Abstract
This essay considers the ontogeny and phylogeny of the cranial neural circuitry producing rhythmic behaviors in vertebrates. These behaviors are characterized by predictable temporal patterns established by a neuronal network variously referred to as either a pacemaker, neural oscillator or central pattern generator. Comparative vertebrate studies have demonstrated that the embryonic hindbrain is divided into segmented compartments called rhombomeres, each of which gives rise to a distinct complement of cranial motoneurons and, as yet, unidentified populations of interneurons. We now propose that novel rhythmic circuits were innovations associated with the adoption of cardiac and respiratory pumps during the protochordate-vertebrate transition. We further suggest that the pattern-generating circuits of more recent innovations, such as the vocal, electromotor and extraocular systems, have originated from the same Hox gene-specified compartments of the embryonic hindbrain (rhombomeres 7–8) that gave rise to rhythmically active cardiac and respiratory circuits. Lastly, we propose that the capability for pattern generation by neurons originating from rhombomeres 7 and 8 is due to their electroresponsive properties producing pacemaker oscillations, as best typified by the inferior olive which also has origins from these same hindbrain compartments and has been suggested to establish rhythmic oscillations coupled to sensorimotor function throughout the neuraxis of vertebrates.
Keywords: Rhombomeres, Hindbrain, Hox genes, Pacemaker, Oscillator, Vocalization, Electromotor, Oculomotor, Inferior olive, Cerebellum, Teleost
Introduction
Studies in evolutionary neurobiology can adopt one of two historical vantage points: namely ontogeny, which is the sum total of developmental events defining the life history of a single individual or phylogeny, which ‘is nothing more than the history of changes in an ancestral ontogeny’ [from Northcutt, 1990, citing Garstang, 1922]. This essay addresses the ontogeny and phylogeny of the cranial neural circuitry that generates rhythmic behaviors in vertebrates. These behaviors are repetitive, stereotyped, and have a predictable temporal pattern determined by a network of neurons referred to as either a pacemaker, neural oscillator or central pattern generator [reviews: Delcomyn, 1980; Grillner and Wallén, 1985; Pearson, 1993].
Central to the hypotheses presented here is that rhythmic behavior depends on the intrinsic oscillatory properties of single neurons and local neuronal ensembles: ‘individual neuronal oscillators are inserted into complex synaptic networks that tend to synchronize their activity into coherent population events’ that produce ‘a timing signal that can be used to synchronize the activity of multiple parallel neuronal systems into a coordinated process’ [emphasis added; Llinás and Paré, 1994]. We hypothesize that novel rhythmic circuits, established by the intrinsic oscillatory properties of neurons, were innovations associated with the adoption of cardiac and respiratory pumps during the protochordate-vertebrate transition [see Gans and Northcutt, 1983; Northcutt and Gans, 1983]. We further suggest that the pattern-generating circuits of more recent innovations, such as the vocal, electromotor and extraocular systems, have originated from the same genetically specified compartments of the embryonic hindbrain (rhombomeres 7–8) that gave rise to rhythmically active cardiac and respiratory circuits. Lastly, we will propose that these same hindbrain rhombomeres largely form rhythmic related cerebellar afferents as best typified by inferior olive climbing fibers that directly target cerebellar Purkinje cells.
Segmental Organization of the Embryonic Hindbrain: Rhombomeres
It has been known since the turn of the century that the embryonic hindbrain is divisible into either segments or compartments known as rhombomeres [reviews: Gilland and Baker, 1993; Guthrie, 1995]. Eight rhombomeres are generally recognized, although the caudal boundary of rhombomeres 1, 7 and 8 are often not distinct (fig. 1A). The use of fluorescent carbocyanine dyes as neuroanatomical tracers to identify the positions of embryonic motoneurons forming cranial nerves permitted characterization of the rhombomere-specific origins of motor nuclei [Lumsden and Keynes, 1989; Gilland and Baker, 1993]. An axial-specific expression pattern of homebox genes (see below), together with various experimental manipulations such as gene knockouts and rhombomere transplantations, demonstrates a genetic basis for hindbrain compartmentalization [reviews: Graham, 1992; Holland, 1992; Guthrie, 1995].
Fig. 1.

Hindbrain rhombomeres, sonic motor nuclei and conserved hindbrain segments. A–C Stage 23 quail embryo hindbrains viewed ventrally in either combined bright field/epifluorescence (A) or epifluorescence alone (B, C). A Rhombomeres 1–8 and spinal cord (sc) are indicated. DiI (orange) and DiA (yellow-green) applied, respectively, to right and left sides of dissected spinal cord segments at C2–3 levels revealed retrogradely labelled neurons with distinct medial (ipsilateral to injection) and lateral (contralateral) positions in the hindbrain. B Higher magnification view of panel A showing marked decrease in density of labelled neurons, especially contralaterally projecting ones (orange), at rhombomeres 6–7 boundary. C Following a unilateral C2–3 application of DiQ (red), the rhombomeres 6–7 border was sharply delineated, and small clusters of medial neurons were observed ipsilateral to the injection in rhombomeres 2–6 [methods after Gilland and Baker, 1993]. D General relationship between midbrain mesomere (m), hindbrain rhombomeres (r), spinal cord (sc), cranial nerve ‘motor nuclei’ and pattern of Hox gene (B1–B5) expression. E–G Following biocytin application to a single sonic motor nerve, transneuronal transport labels sonic motor (SMN) and pacemaker (PN) neurons bilaterally in a toadfish (E). Dextran-biotin labelling of a single nerve labels only the ipsilateral SMN (F) whereas biocytin labels the SMN and putative PNs bilaterally (G) in embryonic midshipman [1.2 and 1.8 cm; see Lindholm and Bass, 1993, and Marchaterre et al., 1993, for embryo staging; Bass et al., 1994, for adult phenotype and methods].
The pattern of motoneuron origins from the neuroectoderm is highly conserved across the vertebrates (fig. 1D) [Gilland and Baker, 1993]: The oculomotor (III) and trochlear (IV) motor nuclei arise, respectively, from single midbrain mesomere (m) and hindbrain rhombomere (r0) segments. The abducens motor nucleus (VI) is positioned in two rhombomeres (r5, r6) in teleosts, reptiles and birds but only rhombomere 6 in elasmobranchs and rhombomere 5 in mammals. The entire branchiomotor series of motoneurons (trigeminal, V; facial, VII; glossopharyngeal, IX; vagus, X) has bimeric origins in all groups (i.e. V – r2, r3; VII – r4, r5; IX – r6, r7; X – r7, r8). The occipital/hypoglossal (OC/XII) motor nuclei arise across the hindbrain rhombomere-spinal myelomere junction.
Genetic Specification of Rhombomeric Units: Patterns of Hox Gene Expression
Segmentation of the vertebrate neural tube is correlated with an expression pattern of homebox genes [Graham, 1992; Holland, 1992; Krumlauf, 1992] (fig. 1D). The homeobox, first discovered in Drosophila, is a 180 bp sequence coding for a 60 amino acid DNA-binding, transcription factor called the homeodomain. There are four paralagous clusters of homeobox genes in vertebrates (Hox genes) that exhibit a high degree of similarity with the homeodomain complex of Drosophila. The linear arrangement of homeobox genes along a chromosome encodes the anterior-posterior axis of body segments in Drosophila. Each Hox gene cluster in vertebrates is located on a separate chromosome and also has an expression pattern in register with the anterior-posterior body axis. Each paralagous gene family differs in its anterior expression boundary which delimits pairs of rhombomeres [Graham, 1992] (fig. 1D). For example, Hox B4, originally designated Hox 2.6 [Scott, 1992], has an anterior expression limit at rhombomere 7 (thus pairing rhombomeres 7 and 8), Hox B3/2.7 at rhombomere 5 (pairing r5 and r6) and Hox B2/2.8 at rhombomere 3 (pairing r3 and r4). Hox B1/2.9 is specific to rhombomere 4. Hence, each rhombomere pair, such as r7–r8, could be specified by a combinatorial Hox gene code [Graham, 1992; Krumlauf, 1992].
Hox-encoded rhombomere pairs generally do not correspond to the bimeric origins of motor nuclei (fig. 1D), although they may, or may not, coincide with populations of interneurons forming specific premotor circuits. Experiments labelling descending and ascending axons in embryonic chick hindbrains suggest that, with one exception, rhombomeres have comparable sets of local and projection neurons [Clarke and Lumsden, 1993]. The exception is rhombomeres 7 and 8 which have a distinct neuronal organization. The distribution of retrogradely labelled neurons in the hindbrain following spinal injections of fluorescent dyes in quail embryos also shows a distinct pairing of rhombomeres 7 and 8 at the caudal boundary of rhombomere 6 (fig. 1A–C) [see Glover and Petursdottir, 1991, for chicken]. These results are predictive, as we propose, of a specialized functional phenotype for rhombomeres 7 and 8, namely their involvement in the formation of rhythmic premotor circuitry.
Rhombomeric Origins of Premotor Vocal Circuits
The discovery of rhombomere-specific motor nuclei raises the following issue. How well does the conserved organizational pattern for motoneurons predict conserved patterns of premotor circuitry? We initially focus on vertebrate vocalization which illustrates a diversity of stereotyped, rhythmic behaviors (fig. 2) and underlying circuitry but whose motoneurons share common hindbrain origins. Subsequently, we provide examples encompassing a broader range of pattern-generating circuits in which motoneurons arise at different levels of the neuraxis. The first two hypotheses, regarding vocalization, are:
Fig. 2.
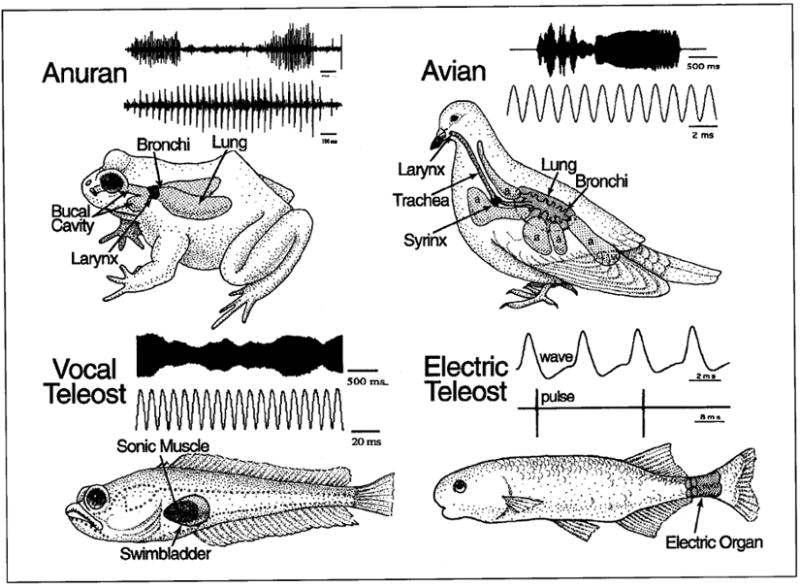
Rhythmically active vocal and electric communication systems of vertebrates. Representative oscillograph records of vocalizations of anuran (advertisement call of gopher frog, Rana capito aesopus), avian (song of loon) and teleost fish (advertisement call, ‘hum’, of plainfin midshipman, Porichthys notatus) species are illustrated; lower trace is on an expanded time scale. Also shown are electric organ discharges from ‘wave’ (Gymnarchus niloticus) and ‘pulse’ (Brienomyrus brachyistius) type, weakly electric teleosts. Line drawings show the position of a sound-generating larynx (anuran), syrinx (avian) and swimbladder (teleost fish), and electric organ [teleost, see text for details; modified from Bass, 1989]. Frog and avian recordings courtesy of D. Bodnar and the Cornell Laboratory of Ornithology, respectively.
Hypothesis 1. The brainstem circuitry responsible for rhythmic vocalization exhibits a conserved pattern of organization evidenced by motoneurons forming vagal, hypoglossal and occipital nerves originating from rhombomeres 7 and 8 that innervate muscles derived from occipital somites.
Hypothesis 2. Rhythmic vocal behavior is largely generated by premotor pacemaker neurons also originating from rhombomeres 7 and 8.
One caveat to our interpretation is that the embryonic origins of premotoneurons associated with the circuits discussed here are generally based on location in an adult phenotype. This assumption is supported by studies in teleost fishes showing that adult brainstem premotoneurons are located close to their positions of origin [Kimmel et al., 1985; Metcalfe et al., 1986; Lee et al., 1993; fig. 1E–G]. Hence, major supporting evidence for our hypotheses has been selected from examples of rhythmic circuitry in teleosts. This further provides a context for ontogenetic studies that directly assess hypotheses 1 and 2 (and 3, below) in all vertebrate groups.
Vocal Muscles
Vocal communication systems share a number of functional and embryological traits, suggesting that common mechanical or developmental factors influence their evolution [Bass, 1989]. Innovations essential to the evolution of vocalization were the larynx of tetrapods, the syrinx of birds, and the sonic swimbladder of teleost fishes (fig. 2). The associated vocal muscles, which are essentially dedicated to sound production, have a common embryonic origin. Among vertebrates, the paraxial head mesoderm exhibits a conserved pattern of seven, partially segmented somitomeres followed by a variable number of completely segmented, occipital somites [reviews: Northcutt, 1990; Noden, 1993]. Occipital somites give rise to laryngeal, glossal and occipitocervical muscles [Noden, 1983]. Syringeal muscles are also considered to be derived from occipital somites, given their shared innervation with glossal/tongue muscles by the hypoglossal nerve [e.g., Nottebohm et al., 1976]. In teleosts, an anterior occipital somite gives rise to the sonic muscle anlage which migrates posteriorly to attach to the lateral walls of the developing swimbladder where myocoytes differentiate into skeletal muscle [Tracy, 1959; Lindholm and Bass, 1993]. Thus, vocal muscles in both fishes and tetrapods originate from occipital somites (table 1).
Table 1.
Cross-systems comparisons: embryological origins of muscles and circuitry (see text for references)
| Muscle | Myocytes | Motor neurons | Premotor neurons1 |
|---|---|---|---|
| Laryngeal2 | oc. somites | r7–8 (nX) | r7–8 |
| Syringeal2 | oc. somites | r8 (nXII) | r7–8 |
| Sonic2 | oc. somites | r8 (oc. nvs.) | r7–8 |
| Abdominal3 | trunk somites | my (spinal nerves) | r7–8 |
| Peri-oral, mouth3 | somitomeres | r2–4 (nV, nVII) | r7–8 |
| Pharyngeal3 | somitomeres oc. somites | r6,7 (nIX, X) | r7–8 |
| Electric organ4 | trunk somites | my (spinal nerves) | r7–8 |
| Extra-ocular | somitomeres | me/r1 (nIII, nIV) r5/r6 (nVI) | r7–8 |
| Neck5 | oc./trunk somites | r8/my (oc./spinal nerves) | r7–8 |
Abbreviations: me=Mesomere; my=myetomere; n=cranial nerve; oc.=occipital; r=rhombomere.
Pacemaker/timing circuit.
Dedicated vocal muscle.
Vocal-associated muscle.
Modified striated muscle.
Gaze-associated postural muscles.
Vocal Motoneurons
Laryngeal, syringeal and sonic swimbladder muscles are innervated, respectively, by neurons in vagal, hypoglossal and sonic/occipital motor nuclei originating in rhombomeres 7 and 8 (table 1) [Bass, 1989]. Laryngeal muscle is innervated by branches of the vagus nerve arising from motoneurons in a subdivision of nucleus ambiguus (X, fig. 1D). Syringeal motoneurons are in a subdivision of the hypoglossal motor nucleus, separate from motoneurons innervating tongue muscles (XII, fig. 1D). The sonic motor nucleus and occipital nerves that innervate the swimbladder ‘drumming’ mucles of teleosts (OC, fig. 1D), are considered homologues of, respectively, the hypoglossal motor nucleus and nerve [Bass, 1989]. The principal evidence supporting this comparison is: (1) swimbladder sonic muscle, like other muscles innervated by the hypoglossal nerve, is derived from occipital somites (above), and (2) the sonic motor nucleus occupies a position in the caudal brainstem (fig. 1E) similar to that of the hypoglossal motor nucleus of tetrapods [e.g., amphibians – Stuesse et al., 1984; birds – Nottebohm et al., 1976; reptiles – Barbas-Henry and Lohman, 1984; mammals – Aldes, 1995].
Vocal Premotoneurons
The central control of tetrapod vocalization involves the precise temporal patterning of multiple neuromuscular systems, primary among which is that underlying ventilation, because the movement of respiratory gases across laryngeal vocal cords and syringeal tympaniform membranes is essential to sound production [Bass, 1989]. Although the avian larynx lacks vocal cords, it likely affects the resonant properties of the vocal tract [e.g., Westneat et al., 1993]. Tetrapod vocalization can also involve pharyngeal, perioral or mouth and beak-opening muscles that could modify the acoustic properties of the vocal tract [e.g., Hauser et al., 1993; Westneat et al., 1993]. These ventilatory and non-ventilatory muscles have diverse embryological origins, including occipital somites, that are innervated by either cranial or spinal nerves (table 1) [Walker, 1986; Noden, 1983].
As discussed below, recent studies of tetrapods suggest that the premotor circuitry providing the timing signal that synchronizes the activity of vocal-related multiple pathways is positioned at brainstem levels comparable to that of the vocal motoneurons in rhombomeres 7 and 8 (table 1). By contrast, teleost vocalization is established by a single, rhythmically-active circuit that, we propose, is comparable to the premotor synchronizing unit of tetrapods. Despite this divergence in complexity, the premotor vocal circuit in the hindbrain of both teleosts and tetrapods appears to be derived from the same caudal rhombomeres. Additional circuitry, positioned at midbrain-isthmal and/or forebrain levels, would then execute higher order command or modulatory functions that adjust the final output of rhythmically active, pacemaker-motoneuron units in the caudal hindbrain (see below).
Anuran Amphibians
In anurans, many of the same oropharyngeal and buccal muscles are used in both vocalization and ventilation [Gans, 1974]. Laryngeal muscles specifically gate airflow across the larynx, which sets the vocal cords into vibration at a basic carrier frequency [Gans, 1974; see Yager, 1992, for alternative mechanism in fully aquatic frogs]. Vagal motoneurons that innervate laryngeal muscles are in a caudal subdivision of a nucleus IX–X complex that corresponds to the nucleus ambiguus of other tetrapods [Stuesse et al., 1984; Simpson et al., 1986]. Schmidt [1992] proposes an isthmal nucleus (pretrigeminal nucleus of the dorsal tegmental area of the medulla, DTAM) and the IX–X region as sites of vocal and ventilatory pattern generators, respectively. The DTAM exhibits rhythmic activity, although given DTAM’s reciprocal connections with IX–X, and inputs to both from the reticular formation lateral to IX–X [Wetzel et al., 1985], the caudal medulla could be the source of DTAM’s rhythmicity. A recent intracellular study identified rhythmic, ventilatory premotoneurons in the area of vagal (caudal IX–X) and hypoglossal (XII) motoneurons [Kogo and Remmers, 1994]. Given that many of these neurons must function in vocalization as well [Gans, 1974], a premotor circuit that synchronizes vocal and ventilatory activities is likely positioned at hindbrain levels comparable to rhombomeres 7 and 8.
Birds
In songbirds, there is a direct relationship between the electromyographic activity of syringeal muscles and the fundamental frequency and duration of a vocalization [e.g., Goller and Suthers, 1995]. Nottebohm et al. [1976] first identified a syringeal control pathway in oscines that included direct projections from a forebrain nucleus robustus archistriatalis (RA) to a tracheosyringeal division of the hypoglossal (XII) motor nucleus that innervates the syrinx. Subsequent studies identified a more extensive vocal motor network in the brainstem and forebrain [reviews: Nottebohm, 1991; Wild, 1994a; also see Streidter, 1994; Wild, 1994b]. Of relevance here are newly identified premotor nuclei in the caudal hindbrain near the hypoglossal nucleus; this includes a nucleus retroambigualis that exhibits rhythmic activity, receives descending inputs from telencephalic and midbrain vocal areas, and projects to syringeal (XII) and ventilatory (spinal) motoneurons [Wild, 1994a]. These findings suggest nucleus retroambigualis as a candidate premotor center synchronizing the rhythmic output of vocal-related neuromuscular pathways. Although lesion and brain stimulation studies demonstrate an important role for the telencephalon in establishing the tempo and order of syllables in learned components of song, caudal brainstem nuclei such as nucleus retroambigualis likely play a principal role in determining the overall temporal structure of vocalizations by providing the final timing signals for the multiple vocal related pathways [see Simpson and Vicario, 1990; Balaban, 1994; Vu et al., 1994].
Quail-chicken chimera experiments support a role for the circuitry descendant from rhombomeres 7 and 8 in avian vocalization [E. Balaban, pers. comm.; but see Balaban, 1994]. Quails, unlike chickens, rhythmically bob their heads up and down during crowing. Quail crows have a characteristic pattern of discrete elements separated by interruptions in airflow that head bob might create by modifying vocal tract mechanics. For chicken hosts with quail transplants of rhombomeres 7–8, the head bobs are given at the onset of crowing. When a transplant includes rostral spinal cord, the head bobs continue throughout the call as in normal quail. This implies that the spinal cord either contains circuitry essential for the entire vocal behavior or assures the integrity of the caudal rhombomeres in the transplant. Importantly, head bobs are less evident in specimens with incomplete transplants of the ventral neural tube in rhombomeres 7–8, an area inclusive of nucleus retroambigualis, the candidate vocal-respiratory pattern generator. These experiments, together with the more recent neuroanatomical ones described above, identify a pivotal role for the caudal hindbrain in the organization of species-typical vocalization.
Mammals
In mammals, as in anurans and birds, the activity of ‘dedicated’ vocal musculature, in this case laryngeal, directly influences the physical attributes of species-specific acoustic signals. This relationship is best typified by studies in horseshoe bats which demonstrate a direct relationship between the discharge frequency of the superior laryngeal (X) nerve that innervates the larynx’ cricothyroid muscles and the constant frequency component of the echolocation call; the activity of the recurrent laryngeal nerve that innervates other intrinsic laryngeal muscles determines call duration. Each nerve originates from a distinct population of motoneurons in nucleus ambiguus, suggesting a functional segregation within the central pathway related to the determination of different call parameters, [Schuller and Rübsamen, 1981; Rübsamen and Schuller, 1981; Schweizer et al., 1981; Rübsamen and Betz, 1986].
As in anurans and birds, various studies also emphasize the importance of ventilatory and other non-laryngeal muscles in mammalian vocalization [e.g., Hauser et al., 1993; Gracco and Löfqvist, 1994; Kirzinger and Jürgens, 1994; Lancaster et al., 1995]. Holstege [1989] has identified nucleus retroambiguus as the premotor nucleus synchronizing the various vocal-related neuromuscular pathways in mammals [also Rübsamen and Schweizer, 1986]. Brain stimulation of the caudal medulla supports a role for this region in the premotor programming of species-typical vocalizations [Jürgens and Pratt, 1979; Yajima et al., 1981]. Nucleus retroambiguus, like its avian homologue nucleus retroambigualis, is located near vagal (X) and hypoglossal (XII) motoneurons at levels comparable to rhombomeres 7 and 8.
Teleost Fishes
Teleosts have a brainstem vocal-acoustic network that includes pacemaker neurons with oscillatory-like activity [Bass and Baker, 1990; Bass et al., 1993,1994] (fig. 1E, 3A). To our knowledge, the studies in vocal fishes represent the only case where intracellular recording and staining has identified individual hindbrain neurons that determine the characteristic properties of a vocalization. A straightforward neural-mechanical translation occurs, whereby the pacemaker discharge frequency determines motoneuron firing rate and, in turn, the contraction rate of swimbladder sonic muscles that establishes the fundamental frequency and duration of vocalizations [Bass and Baker, 1990, 1991; Brantley and Bass, 1994]. Pacemaker neurons are distributed along the ventrolateral margin of the sonic motor nucleus which extends from the caudal hindbrain into the rostral spinal cord (fig. 1E). A newly identified ventromedullary nucleus lies just anterior to pacemaker neurons and gives rise to an extensive commissural bundle linking the pacemaker circuitry bilaterally. Surgical isolation of the ventromedullary-pacemaker-motoneuron network demonstrates that it is both necessary and sufficient for generating a rhythmic vocal output [Weiser et al., 1986; A.H. Bass, unpubl. observ.]. Developmental studies suggest that vocal pacemaker neurons arise at rostral levels of the sonic motor nucleus (fig. 1F, G), consistent with the hypothesis that a vocal pacemaker-motoneuron circuit originates, as does analogous circuitry in tetrapods (see above), from caudal rhombomeres.
Fig. 3.
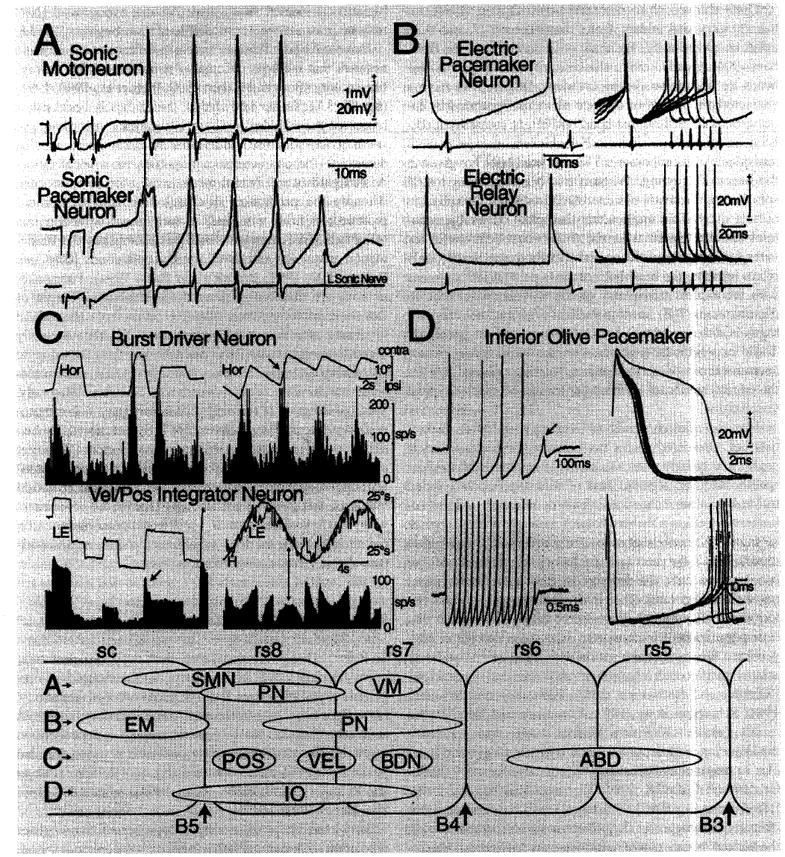
Rhythmically-active vertebrate circuitry. A Vocal circuitry. Intracellular records from identified sonic motor and pacemaker neurons following midbrain stimulation in the plainfin midshipman, Porichthys notatus. Each trace is average of four sweeps; top is intracellular record, and bottom is intracranial record from left sonic occipital nerve. Small arrows at the beginning of the lower traces indicate midbrain stimulus onset. The nerve discharges were highly synchronous and aligned (vertical lines) to illustrate the relative timing between pacemaker and motoneuron firing. Time scale and direction of polarity for all records are indicated. Modified from Bass and Baker [1990]. B Electromotor circuitry. Intracellular recordings from electric pacemaker (top) and relay (bottom) neurons in a pulse gymnotoid, Hypopomus. Pacemaker cell (top) fires after the gradually rising membrane potential reaches a critical threshold. A relay cell (bottom) responds by firing a sudden action potential. The corresponding EOD is shown below each trace. Top and bottom records on the right show, respectively, superimposed traces of pacemaker and relay cells during a smooth rise in discharge frequency. Calibrations are indicated. Modified from Kawasaki and Heiligenberg [1989]. C Eye movement circuitry. Bottom: Velocity to position (Vel/Pos) integrator neurons of goldfish, Carassius auratus. The left histogram indicates firing rate (FR) during spontaneous eye movements correlated with horizontal, left eye position during fixation (LE). Neuron also exhibits sensitivity associated with fast phase of vestibular nystagmus (arrow). The right histogram shows FR of a purely position related neuron during sinusoidal head rotation (Ḣ) in the dark. Head velocity is inverted to facilitate comparison to eye velocity. The FR correlates with eye position but not eye velocity (LĖ) as illustrated by the 90° phase lag of Ḣ and LĖ (arrow) relative to FR. Calibrations are indicated. Modified from Pastor et al. [1994]. Top: Burst-driver neurons (BDNs) in cat (positioned rostral to eye velocity area II in goldfish, see bottom panel). Left histogram indicates FR during spontaneous saccadic eye movements correlated with horizontal eye position (HOR). The right histogram shows a burst of activity in association with fast phase of vestibular nystagmus (arrow). Calibrations are indicated. Modified from Kitama et al. [1992]. Abbreviations: SMN = Sonic motoneurons; PN = vocal pacemaker neurons; VM = ventral medullary nucleus [also see Bass et al., 1994]; EM = electromotoneurons; PN = electric pacemaker neurons; POS = eye position integrator neurons; VEL = eye velocity integrator neurons; BDN = burst driver neurons [also see Pastor et al., 1994]; IO = inferior olive. Anterior expression limits for Hox genes are also indicated B3–B5. D Inferior olive of guinea pig. Spontaneous bursts of spikes were recorded intracellularly from an inferior olive neuron and displayed at different sweep speeds. Top left: The neuron fired four action potentials, with the fifth (arrow) corresponding to a subthreshold somatic Ca2+ spike. Bottom left: A longer burst of spikes is shown at slower sweep speed. Top right: The action potentials shown in bottom left are superimposed at a faster sweep speed. The first action potential, which arises from the resting membrane potential level, had a slightly higher amplitude and a rather prolonged after-depolarization that was followed by a prolonged after-hyperpolarization (also see other traces). The other spikes in the train became progressively shorter until failure of spike generation occurred, terminating the burst. Bottom right: The same set of records as in bottom left showing a somatic Ca2+ spike arising from the after-hyperpolarization and the range of spike intervals in the train. Modified from Llinás and Yarom [1986]. Bottom panel: Proposed embryonic distribution of nuclei forming rhythmically-active circuits superimposed on schematic representations of rhombomere (rs) – spinal (sc) template (see fig. 1D).
Non-Vocal Rhythmic Circuitry
It is perhaps not surprising that rhythmic premotor, vocal circuitry would be located in hindbrain regions comparable to rhombomeres 7 and 8, given two considerations: (1) vocal motoneurons originate from these same hindbrain segments, and (2) vocalization in tetrapods is dependent on respiratory mechanisms whose premotor neurons are also positioned at this hindbrain level [e.g., amphibians – Kogo and Remmers, 1994; birds – Wild, 1994a; mammals – Dobbins and Feldman, 1994].
However, we propose a third hypothesis extending our prediction to non-vocal behaviors:
Hypothesis 3. The brainstem premotor circuitry responsible for other rhythmic behaviors, e.g. electromotor and oculomotor, is also largely derived from rhombomeres 7 and 8. In contrast to vocal pacemaker circuitry, these behaviors are produced by motoneurons not originating in rhombomeres 7–8 and by muscles not derived from occipital somites.
This issue was introduced earlier in the context of spinal/ventilatory motor nuclei of birds and mammals that receive premotor input from caudal brainstem nuclei – retroambigualis and retroambiguus. However, to more completely assess the third hypothesis, two other rhythmic, non-vocal behaviors are discussed: the electric organ discharge (EOD) of teleost fishes and eye movements that are common to all vertebrates.
Electromotor Circuits
Mormyriforms and gymnotiforms are two groups of teleosts that have independently evolved a mechanism for the production of EODs important in social communication and electrolocation [reviews: Bennett, 1971; Bass, 1986; Heiligenberg, 1991]. Electric fish are designated as ‘pulse’ or ‘wave’ species, depending on the appearance of their EOD. In pulse fishes, the interval between each EOD wave-form pulse (equivalent to a single muscle contraction) is longer than the duration of the pulse itself; in wave fishes, the electric pulse has a sinusoidal-like appearance because EOD pulses occur at intervals less than or equal to the duration of the pulse (fig. 2D). Electric organs in mormyriforms and gymnotiforms are modified striated muscle derived from trunk somites that are innervated by spinal motoneurons. A pacemaker nucleus is located in the medulla in both pulse and wave species at levels comparable to rhombomeres 7 and 8 [Bennett, 1971; Bell and Szabo, 1986; Dye and Meyer, 1986; Kawasaki, 1994]. Pacemaker neurons determine the fundamental discharge frequency of the electric organ, just as pacemaker neurons in vocal fishes establish the discharge frequency of sonic muscles. The pacemaker in gymnotoids includes relay and pacemaker neurons, each of which fire rhythmically and can be independently modulated by afferent inputs (fig. 3B) [Dye et al., 1989; Kawasaki and Heiligenberg, 1990]. In pulse mormyriforms (mormyrids), the pacemaker includes command and relay nuclei, both of which generate oscillatory-like activity [Bennett, 1971; Grant et al., 1986]. In wave mormyriforms (gymnarchids), pacemaker neurons are linked to relay neurons by a third cell group – the lateral relay nucleus, all of which generate oscillatory-like potentials [Kawasaki, 1994].
Thus, two groups of electric fishes have independently evolved a pacemaker circuit in a hindbrain location comparable to that of rhombomeres 7–8 and the vocal pacemaker of other theleosts and probably other vertebrates (table 1). The vocalizations and EODs of teleosts are examples of simple, rhythmic behaviors determined by either a central timing unit or pacemaker that establishes the oscillatory-like activity of one neuromuscular system, be it vocal muscles or electric organs. Each pacemaker produces an entire behavior, for example the quasi-sinusoidal-like advertisement call (hum) of the plainfin midshipman or the EOD of wave gymnotoids (fig. 2). These premotor circuits appear to provide simultaneously all the timing information for the spatiotemporal pattern of motoneuron activity. This arrangement may be comparable to the entire premotor unit of other motor systems that synchronizes the coordinated action of multiple pacemaker-motoneuron circuits operating in parallel, as now discussed for eye movement.
Eye Movement Circuits
The number of extraocular muscles and motor nuclei is highly conserved among all vertebrates [Baker, 1992]. The six extraocular muscles are derived from somitomeres, whereas their motoneurons originate from the midbrain and hindbrain (rhombomeres 1, 5–6; fig. 1D) [Noden, 1983; Lumsden and Keynes, 1989; Gilland and Baker, 1993; Wahl et al., 1994].
The extraocular motor system shares several functional traits with vocal and electromotor systems, including rapid speed of contraction (or EOD discharge rate in electric fishes) and a direct relationship between the firing rate of premotoneurons and the behavior itself, in this case eye movements. Eye movements can be divided into two essential types, fast (saccadic) and slow (visuovestibular), based both on speed and premotor neuron circuits. In particular, four premotor, eye and head movement/gaze-related areas (I–IV), which are serially aligned in a hindbrain region equivalent to rhombomeres 7 and 8, have been described in goldfish [Pastor et al., 1994; Gilland and Baker, 1994]. Areas I and II are comparable to mammalian perihypoglossal nuclei [Baker and Berthoz, 1975] based on distinct connectivity with the vestibular nuclei and cerebellum and physiological traits necessary for establishing eye position (fig. 3C bottom) and eye velocity (vestibulo-ocular and visuomotor). Interspersed between areas I–IV are subsets of burst-related neurons generating saccadic and fast phase eye velocity (fig. 3C bottom, arrow). A particularly notable example is that of ‘burst driver neurons’, BDNs, analagous to those described in mammals: BDNs are afferent to ‘burst neurons’ that establish the spatiotemporal activity of extraocular motoneurons during saccades and fast phases of optokinetic and vestibular nystagmus (fig. 3C, top) [Sasaki and Shimazu, 1981; Kitama et al., 1992]. The BDNs in all species appear to act as unique integrator units in which multiple inputs (e.g. visual, vestibular, somatosensory) can, either individually or in concert, provide the appropriate timing signal to burst neurons that control individual groups of motoneurons, in this case generating horizontal saccadic/fast phase eye velocity. As a result, the amplitude, velocity and duration of saccades are always calibrated with respect to both current and desired eye-movement. The BDNs apparently operate in parallel with subsystems of premotor circuitry generating both slow and fast eye movements so that each type of eye movement is independent of any change in the operation of an individual subsystem. Moreover, these circuits or signals also could be utilized to achieve eye/head coordination, that is gaze control. Analagous circuitry, which may also arise from rhombomeres 7 and 8, is described for other behaviors with rhythmic components that necessitate the synchronization of parallel neuromuscular systems [e.g., mastication – Nozaki et al., 1986; Donga and Lund, 1991; respiration – Feldman et al., 1988; acoustic startle response – Lingenhohl and Friauf, 1994] and must also be operative in tetrapod vocalization.
The Inferior Olive: Highest Order Pacemaker Circuit
So far, evidence has been presented to support three hypotheses proposing that rhombomeres 7 and 8 are sites of origin for circuitry providing a timing signal for a variety of rhythmic vertebrate behaviors. This includes species-typical electromotor, vocalization and eye movement behaviors, the latter two of which share many features across the vertebrates. Another rhythmically active network common to all vertebrates and intimately involved in the regulation of premotoneuron activity is the olivo-cerebellar system. Climbing fiber axons from the inferior olive project onto cerebellar Purkinje cells [Llinás, 1969]. The inferior olive of mammals generates a rhythmic oscillation of about 10 Hz that is dependent on two traits: the pacemaker properties of individual neurons and electrotonic coupling within the entire population [Llinás et al., 1974; Llinás and Yarom, 1981, 1986] (fig. 3D). Inferior olive oscillation is proposed to serve as a ‘timing signal used in motor control’ that spreads, via cerebellar efferents, to all other levels of the neuraxis [Llinás and Paré, 1994].
Mossy fiber inputs to the cerebellum arise mainly from two other hindbrain regions: the vestibular nuclei and the lateral and paramedian nuclei of the caudal hindbrain. These areas are well delineated in teleost fishes [e.g., Wullimann and Northcutt, 1988; Pastor et al., 1994]. Together, the data suggest that nearly all rhythmic-related cerebellar afferents, i.e. climbing and mossy fibers from the caudal hindbrain, arise from descendent circuitry of rhombomeres 7–8.
The results of quail-chick chimera experiments are consistent with the third hypothesis and demonstrate a contribution of rhombomeres 7 and 8 to the inferior olive [L. Puelles, pers. comm.]. Rhombomeres 2–6 do not contribute to the inferior olive [Marin and Puelles, 1995]. Transplants of rhombomeres 3 through rostral 8 show a contribution to the rostral portion of the olive [Tan and Le Douarin, 1991]. Therefore, the inferior olive must largely originate from rhombomeres 7 and 8, especially from the caudal portion of rhombomere 8 and not excluding rostral myelomeres.
Evolutionary Origins of Rhythmic Neural Circuits in Vertebrates
The protochordate-vertebrate transition is proposed to have included the adoption of two mechanisms permitting increased oxygen consumption: (1) a gill perfusion pump for directing water flow across a respiratory surface and (2) a central circulatory pump (heart) for delivery of oxygen and nutrients to organ systems [Gans and Northcutt, 1983; Northcutt and Gans, 1983]. We now extend this hypothesis by proposing that novel respiratory and cardiovascular mechanisms were dependent on the innovation of central pacemaker neurons and circuits to ensure their rhythmic operation. We also suggest that such novel circuits originated from regions homologous to rhombomeres 7 and 8, the same sites candidate for the position of premotor respiratory and cardiac pacemakers in adult, extant vertebrates [fishes – Kawasaki, 1984; Barrett and Taylor, 1985a, b; amphibians – Kogo and Remmers, 1994; birds – Wild, 1994a; mammals – Smith et al., 1991; Hopkins and Ellenberger, 1994; Standish et al., 1995]. We thus hypothesize that:
The pattern-generating circuits primarily responsible for the rhythmic operation of novel respiratory and cardiovascular pumps associated with the protochordate-vertebrate transition originate from rhombomeres 7 and 8 and are as innovative as the pumps themselves!
More recent neural innovations, e.g. vocal, electromotor, extraocular and olivo-cerebellar systems, have utilized the same rhombomeric compartments in 7 and 8 to build their pattern-generating circuitry.
Each rhombomere therefore has its own genetically specified ontogenetic and phylogenetic history. Rhombomeres 7 and 8 are the hindbrain compartments specified for the premotor circuitry of many rhythmic behaviors that distinguish vertebrates, especially gnathostomes, from their protochordate ancestors.
Hence, rhombomeres 7–8 are also largely the origin of rhythmic-related, cerebellar afferents (both climbing and mossy fibers), further suggesting that the cerebellum has also been specified for the regulation of rhythmic behavior.
The capability for pattern generation by neurons originating from rhombomeres 7 and 8 is due to their electroresponsive properties producing pacemaker-like oscillations as best typified by the intrinsic rhythmicity of inferior olive neurons that directly target cerebellar Purkinje cells.
From a phylogenetic perspective, the caudal rhombomeres exhibit a remarkable degree of plasticity in phenotypic specification. Consider, alone, the diversity of vocal control pathways described here. The potential for such diversity may derive from an intrinsically segmented hindbrain coupled to ‘axial-level specific neural architecture’ [Guthrie, 1995]. Examples of segment-specific design would then be rhythmic vocal, electromotor, eye movement and olivocerebellar circuits arising from rhombomeres 7 and 8.
Segmentation appears to be an intrinsic property of the hindbrain but not the spinal cord [see Guthrie, 1995]. Despite this distinction, studies in chickens and zebrafish imply that rhombomeres 7 and 8 represent modified spinal cord by suggesting they have ‘a transitional organisation’ between that of the hindbrain and that of the spinal cord [Hanneman et al., 1988; Trevarrow et al., 1990; Clarke and Lumsden, 1993]. In part, there is physiological support for this interpretation, given the local spinal circuitry underlying rhythmic locomotion [Grillner and Matsushima, 1991] and our proposal that rhombomeres 7 and 8 are the origins of such cranial pacemaker circuitry. However, we suggest that the apparent convergence in hindbrain and spinal pacemaker-like circuits depends on the shared electroresponsive properties of neurons [Llinás, 1988]. Rhombomeres 7 and 8 are responsible for rhythmic circuits driving highly derived behaviors. The unique and intimate relationship with the cerebellum suggests a specific purpose in motor performance, adaptation and cranial rhythmicity. Hence, the organization of rhombomeres 7 and 8, as is that of the more anterior rhombomeres and more caudal spinal cord, is best characterized as unique and not simply a transition between these two regions of the neural tube.
Recent evidence suggests that Hox gene specification of positional/segmental information within the neural tube may have been an innovation associated with chordate, rather than just vertebrate, origins [Holland et al., 1994]. Hence, cephalochordates (Amphioxus) have a homeobox gene, AmphiHox3, that is homologous to HoxB3 of vertebrates and has an anterior expression border in the dorsal nerve cord that has been compared to the rhombomere 4/5 boundary (see fig. 1D) [Holland et al., 1992; Gilland and Baker, 1993]. Indeed, we expect that the nerve cord of cephalochordates also forms rhythmic pacemaker circuitry, given the widespread occurrence of neuronal electroresponsive properties among vertebrates and invertebrates [Llinás, 1988; Getting, 1989]. However, we propose that an innovation associated with the origin of vertebrates is phenotypic specification by a combinatorial Hox gene code of hindbrain compartments/rhombomeres to form descendent pacemaker circuitry controlling a variety of rhythmic behaviors.
Acknowledgments
This paper is dedicated to Walter Heiligenberg whose fearless approach to science and love for fish and discovery was and remains an inspiration to us all. The authors thank the Scripps Institution of Oceanography and Professors Ted Bullock and Glenn Northcutt for organizing this symposium in honor of Walter in January 1995 (references are current through July 1995). Thanks also to H. Baker, E. Balaban, J. Fetcho and E. Gilland for comments on the manuscript and E. Balaban and L. Puelles for sharing their unpublished findings. The authors’ work is supported by grants from NSF and NIH.
References
- Aldes LD. Subcompartmental organization of the ventral (protrusor) compartment in the hypoglossal nucleus of the rat. J Comp Neurol. 1995;353:89–108. doi: 10.1002/cne.903530109. [DOI] [PubMed] [Google Scholar]
- Baker R. A contemporary view of the phylogenetic history of eye muscles and motoneurons. In: Shimazu H, Shinoda Y, editors. Vestibular and Brain Stem Control of Eye, Head and Body Movements. Japan Scientific Societies Press; Tokyo/S. Karger, Basel: 1992. pp. 3–19. [Google Scholar]
- Baker R, Berthoz A. Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Res. 1975;86:191–127. doi: 10.1016/0006-8993(75)90643-5. [DOI] [PubMed] [Google Scholar]
- Balaban E. Sex differences in sounds and their causes. In: Shorrt RV, Balaban E, editors. The Differences Between the Sexes. Cambridge University Press; Cambridge: 1994. pp. 243–273. [Google Scholar]
- Barbas-Henry HA, Lohman AHM. The motor nuclei and primary projections of the IXth, Xth, XIth, and XIIth cranial nerves in the monitor lizard, Varanus exanthematicus. J Comp Neurol. 1984;226:565–579. doi: 10.1002/cne.902260409. [DOI] [PubMed] [Google Scholar]
- Barrett DJ, Taylor EW. Spontaneous efferent activity in branches of the vagus nerve controlling heart rate and ventilation in the dogfish. J Exp Biol. 1985;117:433–448. doi: 10.1242/jeb.117.1.433. [DOI] [PubMed] [Google Scholar]
- Bass A. Evolution of a vertebrate communication and orientation organ. In: Bullock TH, editor. Electric Organs Revisited. John Wiley & Sons, Inc.; New York: 1986. pp. 13–70. [Google Scholar]
- Bass AH. Evolution of vertebrate motor systems for acoustic and electric communication: peripheral and central elements. Brain Behav Evol. 1989;33:237–247. doi: 10.1159/000115931. [DOI] [PubMed] [Google Scholar]
- Bass AH, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified neurons. J Neurobiol. 1990;21:1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- Bass A, Baker R. Evolution of homologous vocal control traits. Brain Behav Evol. 1991;38:240–254. doi: 10.1159/000114391. [DOI] [PubMed] [Google Scholar]
- Bass AH, Marchaterre MA, Baker R. Transneuronal biocytin transport del-kineates species differences in a brainstem vocal-acoustic circuit in sound producing fish. Soc Neurosci Abstr. 1993;19:1202. [Google Scholar]
- Bass AH, Marchaterre MA, Baker R. Vocal-acoustic pathways in a teleost fish. J Neurosci. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, Szabo T. Electroreception in mormyrid fish. In: Bullock TH, Heiligenberg W, editors. Electroreception. Wiley; New York: 1986. pp. 375–452. [Google Scholar]
- Bennett MVL. Electric organs. In: Hoar WS, Randall DJ, editors. Fish Physiology. Academic Press; New York: 1971. pp. 347–491. [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- Clarke JDW, Lumsden A. Segmental repetition of neuronal phenotype sets in the chick embryo hindbrain. Development. 1993;118:151–162. doi: 10.1242/dev.118.1.151. [DOI] [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brain-stem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Donga R, Lund JP. Discharge patterns of trigeminal commissural last-order inter-neurons during fictive mastication in the rabbit. J Neurophysiol. 1991;66:1564–1579. doi: 10.1152/jn.1991.66.5.1564. [DOI] [PubMed] [Google Scholar]
- Dye J, Heiligenberg W, Keller CH, Kawasaki M. Different classes of glutamate receptors mediate distinct behaviors in a single brainstem nucleus. Proc Natl Acad Sci USA. 1989;86:8993–8997. doi: 10.1073/pnas.86.22.8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye JC, Meyer JH. Central control of the electric organ discharge in weakly electric fish. In: Bullock TH, Heiligenberg W, editors. Electroreception. John Wiley & Sons; New York: 1986. pp. 71–102. [Google Scholar]
- Feldman JL, Smith JC, McCrimmon DR, Ellenberger HH, Speck DF. Generation of respiratory pattern in mammals. In: Cohen AH, Rosignol S, Grillner S, editors. Neural Control of Rhythmic Movements in Vertebrates. John Wiley & Sons; New York: 1988. pp. 73–100. [Google Scholar]
- Gans C. Biomechanics: An Approach to Vertebrate Biology. J.B Lippincott Company; Philadelphia, Toronto: 1974. [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–274. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Garstang W. Zool J Linn Soc. Vol. 35. London: 1922. The theory of recapitulation: a critical restatement of the biogenic law; pp. 81–101. [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Ann Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Gilland E, Baker R. Conservation of neuroepithelial and mesodermal segments in the embryonic vertebrate head. Acta Anat. 1993;148:110–123. doi: 10.1159/000147530. [DOI] [PubMed] [Google Scholar]
- Gilland E, Baker R. Phylogenetic diversification within embryonic hindbrain rhombomeres and the neuronal organization underlying postural control. Soc Neurosci Abstr. 1994;20:1274. [Google Scholar]
- Glover JC, Petursdottir G. Regional specificity of developing reticulospinal, vestibulospinal, and vestibulo-ocular projections in the chicken embryo. J Neurobiol. 1991;22:353–376. doi: 10.1002/neu.480220405. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Implications for lateralization of bird song from unilateral gating of bilateral motor patterns. Nature. 1995;373:63–66. [Google Scholar]
- Gracco VL, Löfqvist A. Speech motor coordination and control: evidence from lip, jaw, and laryngeal movements. J Neurosci. 1994;14:6585–6597. doi: 10.1523/JNEUROSCI.14-11-06585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A. Patterning the rostrocaudal axis of the hindbrain. Sem Neurosci. 1992;4:307–315. [Google Scholar]
- Grant K, Bell CC, Clausse S, Ravaille M. Morphology and physiology of the brainstem nuclei controlling the electric organ discharge in mormyrid fish. J Comp Neurol. 1986;245:514–530. doi: 10.1002/cne.902450407. [DOI] [PubMed] [Google Scholar]
- Grillner S, Matsushima T. The neural network underlying locomotion in lamprey – synaptic and cellular mechanisms. Neuron. 1991;7:1–15. doi: 10.1016/0896-6273(91)90069-c. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P. Central pattern generators for locomotion, with special reference to vertebrates. Ann Rev Neurosci. 1985;8:233–261. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- Guthrie S. The status of the neural segment. Trends Neurosci. 1995;18:74–79. doi: 10.1016/0166-2236(95)80027-y. [DOI] [PubMed] [Google Scholar]
- Hanneman E, Trevarrow B, Metcalfe WK, Kimmel CB, Westerfield M. Segmental pattern of development of the hindbrain and spinal cord of the zebrafish embryo. Development. 1988;103:49–58. doi: 10.1242/dev.103.1.49. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Evans CS, Marler P. The role of articulation in the production of rhesus monkey, Macaca mulatta, vocalizations. Anim Behav. 1993;45:423–433. [Google Scholar]
- Heiligenberg W. The neural basis of behavior: a neuroethological view. Annu Rev Neurosci. 1991;14:247–267. doi: 10.1146/annurev.ne.14.030191.001335. [DOI] [PubMed] [Google Scholar]
- Holland P. Homeobox genes in vertebrate evolution. BioEssays. 1992;14:267–273. doi: 10.1002/bies.950140412. [DOI] [PubMed] [Google Scholar]
- Holland PWH, Holland LZ, Williams NA, Holland ND. An amphioxus homeobox gene: sequence conservation, spatial expression during development and insights into vertebrate evolution. Development. 1992;116:653–661. doi: 10.1242/dev.116.3.653. [DOI] [PubMed] [Google Scholar]
- Holland PWH, García-Fernández J, Williams NA, Sidow A. Gene duplications and the origins of vertebrate development. Development. 1994;1994(Suppl):125–133. [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Ellenberger HH. Cardiorespiratory neurons in the medula oblongata: input and output relationships. In: Armour JA, Ardell JL, editors. Neurocardiology. Oxford University Press; New York, Oxford: 1994. pp. 277–307. [Google Scholar]
- Jürgens U, Pratt R. Role of the periaqueductal grey in vocal expression of emotion. Brain Res. 1979;167:367–378. doi: 10.1016/0006-8993(79)90830-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki R. Breathing rhythm-generation mechanism in the adult lamprey (Lampetra japonica) Jap J Physiol. 1984;34:319–335. doi: 10.2170/jjphysiol.34.319. [DOI] [PubMed] [Google Scholar]
- Kawasaki M. The African wave-type electric fish, Gymnarchus niloticus, lacks corollary discharge mechanisms for electrosensory gating. J Comp Physiol A. 1994;174:133–144. doi: 10.1007/BF00193781. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Heiligenberg W. Distinct mechanisms of modulation in a neuronal oscillator generate different social signals in the electric fish Hypopomus. J Comp Physiol A. 1989;165:731–741. doi: 10.1007/BF00610872. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Heiligenberg W. Different classes of glutamate receptors and GABA mediate distinct modulations of a neuronal oscillator, the medullary pacemaker of a gymnotiform electric fish. J Neurosci. 1990;10:3896–3904. doi: 10.1523/JNEUROSCI.10-12-03896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Metcalfe WK, Schabtach E. T Reticular interneurons: a class of serially repeating cells in the zebrafish hindbrain. J Comp Neurol. 1985;233:365–376. doi: 10.1002/cne.902330306. [DOI] [PubMed] [Google Scholar]
- Kirzinger A, Jürgens U. Role of extralaryngeal muscles in phonation of subhuman primates. J Comp Physiol A. 1994;175:215–222. doi: 10.1007/BF00215117. [DOI] [PubMed] [Google Scholar]
- Kitama T, Shimazu H, Tanaka M, Yoshida K. Vestibular and visual interaction in generation of rapid eye movements. Ann N Y Acad Sci. 1992;656:396–407. doi: 10.1111/j.1749-6632.1992.tb25224.x. [DOI] [PubMed] [Google Scholar]
- Kogo N, Remmers JE. Neural organization of the ventilatory activity in the frog, Rana catesbeiana. II. J Neurobiol. 1994;25:1080–1094. doi: 10.1002/neu.480250905. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Evolution of the vertebrate Hox homeobox genes. BioEssays. 1992;14:245–252. doi: 10.1002/bies.950140408. [DOI] [PubMed] [Google Scholar]
- Lancaster WC, Henson OW, Jr, Keating AW. Respiratory muscle activity in relation to vocalization in flying bats. J Exp Biol. 1995;198:175–191. doi: 10.1242/jeb.198.1.175. [DOI] [PubMed] [Google Scholar]
- Lee RKK, Eaton RC, Zottoli SJ. Segmental arrangement of reticulospinal neurons in the goldfish hindbrain. J Comp Neurol. 1993;329:539–556. doi: 10.1002/cne.903290409. [DOI] [PubMed] [Google Scholar]
- Lindholm MM, Bass AH. Early events in myofibrillogenesis and innervation of skeletal, sound-generating muscle in a teleost fish. J Morphol. 1993;216:225–239. doi: 10.1002/jmor.1052160209. [DOI] [PubMed] [Google Scholar]
- Lingenhohl K, Friauf E. Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? J Neurosci. 1994;14:1176–1194. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. Neurobiology of Cerebellar Evolution and Development. American Medical Association, Education and Research Foundation; Chicago: 1969. [Google Scholar]
- Llinás R. The intrinsic electrophysiological properties of mammalian neurons: a new insight into CNS function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R, Paré D. Role of intrinsic neuronal oscillations and network ensembles in the genesis of normal and pathological tremors. In: Findley LJ, Koller WC, editors. Handbook of Tremor Disorders. Marcel Dekker, Inc.; New York, Basel, Hong Kong: 1994. pp. 7–36. [Google Scholar]
- Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurons and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Baker R, Sotelo C. Electronic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Marchaterre MA, Lindholm M, Bass AH. Ontogeny of motoneurons, acetylcholine and muscle fibers in the vocal motor circuit of a teleost fish. Soc Neurosci Abstr. 1993;18:1303. [Google Scholar]
- Marin F, Puelles L. Morphological fate of Rhombomeres in quail/chick chimeras: a segmental analysis of hindbrain nuclei. Europ J Neurosci. 1995;7:1714–1738. doi: 10.1111/j.1460-9568.1995.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK, Mendelson B, Kimmel CB. Segmental homologies among reticulospinal neurons in the hindbrain of the zebrafish larva. J Comp Neurol. 1986;251:147–159. doi: 10.1002/cne.902510202. [DOI] [PubMed] [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Noden DM. Spatial integration among cells forming the cranial peripheral nervous system. J Neurobiol. 1993;24:248–261. doi: 10.1002/neu.480240210. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Ontogeny and phylogeny: a re-evaluation of conceptual relationships and some applications. Brain Behav Evol. 1990;36:116–140. doi: 10.1159/000115302. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Reassessing the mechanisms and origins of vocal learning in birds. Trends Neurosci. 1991;14:206–211. doi: 10.1016/0166-2236(91)90107-6. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Iriki A, Nakamura Y. Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol. 1986;55:806–825. doi: 10.1152/jn.1986.55.4.806. [DOI] [PubMed] [Google Scholar]
- Pastor AM, De La Cruz RR, Baker R. Eye position and eye velocity integrators reside in separate brainstem nuclei. Proc Natl Acad Sci USA. 1994;91:807–811. doi: 10.1073/pnas.91.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci. 1993;16:265–297. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- Rübsamen R, Betz M. Control of echolocation pulses by neurons of the nucleus ambiguus in the rufous horseshoe bat, Rhinolophus rouxi. J Comp Physiol A. 1986;159:675–687. doi: 10.1007/BF00612040. [DOI] [PubMed] [Google Scholar]
- Rübsamen R, Schuller G. Laryngeal nerve activity during pulse emission in the CF-FM Bat, Rhinolophus ferrumequinum. II. The recurrent laryngeal nerve. J Comp Physiol. 1981;143:323–327. [Google Scholar]
- Rübsamen R, Schweizer H. Control of echolocation pulses by neurons of the nucleus ambiguus in the rufous horseshoe bat (Rhinolophus rouxi). II. Afferent and efferent connections of the ventral motor nucleus of the laryngeal nerves. J Comp Physiol A. 1986;159:689–699. doi: 10.1007/BF00612041. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shimazu H. Reticulo-vestibular organization participating in generation of horizontal fast eye movement. Ann N Y Acad Sci. 1981;374:130–143. doi: 10.1111/j.1749-6632.1981.tb30866.x. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Neural correlates of frog calling: production by two semi-independent generators. Behav Brain Res. 1992;50:17–30. doi: 10.1016/s0166-4328(05)80284-0. [DOI] [PubMed] [Google Scholar]
- Schuller G, Rübsamen R. Laryngeal nerve activity during pulse emission in the CF-FM Bat, Rhinolophus ferrumequinum. I. Superior laryngeal nerve (external motor branch) J Comp Physiol. 1981;143:317–321. [Google Scholar]
- Schweizer H, Rübsamen R, Ruehle C. Localization of brain stem motoneurons innervating the laryngeal muscles in the Rufous horseshoe bat, Rhinolophus rouxi. Brain Res. 1981;230:41–50. doi: 10.1016/0006-8993(81)90390-5. [DOI] [PubMed] [Google Scholar]
- Scott MP. Vertebrate homeobox gene nomenclature. Cell. 1992;71:551–553. doi: 10.1016/0092-8674(92)90588-4. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Tobias ML, Kelley DB. Origin and identification of fibers in the cranial nerve IX–X complex of Xenopus laevis: Lucifer yellow backfills in vitro. J Comp Neurol. 1986;244:430–444. doi: 10.1002/cne.902440403. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990;10(5):1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streidter GF. The vocal control pathways in budgerigars differ from those in songbirds. J Comp Neurol. 1994;343:35–56. doi: 10.1002/cne.903430104. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Cruce WLR, Powell KS. Organization within the cranial IX–X complex in ranid frogs: a horseradish peroxidase transport study. J Comp Neurol. 1984;222:358–365. doi: 10.1002/cne.902220304. [DOI] [PubMed] [Google Scholar]
- Tan K, Le Douarin NM. Development of the nuclei and cell migration in the medulla oblongata. Application of the quail-chick chimera system. Anat Embryol. 1991;183:321–343. doi: 10.1007/BF00196834. [DOI] [PubMed] [Google Scholar]
- Tracy HC. Stages in the development of the anatomy of motility of the toadfish (Opsanus tau) J Comp Neurol. 1959;11:27–81. [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:690–697. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo Y-C. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl CM, Noden DM, Baker R. Developmental relations between sixth nerve motor neurons and their targets in the chick embryo. Dev Dynam. 1994;201:191–202. doi: 10.1002/aja.1002010209. [DOI] [PubMed] [Google Scholar]
- Walker WF. Vertebrate Dissection. Saunders College Publishing; Philadelphia: 1986. [Google Scholar]
- Weiser M, Bennett MVL, Baker R. Toadfish sonic motor system. III. Localization of the command nucleus. Bio Bull. 1986;171:498–499. [Google Scholar]
- Westneat MW, Long JH, Hoese W, Nowicki S. Kinematics of birdsong: functional correlation of cranial movements and acoustic features in sparrows. J Exp Biol. 1993;182:147–171. doi: 10.1242/jeb.182.1.147. [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Haerter UL, Kelley DB. A proposed neural pathway for vocalization in South African clawed frogs, Xenopus laevis. J Comp Physiol A. 1985;257:749–761. doi: 10.1007/BF01350072. [DOI] [PubMed] [Google Scholar]
- Wild JM. The auditory-vocal-respiratory axis in birds. Brain Behav Evol. 1994a;44:192–209. doi: 10.1159/000113577. [DOI] [PubMed] [Google Scholar]
- Wild JM. Visual and somatosensory inputs to the avian song system via nucleus uvaeformis (Uva) and a comparison with the projections of a similar thalamic nucleus in a nonsongbird, Columba livia. J Comp Neurol. 1994b;349:512–535. doi: 10.1002/cne.903490403. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Northcutt RG. Connections of the corpus cerebelli in the green sunfish and the common goldfish: a comparison of perciform and cypriniform teleosts. Brain Behav Evol. 1988;32:293–316. doi: 10.1159/000116558. [DOI] [PubMed] [Google Scholar]
- Yager DD. A unique sound production mechanism in the pipid anuran Xenopus borealis. Zool J Linn Soc. 1992;104:351–375. [Google Scholar]
- Yajima Y, Hayashi Y, Yoshii N. Identification of ultrasonic vocalization substrates determined by electrical stimulation applied to the medulla oblongata in the rat. Brain Res. 1981;229:353–362. doi: 10.1016/0006-8993(81)90999-9. [DOI] [PubMed] [Google Scholar]


