Abstract
Reactive oxygen species (ROS) are important mediators of cellular signal transduction cascades such as proliferation, migration, and apoptosis. Chronic exposure of isolated β-cells to proinflammatory cytokines elevates intracellular oxidative stress leading to the demise of pancreatic β-cells culminating in the onset of diabetes. Although the mitochondrial electron transport chain is felt to be the primary source of ROS, several lines of recent evidence suggest that phagocyte-like NADPH oxidase plays a central role in cytokine-mediated ROS generation and apoptosis of β-cells. However, the precise mechanisms underlying the regulation of NADPH oxidase remain unknown. To address this, insulin-secreting INS 832/13 cells were treated with cytomix (IL-1β, IFN-γ, and TNF-α; 10 ng/ml each) for different time intervals (0–24 h). A significant, time-dependent increase in NADPH oxidase activation/intracellular ROS production, p47phox subunit, but not p67phox subunit, expression of the phagocyte-like NADPH oxidase were demonstrable under these conditions. Furthermore, siRNA-p47phox transfection or exposure of INS 832/13 cells to apocynin, a selective inhibitor of NADPH oxidase, markedly attenuated cytomix-induced ROS generation in these cells. Cytomix-mediated mitochondrial dysfunction in INS 832/13 cells was evident by a significant loss of mitochondrial membrane potential (MMP) and upregulated caspase 3 activity. Cytomix treatment also caused a transient (within 15 min) activation of Rac1, a component of the NADPH oxidase holoenzyme. Furthermore, GGTI-2147 and NSC23766, known Rac1 inhibitors, not only attenuated the cytomix-induced Rac1 activation but also significantly prevented loss of MMP (NSC23766 > GGTI-2147). However, NSC23766 had no effect on cytomix-induced NO generation or caspase 3 activation, suggesting additional regulatory mechanisms might underlie these signaling steps. Together, these findings suggested that Rac1-mediated regulation of phagocyte-like NADPH oxidase contributes to cytokine-mediated mitochondrial dysfunction in the β-cell.
Keywords: T-lymphoma invasion, metastasis 1, geranylgeranylation, mitochondrial membrane potential, pancreatic β-cell
type-1 diabetes is characterized by an absolute insulin deficiency arising from progressive autoimmune destruction of pancreatic β-cells (2–3, 8, 22). During the progression of the disease, proinflammatory cytokines, particularly IL-1β, TNF-α, and IFN-γ, are released into islets of Langerhans by infiltrated, activated T cells and macrophages (8, 25, 27). However, the exact cellular mechanisms by which cytokines induce β-cell demise is only partially understood (3). Though cytokines modulate the activity of several destructive signaling cascades, apoptosis is considered as the primary mode of cell death in human and mouse models (2, 21–22). Apoptosis is a highly regulated, genetically encoded, and energy-dependent cell death process activated by extracellular signals (11, 13, 24). Caspases, a family of cysteine proteases, play a critical role in apoptosis. In the presence of apoptotic stimuli, caspase signaling axis is activated, in which activation of initiator caspases (i.e., caspases 8 and 9) leads to the downstream activation of executioner caspases (e.g., caspase 3). It is well established that once activated, caspase 3 cleaves ∼40 different cellular substrates (3, 6, 11, 24).
There are three possible mechanisms by which cells undergo death via apoptosis. Recent studies indicate cytokines may signal apoptosis via an intrinsic apoptotic pathway, which involves damage to the mitochondrial membrane and subsequent release of cytochrome c from the intermembranous space into cytosol, leading to the activation of caspase cascade (6, 27). A growing body of recent evidence suggests upregulated oxidative stress from reactive oxygen species (ROS), and nitric oxide (NO) contributes to the damage in mitochondrial membrane, eventually causing defects in the membrane potential. In contrast with most other mammalian cell types, β-cells comprise relatively lower levels of redox-regulating enzymes, making them more vulnerable to oxidative damage (7). Recently, members of the NADPH oxidase family have emerged as one of the sources of redox signaling and pathological oxidative stress. Under basal conditions, this multicomponent enzyme system is inactive, and its respective subunits are dispersed between the soluble and membranous compartments. The membrane-bound catalytic core consists of flavocytochrome b558 components p22phox and gp91phox and small G-protein Rap1. The regulatory core consisting of p47phox, p67phox, and p40phox subunits and the small G-protein Rac1 reside in the cytosol. Upon stimulation, the cytosolic components are translocated to the membrane for holoenzyme assembly and activation of the enzyme (7). It has also been suggested that functional activation of Rac1 (i.e., GTP-Rac) is vital for the NADPH holoenzyme assembly (9).
Several recent studies have demonstrated localization and functional activation of the phagocyte-like NADPH in clonal β-cells, normal rat islets, and human islets under the duress of various stimuli, including elevated levels of glucose, saturated fatty acids, and proinflammatory cytokines (14). It has also been documented that pharmacological inhibition of NADPH oxidase by diphenyleneiodonium chloride (DPI) or anti-sense oligonucleotides for p47phox markedly attenuate glucose-induced ROS production and oxidative stress, suggesting critical involvement of NADPH oxidase in the metabolic dysfunction induced by long-term exposure to elevated glucose (14, 15). These data implicated a significant contributory role for NADPH oxidase in the onset of metabolic dysfunction of the β-cell under a condition of oxidative stress (17, 19–20, 26). Despite the aforementioned compelling lines of evidence, very little has been studied with regard to the potential contributory roles of Rac1 in the cascade of events leading to cytokine-induced NADPH oxidase-mediated superoxide generation and mitochondrial dysfunction in pancreatic β-cells. On the basis of this reasoning, we undertook the current study to test the hypothesis that cytokines induce ROS generation and oxidative stress in pancreatic β-cells by promoting Rac1 activation, which represents one of the signaling events necessary for the functional regulation of the endogenous phagocyte-like NADPH oxidase holoenzyme assembly and its catalytic activity. Herein, we describe the first evidence to suggest a critical modulatory role for Tiam1, a guanine nucleotide exchange factor (GEF) for Rac1 (30), in this signaling pathway leading to the onset of mitochondrial dysfunction. We also report that posttranslational prenylation of Rac1 is also necessary for the optimal activation of NADPH oxidase elicited by cytomix in insulin-secreting cells.
MATERIALS AND METHODS
Materials.
Interleukin-1β, IFN-γ, and TNF-α were obtained from R&D Systems (Minneapolis, MN). Rac1 activation assay kit was obtained from Cytoskeleton (Denver, CO). JC-1 mitochondrial membrane potential detection kit was obtained from Cell Technology (Mountain View, CA). Caspase 3 antiserum was obtained from Cell Signaling Technology (Danvers, MA). p47phox siRNA, p47phox, and p67phox antisera were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). NSC23766 and GGTI-2147 were obtained from Calbiochem (San Diego, CA). Apocynin was obtained from Sigma-Aldrich (St. Louis, MO).
Cell lines and culture conditions.
INS 832/13 cells (kindly provided by Dr. Chris Newgard, Duke University Medical Center, Durham, NC) were cultured in RPMI 1640 medium containing 10% heat-inactivated FBS supplemented with 100 IU/ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, and 10 mM HEPES (pH 7.4). The cultured cells were subcloned twice weekly following trypsinization and passages 53–61 were used for the study. For the inhibitor studies, INS 832/13 cells were cultured up to 70–80% confluence in RPMI medium supplemented with 10% heat-inactivated FBS prior to inhibitor exposure. Cells were then incubated overnight with low serum-low glucose (LS-LG) media in the presence or absence of NSC23766 and GGTI-2147 at 20 μM and 10 μM, respectively.
Quantitation of cytokine-induced NO release.
INS 832/13 cells were incubated with cytomix (IL-1β, IFN-γ, TNF-α; 10 ng/ml; each) or IL-1β (25 ng/ml), for 12 and 24 h in the presence and absence of inhibitors, as indicated in the text. At the end of incubation period, the medium was collected and centrifuged at 1,000 g for 5 min. Equal volumes of media and Griess reagent were mixed, and absorbance (540 nm) was measured using microplate reader (Molecular Devices, Sunnyvale, CA).
Quantitation of ROS.
INS 832/13 cells were seeded in a 6-well plate and treated with either diluent or cytomix in the presence and absence of inhibitors (i.e., NSC23766, GGTI-2147) for a 12- and 24-h period, as indicated in the text. Following that, media were removed, and cells were incubated further in 2′,7′-dichlorofluorescein diacetate (DCHF-DA) (10 μM) at 37°C for 30 min. DCHF-DA is a nonpolar compound that diffuses rapidly into the cells and hydrolyzes readily by cellular esterases into polar DCFH. In the presence of ROS, DCFH is readily oxidized to fluorescent DCF (1, 6). The cells were washed twice with ice-cold PBS and harvested; equal amounts of proteins (50 μg) were taken, and fluorescence was measured (Em: 485 nm and Ex: 535 nm) using a luminescence spectrophotometer (PerkinElmer, Waltham, MA).
Molecular biological or pharmacological inhibition of NADPH oxidase activity.
INS 832/13 cells were seeded in a 24-well plate up to 50% confluence and transfected with mock or antisense siRNA-p47phox (150 nM) and allowed to grow up to 80% or higher confluence. Then the cells were treated either with diluent or cytomix for a 12-h period. Following this, culture medium was removed, and cells were incubated further in DCHF-DA (10 μM) at 37°C for 30 min, washed twice with ice-cold PBS, and harvested; equal amounts of proteins (50 μg) were taken, and fluorescence was measured (Ex: 485 nm and Em: 535 nm) using luminescence spectrophotometer (PerkinElmer, Waltham, MA). Alternatively, NADPH oxidase was inhibited via a pharmacological approach by treating INS 832/13 cells with either diluent or cytomix for 12 or 24 h in the absence or presence of apocynin (75 μM), and NADPH activity was measured with DCFH-DA assay, as described above.
Rac1 activation assay.
The relative degree of Rac1 activation (GTP-bound form) was determined using Rac1 pull-down assay, as described by Syed et al. (23). In brief, INS 832/13 cells were pretreated with either the diluent or pharmacological inhibitors followed by treatments with either diluent or Cytomix for 15 min in the absence and presence of NSC23766 (20 μM) or GGTI-2147 (10 μM). Cell lysates (∼250–300 μg) were clarified by centrifugation. Then PAK-PBD (p21-activated kinase-p21-binding domain) beads (20 μl) were added to the supernatant, rotated for 1 h at 4°C, and pelleted. The resultant pellet was washed and reconstituted in Laemmli buffer. Proteins were resolved by SDS-PAGE and immunoblotted for Rac1.
Determination of mitochondrial membrane potential.
INS 832/13 cells were plated on sterile glass cover slips placed in 6-well plates and pretreated with cytomix for 12 and 24 h in the presence and absence of NSC23766 (20 μM) or GGTI-2147 (10 μM). At the end of treatment, cells were incubated with JC-1 (1: 200) dye for 15 min at 37°C in a 5% CO2 incubator. Cells were washed with assay buffer, mounted onto glass slides, and observed under IX71 inverted fluorescence microscope (X100, Olympus America, Center Valley, Pennsylvania), as we described previously (23). The ratios of red-to-green fluorescence emissions were quantitated to further estimate the extent of mitochondrial membrane damage.
Western blot analysis.
Treated INS 832/13 cells were harvested and homogenized in mannitol-protease inhibitor cocktail buffer (250 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EGTA, 1 mM DTT, and protease inhibitor cocktail). Protein samples (∼20–30 μg) were resolved by SDS-PAGE and transferred onto nitrocellulose membrane. The blots, after blocking with 5% BSA in 20 mM Tris-HCl, pH 7.5, 137 mM NaCl, and 0.1% Tween 20, were immunoprobed with corresponding primary antibody followed by secondary polyclonal rabbit/mouse antibody conjugated to horseradish peroxidase (1:1,000). The protein signal band was detected with enhanced chemiluminescence system (ECL, Amersham Biosciences, Little Chalfont, UK) and developed using Kodak Pro Image 400 R (New Haven, CT). The blots were stripped and reprobed for β-actin to ensure equal loading and transfer of proteins.
Statistical analyses.
Data are presented as means ± SE. Statistically significant differences between values were evaluated by Student's t-test or ANOVA where appropriate. P < 0.05 was considered to be statistically significant.
RESULTS
Cytomix induces phagocyte-like NADPH oxidase activation in INS 832/13 cells.
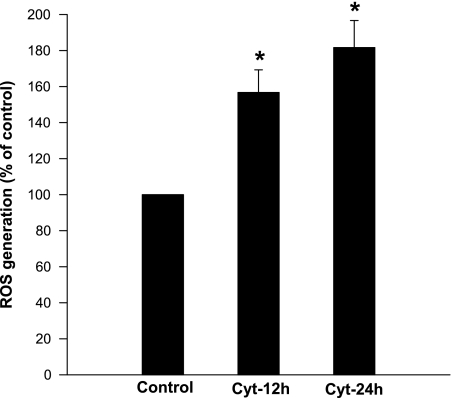
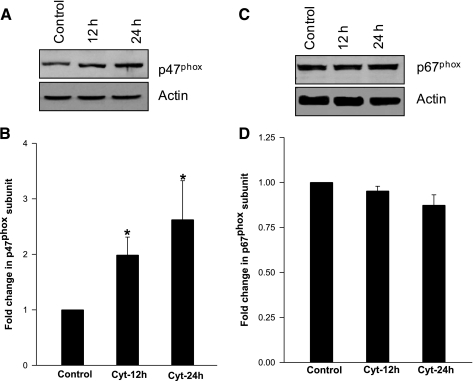
At the outset, we quantitated NADPH oxidase activity in INS 832/13 cells exposed to cytomix (i.e., IL-1β, IFN-γ, TNF-α; 10 ng/ml each). The amount of ROS generation and the degree of expression of NADPH subunits (p47phox and p67phox) were determined following a 12-h or 24-h incubation of these cells with cytomix. Data in Fig. 1 showed a significant increase in ROS generation at these time points (∼60 and 85% above the control at 12 h and 24 h, respectively). Compatible with these findings are data presented in Fig. 2, A and B, indicating a significant increase in the expression of p47phox in these cells following exposure to cytomix. However, no effect of cytomix on the expression of p67phox was demonstrable under these conditions (Fig. 2, C and D).
Fig. 1.
Incubation of INS 832/13 cells with cytomix leads to a time-dependent increase in reactive oxygen species (ROS). INS 832/13 cells were incubated with either diluent or cytomix for 12 or 24 h, as indicated in the figure, and ROS generation was measured using 2′,7′-dichlorofluorescein diacetate (DCFH-DA) assay. Intracellular levels of ROS in treated cells were expressed as a percent of control cells. Data represent means ± SE from four independent experiments. *Significantly different (P < 0.05) from control.
Fig. 2.
Incubation of INS 832/13 cells with cytomix increases expression of p47phox, but not p67phox subunits of NADPH-oxidase. A: INS 832/13 cells were exposed to cytomix for 12 or 24 h as indicated in the figure. Relative degree of expression of p47phox was determined by Western blot analysis. p47phox expression was normalized to actin content in individual lanes. Pooled data from three independent experiments are provided in B. *Significantly different (P < 0.05) from control. C: INS 832/13 cells were exposed to cytomix for 12 or 24 h as indicated in the figure. Relative degree of expression of p67phox was measured by Western blot analysis. p67phox expression was normalized to actin content in individual lanes. Pooled data from three independent experiments are provided in D.
To further assess whether the cytomix-induced ROS are derived from NADPH oxidase, we quantitated cytomix-induced ROS generation following inhibition of NADPH oxidase via two independent approaches. In the first approach, we used apocynin, a selective inhibitor of NADPH oxidase. Data from these studies indicated a marked inhibition in cytomix-induced ROS generation by apocynin. The values for cytomix-mediated ROS generation represented 154.0 ± 3.9% and 167.8 ± 6.5% at 12 h and 24 h, respectively. The corresponding values in the presence of apocynin reached basal levels (i.e., 98.1 ± 5.2% and 106.6 ± 9.1% at 12 and 24 h, respectively; n = 3 experiments in each case; P < 0.05). In the second approach, endogenous expression of the p47phox was knocked down by transfecting cells with siRNA-p47phox. Under the current experimental conditions employed in the study (see materials and methods), we were able to reduce p47phox expression by ∼60–70% in siRNA-p47phox-transfected cells. Furthermore, the ability of cytomix to induce ROS generation (following 12 h of incubation) was completely abolished in siRNA-p47phox-transfected cells (i.e., 102.0 ± 5.5% of control; n = 3 experiments), suggesting that NADPH oxidase might be the principal contributor in cytomix-induced generation of ROS.
Cytomix transiently increases Rac1 activation in INS 832/13 cells: potential requirement for Tiam1 as a guanine nucleotide exchange factor for Rac1.
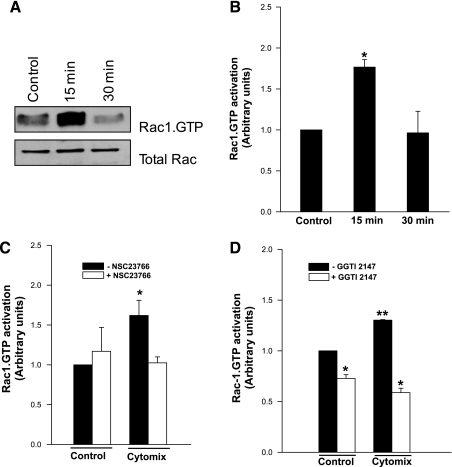
As stated above, Rac1, a small G protein, is one of the components of the NADPH oxidase holoenzyme assembly. Therefore, we next examined whether cytomix-induced activation of NADPH oxidase is mediated via activation of Rac1. This was accomplished by quantitating the GTP-bound Rac1 (active configuration) by a pull-down assay (see materials and methods for additional details). Data depicted in Fig. 3 suggested a significant (∼1.7-fold), but transient, activation of Rac1 (within 15 min) in INS 832/13 cells following exposure to cytomix. Rac1.GTP levels reached basal levels at 30 min of exposure. These data implicate Rac1 activation as one of the signaling steps involved in cytomix-mediated effects on isolated β-cells.
Fig. 3.
Cytomix induces transient activation of Rac1 in INS 832/13 cells: inhibition of this signaling step by NSC23766 and GGTI-2147. A: cytomix causes transient activation of small G-protein Rac1 in INS 832/13 cells, as determined by the pull-down assay followed by Western blot analysis (see materials and methods). Total Rac1 in the lysates is also provided as a loading control. A representative blot of three independent experiments is shown here. B: pooled activation data from three independent experiments are shown here. C: NSC23766 inhibition of cytomix-induced activation of Rac1. Pooled data from three independent studies are depicted in the figure. D: GGTI-2147 inhibits cytomix-induced Rac1 activation in INS 832/13 cells. Pooled data from three independent studies are depicted in the figure. *Significantly different (P < 0.05) from control. *, **Different symbols represent the values that are significantly different at P < 0.05.
Recently, we reported the expression and functional activation of GEFs for small G proteins in pancreatic β-cells (29). The primary function of these proteins is to facilitate GTP/GDP exchange. Our findings in INS 832/13 cells and primary rat islets have indicated that Tiam1 serves as a GEF for Rac1 (29). In the current study, we investigated whether Tiam1 is required for cytomix-induced activation of Rac1 in INS 832/13 cells. This was accomplished using pharmacological inhibitor, NSC23766, which selectively inhibits Tiam1-mediated activation of Rac1, but not Cdc42 or Rho in insulin-secreting β-cells (5). Data in Fig. 3C suggested a significant reduction in cytomix-induced activation of Rac1 by NSC23766 in INS 832/13 cells. These findings support the viewpoint that Tiam1 plays a key regulatory role in Rac1 activation elicited by cytomix in insulin-secreting cells.
It is well established that posttranslational geranylgeranylation is necessary for optimal activation of Rac1 in pancreatic β-cells (see Ref. 12, for a review). Therefore, we examined whether geranylgeranylation of Rac1 is necessary for cytomix-induced activation of Rac1. This was accomplished via a pharmacological approach, which involved quantitation of cytomix-induced activation of Rac1 in cells exposed to diluent or in the presence of GGTI-2147, a known inhibitor Rac1 geranylgeranylation (12, 28). Data in Fig. 3D showed a marked reduction in cytomix-induced Rac1 activation in cells exposed to GGTI-2147. Together, data depicted in Fig. 3, A–D suggested that cytomix induces Rac1 activation in INS 832/13 cells, which is sensitive to inhibition of Tiam1 activation and posttranslational geranylgeranylation.
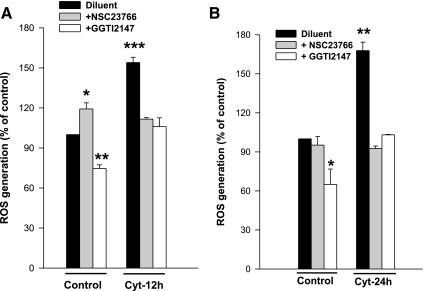
NSC23766 and GGTI-2147 markedly reduce cytomix-induced ROS generation in INS 832/13 cells.
As a logical extension to the above studies, we asked whether inhibitors of Rac1 attenuate cytomix-induced ROS generation. Data shown in Fig. 4 indicate a marked reduction in cytomix-induced ROS generation at both 12- and 24-h time points by NSC23766 and GGTI-2147. It is noteworthy that GGTI-2147, but not NSC23766, also reduced the ROS generated under basal conditions (Fig. 4, A and B). Taken together, our findings establish a direct role for Tiam1-dependent, prenylation-sensitive Rac1 activation in the signaling cascade leading to cytomix-induced NADPH oxidase and ROS generation in INS 832/13 cells.
Fig. 4.
Cytomix-induced ROS generation is inhibited by NSC23766 and GGTI-2147 in INS 832/13 cells. INS 832/13 cells were treated with either diluent or cytomix in the presence and absence of NSC23766 (20 μM) or GGTI-2147 (10 μM) for 12 h (A) and 24 h (B), as indicated in the figure and intracellular levels of ROS was measured using DCHF-DA assay. Data are representative of three independent experiments, expressed as a percentage of control cells and represent means ± SE. Bars with different symbols (*, **, ***) are significantly different at P < 0.05.
Inhibitors of Rac1 activation reduce cytomix-induced mitochondrial defects in INS 832/13 cells.
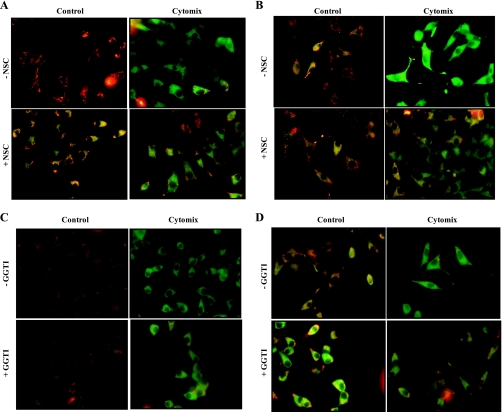
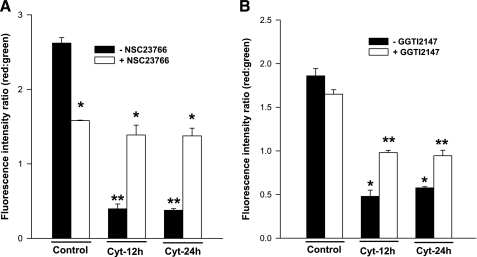
It is widely felt that cytokine-mediated effects on pancreatic β-cells may, in part, be mediated via alterations in mitochondrial membrane properties, including loss of MMP leading to cytochrome-c release and caspase 3 activation (2, 16, 30). Therefore, we examined whether inhibitors of Rac1 activation exert protective effects on cytomix-induced loss in MMP. This was accomplished using JC-1 (5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazolyl-carbocyanine iodide) assay. JC-1 is a lipophilic dye, which fluoresces red when aggregated above the critical concentration within mitochondria. In cells in which mitochondrial membrane is damaged, JC-1 remains in the cytoplasm, as a green fluorescence monomer. Data from these studies, which are depicted in Fig. 5, suggested a significant loss of MMP in INS 832/13 cells treated with cytomix following a 12- or 24-h exposure. Coprovision of NSC23766, a Tiam1 inhibitor, and GGTI-2147, a prenylation inhibitor, modestly, but significantly protected these cells against cytomix-induced damage to the mitochondrial potential (Fig. 5). Quantitation of fluorescence intensity ratios of red to green further confirmed these conclusions (Fig. 6). Further, these data also suggested that the protective effects were more prominent in the case of NSC23766 compared with GGTI-2147 (Fig. 5, A–D, Fig. 6, A and B). It should be mentioned that NSC23766 exerted inhibitory effects on MMP in control (i.e., diluent-treated cells). Regardless of this inhibitory effect, it markedly prevented cytomix-induced loss in MMP at both time points. Together, these data indicate that Rac1 activation might be requisite for cytomix-induced mitochondrial defects in pancreatic β-cells.
Fig. 5.
Cytomix-induced loss in mitochondrial membrane potential is partially prevented by NSC23766 and GGTI-2147. INS 832/13 cells were treated with either diluent alone or cytomix for 12 (A and C) and 24 h (B and D) in the presence and absence of NSC23766 (20 μM) or GGTI-2147 (10 μM), as indicated in the figure. The mitochondrial membrane potential was measured with JC-1 assay kit. Data are representative of three independent experiments with comparable results.
Fig. 6.
Cytomix-induced loss in mitochondrial membrane potential is partially prevented by NSC23766 and GGTI-2147. Cytomix induced changes in mitochondrial membrane potential was measured with JC-1 assay kit, as described in Fig. 5 and red: green fluorescence ratio was calculated by sampling (n = 10 data points per image) for three independent experiments with comparable results. *, **Bars with different symbols are significantly different P < 0.05. A: data from cells treated with NSC23766 (20 μM). B: data from cells treated with GGTI-2147 (10 μM).
Tiam1/Rac1 signaling axis is not necessary for cytomix-induced caspase 3 activation in INS 832/13 cells.
The observed protective effects of NSC23766 against cytomix-induced loss in MMP (Figs. 5 and 6) prompted us to investigate whether caspase 3 activation, which is a hallmark of cellular apoptosis, is inhibited by Tiam1-mediated activation of Rac1. To accomplish this, INS 832/13 cells were treated with cytomix (as above) or IL-1β alone (25 ng/ml) for 12 or 24 h. Activated caspase 3 in the lysates was determined by Western blot analysis using an antiserum that identifies both the native procaspase and degradative product of caspase 3. Under these conditions we noticed no significant effects of NSC23766 on either cytomix-induced or IL-1β-mediated caspase 3 activation at either time points. Cytomix-induced caspase 3 activation represented 1.55 ± 0.11 units and 1.83 ± 0.24 units at 12 and 24 h, respectively. The corresponding values in the presence of NSC23766 were 1.40 ± 0.14 units and 2.06 ± 0.32 units, respectively (n = 3 determinations in each case, not significantly different from each other). Likewise, IL-1β-induced caspase 3 activation represented 1.27 ± 0.10 units and 1.65 ± 0.23 units at 12 and 24 h, respectively. The corresponding values in the presence of NSC23766 were 1.23 ± 0.09 units and 1.71 ± 0.22 units, respectively (n = 3 determinations in each case, not significantly different from each other). Together, these data indicate that additional mechanisms might underlie caspase 3 activation in these cells elicited by cytokines.
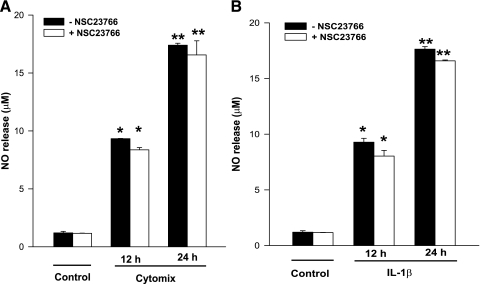
Evidence to further suggest that the Tiam1/Rac1 signaling step may not be required for cytokine-induced NO release from INS 832/13 cells.
It is well established that cytokine-mediated effects on isolated β-cells are mediated via inducible nitric oxide synthase (iNOS) expression and associated NO release. It has also been suggested that NO exerts damaging effects on mitochondria leading to caspase 3 activation. Therefore, in the last set of studies, we investigated whether Tiam1/Rac1 activation is necessary for cytokine-induced NO release in INS 832/13 cells. Data in Fig. 7 demonstrated no significant effect of NSC23766 on either IL-1β or cytomix-induced NO release in INS 832/13 cells either at 12 or 24 h. Together, the above data suggest that Tiam1/Rac1 signaling step is not involved in cytokine-induced NO release and caspase 3 activation and that additional regulatory steps might be necessary for these to occur (see the proposed model below).
Fig. 7.
NSC23766 fails to inhibit cytomix-induced NO release in INS 832/13 cells. INS 832/13 cells were treated with diluent, cytomix (A) or IL-1β (B) for 12 or 24 h. NO released into the medium was measured using Griess assay. Data are expressed as means ± SE from 3 independent experiments. *, **Bars with different symbols represent the values that are significantly different.
DISCUSSION
A growing body of evidence supports the hypothesis that damaging effects of elevated glucose, saturated fatty acids (e.g., palmitate), or proinflammatory cytokines on isolated β-cells may, in part, be due to their ability to increase the generation of superoxides and lipid peroxides leading to increased intracellular oxidative stress culminating in mitochondrial dysfunction and the demise of the effete β-cell (10, 14, 20). More recent evidence (14, 18) suggests that intracellular oxidative stress is largely due to the activation of phagocyte-like NADPH oxidase in these cells. Such a postulation was further supported by pharmacological (e.g., DPI) and molecular biological (e.g., antisense p47phox) approaches. Using apocynin and siRNA-p47phox, we have demonstrated herein that the majority of ROS generated in INS 832/13 cells in the presence of cytomix is derived via the activation of NADPH oxidase. Furthermore, as described in the following sections, our current findings provide additional novel insights into regulatory mechanisms underlying the regulation of NADPH oxidase by cytokines in the islet β-cell.
Our findings implicate a requirement for Tiam1 in the cascade of events leading to cytokine-induced activation of Rac1 in insulin-secreting cells. Using a selective inhibitor of Tiam1-mediated activation of Rac1 (i.e., NSC23766), we have been able to demonstrate that Tiam1 serves as a GEF for Rac1 activation induced by cytomix. These data were further substantiated by our observations to demonstrate a significant reduction in cytomix-induced ROS generation/NADPH oxidase activity by NSC23766. It should be noted in this context that Rac1 activation is transient in nature and that it might be adequate to “initiate” signaling cascade leading to activation of NADPH oxidase. It appears that activation of Rac1 by cytomix seen in the current studies may be attributable primarily to the effects of IL-1β in the cytomix, since we observed a significant (and transient) activation of Rac1 (2 ± 0.4-fold stimulation; n = 3 determinations) in INS 832/13 cells exposed to IL-1β (25 ng/ml). No significant effects were observed with either TNF-α (10 ng/ml) or IFN-γ (10 ng/ml) under these conditions (additional data not shown). Our findings also suggest that IL-1β-mediated Tiam1/Rac1 signaling pathway may not be necessary for iNOS expression and NO release since NSC23766 failed to exert any significant effects on IL-1β (or cytomix)-induced NO release in INS 832/13 cells. Lastly, in the context of transient activation of Rac1 by cytokines, it must be noted that earlier studies from our laboratory and others have demonstrated a significant translocation and membrane association of Rac1 following its activation (see Ref. 12, for a recent review). Therefore, it is likely that the activated Rac1 translocates to the membrane fraction for the NADPH oxidase holoenzyme assembly and activation. However, this remains to be verified.
Our findings provide the first evidence to suggest that prenylation of Rac1 is necessary for cytokine-mediated activation of Rac1 and subsequently the NADPH oxidase. We found that GGTI-2147, a selective inhibitor of protein geranylgeranylation, but not farnesylation, markedly attenuated Rac1 and NADPH oxidase activation mediated by cytokines. In this context, using molecular biological (e.g., dominant-negative Rac1 mutant or siRNA-Rac1) and pharmacological (e.g., GGTI-2147 and 3-allyl or vinyl geranyl geraniols), we have shown recently that geranylgeranylation of Rac1 is necessary for its optimal activation and membrane association in clonal β-cells and normal rats islets (28). It must be pointed out that the NADPH oxidase membrane core component is comprised of Rap1, which also undergoes geranylgeranylation like Rac1. Therefore, the inhibitory effects of GGTI-2147 on cytokine-induced NADPH oxidase may, in part, be due to inhibition of geranylgeranylation of Rac1 and Rap1. Our findings accrued from NSC23766 studies directly support the involvement for Taim1/Rac1 in this signaling cascade since Tiam1 serves as a GEF for Rac1, but not other small G proteins. Taken together, on the basis of the current data accrued from NSC23766 and GGTI-2147 studies, we propose that Tiam1-mediated and geranylgeranylation-sensitive activation of Rac1 is necessary for cytokine-mediated effects of NADPH oxidase and generation of oxidative stress in the islet β-cell.
The currently described evidence (Figs. 5 and 6) is also suggestive of protective effects of the Rac1 inhibitors against cytokine-induced loss in MMP. Our data suggested that NSC23766 affords a better protection compared with GGTI-2147. Therefore, it appears that additional signaling mechanisms might be controlling mitochondrial membrane potential, which are distinct from NADPH oxidase-derived ROS. Compatible with these observations are our findings that demonstrated relative lack of effects of NSC23766 on caspase 3 activation. As in the context of Rac1 activation mentioned above, such steps may be related to direct metabolic effects of IL-1β, but not TNF-α or IFN-γ (also present in the cytomix), since IL-1β-mediated caspase 3 activation and NO release were not affected by Tiam1 inhibition.
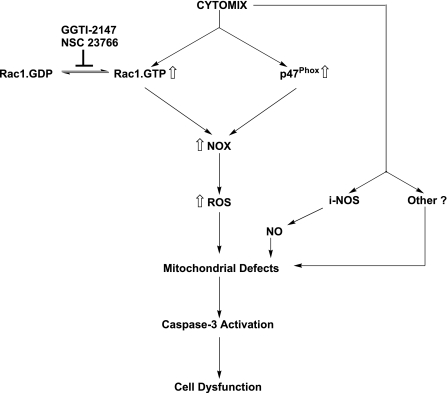
On the basis of these observations, we propose a model for cytokine-mediated effects on islet β-cell as they relate to NADPH oxidase and ROS generation (Fig. 8). Cytomix induces NADPH oxidase activation by promoting the expression of p47phox and activation of Rac1. The Rac1 activation step not only requires the intermediacy of Tiam1, but also prenylation as evidenced by inhibition of the signaling step by GGTI-2147. NADPH oxidase activation leads to an increase in the oxidative stress, resulting in alterations in mitochondrial membrane properties. IL-1β-mediated effects also include an increase in the expression of iNOS and subsequent release of NO, which has been shown to affect mitochondrial function directly leading to further damage and release of cytochrome c followed by activation of caspase 3. Please note that iNOS expression and NO release were found to be independent of Tiam1/Rac1 signaling pathway. It is likely that combined effects of intracellularly generated NO (via activation of iNOS) and ROS (via activation of NADPH oxidase) contribute to maximal damage of the mitochondrial membrane properties leading to caspase 3 activation and metabolic dysfunction of the β-cell.
Fig. 8.
A model for Rac1-dependent NADPH-oxidase-mediated cytomix-induced mitochondrial dysfunction in pancreatic β-cells. On the basis of the data accrued from the current studies, we propose a model for the Rac1-mediated regulation of NADPH oxidase activity under the duress of cytokines. Cytomix induces NADPH oxidase activation by promoting the expression of p47phox and activation of Rac1. The Rac1 activation step not only requires the intermediacy of Tiam1 (i.e., inhibition by NSC23766), but also prenylation, as evidenced by inhibition of the signaling step by GGTI-2147. NADPH oxidase activation leads to an increase in the oxidative stress culminating in loss of mitochondrial membrane potential. IL-1β-mediated effects also include an increase in the expression of iNOS and subsequent release of NO, which has been shown to affect mitochondrial function directly leading to further damage and release of cytochrome c followed by activation of caspase 3. Our findings suggested that iNOS expression and NO release are independent of Tiam1/Rac1 signaling pathway. It is likely that combined effects of intracellularly generated NO (via activation of iNOS) and ROS (via activation of NADPH oxidase) contribute to maximal damage of the mitochondrial membrane properties leading to caspase 3 activation and metabolic dysfunction of the β-cell. NOX, phagocyte-like NADPH oxidase; iNOS, inducible nitric oxide synthase; NO, nitric oxide.
It may be germane to point out that additional regulatory mechanisms might underlie cytokine-mediated stimulatory effects on NADPH oxidase. These include phosphorylation of the cytosolic p47phox, which appears to be necessary for its translocation to the membrane. Published evidence implicates PKC in the phosphorylation of this protein (4). Indeed, studies by Morgan et al. (14) have demonstrated partial restoration of IL-1β-induced ROS to normal levels following exposure to GF109203X, a known inhibitor of PKC. Other regulatory mechanisms might also include regulation of Tiam1/Rac1/NADPH oxidase signaling cascade by sphingolipids, such as ceramide. In this context, we have recently reported palmitic acid-mediated activation of Tiam1/Rac1 and associated increase in NADPH oxidase activation in insulin-secreting β-cells (23). Palmitate effects were inhibited by fumonisin-B1, a known inhibitor of de novo biosynthesis of ceramide from palmitate. In addition, we observed that C2-ceramide, a cell-permeable analog of ceramide exerted similar stimulatory effects on NADPH oxidase in a NSC23766-sensitive manner (23). Additional studies are needed to determine whether intracellular generation of ceramide represents a regulatory mechanism in cytokine-challenged β-cells. Together, these data accrued in the current studies suggest that Rac1-mediated regulation of NADPH oxidase function contributes to cytokine-mediated mitochondrial dysfunction in the β-cell.
Perspectives and Significance
A growing body of evidence supports the hypothesis that damaging effects of elevated proinflammatory factors on isolated β-cells involves increased oxidative stress leading to demise of the β-cell. This may, in part, be due to activation of phagocyte-like NADPH oxidase endogenous to the islet β-cell. On the basis of the extant data and our current findings, it is reasonable to speculate that NADPH oxidase-derived oxidative stress exerts cytotoxic effects on the islet β-cell under the duress of noxious stimuli, including chronically elevated glucose, saturated fatty acids, ceramides, and cytokines. From these studies, it is becoming increasingly evident that Rac1 signaling axis plays a critical role in the functional regulation of NADPH oxidase. Further, it appears that inhibition of Tiam1-mediated activation (using NSC23766) or inhibition of posttranslational geranylgeranylation of Rac1 (using GGTI-2147) restores some of these toxic effects. Unfortunately, however, these signaling steps/enzymes cannot be used as drug targets since they have been shown to play key roles in the normal functioning of the islet β-cells, including glucose-stimulated insulin secretion. Therefore, additional studies are needed to develop novel tools/probes to prevent the constitutive/chronic activation of NADPH oxidase and generation of oxidative stress following exposure of the islet β-cell to aforestated stimuli and prevent the associated metabolic dysfunction, loss of β-cell mass, and the onset of diabetes.
GRANTS
This research was supported by a Merit Review Award from the Department of Veterans Affairs, the National Institutes of Health (DK 74921), and a Research Award from the Juvenile Diabetes Research Foundation (1-2006-4). A. Kowluru is also the recipient of the Senior Research Career Scientist Award from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Zelinette Rodriguez for excellent technical assistance.
REFERENCES
- 1. Chen T, Wong YS. Selenocystine induces apoptosis of A375 human melanoma cells by activating ROS-mediated mitochondrial pathway and p53 phosphorylation. Cell Mol Life Sci 65: 2763–2775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54 Suppl 2: S97–S107, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Collier JJ, Fueger PT, Hohmeier HE, Newgard CB. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes 55: 1398–1406, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Dang PMC, Fontayne A, Hakim J, El Benna J, Perianin A. Protein kinase C ζ phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol 166: 1206–1213, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 101: 7618–7623, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, Storling J, Rosenberg L, Billestrup N, Maysinger D, Mandrup-Poulsen T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes 58: 1807–1815, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guichard C, Moreau R, Pessayre D, Epperson TK, Krause KH. NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem Soc Trans 36: 920–929, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Gurzov EN, Ortis F, Cunha DA, Gosset G, Li M, Cardozo AK, Eizirik DL. Signaling by IL-1beta+IFN-gamma and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ 16: 1539–1550, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98: 453–462, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939–1945, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Kim KW, Chung HH, Chung CW, Kim IK, Miura M, Wang S, Zhu H, Moon KD, Rha GB, Park JH, Jo DG, Woo HN, Song YH, Kim BJ, Yuan J, Jung YK. Inactivation of farnesyltransferase and geranylgeranyltransferase I by caspase-3: cleavage of the common alpha subunit during apoptosis. Oncogene 20: 358–366, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev 31: 52–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li XL, Xu G, Chen T, Wong YS, Zhao HL, Fan RR, Gu XM, Tong PC, Chan JC. Phycocyanin protects INS-1E pancreatic beta cells against human islet amyloid polypeptide-induced apoptosis through attenuating oxidative stress and modulating JNK and p38 mitogen-activated protein kinase pathways. Int J Biochem Cell Biol 41: 1526–1535, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Xu G, Bordin S, Curi R, Newsholme P, Carpinelli AR. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia 50: 359–369, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Morgan D, Rebelato E, Abdulkader F, Graciano MF, Oliveira-Emilio HR, Hirata AE, Rocha MS, Bordin S, Curi R, Carpinelli AR. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology 150: 2197–2201, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112: 481–490, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Newsholme P, Keane D, Welters HJ, Morgan NG. Life and death decisions of the pancreatic beta-cell: the role of fatty acids. Clin Sci (Lond) 112: 27–42, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HC, Procopio J, Curi R, Carpinelli A. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 52: 2489–2498, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes 52: 1457–1463, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Piro S, Anello M, Di Pietro C, Lizzio MN, Patane G, Rabuazzo AM, Vigneri R, Purrello M, Purrello F. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism 51: 1340–1347, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Sarkar SA, Kutlu B, Velmurugan K, Kizaka-Kondoh S, Lee CE, Wong R, Valentine A, Davidson HW, Hutton JC, Pugazhenthi S. Cytokine-mediated induction of anti-apoptotic genes that are linked to nuclear factor κB (NF-κB) signalling in human islets and in a mouse beta cell line. Diabetologia 52: 1092–1101, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Souza KL, Gurgul-Convey E, Elsner M, Lenzen S. Interaction between pro-inflammatory and anti-inflammatory cytokines in insulin-producing cells. J Endocrinol 197: 139–150, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Syed I, Jayaram B, Subasinghe W, Kowluru A. Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol 80: 874–883, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tawa P, Hell K, Giroux A, Grimm E, Han Y, Nicholson DW, Xanthoudakis S. Catalytic activity of caspase-3 is required for its degradation: stabilization of the active complex by synthetic inhibitors. Cell Death Differ 11: 439–447, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes 51: 311–316, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Uchizono Y, Takeya R, Iwase M, Sasaki N, Oku M, Imoto H, Iida M, Sumimoto H. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci 80: 133–139, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Veluthakal R, Kaur H, Goalstone M, Kowluru A. Dominant-negative alpha-subunit of farnesyl- and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes 56: 204–210, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A. Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem Pharmacol 77: 101–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veluthakal R, Palanivel R, Zhao Y, McDonald P, Gruber S, Kowluru A. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis 10: 841–850, 2005 [DOI] [PubMed] [Google Scholar]