benefits with respect to risks, first by the FDA, then subsequently by the prescribing physician. A vast amount of clinical evidence suggests that, for statins in general, and rosuvastatin in particular, this ratio is large.
JUPITER reported a rise in the number of cases of diabetes in the treatment group, amounting to 270 events, or about a 25% increased risk of developing diabetes. In about 1.4% of prescriptions, rosuvastatin has to be discontinued due to adverse reactions, with the Package Insert quoting the incidence of myalgia at 3.1%, abdominal discomfort, 2.6%, asthenia, 2.5%, and nausea at 2.2%. The frequency of overt myotoxicity, with diagnostic elevations in creatine kinase (CK) 10-fold higher than the upper limit of normal and requiring inpatient care, is in the order of 0.4 per 10,000 person-years.349,350 Less severe myalgia, however, may be responsible for discontinuing statins in 5% to 10% of statin users.351,352 Both myotoxicity and an increase in the incidence of diabetes, as noted in JUPITER, are currently of greatest interest.
Statins raise incidence of diabetes
In a study of 345,417 men, Sukhija et al353 reported that a decrease in insulin sensitivity was a class effect of statin drugs, and found that the change in fasting plasma glucose level in nondiabetic statin users was 7 mg/dL (versus 5 mg/dL in patients not using statins; P < 0.0001), and in diabetic statin users, the rise was 39 mg/dL (versus 32 mg/dL in patients not using statins; P < 0.0001). The effect has subsequently been confirmed for atorvastatin354 and for rosuvastatin,355 which produced a dose-dependent rise in insulin resistance. Using data from the Cholesterol Treatment Trialists’ Collaborators,356 Sattar and colleagues357 conducted a meta-analysis of 13 randomized statin trials and studied the incidence of diabetes among 91,140 participants. Statin therapy was associated with a 9% increased risk for diabetes, translating to 1 extra case of diabetes per 255 patients treated over 4 years. Absolute risk was low, at 1 case per 1000 patient-years of treatment. During those 4 years, however, 5.4 MIs or cardiovascular deaths and 5.4 coronary revascularizations and strokes would be prevented, amounting to an overall benefit of 9:1 events. Their analysis also indicated that lowering glucose tolerance was a class effect of statins, in agreement with a review of several statin trials prior to JUPITER. 310 Baker et al358 systematically reviewed 16 studies and determined there was no class effect of statins upon glucose tolerance in patients without diabetes, and that differences between statins may have accounted for prior findings.
Some side effects of statins are obligatory consequences of HMGR inhibition of the mevalonate pathway used for cholesterol synthesis (Figure 2), and are molecularly unavoidable, extending throughout the metabolome. Among the intermediate products of this pathway are the 15-carbon farnesyl pyrophosphate and 20-carbon geranylgeranyl pyrophosphate molecules, used to add an isoprenoid group to over 100 signaling proteins in a fundamental post-translational modification known as prenylation.359 This addition of an isoprenoid group is necessary for function of small Rho-family GTPases such as Rac, Rho, and Rab. Some of these GTPases serve as molecular switches, and others are involved in intracellular membrane trafficking, using the lipophilic prenyl group to anchor in membranes.360 Many desirable pleiotropic actions of statins, such as the antioxidant, anti-inflammatory, and antiproliferative properties361–363 may be related to decreased ability to prenylate signaling molecules. Inhibition of Rho and its downstream target Rho kinase (ROCK) function is especially important, since elevated ROCK activity may occur in association with elevated CRP levels364 and contribute to atherosclerosis.365,366 Less desirable consequences of HMGR inhibition may arise from deficiencies in downstream metabolites of the mevalonate pathway such as ubiquinone and dolichol, which are, respectively, ignored and largely unknown. Dolichol, a family of linear polyisoprenols containing 16 to 22 isoprene units, are carriers involved in N-glycosylation of nascent polypeptides, and serve as sites for assembly of oligosaccharides during the formation of glycoproteins, ensuring the membrane fluidity and permeability required for glycoprotein maturation and secretion.
Figure 2.
Cholesterol is synthesized via the mevalonate pathway. Acetyl-CoA forms 3-hydroxyl-3-methylglutaryl CoA (HMG-CoA) in several steps. The conversion of 3-hydroxy-3-methylglutaryl (HMG)-CoA to mevalonate, the rate-limiting step in cholesterol synthesis, is catalyzed by HMG-CoA reductase (HMGR), an enzyme within the endoplasmic reticulum. Rosuvastatin is an efficient competitive inhibitor of HMGR, reducing not only mevalonate levels, but also prenylated downstream products. The post-translational process of prenylation is needed for the function of small G proteins, including geranylgeranylation of Rho, Ras, and Rab, necessary for cellular signaling, transduction, and intermembrane translocation. As beneficial as statins are, there are obligatory molecular consequences inherent in their use, some related to their beneficial pleiotropic actions, but also to their side effects. Many steps and enzymes are omitted for clarity.
There are several signaling steps between the insulin receptor and translocation of the glucose transporter GLUT4 from specialized intracellular compartments to the plasma membrane. Individual statins may have different effects upon these steps, and which of them predominate may account for differences on overall glucose tolerance.367 Thus, in one preparation, atorvastatin may improve glucose tolerance, possibly by lowering membrane cholesterol content368 yet in another report atorvastatin appeared to attenuate the expression of GLUT-4 in adipocytes to lower glucose tolerance.369 In addition, there may be differential effects of statins upon levels of adiponectin, leptin, and other inflammatory mediators that affect glucose intolerance. Differences in lipophilicity among the statins may contribute both to the effects on insulin sensitivity and myotoxicity. Pravastatin, which improves glucose tolerance when compared with atorvastatin, has a relatively favorable effect on beta-cell function.370,371 It has been suggested that pravastatin, hydrophilic but forming weak steric interactions with HMGR, has limited ability to block the mevalonate pathway in cells outside the liver.358 Simvastatin, which worsens glucose tolerance, has a greater affinity for cell membranes due to its lipophilicity, and may block L-type calcium channels, interfering with glucose-induced cytosolic calcium signaling and insulin secretion in beta cells.372 Another possible explanation for statin-induced glucose intolerance includes dysfunctional insulin receptors and/or insulin-like growth factor receptors as a result of impaired glycosylation (see above).373 Finally, by upregulating hepatic LDL receptors, a greater number of triglyceride-rich particles returning to the liver may induce hepatic insulin resistance.374
In view of the large reduction in major vascular events associated with use of statins in diabetics,356 the small increase in diabetes discussed above has not changed clinical practice,375 and further work is needed to delineate its clinical significance.
Statin-induced myopathy
Statin myotoxicity is a major concern of practitioners; part of the reluctance to titrate LDL levels down to guideline targets is a fear of myopathy. Although this phenomenon has received enormous attention from researchers, clinicians, the lay press, and on the internet, its mechanism and optimal management remain uncertain. The spectrum of myopathy or myotoxicity includes myalgia (muscle ache or weakness without CK elevation), myositis (muscle symptoms with raised CK levels), and rhabdomyolysis (symptoms with CK over the upper limit of normal by a factor of at least 10).376 Myalgia is noted in randomized statin trials in the order of <3%, compared to the incidence in outpatient lipid clinics, which varies between 5% and 10%.377 Underreporting is commonly assumed, but individuals with predisposing conditions are carefully screened, and many randomized trials include a run-in period, then fail to randomize candidates with myopathic symptoms.
During vigorous exercise, the phenomenon is accentuated, raising the reported incidence of myalgia up to 25%.378 Statin intolerance among athletes is well known. Factors that predispose to myopathy are increasing age, female gender, hepatic or renal disease, diabetes, polypharmacy, and any condition that diminishes muscle reserve (small frame, frailty, sarcopenia), or causes muscle pathology, including hypothyroidism or hyperthyroidism, metabolic muscle disease, polymyalgia rheumatica, autoimmune diseases with myositis, postoperative status, hypovitaminosis D, chronic alcohol use, and others.
Some adverse reactions may be minimized by avoiding negative drug–drug interactions. As mentioned above, since rosuvastatin is not eliminated through the CYP3A4 pathway, only minimally metabolized by the CYP2C9 enzyme, and does not interfere with P-gp, drugs that inhibit OATP1B1, a transporter that facilitates rosuvastatin uptake in the liver, may clinically be more important offenders than cytochrome P450 inhibitors or substrates. Common drugs usually mentioned that may raise the likelihood of myotoxicity are cyclosporine, gemfibrozil, itraconazole, ketoconazole, fluconazole, lopinavir/rotinavir, atazanavir/ritonavir, fosamprenavir/ritonavir, warfarin, and amiodarone. Some of the quantitative effects of coadministered drugs are summarized in Tables 8 and 9. As noted in Table 9, digoxin coadministered with rosuvastatin only raises the AUC 4%, reflecting the statin is not a P-gp substrate. Genetic polymorphism within SLCO1B1, the gene that encodes OATP1B1, produces a variant which increases susceptibility to statin-induced myopathy.379
Table 8.
Effect of coadministered drugs on rosuvastatin systemic exposure
| Coadministered drug and dosing regimen | Rosuvastatin |
||
|---|---|---|---|
| Dose (mg)a | Change in AUCb | Change in Cmaxb | |
| Cyclosporine – stable dose required (75–200 mg twice daily) | 10 mg daily for 10 days | ↑ 7-foldc | ↑ 11-foldc |
| Gemfibrozil 600 mg twice daily for 7 days | 80 mg | ↑ 1.9-foldc | ↑ 2.2-foldc |
| Lopinavir/ritonavir combination 400 mg/100 mg twice daily for 10 days | 20 mg daily for 7 days | ↑ 2-foldc | ↑ 5-foldc |
| Atazanavir/ritonavir combination 300 mg/100 mg daily for 7 days | 10 mg | ↑ 3-foldc | ↑ 7-foldc |
| Tipranavir/ritonavir combination 500 mg/200 mg twice daily for 11 days | 10 mg | ↑ 26% | ↑ 2-fold |
| Fosamprenavir/ritonavir 700 mg/100 mg twice daily for 7 days | 10 mg | ↑ 8% | ↑ 45% |
| Fenofibrate 67 mg 3 times daily for 7 days | 10 mg | ↑ 7% | ↑ 21% |
| Aluminum and magnesium hydroxide combination antacid, administered simultaneously | 40 mg | ↓ 54%c | ↓ 50%c |
| The above, administered 2 hours apart | 40 mg | ↓ 22% | ↓ 16% |
| Erythromycin 500 mg 4 times daily for 7 days | 80 mg | ↓ 20% | ↓ 31% |
| Ketoconazole 200 mg twice daily for 7 days | 80 mg | ↑ 2% | ↓ 5% |
| Itraconazole 200 mg daily for 5 days | 10 mg 80 mg |
↑ 39% ↑ 28% |
↑ 36% ↑ 15% |
| Fluconazole 200 mg daily for 11 days | 80 mg | ↑ 14% | ↑ 9% |
Notes: Single dose unless otherwise noted;
Mean ratio (with/without coadministered drug and no change = 1-fold) or % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively;
Clinically significant.
Abbreviations: AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration.
Table 9.
Effect of rosuvastatin coadministration with warfarin, digoxin, and an oral contraceptive
| Rosuvastatin dosage regimen | Coadministered drug |
||
|---|---|---|---|
| Name and dose | Change in AUC | Change in Cmax | |
| 40 mg daily for 10 days | Warfarin 25 mg, single dose | R-Warfarin ↑ 4% S-Warfarin ↑ 6% |
R-Warfarin ↓ 1% S-Warfarin 0% |
| 40 mg daily for 12 days | Digoxin 0.5 mg, single dose | ↑ 4% | ↑ 4% |
| 40 mg daily for 28 days | Oral contraceptive (EE 0.035 mg and NG 0.180, 0.215, and 0.250 mg) daily for 21 days | EE ↑ 26% NG ↑ 34% |
EE ↑ 25% NG ↑ 23% |
Abbreviations: AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; EE, ethinyl estradiol; NG, norgestrel; R-warfarin and S-warfarin, referring to the stereoisomers.
Since statins affect many different molecules and signaling systems, the mechanism of statin myopathy also remains uncertain. A relationship to a deficiency of prenylating moieties, farnesyl pyrophosphate and geranylgeranyl pyrophosphate, is again likely. Inability to prenylate seleno-proteins, dolichols, and small GTPases Rho, Ras, and Rac is believed to disturb intracellular trafficking of proteins between membranes needed for muscle growth and repair. Statins specifically activate mechanisms that lead to muscle atrophy in a number of circumstances, mediated by overexpression of atrogin-1, a muscle-specific E3 ubiquitin ligase in the ubiquitin proteasome proteolytic pathway.380–382 Induction of atrogin-1 appears to be significant in the pathogenesis of statin myopathy, but is by no means the only mechanism. Experimental overexpression of peroxisome proliferator- activated receptor-gamma coactivator-1α (PPARγ coactivator 1α or PGC-1α), coactivator of many transcription factors and a regulator of mitochondrial number and function, attenuates statin-induced atrogin-1 expression, protects against statin myotoxicity, and may lead to a therapeutic target.
Skeletal muscle expresses drug transporters such as OATP2B1, high-affinity uptake transporters for both atorvastatin and rosuvastatin.383 A number of efflux transporters, multidrug-resistance-associated proteins 1, 4, and 5, are also expressed on the sarcolemmal membrane of skeletal muscle. Susceptibility to myotoxicity may depend not only on the serum concentration of rosuvastatin, but also locally on the balance between activities of muscle fiber uptake and efflux transporters. For example, in one experimental model, probenecid, which inhibits efflux transporter MRP1, raised myocyte rosuvastatin levels and promoted myotoxicity.384
Lipophilic statins, such as lovastatin or simvastatin, inhibit protein synthesis in skeletal myotubules to a greater extent than hydrophilic statins, such as pravastatin.385 Statins in higher doses may decrease the ability of muscle progenitor cells (satellite cells) to repair and regenerate skeletal muscle. 386 Only in part because they severely reduce ubiquinone (coenzyme Q10) levels, statins are associated with multiple defects in muscle mitochondrial anatomy and function.387 Lower mitochondrial volume, mitochondrial DNA loss, reduction in mitochondrial membrane potential, inefficient oxidative phosphorylation, anatomical fractionation, leakage of pro-apoptotic chemicals through mitochondrial membranes, and apoptosis have all been reported. Apoptosis of muscle cells – direct myotoxicity – has been described after acute statin exposure.383,388 Intracellular calcium overload and activation of caspase-3 to initiate apoptosis is yet another proposed mechanism.389 Dolichol is needed for synthesis of α-dystroglycan, a major protein in the skeletal muscle dystrophin–glycoprotein complex, and deficiencies have been linked to muscle dysfunction. Finally, some evidence also implicates immune involvement, which may persist after statin therapy is discontinued.390,391 Four detailed, well-referenced recent reviews of statin myotoxicity are available.392–395
In summary, it appears that the side effects of statins are just as multimechanistic, or “pleiotropic”, as the beneficial actions. Despite these seemingly complex biochemical effects, in practice, the incidence of myopathy and glucose intolerance is far outweighed by the overall benefits of statins in general, and rosuvastatin in particular.396,397 Most serious events may be averted with careful monitoring and avoidance of drug interactions, but individual and genetic susceptibility can unfortunately not yet be predicted clinically. The overwhelming majority of patients tolerate rosuvastatin well. In JUPITER, there were no significant differences in myotoxicity between treatment and control groups.
Conclusion
The challenge of a rising sea of cardiovascular risk, threatening to reverse improved coronary death rates, is immense. The reality is that few individuals will sustain the necessary lifestyle and behavioral changes to lower their risk, and this is amply reflected by the dismal success in weight control. Obesity drives the dual epidemics of diabetes and CHD. Primary prevention, sometimes regarded as an illusion, is only effective if it is done early and intensively. When lifestyle modification fails, reduction of elevated risk factors through pharmacological means is imperative, because by the time they are evident, atherosclerotic processes are already advanced. If we are to stem the tide, simply waiting for a cardiovascular event or diagnosis will no longer suffice.
Asymptomatic individuals randomized in the JUPITER study, many similar to those in the general population, bore a considerable cardiovascular burden, even though they were free from a formal diagnosis of heart disease, diabetes and dyslipidemia. Studies other than JUPITER confirm that higher values of CRP are associated with greater cardiovascular risk, which may assist in the decision of whether to treat or not. With the FDA approval for the use of rosuvastatin in JUPITER-eligible patients with at least one additional cardiovascular risk factor, an additional choice became available to clinicians.
The prevalence of metabolic syndrome in JUPITER participants was about the same as in US adults at the present time. Jupiter demonstrated that within this population, those persons with elevations in CRP levels benefited from rosuvastatin therapy. While, as mentioned, some critics, observing that 41% of JUPITER participants had metabolic syndrome, suggested this diminished the value of the study. However, one can argue that the opposite was true, in that JUPITER demonstrated that rosuvastatin therapy in this syndrome, when CRP was elevated, was effective therapy. The prevalence of metabolic syndrome more closely follows the rise in level of visceral adiposity within the population, now averaging 53%.398 At the moment, the highest prevalence of metabolic syndrome reported in an US subpopulation also stands at 53%.127 The evidence that inflammation plays a critical role in the pathogenesis of components of metabolic syndrome is of high quality.198,199 A recent systematic review and meta-analysis of 87 studies involving 951,083 patients found that metabolic syndrome is associated with a 2-fold increase in risk of CVD, cardiovascular mortality, and stroke, and a 1.5-fold rise in all-cause mortality.399
DeMazumder et al400 noted that the use of global risk assessment tools may result in denial of statin therapy to individuals at low risk when current guidelines are employed, while others with a high absolute risk may not benefit from statin therapy. These authors therefore suggested that statin therapy might be better allocated on the basis of randomized trial evidence rather than global risk scores. Nambi and Ballantyne401 proposed a formal protocol using the FRS, lifetime risk, and CRP, CIMT, calcium artery calcification burden, or genetic risk markers for progressive refinement of stratification. CRP measurements may be repeated easily, and are less involved than imaging, with no radiation exposure.
The reaction to JUPITER, many physicians will agree, has been most unusual. The rationale and findings are bracketed by many other independent investigations, but the final interpretation of the data presented is up to each physician. A repeat of JUPITER will probably not occur. The study is not perfect, and limitations are clear and well known. Controversies in medicine may endure for years without resolution. In the individual case, certainly other methods for risk stratification, such as CIMT measurement and coronary artery calcium score, are available. Processes bringing about alterations in each risk factor are different but overlap, and the information their changes bring to the physician’s desk are complementary, not identical. Eventually a place for each will be found in the clinician’s toolbox, but ultimately, the individual practitioner has the choice of where to reach in his armamentarium, the essence of the “art of medicine”. It is encouraging to note that in blog comments written by individuals critical of some results and potential applications of JUPITER, when presented with specific cases, rosuvastatin is in fact being prescribed for high-risk individuals with elevated CRP values in primary prevention, indicating that patients’ needs are being valued with higher priority than personal views.
Almost all JUPITER-eligible patients who would benefit from rosuvastatin have an alternative. Weight loss, especially in conjunction with exercise and dietary changes, are powerful techniques to lower cardiac risk and CRP levels197,213–222 and in fact would be preferred. The pervasive condition for which rosuvastatin has been found effective in JUPITER differs significantly from other diseases. This “illness” of unrecognized cardiovascular risk is diffuse, significant, difficult to quantitate, but deadly nonetheless. However, it is also largely self-inflicted, with a common cause in poor personal lifestyle choices. A recurring comment that ostensibly healthy people are being subjected to testing and side effects may be, in part, rooted in erroneously equating “asymptomatic” with ideal cardiovascular health – free of risk factors and a likelihood that none will appear in the near future402 – by the public. Perception of risk by patients is commonly inaccurate, and educational programs have been repeatedly recommended.403
The broader message of JUPITER is, first, it reminds us that the level of cardiovascular risk is currently oppressive, and greater than previously recognized when evaluated by LDL and global risk scores. Second, physicians must go on the offensive, delve into the population, and ferret out the risk, rather than wait for the risk to open their office door.
Today the clinician has a much greater amount of information and a wider variety of techniques from which to choose in order to evaluate individuals for primary prevention. Rosuvastatin will lower risk in JUPITER-eligible subpopulations, but also in many others selected differently for risk, even if one uses age for stratification, simply because the current level of risk is so high. Whatever markers or techniques are chosen, a fresh view of preventive care is before us, and action is now imperative. New guidelines in preparation will hopefully provide even greater direction in maximizing returns in primary prevention and improve future outcomes in our patients.
Acknowledgment
The author thanks Michelle Delaney for her suggestions, computer skills, and astuteness in the preparation of this manuscript.
Footnotes
Disclosure
The author states that he has not received any funds or favors from any private or public entity, corporation, agency, or other source related to this work.
References
- 1.USDHHS, National Center for Health Statistics. Second National Health and Nutrition Examination Survey (NHANES II) Hyattsville MD: CDC; pp. 1976–1980. [Google Scholar]
- 2.USDHHS, National Center for Health Statistics. Second National Health and Nutrition Examination Survey (Continuous NHANES) Hyattsville MD: CDC; pp. 1999–2002. [Google Scholar]
- 3.Young F, Capewell S, Ford ES, Critchley JA. Coronary mortality declines in the US between 1980 and 2000. Quantifying the contributions from primary and secondary prevention. Am J Prev Med. 2010;39:228–234. doi: 10.1016/j.amepre.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the US from 1980 to 2002: concealed leveling or mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 5.CDC National Center for Health Statistics. CDC mortality data, 2008: CDC latest release mortality data, 2005. [Accessed 2010 Oct 7]. www.cdc.gov/nchs/fastats/deaths.htm.
- 6.Sacker A, Head J, Bartley M. Impact of coronary heart disease on health functioning in an aging population: are there differences according to socioeconomic position. Psychosom Med. 2008;70:133–140. doi: 10.1097/PSY.0b013e3181642ef5. [DOI] [PubMed] [Google Scholar]
- 7.Allender S, Scarborough P, Peto V, et al. European cardiovascular disease statistics 2008. European Heart Network, 2008. [Accessed 2010 Oct 7]. www.heart-stats.org/uploads/documents%5Cproof30NOV2007.pdf.
- 8.WHO Statistical Information System. Mortality data for ICD 10 codings. [Accessed 2010 Oct 7]. www.who.int/whosis/database/mort/download/ftp/morticd10.zip.
- 9.Ridker PM, Danielson E, Fonseca FA, et al. for JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 10.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7:169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 11.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 13.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 14.Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 16.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 17.Libby P, Ridker PM. Inflammation and atherothrombosis: from population biology and bench research to clinical practice. J Am Coll Cardiol. 2006;48:A33–46. [Google Scholar]
- 18.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 19.Libby P, Nahrendorf M, Pittet MJ, Swirski FK. Diversity of denizens of the atherosclerotic plaque: not all monocytes are created equal. Circulation. 2008;117:3168–3170. doi: 10.1161/CIRCULATIONAHA.108.783068. [DOI] [PubMed] [Google Scholar]
- 20.Mach F, Schoenbeck U, Bonnefoy JY, Pober J, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40. Induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96:396–399. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- 21.Spagnoli LG, Bonanno E, Mauriello A, et al. Multicentric inflammation in epicardial coronary arteries of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2002;40:1579–1588. doi: 10.1016/s0735-1097(02)02376-8. [DOI] [PubMed] [Google Scholar]
- 22.Abbate A, Bonanno E, Mauriello A, et al. Widespread myocardial inflammation and infarct-related artery patency. Circulation. 2004;110:46–50. doi: 10.1161/01.CIR.0000133316.92316.81. [DOI] [PubMed] [Google Scholar]
- 23.Lombardo A, Biasucci LM, Lanza GA, et al. Inflammation as a possible link between coronary and carotid plaque instability. Circulation. 2004;109:3158–3163. doi: 10.1161/01.CIR.0000130786.28008.56. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Schulte S, Warrington KJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 25.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 26.Esposito K, Giugliano D. Diet and inflammation: a link to metabolic and cardiovascular diseases. Eur Heart J. 2006;27:15–20. doi: 10.1093/eurheartj/ehi605. [DOI] [PubMed] [Google Scholar]
- 27.Freedman JE, Loscalzo J. Platelet–monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105:2130–2132. doi: 10.1161/01.cir.0000017140.26466.f5. [DOI] [PubMed] [Google Scholar]
- 28.Steinhubl SR. Platelets as mediators of inflammation. Hematol Oncol Clin North Am. 2007;21:115–121. doi: 10.1016/j.hoc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Nettleton JA, Matijevic N, Follis JL, Folsom AR, Boerwinkle E. Associations between dietary patterns and flow cytometry-measured biomarkers of inflammation and cellular activation in the Atherosclerosis Risk in Communities (ARIC) Carotid Artery MRI Study. Atherosclerosis. 2010;212:260–267. doi: 10.1016/j.atherosclerosis.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(Suppl 1):332–339. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 32.Brugaletta S, Biasucci LM, Pinnelli M, et al. Novel anti-inflammatory effect of statins: reduction of CD4+CD28null T lymphocyte frequency in patients with unstable angina. Heart. 2006;92:249–250. doi: 10.1136/hrt.2004.052282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taubes G. Does Inflammation cut to the heart of the matter. Science. 2002;296:242–245. doi: 10.1126/science.296.5566.242. [DOI] [PubMed] [Google Scholar]
- 34.Williams KJ, Tabas I. Atherosclerosis and inflammation. Science. 2002;297:521–522. doi: 10.1126/science.297.5581.521. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 36.Packard RRS, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;43:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 37.Altman R. Risk factors in coronary atherosclerosis ather-inflammation: the meeting point. Thrombosis J. 2003;1:4. doi: 10.1186/1477-9560-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vorchheimer DA, Fuster V. Inflammatory markers in coronary artery disease. Let prevention douse the flames. JAMA. 2001;286:2154–2156. doi: 10.1001/jama.286.17.2154. [DOI] [PubMed] [Google Scholar]
- 39.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 40.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mono-nuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 41.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 42.Liuzzo G, Biasucci LM, Gallimore JL, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 43.Biasucci LM, Vitelli A, Liuzzo G, et al. Elevated level of interleukin-6 in unstable angina. Circulation. 1996;94:874–877. doi: 10.1161/01.cir.94.5.874. [DOI] [PubMed] [Google Scholar]
- 44.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 45.Wu KK, Aleksic N, Ballantyne ChM, Ahn Ch, Juneja H, Boerwinkle E. Interaction between soluble thrombomodulin and Intercellular Adhesion Molecule-1 in predicting risk of coronary heart disease. Circulation. 2003;107:1729–1732. doi: 10.1161/01.CIR.0000064894.97094.4F. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt DL, Topol EJ. Need to test the arterial inflammation hypothesis. Circulation. 2002;106:136–140. doi: 10.1161/01.cir.0000021112.29409.a2. [DOI] [PubMed] [Google Scholar]
- 47.Kereiakes DJ. The fire that burns within. C-reactive protein. Circulation. 2003;107:373–374. doi: 10.1161/01.cir.0000053942.27259.ba. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J. 2004;148:S19–26. doi: 10.1016/j.ahj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 49.Dhingra R, Gona P, Nam B-H, et al. C-reactive protein, inflammatory conditions, and cardiovascular disease risk. Am J Med. 2007;120:1054–1062. doi: 10.1016/j.amjmed.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Atheroscler Thromb Vasc Biol. 2007;27:2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Yu H, Zhang Y, Zhao Y. TLRs are important inflammatory factors in atherosclerosis and may be a therapeutic target. Med Hypoth. 2008;70:314–316. doi: 10.1016/j.mehy.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 52.Virani SS, Polsani VR, Nambi ZV. Novel markers of inflammatioin in atherosclerosis. Curr Atheroscler Rep. 2008;10:164–170. doi: 10.1007/s11883-008-0024-0. [DOI] [PubMed] [Google Scholar]
- 53.Peisajovich A, Marnell L, Mold C, Du Clos TW. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev Clin Immunol. 2008;4:379–390. doi: 10.1586/1744666X.4.3.379. [DOI] [PubMed] [Google Scholar]
- 54.Ikonomidis I, Stamatelopoulos K, Lekakis J, Vamvakou GD, Kremastinos DT. Inflammatory and non-invasive vascular markers: the multimarker approach for risk stratification in coronary artery disease. Atherosclerosis. 2008;199:3–11. doi: 10.1016/j.atherosclerosis.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Otake H, Shite J, Shinke T, et al. Relation between plasma adiponectin, high sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. Am J Cardiol. 2008;101:1–7. doi: 10.1016/j.amjcard.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 56.Chamberlain J, Francis S, Brookes Z, et al. Interleukin-1 regulates multiples atherogenic mechanisms in response to fat feeding. PLoS ONE. 2009;4:e5073. doi: 10.1371/journal.pone.0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 58.Ikeda U. Inflammation and coronary artery disease. Curr Vasc Pharmacol. 2003;1:65–70. doi: 10.2174/1570161033386727. [DOI] [PubMed] [Google Scholar]
- 59.Heinisch RH, Zanetti CR, Comin F, Fernandes JL, Ramires JA, Serrano CV., Jr Serial changes in plasma levels of cytokines in patients with coronary artery disease. Vasc Health Risk Manag. 2005;1:245–250. [PMC free article] [PubMed] [Google Scholar]
- 60.Ridker PM. Inflammation, high-sensitivity C-reactive protein, and vascular protection. Tex Heart Inst J. 2010;37:40–41. [PMC free article] [PubMed] [Google Scholar]
- 61.Curtiss LK. Reversing atherosclerosis. N Eng J Med. 2009;360:1144–1146. doi: 10.1056/NEJMcibr0810383. [DOI] [PubMed] [Google Scholar]
- 62.Tillett WS, Francis T., Jr Serological reactions in pneumonia with a non-protein fraction of pneumococcus. J Exp Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norata GD, Marchesi P, Pulakazhi VK, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 64.Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 65.Abou-Raya A, Abou-Raya S. Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun Rev. 2006;5:331–337. doi: 10.1016/j.autrev.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Sun J, Hartvigsen K, Chou M-Y, et al. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation. 2010;122:808–820. doi: 10.1161/CIRCULATIONAHA.109.891887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okemefuna AI, Nan R, Miller A, Gor J, Perkins SJ. Complement factor H binds at two independent sites to C-reactive protein in acute phase concentrations. J Biol Chem. 2010;285:1053–1065. doi: 10.1074/jbc.M109.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bodman-Smith KB, Melendez AJ, Campbell I, Harrison PT, Allen JM, Raynes JG. C-reactive protein-mediated phagocytosis and phospholipase D signaling through the high-affinity receptor for immunoglobulin G (FcγRI) Immunology. 2002;207:252–260. doi: 10.1046/j.1365-2567.2002.01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Eng J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 70.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–1357. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 72.Brull DJ, Serrano N, Zito F, et al. Human CRP gene polymorphism influences CRP levels: implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:2063–2069. doi: 10.1161/01.ATV.0000084640.21712.9C. [DOI] [PubMed] [Google Scholar]
- 73.Kathiresan S, Larson MG, Vasan RS, et al. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to inter-individual variability in serum C-reactive protein level. Circulation. 2006;113:1415–1423. doi: 10.1161/CIRCULATIONAHA.105.591271. [DOI] [PubMed] [Google Scholar]
- 74.Shah T, Newcombe P, Smeeth L, et al. Ancestry as a determinant of mean population C-reactive protein values: implications for cardiovascular risk prediction. Circ Cardiovasc Genet. 2010;3:436–444. doi: 10.1161/CIRCGENETICS.110.957431. [DOI] [PubMed] [Google Scholar]
- 75.Rückerl R, Greven S, Ljungman P, et al. for the Airgene study. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007;115:1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.King DE, Egan BM, Woolson RF, Mainous AG, III, Al-Solaiman Y, Jesri A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502–506. doi: 10.1001/archinte.167.5.502. [DOI] [PubMed] [Google Scholar]
- 77.Bo S, Durazzo M, Guidi S, et al. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am J Clinical Nutrition. 2006;84:1062–1069. doi: 10.1093/ajcn/84.5.1062. [DOI] [PubMed] [Google Scholar]
- 78.King DE, Mainous AG, III, Egan BM, Woolson RF, Geesey ME. Effect of psyllium fiber supplementation on C-reactive protein: the trial to reduce inflammatory markers (TRIM) Ann Fam Med. 2008;6:100–106. doi: 10.1370/afm.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. 2004;134:1181–1185. doi: 10.1093/jn/134.5.1181. [DOI] [PubMed] [Google Scholar]
- 80.Zampelas A, Panagiotakos DB, Pitsavos C, et al. Associations between coffee consumption and inflammatory markers in healthy persons: the ATTICA study. Am J Clin Nutr. 2004;80:862–867. doi: 10.1093/ajcn/80.4.862. [DOI] [PubMed] [Google Scholar]
- 81.Cavicchia PP, Steck SE, Hurley TF, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masters RC, Liese AD, Haffner SM, Wagenknecht LE, Hanley AJ. Whole and refined grain intakes are related to inflammatory protein concentrations in human plasma. J Nutr. 2010;140:587–594. doi: 10.3945/jn.109.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esmaillzadah A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. 2006;84:1489–2497. doi: 10.1093/ajcn/84.6.1489. [DOI] [PubMed] [Google Scholar]
- 84.Micallef MA, Munro IA, Garg ML. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr. 2009;63:1154–1156. doi: 10.1038/ejcn.2009.20. [DOI] [PubMed] [Google Scholar]
- 85.Yen ML, Yang CY, Yen BL, et al. Increased high sensitivity C-reactive protein and neutrophil count are related to increased standard cardiovascular risk factors in healthy Chinese men. Int J Cardiol. 2006;110:191–198. doi: 10.1016/j.ijcard.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 86.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 87.Faber DR, van der Graaf Y, Westerink J, Visseren FLJ. Increased visceral adipose tissue mass is associated with increased C-reactive protein in patients with manifest vascular diseases. Atherosclerosis. 2010;212:274–280. doi: 10.1016/j.atherosclerosis.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 88.Imhof A, Froehlich M, Brenner H, et al. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 89.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 90.Dreon DM, Slavin JL, Phinney SD. Oral contraceptive use and increased plasma concentration of C-reactive protein. Life Sci. 2003;73:1245–1252. doi: 10.1016/s0024-3205(03)00425-9. [DOI] [PubMed] [Google Scholar]
- 91.Frohlich M, Muhlberger N, Hanke H, et al. Markers of inflammation in women on different hormone replacement therapies. Ann Med. 2003;35:353–361. doi: 10.1080/07853890310007090. [DOI] [PubMed] [Google Scholar]
- 92.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 93.McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr. 2009;89:1237–1245. doi: 10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Russell AI, Cunninghame Graham DS, Shepherd C, et al. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–147. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jönsen A, Gunnarsson I, Gullstrand B, et al. Association between SLE nephritis and polymorphic variants of the CRP and Fcgamma;RIIIa genes. Rheumatology (Oxford) 2007;46:1417–1421. doi: 10.1093/rheumatology/kem167. [DOI] [PubMed] [Google Scholar]
- 96.Edberg JC, Wu J, Langefeld CD, et al. Genetic variation in the CRP promoter: association with systemic lupus erythematosus (SLE) Hum Mol Genet. 2008;17:1147–1455. doi: 10.1093/hmg/ddn004. [DOI] [PubMed] [Google Scholar]
- 97.Graf J, Scherzer R, Grunfeld C, Imboden J. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS ONE. 2009;4:e6242. doi: 10.1371/journal.pone.0006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coventry BJ, Ashdown ML, Quinn MA, Markovic SN, Yatomi-Clarke SL, Robinson AP. CRP identifies homeostatic immune oscillations in cancer patients: a potential treatment targeting tool. J Transl Med. 2009;7:102. doi: 10.1186/1479-5876-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4649–4654. doi: 10.1210/jc.2002-021688. [DOI] [PubMed] [Google Scholar]
- 100.Tosi F, Dorizzi R, Castello R, et al. Body fat and insulin resistance independently predict increased serum C-reactive protein in hyperandrogenic women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161:737–745. doi: 10.1530/EJE-09-0379. [DOI] [PubMed] [Google Scholar]
- 101.Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. J Clin Endocrinol Metab. 2009;94:103–108. doi: 10.1210/jc.2008-1750. [DOI] [PubMed] [Google Scholar]
- 102.Hoeger KM. Polycystic ovary syndrome, inflammation and statins: do we have the right target. J Clin Endocrinol Metab. 2009;94:35–37. doi: 10.1210/jc.2008-2376. [DOI] [PubMed] [Google Scholar]
- 103.De Beer F, Soutar AK, Baltz ML, Trayner IM, Feinstein A, Pepys MB. Low density lipoprotein and very low density lipoprotein are selectively bound by aggregated C-reactive protein. J Exp Med. 1982;156:230–242. doi: 10.1084/jem.156.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang YX, Cliff WJ, Schoefl GI, Higgins G. Coronary C-reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis. 1999;145:375–379. doi: 10.1016/s0021-9150(99)00105-7. [DOI] [PubMed] [Google Scholar]
- 105.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 106.Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094–2099. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 107.Devaraj S, Davis B, Simon SI, Jialal I. CRP promotes monocyte-endothelial cell adhesion via Fcgamma;receptors in human aortic endothelial cells under static and shear flow conditions. Am J Physiol Heart Circ Physiol. 2006;291:H1170–1176. doi: 10.1152/ajpheart.00150.2006. [DOI] [PubMed] [Google Scholar]
- 108.Rocker C, Manolov DE, Kuzmenkina EV, et al. Affinity of C-reactive protein toward FcgammaRI is strongly enhanced by the gamma-chain. Am J Pathol. 2007;170:755–763. doi: 10.2353/ajpath.2007.060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartz R, Osborne-Lawrence S, Hahner L, et al. C-reactive protein downregulates endothelial NO synthase and attenuates reendothelialization in vivo in mice. Circ Res. 2007;100:1452–1459. doi: 10.1161/01.RES.0000267745.03488.47. [DOI] [PubMed] [Google Scholar]
- 110.Szmitko PE, Verma S. C-reactive protein and reendothelialization: NO involvement. Circ Res. 2007;100:1405–1407. doi: 10.1161/01.RES.0000269683.67849.b1. [DOI] [PubMed] [Google Scholar]
- 111.Xing D, Hage FG, Chen YF, et al. Exaggerated neointima formation in human C-reactive protein transgenic mice is IgG Fc receptor type 1 (Fc gamma RI)-dependent. Am J Pathol. 2008;172:22–30. doi: 10.2353/ajpath.2008.070154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salio M, Chimenti S, de Angelis N, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 113.Van Vré EA, Bult H, Hoymans VY, van Tendeloo FI, Vrints CJ, Bosmans JM. Human C-reactive protein activates monocyte-derived dendritic cells and induces dendritic cell-mediated T-cell activation. Arterioscler Thromb Vasc Biol. 2008;28:511–518. doi: 10.1161/ATVBAHA.107.157016. [DOI] [PubMed] [Google Scholar]
- 114.Fujita Y, Kakino A, Nishimichi N, et al. Oxidized LDL receptor LOX-1 binds to C-reactive protein and mediates its vascular effects. Clin Chem. 2009;55:285–294. doi: 10.1373/clinchem.2008.119750. [DOI] [PubMed] [Google Scholar]
- 115.Filep JG. Platelets affect the structure and function of C-reactive protein. Circ Res. 2009;105:109–111. doi: 10.1161/CIRCRESAHA.109.202010. [DOI] [PubMed] [Google Scholar]
- 116.Jin C, Lu L, Zhang RY, et al. Association of serum glycated albumin, C-reactive protein and ICAM-1 levels with diffuse coronary artery disease in patients with type 2 diabetes mellitus. Clin Chim Acta. 2009;408:45–49. doi: 10.1016/j.cca.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Ridker PM. C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem. 2009;55:209–215. doi: 10.1373/clinchem.2008.119214. [DOI] [PubMed] [Google Scholar]
- 118.Geluk CA, Post WJ, Hillege HL, et al. C-reactive protein and angiographic characteristics of stable and unstable coronary artery disease: data from the prospective PREVEND cohort. Atherosclerosis. 2008;196:372–382. doi: 10.1016/j.atherosclerosis.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 119.Nabata A, Kuroki M, Ueba H, et al. C-reactive protein induces endothelial cell apoptosis and matrix metalloproteinase-9 production in human mononuclear cells: implications for the destabilization of atherosclerotic plaque. Atherosclerosis. 2008;196:129–135. doi: 10.1016/j.atherosclerosis.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 120.Scirica BM, Cannon CP, Sabatine MS, et al. Concentrations of C-reactive protein and B-type natriuretic peptide 30 days after acute coronary syndromes independently predict hospitalization for heart failure and cardiovascular death. Clin Chem. 2009;55:265–273. doi: 10.1373/clinchem.2008.117192. [DOI] [PubMed] [Google Scholar]
- 121.Scirica BM, Morrow DA, Cannon CP, et al. Thrombolysis in Myocardial Infarction (TIMI) Study Group. Clinical application of C-reactive protein across the spectrum of acute coronary syndromes. Clin Chem. 2007;53:1800–1807. doi: 10.1373/clinchem.2007.087957. [DOI] [PubMed] [Google Scholar]
- 122.Scirica BM, Morrow DA. Is C-reactive protein an innocent bystander or proatherogenic culprit? The verdict is still out. Circulation. 2006;113:2128–2134. doi: 10.1161/CIRCULATIONAHA.105.611350. [DOI] [PubMed] [Google Scholar]
- 123.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–2150. [PubMed] [Google Scholar]
- 124.Jialal I, Verma S, Devaraj S. Inhibition of endothelial nitric oxide synthase by C-reactive protein: clinical relevance. Clin Chem. 2009;55:206–208. doi: 10.1373/clinchem.2008.119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guan H, Wang P, Hui R, Edin ML, Zeldin DC, Wang DW. Adeno-associated virus-mediated human C-reactive protein gene delivery causes endothelial dysfunction and hypertension in rats. Clin Chem. 2009;55:274–284. doi: 10.1373/clinchem.2008.115857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Radjesh J, Bisoendial S, Boekholdt M, Vergeer M, Stroes ESG, Kastelein JJP. C-reactive protein is a mediator of cardiovascular disease. Eur Heart J. 2010;31:2087–2091. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- 127.Sinclair KA, Bogart A, Buchwald D, Henderson JA. The prevalence of metabolic syndrome and associated risk factors in Northern Plains and Southwest American Indians. Diabetes Care. 2010 Sep 23; doi: 10.2337/dc10-0221. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kearney MT. Targeting the endothelium to prevent diabetes-related atherosclerosis. Diab Vasc Dis Res. 2010;7:177. doi: 10.1177/1479164110374902. [DOI] [PubMed] [Google Scholar]
- 129.Ford ES, Giles WH, Myers GL, Mannino DM. Population distribution of high-sensitivity C-reactive protein among US men: findings from national health and nutrition examination survey 1999–2000. Clin Chem. 2003;49:686–690. doi: 10.1373/49.4.686. http://www.clinchem.org/cgi/content/full/49/4/686. [DOI] [PubMed] [Google Scholar]
- 130.Centers for Disease Control and Prevention. NHANES 1999–2000 public data release file documentation. [Accessed 2010 Oct 18]. http://www.cdc.gov/nchs/data/nhanes/gendoc.pdf.
- 131.Rifai N, Ridker PM. Population distributions of C-reactive protein in apparently healthy men and women in the United States: implication for clinical interpretation. Clin Chem. 2003;49:666–669. doi: 10.1373/49.4.666. [DOI] [PubMed] [Google Scholar]
- 132.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Eng J Med. 2005;352:1611–1613. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]
- 133.Kushner I, Rzewnicki W, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:166.e17–166.e28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 134.Kao PC, Shiesh S-C, Wu TJ. Serum C-reactive protein as a marker for wellness assessment. Ann Clin Lab Sci. 2006;36:163–169. [PubMed] [Google Scholar]
- 135.Koenig W, Sund M, Frohlich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 136.Miller M, Zhan M, Havas S. High attributable risk of elevated C-reactive protein level to conventional coronary heart disease risk factors: the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2005;165:2063–2068. doi: 10.1001/archinte.165.18.2063. [DOI] [PubMed] [Google Scholar]
- 137.Greenfield JR, Samaras K, Jenkins AB, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109:3022–3028. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 138.Currie CJ, Poole CD, Conway P. Evaluation of the association between the first observation and the longitudinal change in C-reactive protein, and all-cause mortality. Heart. 2008;94:457–462. doi: 10.1136/hrt.2007.118794. [DOI] [PubMed] [Google Scholar]
- 139.Marsik C, Kazemi-Shirazi L, Schickbauer T, et al. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008;54:343–349. doi: 10.1373/clinchem.2007.091959. [DOI] [PubMed] [Google Scholar]
- 140.Kravitz BA, Corrada MM, Kawas CH. High levels of serum C-reactive protein are associated with greater risk of all-cause mortality, but not dementia, in the oldest-old: results from the 90+ study. J Am Ger Soc. 2009;57:641–646. doi: 10.1111/j.1532-5415.2009.02169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wassel CL, Barrett-Connor E, Laughlin GA. Association of circulating C-reactive protein and interleukin-6 with longevity into the 80s and 90s: The Rancho Bernardo Study. J Clin Endocrinol Metab. 2010;95:4748–4755. doi: 10.1210/jc.2010-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 143.Diaz JJ, Arguelles J, Malaga I, et al. C-reactive protein is elevated in the offspring of parents with essential hypertension. Arch Dis Child. 2007;92:304–308. doi: 10.1136/adc.2006.094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mancia G, de Backer G, Dominiczak A, et al. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 145.Ridker PM, Danielson E, Rifai N, Glynn RJ. Valsartan, blood pressure reduction, and C-reactive protein: primary report of the Val-MARC trial. Hypertension. 2006;48:73–79. doi: 10.1161/01.HYP.0000226046.58883.32. [DOI] [PubMed] [Google Scholar]
- 146.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 147.Sathiyapriya V, Nandeesha H, Selvaraj N, Bobby Z, Agrawal A, Pavithran P. Association between protein-bound sialic acid and high-sensitivity C-reactive protein in essential hypertension: a possible indication of underlying cardiovascular risk. Angiology. 2009;59:721–726. doi: 10.1177/0003319708314246. [DOI] [PubMed] [Google Scholar]
- 148.Lande MB, Pearson TA, Vermilion RP, Auinger P, Fernandez ID. Elevated blood pressure, race/ethnicity, and C-reactive protein levels in children and adolescents. Pediatrics. 2008;122:1252–1257. doi: 10.1542/peds.2007-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Black GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108:2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- 150.Perticone F, Maio R, Sciacqua A, et al. Enodthelial dysfunction and C-reactive protein are risk factors for diabetes in essential hypertension. Diabetes. 2008;57:167–171. doi: 10.2337/db07-1189. [DOI] [PubMed] [Google Scholar]
- 151.Vardas PE, Marketou ME. CRP in non-dippers: new perspectives and old queries. J Human Hypertension. 2008;22:447–449. doi: 10.1038/jhh.2008.33. [DOI] [PubMed] [Google Scholar]
- 152.Veerabhadrappa P, Diaz KM, Feairheller DL, et al. Enhanced blood pressure variability in a high cardiovascular risk group of African Americans: FIT4 Life Study. J Am Soc Hypertens. 2010;4:187–195. doi: 10.1016/j.jash.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iijima R, Byrne RA, Ndrepepa G, et al. Pre-procedural C-reactive protein levels and clinical outcomes after percutaneous coronary interventions with and without abciximab: pooled analysis of four ISAR trials. Heart. 2009;95:107–112. doi: 10.1136/hrt.2008.153635. [DOI] [PubMed] [Google Scholar]
- 154.Ishii H, Toriyama T, Aoyama T, et al. Prognostic values of C-reactive protein levels on clinical outcome after implantation of sirolimuseluting stents in patients on hemodialysis. Circ Cardiovasc Interv. 2009;2:513–518. doi: 10.1161/CIRCINTERVENTIONS.109.889915. [DOI] [PubMed] [Google Scholar]
- 155.Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA, et al. Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning. Heart. 2006;92:1821–1826. doi: 10.1136/hrt.2006.089060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kangasniemi OP, Biancari F, Luukkonen J, et al. Preoperative C-reactive protein is predictive of long-term outcome after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;29:983–985. doi: 10.1016/j.ejcts.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 157.Wypasek E, Undas A, Sniezek-Maciejewska M, et al. The increased plasma C-reactive protein and interleukin-6 levels in patients under-going coronary artery bypass grafting surgery are associated with the interleukin-6-174G. C gene polymorphism. Ann Clin Biochem. 2010;47:343–349. doi: 10.1258/acb.2010.090305. [DOI] [PubMed] [Google Scholar]
- 158.Lo B, Fijnheer R, Nierich AP, Bruins P, Kalkman CJ. C-reactive protein is a risk indicator for atrial fibrillation after myocardial revascularization. Ann Thorac Surg. 2005;79:1530–1535. doi: 10.1016/j.athoracsur.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 159.Kardys I, Knetsch AM, Bleumink GS, et al. C-reactive protein and risk of heart failure. The Rotterdam Study. Am Heart J. 2006;152:514–520. doi: 10.1016/j.ahj.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 160.Williams ES, Shah SJ, Ali S, Na BY, Schiller NB, Whooley MA. C-reactive protein, diastolic dysfunction, and risk of heart failure in patients with coronary disease: Heart and Soul Study. Eur J Heart Fail. 2008;10:63–69. doi: 10.1016/j.ejheart.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 162.Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010;15:331–341. doi: 10.1007/s10741-009-9140-3. [DOI] [PubMed] [Google Scholar]
- 163.Araujo JP, Lourenco P, Azevedo A, et al. Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Cardiac Fail. 2009;15:256–266. doi: 10.1016/j.cardfail.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 164.Collinson PO. Concentrations of C-reactive protein and B-type natriuretic peptide 30 days after acute coronary syndromes independently predict hospitalization for heart failure and cardiovascular death: just another brick in the wall. Clin Chem. 2009;55:203–205. doi: 10.1373/clinchem.2008.119198. [DOI] [PubMed] [Google Scholar]
- 165.Lamblin N, Mouquet F, Hennache B, et al. High-sensitivity C-reactive protein: potential adjunct for risk stratification in patients with stable congestive heart failure. Eur Heart J. 2005;26:2245–2250. doi: 10.1093/eurheartj/ehi501. [DOI] [PubMed] [Google Scholar]
- 166.Anand IS, Latini R, Florea VG, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–1434. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 167.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 168.Conen D, Rexrode KM, Creager MA, Ridker PM, Pradhan AD. Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women: a prospective study. Circulation. 2009;120:1041–1047. doi: 10.1161/CIRCULATIONAHA.109.863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of insulin resistance and inflammation with peripheral arterial disease: the National Health and Nutrition Examination Survey, 1999 to 2004. Circulation. 2008;118(1):33–41. doi: 10.1161/CIRCULATIONAHA.107.721878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schlager O, Exner M, Mlekusch W, et al. C-reactive protein predicts future cardiovascular events in patients with carotid stenosis. Stroke. 2007;38:1263–1268. doi: 10.1161/01.STR.0000259890.18354.d2. [DOI] [PubMed] [Google Scholar]
- 171.Albert CM, Ma J, Rifai N, et al. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;4(105):2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 172.Spooner PM, Zipes DP. Sudden death predictors: an inflammatory association. Circulation. 2002;105:2574–2576. doi: 10.1161/01.cir.0000017821.98250.25. [DOI] [PubMed] [Google Scholar]
- 173.Beri A, Contractor T, Khasnis A, Thakur R. Statins and the reduction of sudden cardiac death: antiarrhythmic or anti-ischemic effect. Am J Cardiovasc Drugs. 2010;10:155–164. doi: 10.2165/11536690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 174.Corbalán R, Braun S, Pereira J, Navarrete C, Gonzalez I. C-reactive protein and atrial fibrillation: evidence for the presence of inflammation in the perpetuation of the arrhythmia. Int J Cardiol. 2006;108:326–331. doi: 10.1016/j.ijcard.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 175.Conen D, Ridker PM, Everett BM, et al. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–1736. doi: 10.1093/eurheartj/ehq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marott SCW, Nordestgaard BG, Zacho J, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56:789–795. doi: 10.1016/j.jacc.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 177.Blangy H, Sadoul N, Dousset B, et al. Serum BNP, hs-C-reactive protein, procollagen to assess the risk of ventricular tachycardia in ICD recipients after myocardial infarction. Europace. 2007;9:724–729. doi: 10.1093/europace/eum102. [DOI] [PubMed] [Google Scholar]
- 178.Jeevanantham V, Singh N, Izuora K, D’souza JP, His DH. Correlation of high sensitivity C-reactive protein and calcific aortic valve disease. Mayo Clin Proc. 2007;82:171–174. doi: 10.4065/82.2.171. [DOI] [PubMed] [Google Scholar]
- 179.Jones P, Davidson M, Stein E, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 180.Ay C, Tengler T, Vormittag R, et al. Venous thromboembolism a manifestation of the metabolic syndrome. Haematologica. 2007;92:374–380. doi: 10.3324/haematol.10828. [DOI] [PubMed] [Google Scholar]
- 181.Quarck R, Nawrot T, Meyns B, Delcroix M. C-reactive protein a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;53:1211–1218. doi: 10.1016/j.jacc.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 182.Soinio M, Marniemi J, Laakso M, et al. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care. 2006;29:329–333. doi: 10.2337/diacare.29.02.06.dc05-1700. [DOI] [PubMed] [Google Scholar]
- 183.Marcovecchio ML, Giannini C, Widmer B, et al. C-reactive protein in relation to the development of microalbuminuria in type 1 diabetes. Diabetes Care. 2008;31:974–976. doi: 10.2337/dc07-2101. [DOI] [PubMed] [Google Scholar]
- 184.Dehghan A, Kardys I, de Maat MPM, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56:872–878. doi: 10.2337/db06-0922. [DOI] [PubMed] [Google Scholar]
- 185.Kahn SE, Zinman B, Haffner SM, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–2364. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- 186.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94:3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 187.Ridker PM, Pare G, Parker A, et al. Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Genet. 2008;82:1185–1192. doi: 10.1016/j.ajhg.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14,719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 189.Moreno-Navarrete JM, Martinez-Barricarte R, Catalan V, et al. Complement factor H is expressed in adipose tissue in association with insulin resistance. Diabetes. 2010;59:200–209. doi: 10.2337/db09-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jialal I. The role of the laboratory in the diagnosis of the metabolic syndrome. Am J Clin Pathol. 2009;132(2):161–162. doi: 10.1309/AJCP1B0DHHSAIJJR. [DOI] [PubMed] [Google Scholar]
- 191.Steinberger J, Daniels SR, Eckel RH, et al. American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 192.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Suzuki T, Katz R, Jenny NS, et al. Metabolic syndrome, inflammation, and incident heart failure in the elderly: the Cardiovascular Health Study. Circ Heart Fail. 2008;1:242–248. doi: 10.1161/CIRCHEARTFAILURE.108.785485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.You T, Nicklas BJ, Ding J, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63:414–419. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dai DF, Lin JW, Kao JH, et al. The effects of metabolic syndrome versus infectious burden on inflammation, severity of coronary atherosclerosis, and major adverse cardiovascular events. J Clin Endocrinol Metab. 2007;92:2532–2537. doi: 10.1210/jc.2006-2428. [DOI] [PubMed] [Google Scholar]
- 196.Ye X, Yu Z, Li H, Franco OH, Liu Y, Lin X. Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol. 2007;49:1798–1805. doi: 10.1016/j.jacc.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 197.Pitsavos C, Panagiotakos DB, Tzima N, et al. Diet, exercise, and C-reactive protein levels in people with abdominal obesity: the ATTICA epidemiological study. Angiology. 2007;58:225–233. doi: 10.1177/0003319707300014. [DOI] [PubMed] [Google Scholar]
- 198.Devaraj S, Valleggi D, Siegel D, Jialal I. Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep. 2010;12:110–118. doi: 10.1007/s11883-010-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang A, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Alyan O, Metin F, Kacmaz F, et al. High levels of high sensitivity C-reactive protein predict the progression of chronic rheumatic mitral stenosis. J Thromb Thrombolysis. 2009;28:63–69. doi: 10.1007/s11239-008-0245-7. [DOI] [PubMed] [Google Scholar]
- 201.Lee HM, Le TV, Lopez VA, Wong ND. Association of C-reactive protein with reduced forced vital capacity in a nonsmoking US population with metabolic syndrome and diabetes. Diabetes Care. 2008;31:2000–2002. doi: 10.2337/dc08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Magnussen H, Watz H. Systemic inflammation in chronic obstructive pulmonary disease and asthma: relation with comorbidities. Proc Am Thorac Soc. 2009;6:648–651. doi: 10.1513/pats.200906-053DP. [DOI] [PubMed] [Google Scholar]
- 203.Eickhoff P, Valipour A, Kiss D, et al. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 204.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 205.Chen HY, Chiu YL, Hsu SP, et al. Elevated C-reactive protein level in hemodialysis patients with moderate/severe uremic pruritus: a potential mediator of high overall mortality. QJM. 2010;103:837–846. doi: 10.1093/qjmed/hcq036. [DOI] [PubMed] [Google Scholar]
- 206.Lahrach H, Ghalim N, Taki H, et al. Serum paraoxonase activity, high-sensitivity C-reactive protein, and lipoprotein disturbances in end-stage renal disease patients on long-term hemodialysis. J Clin Lipidol. 2008;2:43–50. doi: 10.1016/j.jacl.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 207.Lui MM, Lam JC, Mak HK-F, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–956. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 208.Schiza SE, Mermigkis C, Panagiotis, et al. C-reactive protein evolution in obstructive sleep apnoea patients under CPAP therapy. Eur J Clin Invest. 2010;40:968–975. doi: 10.1111/j.1365-2362.2010.02348.x. [DOI] [PubMed] [Google Scholar]
- 209.Zeka A, Sullivan JR, Vokonas PS, Sparrow D, Schwartz J. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. Int J Epidemiol. 2006;35:1347–1354. doi: 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]
- 210.Dixon JB, Hayden MJ, Lambert GW, et al. Raised CRP Levels in obese patients: symptoms of depression have an independent positive association. Obesity. 2008;16:2010–2015. doi: 10.1038/oby.2008.271. [DOI] [PubMed] [Google Scholar]
- 211.Hak AE, Stehouwer CD, Bots ML, et al. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler Thromb Vasc Biol. 1999;19:1986–1991. doi: 10.1161/01.atv.19.8.1986. [DOI] [PubMed] [Google Scholar]
- 212.McLaughlin T, Abbasi F, Lamendola C, et al. Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation. 2002;106:2908–2912. doi: 10.1161/01.cir.0000041046.32962.86. [DOI] [PubMed] [Google Scholar]
- 213.Wee CC, Mukamal KJ, Huang A, Davis RB, McCarthy EP, Mittleman MA. Obesity and C-reactive protein levels among white, black, and hispanic US adults. Obesity. 2008;16:875–880. doi: 10.1038/oby.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–e809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Puglisi MJ, Fernandez ML. Modulation of C-reactive protein, tumor necrosis factor α, and adiponectin by diet, exercise, and weight loss. J Nutr. 2008;138:2293–2296. doi: 10.3945/jn.108.097188. [DOI] [PubMed] [Google Scholar]
- 216.Sacheck J. Pediatric obesity: an inflammatory condition. JPEN J Parenter Enteral Nutr. 2008;32:633–637. doi: 10.1177/0148607108324876. [DOI] [PubMed] [Google Scholar]
- 217.Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007;26:163–169. doi: 10.1080/07315724.2007.10719598. [DOI] [PubMed] [Google Scholar]
- 218.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 219.Pope CA., III Particulate air pollution, C-reactive protein, and cardiac risk. Eur Heart J. 2001;22:1149–1150. doi: 10.1053/euhj.2001.2593. [DOI] [PubMed] [Google Scholar]
- 220.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–1209. doi: 10.1503/cmaj.1040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gentile M, Panico S, Rubba F, et al. Obesity, overweight, and weight gain over adult life are main determinants of elevated hs- CRP in a cohort of Mediterranean women. Eur J Clin Nutr. 2010;64:873–878. doi: 10.1038/ejcn.2010.69. [DOI] [PubMed] [Google Scholar]
- 222.Hamer M, Steptoe A. Prospective study of physical fitness, adiposity, and inflammatory markers in healthy middle-aged men and women. Am J Clin Nutr. 2009;89:85–89. doi: 10.3945/ajcn.2008.26779. [DOI] [PubMed] [Google Scholar]
- 223.Welsh P, Polisecki E, Robertson M, et al. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab. 2010;95:93–99. doi: 10.1210/jc.2009-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hubel CA, Powers RW, Snaedal S, et al. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension. 2008;51:1499–1505. doi: 10.1161/HYPERTENSIONAHA.108.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lau B, Sharrett SR, Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 226.Surtees PG, Wainwright NWJ, Boekholdt SM, Luben RN, Wareham NJ, Khaw KT. Major depression, C-reactive protein, and incident ischemic heart disease in healthy men and women. Psychosom Med. 2008;70:850–855. doi: 10.1097/PSY.0b013e318183acd5. [DOI] [PubMed] [Google Scholar]
- 227.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 228.Pankow JS, Folsom AR, Cushman M, et al. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–689. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 229.Lee CC, You NC, Song Y, et al. Relation of genetic variation in the gene coding for C-reactive protein with its plasma protein concentrations: findings from the Women’s Health Initiative Observational Cohort. Clin Chem. 2009;55(2):351–360. doi: 10.1373/clinchem.2008.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem. 2008;54:1027–1037. doi: 10.1373/clinchem.2007.098996. [DOI] [PubMed] [Google Scholar]
- 231.Smith GD, Ebrahim S. Mendelian randomization: can genetic epidemiology contribute to understanding environmental determinants of disease. Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 232.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 233.Smith DG. Capitalizing on Mendelian randomization to assess the effects of treatments. J R Soc Med. 2007;100:432–435. doi: 10.1258/jrsm.100.9.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 2008;5:e177. doi: 10.1371/journal.pmed.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 237.Emerging Risk, Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Clarke R, Emberson JR, Breeze E, et al. Biomarkers of inflammation predict both vascular and non-vascular mortality in older men. Eur Heart. 2008;29:800–809. doi: 10.1093/eurheartj/ehn049. [DOI] [PubMed] [Google Scholar]
- 240.Heikkila K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 241.Habersberger J, Eisenhardt S, Peter K. C-reactive protein measurement and cardiovascular disease. Original Text. Lancet. 2010;9720:1078. doi: 10.1016/S0140-6736(10)60470-9. [DOI] [PubMed] [Google Scholar]
- 242.Ji SR, Wu Y, Zhu L, et al. Cell membranes and liposomes dissociate C-reactive protein (CRP) to form a new, biologically active structural intermediate: mCRP(m) FASEB J. 2007;21:284–294. doi: 10.1096/fj.06-6722com. [DOI] [PubMed] [Google Scholar]
- 243.Molins B, Pena E, Vilahur G, Mendieta C, Slevin M, Badimon L. C-reactive protein isoforms differ in their effects on thrombus growth. Arterioscler Thromb Vasc Biol. 2008;28:2239–2246. doi: 10.1161/ATVBAHA.108.174359. [DOI] [PubMed] [Google Scholar]
- 244.Eisenhardt SU, Habersberger J, Murphy A, et al. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ Res. 2009;105:128–137. doi: 10.1161/CIRCRESAHA.108.190611. [DOI] [PubMed] [Google Scholar]
- 245.Ji SR, Ma L, Bai CJ, et al. Monomeric C-reactive protein activates endothelial cells via interaction with lipid raft microdomains. FASEB J. 2009;23:1806–1816. doi: 10.1096/fj.08-116962. [DOI] [PubMed] [Google Scholar]
- 246.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 247.Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 248.Ridker PM, Cannon CP, Morrow D, et al. Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT – TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 249.De Beer FC, Hind CR, Fox KM, et al. Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br Heart J. 1982;47:239–243. doi: 10.1136/hrt.47.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Haverkate F, Thompson SG, Pyke SD, et al. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 251.Niccoli G, Biasucci LM, Biscione C, et al. Independent prognostic value of C-reactive protein and coronary artery disease extent in patients affected by unstable angina. Atherosclerosis. 2008;196:779–785. doi: 10.1016/j.atherosclerosis.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 252.Ridker PM, Cushman M, Stampfer MJ, et al. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 253.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 254.Hemingway H, Philipson P, Chen R, Fitzpatrick NK, Damant J, et al. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7:e1000286. doi: 10.1371/journal.pmed.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Boekholdt SM, Hack CE, Sandhu MS, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis. 2006;187:415–422. doi: 10.1016/j.atherosclerosis.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 256.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 257.Genest J, McPherson R, Frohlich J, et al. Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Can J Cardiol. 2009;2009;25:567–579. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Albert MA, Glynn RJ, Ridker PM. Plasma concentration of C-reactive protein and the calculated Framingham Coronary Heart Disease Risk Score. Circulation. 2003;108:161–165. doi: 10.1161/01.CIR.0000080289.72166.CF. [DOI] [PubMed] [Google Scholar]
- 259.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 260.Shah T, Casas JP, Cooper JA, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38:217–231. doi: 10.1093/ije/dyn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wald DS, Kasturiratne A, Bestwick JP. The value of C-reactive protein in screening for future coronary heart disease events. J Med Screen. 2009;16:212–214. doi: 10.1258/jms.2009.009087. [DOI] [PubMed] [Google Scholar]
- 262.Lakoski SG, Greenland P, Wong ND, Schreiner PJ, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the Multi- Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2007;167:2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 263.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 264.Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R, Sr, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1:92–97. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for Men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cook NR. The case for C-reactive protein as a risk marker for coronary heart disease. Ann Intern Med. 2010;152:406. doi: 10.7326/0003-4819-152-6-201003160-00020. [DOI] [PubMed] [Google Scholar]
- 267.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;50:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Am J Cardiol. 2010;106:204–209. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 269.Slejko JF, Page RL, II, Sullivan PW. Implications of Jupiter prevention in adults with elevated C-reactive protein: statin therapy is cost-effective for vascular event. J Am Coll Cardiol. 2010;55:A131.E1229. doi: 10.1185/03007995.2010.516994. [DOI] [PubMed] [Google Scholar]
- 270.Rao AD, Milbrandt EB. To JUPITER and beyond: statins, inflammation, and primary prevention. Critical Care. 2010;14:310. doi: 10.1186/cc9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.MacDonald GP. Cost-effectiveness of rosuvastatin for primary prevention of cardiovascular events according to Framingham Risk Score in patients with elevated C-reactive protein. J Am Osteopath Assoc. 2010;110:427–436. [PubMed] [Google Scholar]
- 272.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 273.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nambi V, Ballantyne CM. Utility of statin therapy using high- sensitivity C-reactive protein as an indicator of coronary heart disease risk. Curr Atheroscler Rep. 2005;7:22–28. doi: 10.1007/s11883-005-0071-8. [DOI] [PubMed] [Google Scholar]
- 275.Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol. 2003;92:17K–22K. doi: 10.1016/s0002-9149(03)00774-4. [DOI] [PubMed] [Google Scholar]
- 276.Roche VF. Antihyperlipidemic statins: a self-contained, clinically relevant medicinal chemistry lesson. Am J Pharm Educ. 2005;69:546–560. [Google Scholar]
- 277.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 278.Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci. 1998;19:26–37. doi: 10.1016/s0165-6147(97)01147-4. [DOI] [PubMed] [Google Scholar]
- 279.McTaggart F. Comparative pharmacology of rosuvastatin. Atheroscler Suppl. 2003;4:9–14. doi: 10.1016/s1567-5688(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 280.Kapur NK. Rosuvastatin: a highly potent statin for the prevention and management of coronary artery disease. Expert Rev Cardiovasc Ther. 2007;5:161–175. doi: 10.1586/14779072.5.2.161. [DOI] [PubMed] [Google Scholar]
- 281.Holtzman CW, Wiggins BS, Spinler SA. Role of P-glycoprotein in statin drug interactions. Pharmacotherapy. 2006;26:1601–1607. doi: 10.1592/phco.26.11.1601. [DOI] [PubMed] [Google Scholar]
- 282.Kostapanos MS, Haralampos J, Elisaf MS. Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drugs. 2010;10:11–28. doi: 10.2165/13168600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 283.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterollowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 284.McKenney JM. Pharmacologic options for aggressive low-density lipoprotein cholesterol lowering: benefits versus risks. Am J Cardiol. 2005;96:60E–66E. doi: 10.1016/j.amjcard.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 285.The Galaxy Program. [Accessed 2010 Oct 18]. http://www.galaxyprogramme.org/about-galaxy/?itemId=2592383.
- 286.Crouse JR, III, Raichlen JS, Riley WA, et al. METEOR study group. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 287.Crouse JR, III, Bots ML, Evans GW, et al. Does baseline carotid intima-media thickness modify the effect of rosuvastatin when compared with placebo on carotid intima-media thickness progression? The METEOR study. Eur J Cardiovasc Prev Rehabil. 2010;17:223–229. doi: 10.1097/HJR.0b013e3283359c38. [DOI] [PubMed] [Google Scholar]
- 288.Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 289.McMurray JJV, Kjekshus J, Gullestad L, et al. CORONA Study Group. Effects of statin therapy according to plasma high-sensitivity C-reactive protein concentration in the Controlled Rosuvastatin Multinational trial in Heart Failure (CORONA). A retrospective analysis. Circulation. 2009;120:2188–2196. doi: 10.1161/CIRCULATIONAHA.109.849117. [DOI] [PubMed] [Google Scholar]
- 290.Gissi-HF Investigators, Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 291.Van der Harst P, de Boer RA. Statins in the treatment of heart failure. Circ Heart Fail. 2010;3:462–464. doi: 10.1161/CIRCHEARTFAILURE.110.956342. [DOI] [PubMed] [Google Scholar]
- 292.Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Eng J Med. 2009;373:1175–1182. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 293.Narla V, Blaha MJ, Blumenthal RS, Michos ED. The JUPITER and AURORA clinical trials for rosuvastatin in special primary prevention populations: perspectives, outcomes, and consequences. Vasc Health Risk Manag. 2009;5:1033–1042. doi: 10.2147/vhrm.s6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 295.Ballantyne CM, Raichlen JS, Nicholls S, et al. ASTEROID Investigators. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation. 2008;117:2458–2466. doi: 10.1161/CIRCULATIONAHA.108.773747. [DOI] [PubMed] [Google Scholar]
- 296.Mack WJ, Xiang M, Selzer RH, Hodis HN. Serial quantitative coronary angiography and coronary events. Am Heart J. 2000;139:993–999. doi: 10.1067/mhj.2000.105702. [DOI] [PubMed] [Google Scholar]
- 297.Buchwald H, Matts JP, Fitch LL, et al. Program on the Surgical Control of the Hyperlipidemias (POSCH) Group. Changes in sequential coronary arteriograms and subsequent coronary events. JAMA. 1992;268:1429–1433. [PubMed] [Google Scholar]
- 298.Fonseca FAH, Izar MCO. Primary prevention of vascular events in patients with high levels of C-reactive protein: the JUPITER Study. Expert Rev Cardiovasc Ther. 2009;7:1041–1056. doi: 10.1586/erc.09.93. [DOI] [PubMed] [Google Scholar]
- 299.Libby P, Ridker PM. Novel inflammatory markers of coronary risk: theory versus practice. Circulation. 1999;100:1148–1150. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- 300.Lemieux I, Pascot A, Prud’homme D, Almeras N, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 301.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 302.Ridker PM JUPITER Study Group. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–2297. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 303.Ridker PM, Fonseca FAH, Genest J, et al. JUPITER Trial Study Group. Baseline characteristics of participants in the JUPITER Trial, a randomized placebo-controlled primary prevention trial of statin therapy among individuals with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein. Am J Cardiol. 2007;100:1659–1664. doi: 10.1016/j.amjcard.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 304.De Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction – final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 305.Rembold CM. To statin or to non-statin in coronary disease – considering absolute risk is the answer. Atherosclerosis. 2007;195:1–6. doi: 10.1016/j.atherosclerosis.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 306.Everett BM, Ridker PM. Using inflammatory biomarkers to guide lipid therapy. Curr Cardiovasc Risk Reports. 2008;2:29–34. [Google Scholar]
- 307.Kritek P, Campion EW. JUPITER Clinical Directions – polling results. N Eng J Med. 2009;360:e14. doi: 10.1056/NEJMe086509. [DOI] [PubMed] [Google Scholar]
- 308.Spatz ES, Canavan ME, Desai MM. From here to JUPITER. Identifying new patients for statin therapy using data from the 1999–2004 National Health and Nutrition Examination Survey. Circ Qual Outcomes. 2009;2:41–48. doi: 10.1161/CIRCOUTCOMES.108.832592. [DOI] [PubMed] [Google Scholar]
- 309.Michos ED, Blumenthal RS. Prevalence of the JUPITER ( Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin) Study. J Am Coll Cardiol. 2009;53:931–935. doi: 10.1016/j.jacc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 310.Ridker PM. The JUPITER trial. Results, controversies, and implications for prevention. Circ Cardiovasc Qual Outcomes. 2009;2:279–285. doi: 10.1161/CIRCOUTCOMES.109.868299. [DOI] [PubMed] [Google Scholar]
- 311.Ridker PM, MacFadyen JG, Fonseca FAH, et al. JUPITER Study Group. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein. Justification for the Use of statins in Prevention: an Intervention Trial Evaluation Rosuvastatin (JUPITER) Circ Cardiovasc Qual Outcomes. 2009;2:616–623. doi: 10.1161/CIRCOUTCOMES.109.848473. [DOI] [PubMed] [Google Scholar]
- 312.Yang EY, Nambi V, Tang Z, et al. Clinical implications of JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) in a US population: insights from the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2009;54:2388–2395. doi: 10.1016/j.jacc.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Vaccarino V, Bremmer JD, Kelley ME. JUPITER. A few words of caution. Circ Cardiovasc Qual Outcomes. 2009;2:286–288. doi: 10.1161/CIRCOUTCOMES.109.850404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kones R. Recent advances in the management of chronic stable angina. II. Anti-ischemic therapy, options for refractory angina, risk factor reduction, and revascularization. Vasc Health Risk Manag. 2010;6:749–774. doi: 10.2147/vhrm.s11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Eyre H, Kahn R, Robertson RM. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Diabetes Care. 2004;27:1812–1824. doi: 10.2337/diacare.27.7.1812. [DOI] [PubMed] [Google Scholar]
- 316.Levi J, Segal LM, Juliano C. Prevention for a healthier America: investments in disease prevention yield significant savings, stronger communities: Trust for America’s Health. [Accessed 2010 Sep 1]. Issue Report 2008 Jul. http://healthyamericans.org/reports/prevention08/
- 317.Heart Protection Study Collaborative Group. Statin cost-effectiveness in the United States for people at different vascular risk levels. Circ Cardiovasc Qual Outcomes. 2009;2:65–72. doi: 10.1161/CIRCOUTCOMES.108.808469. [DOI] [PubMed] [Google Scholar]
- 318.Accad M, Fred HL. Is JUPITER also a God of primary prevention. Tex Heart Inst J. 2010;37:6–7. [PMC free article] [PubMed] [Google Scholar]
- 319.Kappagoda CT, Amsterdam EA. Another look at the results of the JUPITER trial. Am J Cardiol. 2009;104:1603–1605. doi: 10.1016/j.amjcard.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 320.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 321.Despres JP. Bringing JUPITER down to Earth. Lancet. 2009;373:1147–1148. doi: 10.1016/S0140-6736(09)60448-7. [DOI] [PubMed] [Google Scholar]
- 322.Kostapanos MS, Milionis HJ, Elisaf MS. An overview of the extra-lipid effects of rosuvastatin. J Cardiovasc Pharmacol Ther. 2008;13:157–174. doi: 10.1177/1074248408318628. [DOI] [PubMed] [Google Scholar]
- 323.Kendrick M. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? No. BMJ. 2007;334:983. doi: 10.1136/bmj.39202.397488.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Eisenberg T, Wells M. Statins and adverse cardiovascular events in moderate-risk females: a statistical and legal analysis with implications for FDA preemption claims. J Empir Leg Stud. 2008;5:507–550. [Google Scholar]
- 325.Rosenberg H, Allard D. Women and statin use: a women’s health advocacy perspective. Scand Cardiovasc J. 2008;42:268–273. doi: 10.1080/14017430801993180. [DOI] [PubMed] [Google Scholar]
- 326.Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation. 2010;121:1069–1077. doi: 10.1161/CIRCULATIONAHA.109.906479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Duvernoy CS, Blumenthal R. The numbers are in: statins for the primary prevention of cardiovascular disease in women. Circulation. 2010;121:1063–1065. doi: 10.1161/CIR.0b013e3181d731c6. [DOI] [PubMed] [Google Scholar]
- 328.Highlights of prescribing information: Crestor (rosuvastatin calcium) tablets. [Accessed 2010 Apr 16]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021366s016lbl.pdf.
- 329.Kaul S, Morrissey RP, Diamond GA. By Jove! What is a clinician to make of JUPITER. Arch Int Med. 2010;170:1073–1077. doi: 10.1001/archinternmed.2010.189. [DOI] [PubMed] [Google Scholar]
- 330.Jupiter criticism addressed again in more detail. 2010. Oct 1, [Accessed 2010 Oct 15]. http://www.theheart.org/article/1130113.do.
- 331.Bassler D, Briel M, Montori VM, et al. STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303:1180–1187. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- 332.Montori VM, Devereaux PJ, Adhikari NKJ, et al. Randomized trials stopped early for benefit: a systematic review. JAMA. 2005;294:2203–2209. doi: 10.1001/jama.294.17.2203. [DOI] [PubMed] [Google Scholar]
- 333.Pocock SJ. Current controversies in data monitoring for clinical trials. Clin Trials. 2006;3:513–521. doi: 10.1177/1740774506073467. [DOI] [PubMed] [Google Scholar]
- 334.McCatney M. Leaping to conclusions. BMJ. 2008;336:1213–1214. doi: 10.1136/bmj.39589.396840.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Department of Health and Human Services, United States Food and Drug Administration, Center for Drug Evaluation and Research. Transcript for the 2009 Dec 15, Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee. [Accessed 2010 Oct 7]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM200611.pdf.
- 336.De Lorgeril M, Salen P, Abramson J, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med. 2010;170:1032–1036. doi: 10.1001/archinternmed.2010.184. [DOI] [PubMed] [Google Scholar]
- 337.De Lorgeril M. Answers to R. Ridker by M. de Lorgeril. 2010. Jun 30, [Accessed 2010 Aug 15]. http://michel.delorgeril.info/dwnl/JUPITER/2010_Answers-to-Ridker-by-de-Lorgeril.pdf.
- 338.Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ridker PM, Macfadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention – an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol. 2010;55:1266–1273. doi: 10.1016/j.jacc.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 340.Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med. 2010;152:488–496. W174. doi: 10.1059/0003-4819-152-8-201004200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 342.Ridker PM, MacFadyen JG, Børge G, Nordestgaard BG, et al. Rosuvastatin for primary prevention among individuals with elevated high-sensitivity C-reactive protein and 5% to 10% and 10% to 20% 10-year risk: implications of the Justification for Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial for “Intermediate Risk”. Circ Cardiovasc Qual Outcomes. 2010;3:447–452. doi: 10.1161/CIRCOUTCOMES.110.938118. [DOI] [PubMed] [Google Scholar]
- 343.Kones R. Recent advances in the management of chronic stable angina I: approach to the patient, diagnosis, pathophysiology, risk stratification, and gender disparities. Vasc Health Risk Manag. 2010;6:635–656. doi: 10.2147/vhrm.s7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ridker PM, Glynn RJ. The JUPITER Trial: Responding to the critics. Am J Cardiol. 2010;106:1351–1356. doi: 10.1016/j.amjcard.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 345.Harrington R, Ridker PM. The Bob Harrington Show. Episode #26: Dissecting the controversy around JUPITER with Dr Paul Ridker. 2010. Aug 27, http://radio.theheart.org/bob-harrington-show/2010/8/20/episode-26-dissecting-the-controversy-around-jupiter-with-dr-paul-ridker.
- 346. [Accessed 2010 Oct 7]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM193831.pdf. (FDA memorandum, 2009 Nov 12, Appendix, page 11)
- 347.Friedlin B, Korn E. Stopping clinical trials early for benefit: impact on estimation. Clin Trials. 2009;6:119–125. doi: 10.1177/1740774509102310. [DOI] [PubMed] [Google Scholar]
- 348.Korn EL, Friedlin B, Mooney M. Stopping or reporting early for positive results in randomized clinical trials: the National Cancer Institute Cooperative Experience from 1990 to 2005. J Clin Oncol. 2009;27:1712–1721. doi: 10.1200/JCO.2008.19.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients: the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 350.Hsu I, Spinler SA, Johnson NE. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hypercholesterolemia. Ann Pharmacother. 1995;29:743–759. doi: 10.1177/106002809502907-818. [DOI] [PubMed] [Google Scholar]
- 351.Garcia-Rodriguez LA, Masso-Gonzalez EL, Wallander MA, Johansson S. The safety of rosuvastatin in comparison with other statins in over 100,000 statin users in UK primary care. Pharmacoepidemiol Drug Saf. 2008;17:943–952. doi: 10.1002/pds.1603. [DOI] [PubMed] [Google Scholar]
- 352.Garcia-Rodriguez LA, Gonzalez-Perez A, Stang MR, et al. The safety of rosuvastatin in comparison with other statins in over 25,000 statin users in the Saskatchewan Health Databases. Pharmacoepidemiol Drug Saf. 2008;17:953–961. doi: 10.1002/pds.1602. [DOI] [PubMed] [Google Scholar]
- 353.Sukhija R, Prayaga S, Marashdeh M, et al. Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. J Investig Med. 2009;57:495–499. doi: 10.2310/JIM.0b013e318197ec8b. [DOI] [PubMed] [Google Scholar]
- 354.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010;55:1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kostapanos MS, Milionis HJ, Agouridis AD, Rizos CV, Elisaf MS. Rosuvastatin treatment is associated with an increase in insulin resistance in hyperlipidaemic patients with impaired fasting glucose. Int J Clin Pract. 2009;63:1308–1313. doi: 10.1111/j.1742-1241.2009.02101.x. [DOI] [PubMed] [Google Scholar]
- 356.Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,696 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 357.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 358.Baker WL, Talati R, White CM, Coleman CI. Differing effect of statins on insulin sensitivity in non-diabetics: a systematic review and meta-analysis. Diab Res Clin Pract. 2010;87:98–107. doi: 10.1016/j.diabres.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 359.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 360.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 361.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nature Rev Immunol. 2006;6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Chakrabarti R, Engleman EG. Interrelationships between mevalonate metabolism and the mitogenic signaling pathway in T lymphocyte proliferation. J Biol Chem. 1991;266:12216–12222. [PubMed] [Google Scholar]
- 363.Graham I, Atar D, Borch-Johnsen K, et al. Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 364.Liu PY, Chen JH, Lin LJ, Liao JK. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol. 2007;49:1619–1624. doi: 10.1016/j.jacc.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Liao JK. Does it matter whether or not a lipid-lowering agent inhibits Rho kinase. Curr Atheroscler Rep. 2007;9:384–388. doi: 10.1007/s11883-007-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Dong M, Yan BP, Liao JK, Lam YY, Yip GWK, Yu CM. Rho-kinase inhibition: a novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov Today. 2010;15:622–629. doi: 10.1016/j.drudis.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Brozinick JT, Jr, Berkemeier BA, Elmendorf JS. “Acting” on GLUT 4: membrane and cytoskeletal components of insulin action. Curr Diabetes Rev. 2007;3:111–122. doi: 10.2174/157339907780598199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Horvath EM, Tackett L, Elmendorf JS. A novel membrane-based anti-diabetic action of atorvastatin. Biochem Biophys Res Commun. 2008;372:639–643. doi: 10.1016/j.bbrc.2008.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 369.Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycemic control. Diabetologia. 2006;49:1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 370.White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42:963–970. [PubMed] [Google Scholar]
- 371.Mita T, Watada H, Nakayama S, et al. Preferable effect of pravastatin compared to atorvastatin on beta cell function in Japanese early-state type 2 diabetes with hypercholesterolemia. Endocr J. 2007;54:441–447. doi: 10.1507/endocrj.k06-198. [DOI] [PubMed] [Google Scholar]
- 372.Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signaling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet b-cells. Br J Pharmacol. 1999;126:1205–1213. doi: 10.1038/sj.bjp.0702397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Siddals KW, Marshman E, Westwood M, Gibson JM. Abrogation of insulin-like growth factor-I (IGF-I) and insulin action by mevalonic acid depletion: synergy between protein prenylation and receptor glycosylation pathways. J Biol Chem. 2004;279:38353–38359. doi: 10.1074/jbc.M404838200. [DOI] [PubMed] [Google Scholar]
- 374.Otokozawa S, Ai M, van Himbergen T, et al. Effects of intensive atorvastatin and rosuvastatin treatment on apolipoprotein B-48 and remnant lipoprotein cholesterol levels. Atherosclerosis. 2009;205:197–201. doi: 10.1016/j.atherosclerosis.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.American Diabetes Association. Standards of Medical Care in Diabetes – 2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Stroke. 2002;33:2337–2341. doi: 10.1161/01.str.0000034125.94759.41. [DOI] [PubMed] [Google Scholar]
- 377.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 378.Tomlinson SS, Mangione KK. Potential adverse effects of statins on muscle. Phys Ther. 2005;85:459–465. [PubMed] [Google Scholar]
- 379.SEARCH Collaborative Group. Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy – a genomewide study. N Eng J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 380.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Hanai J, Cao P, Tanksale P, et al. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Cao P, Hanai J-i, Tanksale P, Imamura S, Sukhatme VP, Lecker SH. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J. 2009;23:2844–2854. doi: 10.1096/fj.08-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Knauer MJ, Urquhart BL, Meyerzu Schwabedissen HE, et al. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106:297–306. doi: 10.1161/CIRCRESAHA.109.203596. [DOI] [PubMed] [Google Scholar]
- 384.Dorajoo R, Pereira BP, Yu Z, et al. Role of multidrug resistance-associated protein-1 transporter in statin-induced myopathy. Life Sci. 2008;82:823–830. doi: 10.1016/j.lfs.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 385.Masters BA, Palmoski MJ, Glint OP, Gregg RE, Wang-Iverson D, Durham SK. In vitro myotoxicity of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, pravastatin, lovastatin, and simvastatin, using neonatal rat skeletal myocytes. Toxicol Appl Pharmacol. 1995;131:163–174. doi: 10.1006/taap.1995.1058. [DOI] [PubMed] [Google Scholar]
- 386.Thalacker-Mercer A, Baker M, Calderon C, Bamman M. Simvastatin Reduces Human Primary Satellite Cell Proliferation in Culture. Presentation, American Physiological Society, The Integrative Biology of Exercise V; 2008 Sep 24–27; Hilton Head, SC. . [Accessed 2010 Nov 20]. http://www.the-aps.org/press/journal/08/32.htmand http://www.the-aps.org/Video/Clips/AnnaThalacker-Mercer.htm. [Google Scholar]
- 387.Marcoff L, Thompson PD. The role of coenzyme Q10 in statinassociated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–2237. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 388.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol Cell Physiol. 2006;291:C1208–1212. doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- 389.Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol. 2008;8:333–338. doi: 10.1016/j.coph.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 390.Needham M, Fabian V, Knezevic W, et al. Progressive myopathy with upregulation of MHC-I associated with statin therapy. Neuromuscul Disord. 2007;17:194–200. doi: 10.1016/j.nmd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 391.Grable-Esposito P, Katzberg HD, Greenberg SA, et al. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41:185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 392.Vaklavas C, Chatzizisis YS, Ziakas A, Zamboulis C, Giannoglou GD. Molecular basis of statin-associated myopathy. Atherosclerosis. 2009;202:18–28. doi: 10.1016/j.atherosclerosis.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 393.Mas E, Mori TA. Coenzyme Q10 and statin myalgia: what is the evidence. Curr Atheroscler Rep. 2010;12:407–413. doi: 10.1007/s11883-010-0134-3. [DOI] [PubMed] [Google Scholar]
- 394.Vandenberg BF, Robinson J. Management of the patient with statin intolerance. Curr Atheroscler Rep. 2010;12:48–57. doi: 10.1007/s11883-009-0077-8. [DOI] [PubMed] [Google Scholar]
- 395.Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheumatol. 2010;22:644–650. doi: 10.1097/BOR.0b013e32833f0fc7. [DOI] [PubMed] [Google Scholar]
- 396.Thompson PD, Clarkson PM, Rosenson RS. An assessment of statin safety by muscle experts. Am J Cardiol. 2006;97:69C–76C. doi: 10.1016/j.amjcard.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 397.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 398.Ford ES, Li C, Zhao G, Tsai J. Trends in obesity and abdominal obesity among adults in the United States from 1999–2008. Int J Obes (Lond) 2010 Sep 7; doi: 10.1038/ijo.2010.186. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 399.Filion KB, Genest J, Joseph L, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 400.DeMazumder D, Hasan RK, Blumenthal RS, Michos ED, Jones S. Should statin therapy be allocated on the basis of global risk or on the basis of randomized trial evidence. Am J Cardiol. 2010;106:905–909. doi: 10.1016/j.amjcard.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Nambi V, Ballantyne CM. “Risky Business”: ten years is not a lifetime. Circulation. 2009;119:362–364. doi: 10.1161/CIRCULATIONAHA.108.830281. [DOI] [PubMed] [Google Scholar]
- 402.Lloyd-Jones DM, Hong Y, Labarthe D, et al. American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 403.Webster R, Heeley E. Perceptions of risk: understanding cardiovascular disease. Risk Management and Healthcare Policy. 2010;3:49–60. doi: 10.2147/RMHP.S8288. [DOI] [PMC free article] [PubMed] [Google Scholar]