Abstract
Breathing hyperbaric oxygen (HBO2), particularly at pressures above 3 atmospheres absolute, can cause acute pulmonary injury that is more severe if signs of central nervous system toxicity occur. This is consistent with the activation of an autonomic link between the brain and the lung, leading to acute pulmonary oxygen toxicity. This pulmonary damage is characterized by leakage of fluid, protein, and red blood cells into the alveoli, compatible with hydrostatic injury due to pulmonary hypertension, left atrial hypertension, or both. Until now, however, central hemodynamic parameters and autonomic activity have not been studied concurrently in HBO2, so any hypothetical connections between the two have remained untested. Therefore, we performed experiments using rats in which cerebral blood flow, electroencephalographic activity, cardiopulmonary hemodynamics, and autonomic traffic were measured in HBO2 at 5 and 6 atmospheres absolute. In some animals, autonomic pathways were disrupted pharmacologically or surgically. Our findings indicate that pulmonary damage in HBO2 is caused by an abrupt and significant increase in pulmonary vascular pressure, sufficient to produce barotrauma in capillaries. Specifically, extreme HBO2 exposures produce massive sympathetic outflow from the central nervous system that depresses left ventricular function, resulting in acute left atrial and pulmonary hypertension. We attribute these effects on the heart and on the pulmonary vasculature to HBO2-mediated central sympathetic excitation and catecholamine release that disturbs the normal equilibrium between excitatory and inhibitory activity in the autonomic nervous system.
Keywords: hyperbaric oxygen toxicity, cardiogenic pulmonary hypertension, autonomic nervous system, sympathetic excitation, nitric oxide bioactivity
breathing oxygen at elevated pressures (hyperbaric hyperoxia), especially above 3 atmospheres absolute (ATA), can cause severe pulmonary damage in minutes. By contrast, the lung injury caused by inhalation of high concentrations of O2 at 1 ATA (normobaric hyperoxia) develops over hours or days. In hyperbaric oxygen (HBO2), acute pulmonary damage is characterized by transpulmonary leakage of fluid and protein and by focally distributed intra-alveolar hemorrhage (3, 17) and is particularly likely in subjects in which central nervous system (CNS) O2 toxicity has also occurred, as manifested by electroencephalogram (EEG) spikes or motor convulsions (5, 6, 41). Neuroendocrine pathways, especially in the sympathoadrenal system, have been implicated in this abrupt pulmonary damage (reviewed in Ref. 12). Indeed, interruption of these pathways before HBO2 exposure, by hypophysectomy and adrenalectomy (8) or by adrenergic blockade (7), can mitigate pulmonary injury. In our laboratory, for example, we have found that the β-adrenergic antagonist propranolol protects the lungs of conscious rats exposed to 3 ATA O2 (17).
Because adrenergic blockade can diminish or prevent the severe pulmonary injury associated with oxygen seizures, it appears that the sympathetic nervous system links CNS toxicity and pulmonary injury in HBO2. Although it is not known how this occurs, we hypothesize that sympathetic activity, with consequent catecholamine release, potentiates pulmonary O2 toxicity by altering cardiac dynamics and central hemodynamics. Past studies, as well as our own more recent observations, have shown that the severe lung injury seen in rats that exhibit motor convulsions or EGG spikes in HBO2 is characterized by pulmonary edema and extravasation of red blood cells (RBCs) into the alveoli (9, 17, 26). The most straightforward explanation for such a pathological pattern is that pulmonary or left atrial hypertension mechanically compromises the integrity of alveolar-capillary membrane, allowing RBCs to enter the air spaces. Indeed, HBO2 does increase systemic arterial blood pressure (BP) and reduce cardiac output (CO), which together would shift blood from the systemic to the pulmonary circulation and increase pulmonary blood volume (PBV) and pressure. Although these responses to HBO2 may be rapid and transient, any resulting disruption of pulmonary capillaries would persist for a time, allowing protein leak, pulmonary edema, and extravasation of RBCs. The hemodynamic components of pulmonary hypertension in HBO2 and the autonomic discharges that could produce these changes have not been demonstrated experimentally, nor have such changes been explicitly linked to CNS O2 toxicity.
The objectives of this study are to investigate real-time cardiac and hemodynamic responses to HBO2 that may contribute to pulmonary hypertension and acute hydrostatic lung injury and to study the role of the autonomic nervous system in linking CNS O2 toxicity and pulmonary injury. Thus we exposed anesthetized, instrumented rats to HBO2 at 6 ATA to simultaneously monitor the EEG, sympathetic activity, cardiac dynamics, and central and pulmonary hemodynamics. Some animals were vagotomized shortly before HBO2 exposure. In a separate set of experiments, we exposed awake rats, either freely moving or lightly constrained, to HBO2 at 5 ATA to eliminate the confounding effects of the anesthetic and paralytic agents. Some of the awake rats were subjected to left unilateral vagotomy to study the effects of chronic disruption of vagal pathways, or were chronically catheterized to measure hemodynamics and to obtain blood samples to assay for catecholamines.
In all rats, pulmonary damage was assessed immediately after hyperbaric exposure to correlate the severity of lung injury with hemodynamic parameters, neuronal activity, or surgical or pharmacological disruption of autonomic pathways. Results were compared with findings in untreated control animals.
METHODS AND EXPERIMENTAL DESIGN
Surgical preparation for rats exposed to 6 ATA HBO2 under anesthesia.
Male Sprague-Dawley rats weighing 398 ± 19 g were used, as approved by the Duke University Institutional Animal Use and Care Committee (Durham, NC). Anesthesia was induced with urethane (750 mg/kg ip) and α-chloralose (25 mg/kg ip) and maintained by intravenous administration of one-fourth of the initial dose of anesthetic each hour, or as indicated by BP and heart rate (HR) responses.
The left femoral artery and vein were catheterized to monitor BP, withdraw samples, and infuse drugs. For measuring CO, polyethylene tubing (PE 50) was inserted through the right jugular vein into the right atrium, and a thermistor (model 511; Yellow Springs Instruments) was advanced into the ascending aorta via the right carotid artery. To measure ventricular pressures, polyethylene tubing was also inserted into the right ventricle through the right jugular vein or in the left ventricle (LV) through the right carotid artery. The positions of the tubing and thermistor were verified at autopsy.
The trachea was intubated, and anesthetized animals were ventilated with 30% O2 in N2 (termed “air” in this study). Anesthetized rats were given a single dose of pancuronium bromide (0.5 mg/kg iv) to prevent voluntary respiratory movements and to maintain arterial Pco2 at 35 to 40 Torr, by adjusting tidal volume.
To measure regional cerebral blood flow (rCBF), the head of each rat was secured in a stereotaxic frame, the scalp reflected, a craniotomy made, and dura mater opened with a small incision. Then an insulated platinum wire electrode, having a bare conical tip 1 mm in length with an apical diameter of 10–50 μm, was inserted into the striatum under stereotaxic control, with an Ag-AgCl reference electrode clipped to the tail. Two stainless steel cranial screws were positioned over the left and right parietal cortex for EEG recording.
For acutely vagotomized rats, an incision was made in the neck, and either the left vagus or both vagi were exposed and encircled with surgical thread that was then passed through a 22-gauge needle, around which the wound was closed. Approximately 30 min before HBO2 exposure, control measurements were made, and the thread was pulled to transect the nerves.
Central hemodynamic measurements.
Arterial and venous BPs were measured continuously through catheters inserted into the femoral artery and the right atrium, respectively, using pressure transducers (Vigo-Spectramed, Oxnard, CA). The pulse signals were electronically averaged to obtain mean arterial (MABP) or venous BPs. HR was determined from pulse waves in the arterial pressure tracing. CO was calculated from transpulmonary thermodilution curves (13) generated by a 0.075-ml bolus of room temperature glucose solution (2.5%) injected rapidly into the right atrium by an infusion pump (Harvard Apparatus, Holliston, MA) operated remotely from outside the hyperbaric chamber. CO was calculated from temperature changes in the blood of the aortic root as follows:
where CO is in ml/min; Q is the injected volume (0.075 ml); Tb is blood temperature (°C); Ti is the injectate temperature (°C); K is a derived constant (62.14); and the area under curve is in units of °C × seconds. CO was normalized for body weight (ml·min−1·100 g body wt−1).
Cardiopulmonary hemodynamics and ventricular function.
Right (RVP) and LV pressures (LVP) were measured, using catheters inserted directly into the ventricles. Right ventricular systolic pressure (RVSP) and LV end-diastolic pressure (LVEDP), averaged over 10 s, were used as indicators of pulmonary arterial pressure and pulmonary venous pressure, respectively. Pulmonary vascular resistance (PVR) was calculated from these means:
PBV was assessed as described by Abel et al. (2) with the following minor modification: 0.1 ml of ascorbic acid solution (40 mg/ml in 0.9% NaCl solution) was injected into the right ventricle and detected by the polarized platinum electrode (+400 mV vs. Ag-AgCl) in the LV and connected to a polarographic amplifier (model 1900, A-M Systems, Carlsborg, WA). The resulting indicator-dilution curves were used to compute mean transit time (MTT) (42). PBV was then given as:
where CO is in ml·min−1·100 g body wt−1, and MTT is in seconds. Stroke work (the product of stroke volume and mean arterial pressure) was used as an index of LV function (31).
CBF.
rCBF was measured by hydrogen clearance using platinum electrodes inserted into the striatum (15). To initiate a measurement, 2.5% H2 in air was introduced through the respirator for 40 s, and then H2 washout curves were captured using a polarographic amplifier, computer, and WinDaq software (D-1200 AC, DATAQ Instruments). Absolute rCBF (ml·g−1·min−1) was calculated, using Mathematica 3.0 software (Wolfram Research).
EEG and renal sympathetic nerve activity.
The EEG was monitored visually for onset of CNS oxygen toxicity, signaled by a train of multiple EEG spikes repeated every few seconds.
To record renal sympathetic nerve activity (RSNA), the left kidney was exposed through the retroperitoneal space. A branch of the renal nerve was lifted free from fat and connective tissue and placed on a pair of platinum electrodes. A silicone gel (Wacker SilGel 604 A/B) was used to electrically isolate the nerve-electrode junctions from surrounding tissue (29). The RSNA signal was amplified by a low-noise differential amplifier and recorded using WinDaq software. The signal averaged over 2 min after completion of the experiment, with all instruments still running, represented background noise. Peak RSNA above background was averaged over 10 s and expressed as percent change from control levels in animals breathing air at 1 ATA.
Arterial Po2, arterial Pco2, and pH were determined in blood from animals breathing air at 1 ATA before each experiment and then from samples taken immediately after decompression from HBO2 exposure, with animals still breathing 100% O2 (1306 blood gas/pH analyzer, Instrumentation Laboratories). Rectal temperature was monitored continuously and maintained at 37 ± 0.5°C, using a heating pad.
Assessment of bronchoalveolar protein and pulmonary edema.
Bilateral bronchoalveolar lavage (BAL) was performed in control animals, as well as in rats exposed to HBO2, using 10 ml of sterile phosphate-buffered 0.9% NaCl solution. BAL fluid (BALF) protein was used as a marker for lung injury. Total protein content was measured with the bicinchoninic acid assay using bovine serum albumin as a standard (37). As an index of edema, wet-to-dry weight ratio was assessed in freshly excised lungs: the lungs were weighed immediately after excision and then placed in a vacuum oven at 60°C for at least 72 h, or until a stable dry weight was obtained. No corrections were made for intravascular lung water.
HBO2 exposures for anesthetized rats.
Animals were placed in a hyperbaric chamber (Duke Center for Hyperbaric Medicine and Environmental Physiology), along with the stereotaxic frame, respirator, BP transducer, heating pad, and infusion pumps. Electrodes were connected through hermetic wall penetrations to amplifiers outside the chamber. After a 60-min stabilization period, during which the animal breathed 30% O2 in N2, all physiological parameters were recorded three times and averaged to establish control values. The respirator was supplied with 100% O2, and the chamber was pressurized with air to 6 ATA, at 0.6 ATA/min. Temperature and relative humidity in the chamber were maintained at 23 ± 0.5°C and 60 ± 2%, respectively. Exposure to HBO2 was limited to 60 min to avoid prolonged seizures. Decompression to 1 ATA was accomplished at rate of 0.6 ATA/min, as the rats continued to breathe 100% O2. Immediately after animals were euthanized with Nembutal (250 mg/kg), lung lavage was performed.
HBO2 exposure of awake rats at 5 ATA.
To control for the effects of anesthetic and paralytic agents on physiological measurements, we exposed awake Sprague-Dawley male rats, either freely moving or lightly constrained by hammock, to O2 at 5 ATA for 60 min in a small-animal hyperbaric chamber at the Institute of Evolutionary Physiology and Biochemistry, Russian Academy Sciences, St. Petersburg, Russia. These experiments were approved by the Institute, in accord with the Declaration of Helsinki. This level of exposure was chosen because our laboratory had previously found that oxygen seizure latencies in awake animals in 5 ATA HBO2 were similar to those in anesthetized rats at 6 ATA (16).
For chronically vagotomized rats, the left vagus nerve was transected at the cervical level under Nembutal anesthesia (50 mg/kg). Using sterile procedure, an incision was made in the neck, the left vagus was carefully exposed by blunt dissection, and an ∼2-mm section was removed. The wound was closed and sutured, and 7–10 days were allowed for recovery before exposure to hyperoxia.
To prepare chronically catheterized animals, the right carotid artery was exposed under Nembutal anesthesia (50 mg/kg); and polyethylene tubing (PE 50) filled with 0.9% NaCl solution containing glucose (2.5%) and heparin (300 IU/ml) was inserted, secured with a ligature, and then tunneled subcutaneously to exit at the back of the neck; and the wound was closed and sutured. Two stainless steel cranial screws were inserted in the skull for later recording of EEG. Each animal was allowed to emerge from anesthesia and recover from surgery for 7–10 days, during which time the tubing was refilled daily with fresh solution.
Intact or vagotomized rats were placed three to four in a partitioned cage and exposed to 100% O2 at 5 ATA for 60 min. Temperature and humidity were controlled (22–24°C and 60–65%), and CO2 was kept <1%. The animals was observed continuously, and seizure latencies were noted. After decompression, the animals were euthanized with Nembutal (250 mg/kg), and BAL was performed.
Chronically catheterized rats were placed individually in the hyperbaric chamber. During HBO2 exposure, arterial BP and HR were monitored by means of the arterial catheter, and EEG was recorded. Immediately after decompression, arterial blood samples were drawn for norepinephrine assay by HPLC (Coulochem II analyzer, ESA, Chelmsford, MA), as described (39).
Study design.
Six groups of anesthetized rats were used. Because simultaneous insertion of catheters in the aorta and both ventricles could adversely affect hemodynamic function, CO was measured independently. The first group of monitored rats (n = 17) was exposed to O2 at 6 ATA, and CO was measured every 10 min, while RSNA was monitored continuously. Immediately after decompression, protein in BALF and extent of lung edema were assessed. The next two groups of rats were subjected to the above protocol, except that RVP (group 2, n = 9) or LVP (group 3, n = 11) were measured instead of CO. The fourth (n = 14), fifth (n = 7), and sixth (n = 9) groups were used to verify whether autonomic activity is associated with cardiopulmonary dysfunction and lung injury in HBO2. For this, neuronal and hemodynamic parameters were measured in rats treated with propranolol (5 mg/kg iv) 20 min before compression (group 4), or subjected to either unilateral (group 5) or bilateral (group 6) cervical vagotomies 30 min before compression.
Three groups of awake rats were used: freely moving intact rats (n = 12), freely moving, chronically vagotomized rats (n = 13), and lightly-constrained catheterized rats (n = 12). Seizure latencies were determined in all of these animals, and MABP and EEG were recorded in chronically catheterized animals.
Statistical analysis.
Data were analyzed using StatView software (SAS Institute, Cary, NC). Absolute and percent changes in physiological variables measured during HBO2 exposures were compared with baseline values taken in air at 1 ATA, using repeated ANOVA, followed by post hoc comparison with Fisher's exact test. Values from groups of animals are expressed as means ± SE. Intergroup comparisons were made using a Student's t-test, with P < 0.05 accepted as significant.
RESULTS
Seizures, pulmonary O2 toxicity, and sympathetic outflow in awake rats.
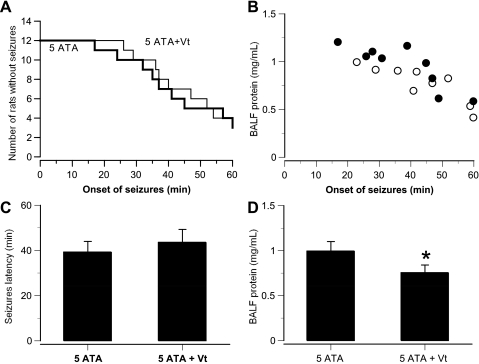
Of the freely moving awake rats exposed to O2 at 5 ATA for 60 min, 9 of 12 exhibited convulsions, with latencies between 17 and 60 min (Fig. 1A). Postmortem assay of BALF in this group of animals revealed pronounced pulmonary protein leakage, inversely proportional to seizure latency (Fig. 1B). The chronically left-vagotomized rats showed decreased pulmonary damage in HBO2: 9 of 13 had seizures, but no difference in seizure latency was observed compared with the intact rats (Fig. 1, C and D). Nine of the twelve chronically catheterized rats also manifested seizures, and patterns of EEG discharge progressed in three pathological stages. Within the first 15–45 min after reaching 5 ATA, amplitude decreased and frequency increased. Next, a desynchronized pattern transformed to slow delta- and theta-waves, with irregular single spikes. Finally, multiple spikes were observed in seven of the nine catheterized animals 1–5 min before seizures occurred.
Fig. 1.
Central nervous system (CNS) O2 toxicity and lung injury in intact and chronically vagotomized (Vt) awake rats in 5-atmospheres absolute (ATA) O2. A: Kaplan-Meier plot of time to onset of motor convulsions in 12 intact and 12 Vt rats during 60-min exposure to 5 ATA O2, beginning at time 0. Nine rats in each group exhibited convulsions. B: bronchoalveolar lavage fluid (BALF) total protein in intact (●) and Vt (○) rats was plotted against seizure latency. C: seizure latency in intact and Vt rats. D: BALF total protein in intact and Vt rats. Values are means ± SE. *P < 0.05 vs. 5 ATA.
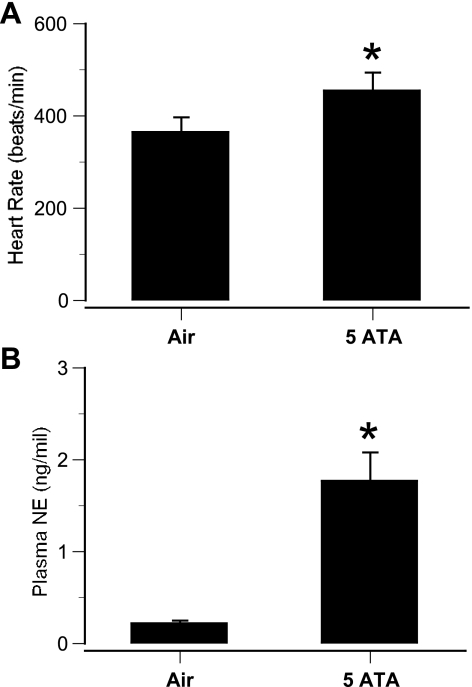
HBO2 was also associated with sympathetic excitation, as indicated by an increase in HR (Fig. 2A). Also, MABP increased from control values of 103 ± 8.3 to 178 ± 13.3 mmHg at the onset of EEG spikes. In addition, plasma norepinephrine levels increased dramatically in rats that had exhibited EEG spikes (Fig. 2B).
Fig. 2.
Sympathetic activity in awake rats in 5 ATA O2. A: heart rate (HR) in awake rats exhibiting seizures in hyperbaric oxygen (HBO2). HR was measured before HBO2 (control) and after the onset of electroencephalogram (EEG) spikes. B: plasma norepinephrine (NE) levels in rats exhibiting EEG spikes. Arterial blood samples from chronically catheterized rats were obtained immediately after 60-min HBO2 exposures. Values are means ± SE. *P < 0.05 vs. air.
EEG seizures and sympathetic outflow in anesthetized rats.
The appearance of EEG, RSNA, and central hemodynamic changes was similar in all anesthetized rats exposed to 6 ATA HBO2. Thus the EEG before seizures was comparable to that seen in awake animals in HBO2, with initial reductions in amplitude and increases in frequency, followed by increases in amplitude just before the first spikes. In the final stage, generalized spikes appeared in ∼80% of the animals, with a mean latency of 47 ± 4.2 min. And 1–3 min before generalized EEG spikes, there was a sudden, statistically significant increase in RSNA (n = 12, P < 0.05), reaching 13.9–51.4% above control levels after 44–60 min from beginning of HBO2 exposure.
Hemodynamic changes in anesthetized rats.
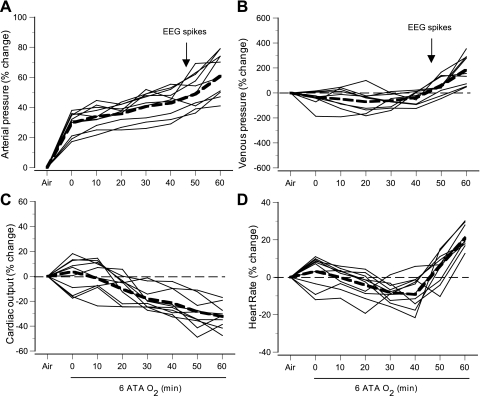
Control values of hemodynamic parameters in anesthetized rats breathing air at 1 ATA are shown in Table 1, and time courses of changes in these parameters in 6 ATA HBO2 are illustrated in Fig. 3. MABP rose initially during compression, followed by slower rise, leading to the appearance of the first EEG spikes (Fig. 3A). By contrast, venous pressures changed little during the first 30 min at 6 ATA, but then increased approximately two- to threefold as EEG spikes appeared (Fig. 3B).
Table 1.
Baseline hemodynamic parameters in anesthetized rats
| Control | Propranolol | Vagotomy | |
|---|---|---|---|
| MABP, mmHg | 104 ± 6 | 98 ± 9 | 109 ± 9 |
| MVBP, mmHg | 1.2 ± 1.4 | 1.4 ± 1.3 | 0.9 ± 1.1 |
| Heart rate, beats/min | 386 ± 23 | 379 ± 25 | 421 ± 27* |
| Cardiac output, ml/min | 178 ± 19 | 166 ± 15 | 191 ± 18 |
| RVSP, mmHg | 38 ± 5 | 35 ± 7 | 39 ± 5 |
| LVEDP, mmHg | 2.1 ± 0.3 | 2.5 ± 0.4 | 1.9 ± 0.3 |
Values are means ± SE. MABP, mean arterial blood pressure; MVBP, mean venous blood pressure; RVSP, right ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure.
P < 0.05 vs. control.
Fig. 3.
Central hemodynamic parameters in anesthetized rats in 6 ATA O2. Changes in mean arterial (A) and venous blood pressures (B), cardiac output (CO; C), and HR (D) in individual rats were plotted for the control period in air at 1 ATA, followed by 6 ATA O2 for 60 min. Compression to 6 ATA was achieved at time 0. Dashed lines are mean values. Arrows indicates the mean time for onset of EEG spikes.
CO fell gradually, decreasing 19–46% by the end of hyperbaric exposure (Fig. 3C). HR decreased progressively during the first 30 min of HBO2 exposure, but then rose to preexposure levels and above, reaching 15–35% (440–520 beats/min) above control levels in air at 1 ATA by the end of the exposure, and peaking near 500 beats/min after the onset of EEG spikes (Fig. 3D).
Cardiopulmonary function in HBO2.
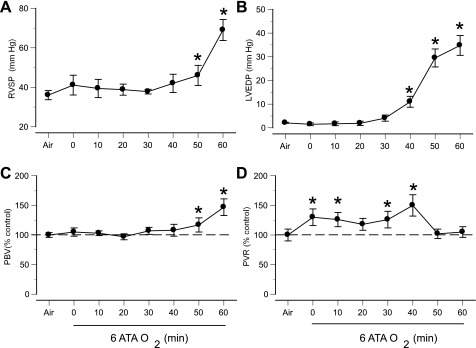
Control values of cardiopulmonary function in rats breathing air at 1 ATA are shown in Table 1. Oxygen at 6 ATA altered cardiovascular function in a time-dependent manner. Systolic RVP rose during compression, returned close to preexposure levels within 30–40 min of reaching 6 ATA, and rose rapidly again as EEG spikes appeared (Fig. 4A). End-diastolic LVP did not change for 30–40 min after 6 ATA was reached, but a sudden increase was observed close to the onset of EEG spikes (Fig. 4B). Both RVSP and LVEDP remained elevated until decompression. PBV did not change significantly for the first 30 min of HBO2, but increased dramatically when EEG spikes appeared (Fig. 4C). Also, calculated PVR was significantly higher within the first 40 min of HBO2 and decreased after the onset of EEG spikes (Fig. 4D).
Fig. 4.
Cardiopulmonary hemodynamic responses in anesthetized rats in 6 ATA O2. A: right ventricular systolic pressure (RVSP). B: left ventricular end-diastolic pressure (LVEDP). C: calculated pulmonary blood volume (PBV). D: calculated pulmonary vascular resistance (PVR). RVSP and LVEDP are expressed as absolute values; PBV and PVR are shown as percentages of preexposure levels (in air at 1 ATA). Compression to 6 ATA was achieved at time 0. Values are means ± SE. *P < 0.05 vs. air.
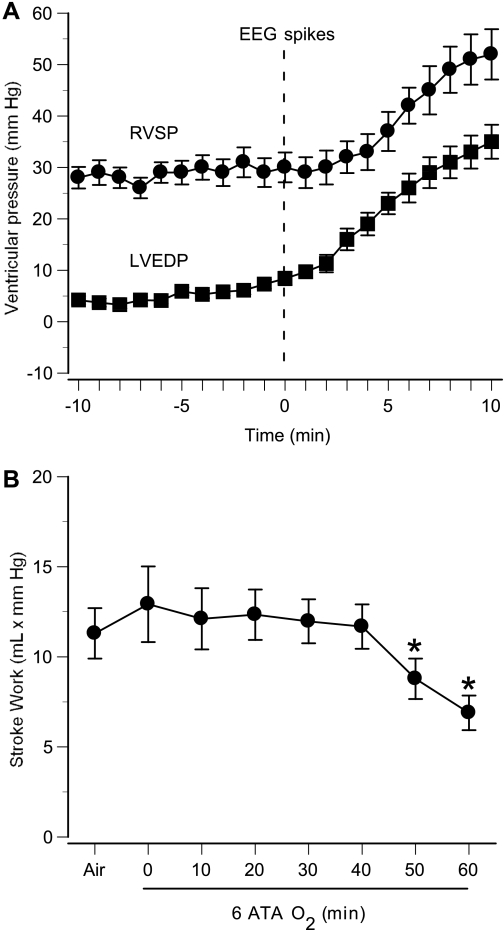
To assess changes in pulmonary arterial and venous pressures in HBO2, we determined temporal profiles of RVSP and LVEDP with respect to EEG discharges, taking the onset of spikes as the zero point. Figure 5A shows that increases began earlier in LVEDP than in RVSP and peaked after the onset of seizures. LV function did not change significantly during the first 40 min of HBO2 at 6 ATA, as indicated by the stroke work curve. After that, stroke work decreased markedly in rats exhibiting seizures, demonstrating substantial LV functional impairment after the massive sympathetic activation associated with seizures (Fig. 5B).
Fig. 5.
Ventricular pressures and left ventricular function in anesthetized rats in 6 ATA O2. Mean values of cardiac parameters are shown for 8 anesthetized animals that demonstrated EEG spikes during 60-min exposure. A: RVSP and LVEDP are shown for 10 min before and after the onset of EEG spikes (time 0). B: stroke work (stroke volume × mean arterial pressure), an index of left ventricular function, was calculated every 10 min. Left ventricular function (stroke work) diminished significantly in these rats, which exhibited EEG spikes. Values are means ± SE. *P < 0.05 vs. air.
Lung injury in HBO2.
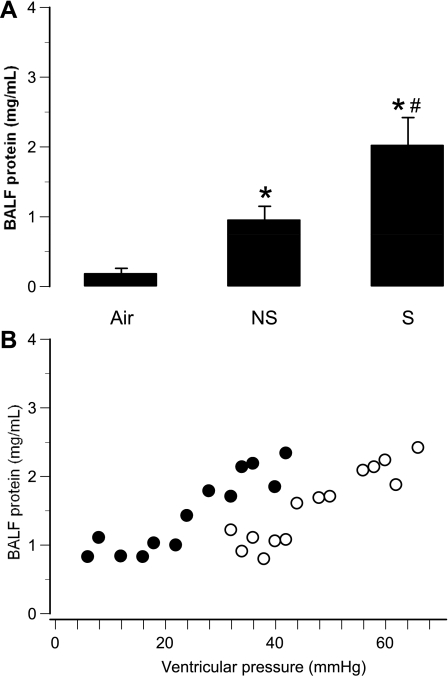
In animals that had exhibited EEG spikes, postmortem inspection of the lungs revealed a patchy distribution of intra-alveolar hemorrhage, as well as pulmonary edema. The mean wet-to-dry weight ratio of rats exposed to HBO2 was 5.2 ± 0.13, compared with 4.8 ± 0.09 in controls (P < 0.01). In rats that did not exhibit EEG spikes in 6 ATA HBO2, total BALF protein was 4 times control levels, whereas it was 10 times control levels in rats that did exhibit spikes (Fig. 6A). Severity of lung injury correlated closely with the magnitude of changes in RVSP or LVEDP, as indicated by the stability of total BALF protein until RVSP rose above 40 mmHg or LVEDP exceeded 20 mmHg (Fig. 6B).
Fig. 6.
BALF total protein and ventricular pressures in anesthetized rats in 6 ATA O2. A: BALF total protein is shown for controls, as well as for rats exhibiting generalized EEG spikes (S) and those not exhibiting spikes (NS). Values are means ± SE. *P < 0.05 vs. air. #P < 0.05 vs. NS. B: peak values of LVEDP (●) or the RVSP (○) were plotted against BALF protein for individual rats.
The effect of β-adrenergic blockade and vagotomy on pulmonary oxygen toxicity.
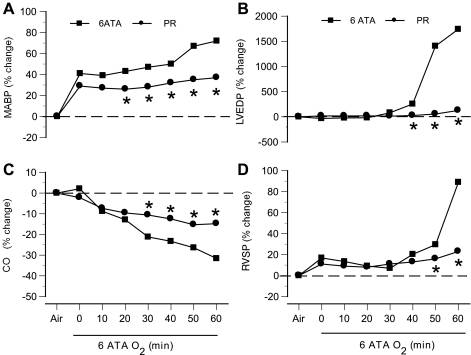
Propranolol lowered CO and slowed HR, but did not alter arterial BP or cardiopulmonary hemodynamics in rats breathing air (Table 1). However, rats exposed to HBO2 after propranolol treatment exhibited changes in systemic and cardiopulmonary hemodynamics, along with a significant decrease in blood flow (23 ± 3.8%) in the striatum (Fig. 7). Rats pretreated with propranolol before exposure to 6 ATA O2 were protected from both CNS O2 toxicity and pulmonary damage: no seizures were observed in any of these animals (n = 14), and mean BALF protein concentration in these animals was 0.41 ± 0.06 vs. 0.94 ± 0.11 mg/ml in untreated animals without seizures and 2.01 ± 0.29 mg/ml in untreated animals with seizures. For comparison, mean BALF protein in control animals breathing air at 1 ATA was 0.18 ± 0.03 mg/ml.
Fig. 7.
Hemodynamic responses of anesthetized rats in 6 ATA HBO2 after pretreatment with propranolol. Changes in mean arterial blood pressure (MABP; A), LVEDP (LVEDP; B), CO (C), and RVSP (RVSP; D) are shown as percentages of control values in air at 1 ATA. Compression to 6 ATA was achieved at time 0. *P < 0.05 vs. untreated rats exposed to 6 ATA O2.
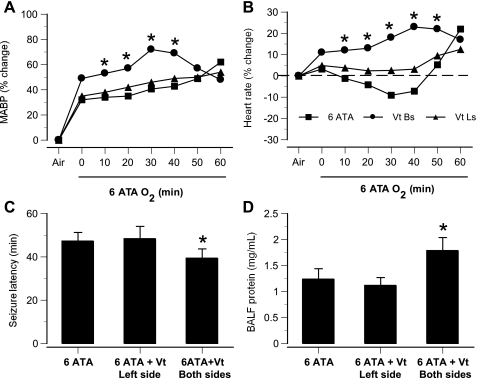
Left vagotomy 30 min before compression did not significantly change the time course of hemodynamic parameters in HBO2 (Fig. 8). However, acute bilateral vagotomy altered hemodynamic parameters in rats breathing air at 1 ATA (Table 1), and, in rats exposed to HBO2 it accelerated both sympathetic excitation and onset of CNS O2 toxicity, as indicated by the time courses of MABP and HR and by seizure latency (Figs. 8, A–C). BAL protein in bilaterally vagotomized rats was also higher (Fig. 8D).
Fig. 8.
CNS-mediated sympathetic excitation and lung injury in anesthetized, intact, unilaterally Vt, and bilaterally Vt rats in 6 ATA O2. Time courses are shown for changes in MABP (A) and mean HR (B) in bilaterally Vt rats (●), left-Vt rats (▴), and intact rats (■). C: seizure latencies in acutely left-Vt rats were not significantly different from those in intact rats, but bilaterally Vt rats had significantly shortened seizure latencies. D: lung damage, as indicated by BALF total protein, was significantly greater in bilaterally Vt rats than in intact animals. *P < 0.05 vs. intact rats.
DISCUSSION
Although it has been recognized for some time, and confirmed here, that HBO2-induced seizures exacerbate pulmonary injury, this is the first study to define the pathophysiological mechanisms responsible for the association of CNS and pulmonary toxicity in HBO2 by simultaneous measurement of cardiopulmonary hemodynamics and autonomic activity. The most novel of our findings is that acute lung injury in HBO2 is associated with massive sympathetic activation, coupled with profound changes in systemic and cardiopulmonary hemodynamics. Thus we find that lung damage in HBO2 is linked to extreme pulmonary hypertension and elevated left atrial pressure of cardiogenic origin. We also found that sympathetic excitation in HBO2 allows an increase in plasma catecholamines that presumably contribute to the pulmonary capillary hypertension and lung injury. Finally, these studies indicate that vagal afferents play an important role in both CNS and pulmonary toxicity in HBO2.
Are CNS and pulmonary HBO2 toxicity pathologically distinct?
The convulsions and pulmonary damage induced by HBO2 have been considered as separate entities with distinct etiologies, with oxygen acting directly on the CNS in the first case and on the alveolar region of the lung in the second (34). As early as 1953, Bean and coworkers (5, 6, 8, 27) demonstrated that HBO2 can act on the lungs indirectly through CNS-mediated sympatho-adreno-medullary pathways. Our demonstration that HBO2-induced pulmonary damage and CNS O2 toxicity are linked by the sympathetic nervous system is consistent with these early findings. In accord with observations that EEG spiking comprises an early stage of CNS oxygen toxicity (24, 32, 38), we observed spikes in 75% of awake rats exposed to O2 at 5 ATA and ∼80% of anesthetized rats exposed over 60 min to 6 ATA O2. All of these animals also exhibited damage to alveolar-capillary membranes, as indicated by pulmonary edema and high levels of BALF protein. By contrast, animals that did not exhibit EEG spikes sustained significantly less pulmonary injury (Fig. 6A), implying that the pulmonary and CNS manifestations of HBO2 toxicity are not independent events and that, in large part, early lung damage in HBO2 is attributable to CNS O2 toxicity. In addition, this pulmonary pathology could not be due to the mechanical effects of motor convulsions, since the lung injury occurred even though the somatic component of seizures was prevented by pancuronium bromide.
Hemodynamic components of lung injury in HBO2.
In rats exposed to O2 at 6 ATA, we observed that acute pulmonary hemorrhage was associated with systemic hypertension, decreases in CO, and dramatic pulmonary hypertension. Although pulmonary vascular pressure was not measured directly, which is technically difficult in small animals under hyperbaric conditions, we infer from our RVSP and LVEDP data that the transpulmonary hydrostatic forces were sufficient to damage the pulmonary microvasculature. Close positive correlation between RVSP and LVEDP and pulmonary hypertension is well established (1, 10, 18, 30, 40). We also found that increases in PBV coincided with massive sympathetic outflow and EEG spikes. And others have measured pulmonary hypertension directly in larger animals, dogs, and rabbits, exposed to O2 at 3 and 4 ATA, respectively (1, 25). The main alternative explanation for this acute pulmonary damage would be increased local vascular permeability, but, in the absence of a direct toxin or noxious agent, such events usually require days (17), whereas we observed red cells in alveolar air spaces within minutes of seizures.
An important question arises from our observations: which pulmonary pressure, arterial or venous, is most important in this hydrostatic lung injury? We found that a pronounced increase in BALF total protein occurred in animals when RVSP exceeded 40 mmHg or LVEDP exceeded 20 mmHg (Fig. 6B), consistent with the concept that pulmonary capillary pressure must reach a critical threshold for lung injury to develop. Although temporal profiles of RVSP and LVEDP throughout HBO2 exposure were very similar (Fig. 5A), LV preload began to rise 2–5 min earlier than right ventricular afterload. Moreover, relative pressure changes in the right ventricle and LV differed significantly: RVSP doubled after the onset of EEG spikes, whereas LVEDP increased to ∼15 times normal levels. Because LVEDP represents left atrial and pulmonary venous pressures (31), we suggest elevated hydrostatic pressure occurs first in the pulmonary veins, and that this likely drives capillary damage. It is well known that pulmonary hypertension is a common cause of pulmonary edema (11, 20), yet we must also mention that increases in pulmonary arterial pressure probably also contribute to hydrostatic lung injury by raising pulmonary capillary pressure, since RVSP nearly doubled after the onset of EEG spikes. This is consistent with our laboratory's previous observations of acute pulmonary capillary damage and extravasation of red cells in rats after oxygen convulsions (17).
Another question about mechanisms by which pulmonary pressure increases in HBO2 concerns the role of altered cardiac dynamics. It is clear that the major source of volume and pressure loading in the pulmonary circulation occurs when pulmonary venous return into the LV exceeds LV output (22, 35). Our study has demonstrated that increases in PBV and LVEDP (LV return) are accompanied by dramatic decreases in LV output at the onset of EEG spikes or sympathetic excitation in rats exposed to O2 at 6 ATA, pointing to LV dysfunction. Our evidence for this includes high levels of LVEDP (lowered LV filling), lowered CO, and a marked decrease in LV function (stroke work) after the onset of EEG spikes. All of this indicates that LV contractility is diminished in animals that exhibit sympathetic overexcitation associated with CNS O2 toxicity.
Initially, at least, LV dysfunction in the face of relatively preserved contractility in the right heart increases pulmonary arterial pressure, blood volume, and pulmonary capillary pressure, leading to hydrostatic failure of the microvessels. The imbalance between right and left CO is characterized by an immediate decrease in aortic flow, as observed by us and by others (1, 28). Therefore, our findings suggest that lung damage in HBO2 is caused by sudden pulmonary capillary hypertension, due to acute LV dysfunction and pulmonary hypertension, followed by mechanical disruption of alveolar capillary membranes, resulting in extravasation of plasma protein and red cells into the air spaces.
Autonomic components of lung injury in HBO2.
Massive sympathetic activation after head trauma, stroke, or epileptic seizures may also produce extreme degrees of pulmonary hypertension and pulmonary edema (4, 11, 35). The idea that acute lung injury in HBO2 may also be related to sympathetic hyperexcitation is supported by observations made more than five decades ago that the neuroendocrine system, particularly the sympathoadrenal axis, is an important causal factor in the pulmonary damage associated with convulsions induced by HBO2 (7). This early work showed that hypophysectomy, adrenalectomy, or adrenergic blocking agents afford appreciable protection against pulmonary damage (see Ref. 12 for review), suggesting a role for sympathetic excitation. Here, we have provided direct evidence for this, as indicated by a pronounced increase in RSNA. We also found that these autonomic discharges are associated with severe damage to the pulmonary microvasculature and determined that sympathetic hyperactivity is accompanied by LV dysfunction, leading to pulmonary hypertension, a major causal factor for lung injury. In addition, the systemic vasoconstriction effects of both hyperoxia and sympathetic activation must play a role. The fact that β-adrenergic blockade by propranolol is protective is strong evidence that sympathetic excitation plays a role in acute pulmonary oxygen toxicity in HBO2.
Protection by propranolol, however, cannot be explained by one mechanism alone, because β-adrenergic blockade also diminished systemic and cardiopulmonary hemodynamic responses to HBO (Table 1). These experiments reveal at least two mechanisms that could play key roles in protection from lung injury in propranolol-treated animals. One mechanism may be related to blockade of myocardial β-adrenergic receptors, resulting in preservation of LV function in HBO2. Another may be stabilization of cerebral O2 delivery, indicated by diminished CBF in treated rats, that limits toxic increases in brain Po2. In fact, a decrease in CBF could be a factor in propranolol-mediated protection against both CNS and pulmonary oxygen toxicity.
Previous studies of the effects of the parasympathetic nervous system on CNS and pulmonary HBO2 toxicity show contradictory results. In the present study, however, lung injury in HBO2 at 5 ATA was significantly reduced in awake rats with chronic left vagotomy, as our laboratory found previously in awake rats in HBO2 at 3 ATA (17). By contrast, acute unilateral vagotomy did not mitigate pulmonary damage, and acute bilateral vagotomy hastened HBO2 seizures and exacerbated pulmonary injury. Protective effects of chronic unilateral vagotomy were also reported long ago (21), whereas acute bilateral vagotomy increased lung damage (36). In cats, acute unilateral vagotomy did not affect pulmonary pathology, but bilateral vagotomy ameliorated pulmonary edema and hemorrhage (33).
The discrepancies among these studies are not easily reconciled. Our observation that chronic vagotomy mitigates lung injury in HBO2, whereas acute vagal transection exacerbates it, could be explained by alterations in nitric oxide (NO) bioactivity. Our laboratory has previously reported that the pathogenesis of acute pulmonary injury in HBO2 involves NO-mediated neuronal pathways in the CNS (14). It has also been shown that 5–20 days after left cervical vagotomy, NO synthesis is markedly enhanced in some vagal nuclei, for example, in the dorsal motor nucleus, the nucleus ambiguous, and the nucleus tractus solitarii (19, 23). However, it is still unknown which vagal afferents or efferents affect the lung damage caused by altered central NO synthase expression. We found that pulmonary damage in rats with acute bilateral vagotomies was more severe than in rats with intact vagi that were pretreated with atropine (unpublished observations), suggesting that afferent nerves have a greater inhibitory effect on pulmonary leakage than their efferent counterparts.
In summary, this study suggests that acute lung injury in HBO2 is caused by an abrupt and substantial increase in pulmonary vascular pressure, producing barotrauma in capillaries and leading to transudation of fluid, plasma protein, and blood cells into the pulmonary interstitial and alveolar air spaces. Our findings show that a key factor in pulmonary damage in HBO2 is massive sympathetic outflow, mediated by the CNS, that depresses LV function, leading to the sudden development of cardiogenic pulmonary edema. It is reasonable to propose, therefore, that LV dysfunction, pulmonary hypertension, and capillary failure in HBO2 can be attributed to the direct effects of CNS-mediated sympathetic excitation and catecholamine release on the myocardium and the cardiopulmonary vasculature. We also hypothesize that autonomic afferents can initiate central sympathetic excitation, leading to reflex pulmonary hydrostatic damage in hyperbaric hyperoxia.
GRANTS
This work was supported by Office of Naval Research Grant N00014-04-1-0171 (to C. A. Piantadosi, B. W. Allen, and I. T. Demchenko), by National Institute of Allergy and Infectious Diseases Grant RO1-AI064789 (to C. A. Piantadosi), and by Russian Foundation for Basic Research Grant 09-04-01439a (to I. T. Demchenko).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1. Abel FL, McNamee JE, Cone DL, Clarke D, Tao J. Effects of hyperbaric oxygen on ventricular performance, pulmonary blood volume, and systemic and pulmonary vascular resistance. Undersea Hyperb Med 27: 67–73, 2000 [PubMed] [Google Scholar]
- 2. Abel FL, Waldhausen JA, Daly WJ, Pearce WL. Pulmonary blood volume in hemorrhagic shock in the dog and primate. Am J Physiol 213: 1072–1078, 1967 [DOI] [PubMed] [Google Scholar]
- 3. Balentine JD. Pathology of Oxygen Toxicity. New York: Academic, 1982 [Google Scholar]
- 4. Baumann A, Audibert G, McDonnell J, Mertes PM. Neurogenic pulmonary edema. Acta Anaesthesiol Scand 51: 447–455, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bean J, Zee D, Thom B. Pulmonary changes with convulsions induced by drugs and oxygen at high pressure. J Appl Physiol 21: 865–872, 1966 [DOI] [PubMed] [Google Scholar]
- 6. Bean JW. Effects of oxygen at increased pressure. Physiol Rev 25: 1–147, 1945 [Google Scholar]
- 7. Bean JW, Johnson PC. Epinephrine and neurogenic factors in the pulmonary edema and CNS reactions induced by O2 at high pressure. Am J Physiol 180: 438–444, 1955 [DOI] [PubMed] [Google Scholar]
- 8. Bean JW, Smith CW. Hypophyseal and adrenocoritcal factors in pulmonary damage induced by oxygen at atmospheric pressure. Am J Physiol 171: 169–174, 1953 [DOI] [PubMed] [Google Scholar]
- 9. Bean JW, Zee D. Influence of anesthesia and CO2 on CNS and pulmonary effects of O2 at high pressure. J Appl Physiol 21: 521–526, 1966 [DOI] [PubMed] [Google Scholar]
- 10. Chen EP, Bittner HB, Craig DM, Davis R, Duane J, Van Trigt P., III Pulmonary hemodynamics and blood flow characteristics in chronic pulmonary hypertension. Ann Thorac Surg 63: 806–813, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Chen HI. Hemodynamic mechanisms of neurogenic pulmonary edema. Biol Signals 4: 186–192, 1995 [PubMed] [Google Scholar]
- 12. Clark JM, Lambertsen CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev 23: 37–133, 1971 [PubMed] [Google Scholar]
- 13. Cooper T, Pinakatt T, Richardson A. The use of the thermal dilution principle for measurement of cardiac output in the rat. Med Biol Eng Comput 1: 61–65, 1963 [Google Scholar]
- 14. Demchenko IT, Atochin DN, Gutsaeva DR, Godfrey RR, Huang PL, Piantadosi CA, Allen BW. Contributions of nitric oxide synthase isoforms to pulmonary oxygen toxicity, local vs. mediated effects. Am J Physiol Lung Cell Mol Physiol 294: L984–L990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demchenko IT, Boso AE, O'Neill TJ, Bennett PB, Piantadosi CA. Nitric oxide and cerebral blood flow responses to hyperbaric oxygen. J Appl Physiol 88: 1381–1389, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Demchenko IT, Bosso AE, Zhiljaev SY, Moskvin AN, Gutsaeva DR, Atochin DN, Bennett PB, Piantadossi KA. [Involvement of nitrogen oxide in the cerebral vasoconstriction during respiration with high pressure oxygen.] Ross Fiziol Zh Im I M Sechenova 86: 1594–15603, 2000 [PubMed] [Google Scholar]
- 17. Demchenko IT, Welty-Wolf KE, Allen BW, Piantadosi CA. Similar but not the same: normobaric and hyperbaric pulmonary oxygen toxicity, the role of nitric oxide. Am J Physiol Lung Cell Mol Physiol 293: L229–L238, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Feng GG, Nishiwaki K, Kondo H, Shimada Y, Ishikawa N. Inhibition of fibrin-induced neurogenic pulmonary edema by previous unilateral left-vagotomy correlates with increased levels of brain nitric oxide synthase in the nucleus tractus solitarii of rats. Auton Neurosci 102: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Arenare F, Spaziani D, Mancia G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension 53: 205–209, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Groshikov MA, Sorokin PA. Pathological changes in the lungs of animals under the influence of high oxygen pressures. In: [The Effect of the Gas Medium and Pressure on Body Functions], edited by Brestkin MP. Jerusalem: Israel Program for Scientific Translations, 1965, p. 106–115 [Google Scholar]
- 22. Grossman W. Diastolic dysfunction and congestive heart failure. Circulation 81: 1–7, 1990 [PubMed] [Google Scholar]
- 23. Hamdy O, Maekawa H, Shimada Y, Feng GG, Ishikawa N. Role of central nervous system nitric oxide in the development of neurogenic pulmonary edema in rats. Crit Care Med 29: 1222–1228, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Harel D, Lavy S. Changes in electrical activity of the brain as a criterion of hyperbaric oxygen toxicity in the central nervous system. TIT J Life Sci 1: 111–114, 1971 [Google Scholar]
- 25. Jacobson JM, Michael JR, Meyers RA, Bradley MB, Sciuto AM, Gurtner GH. Hyperbaric oxygen toxicity: role of thromboxane. J Appl Physiol 72: 416–422, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Jamieson D, Van Den Brenk HA. Pulmonary damage due to high pressure oxygen breathing in rats. Aust J Exp Biol Med Sci 40: 309–314, 1962 [DOI] [PubMed] [Google Scholar]
- 27. Johnson PC, Bean JW. Effect of sympathetic blocking agents on the toxic action of O2 at high pressure. Am J Physiol 188: 593–598, 1957 [DOI] [PubMed] [Google Scholar]
- 28. Kioschos JM, Behar VS, Saltzman HA, Thompson HK, Myers NE, Smith WW, McIntosh HD. Effect of hyperbaric oxygenation on left ventricular function. Am J Physiol 216: 161–166, 1969 [DOI] [PubMed] [Google Scholar]
- 29. Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 291: H2847–H2856, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Molenat F, Boussuges A, Grandfond A, Rostain JC, Sainty JM, Robinet C, Galland F, Meliet JL. Haemodynamic effects of hyperbaric hyperoxia in healthy volunteers: an echocardiographic and Doppler study. Clin Sci (Lond) 106: 389–395, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Pilati CF, Bosso FJ, Maron MB. Factors involved in left ventricular dysfunction after massive sympathetic activation. Am J Physiol Heart Circ Physiol 263: H784–H791, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Rucci FS, Giretti ML, La Rocca M. Changes in electrucal activity of the cerebral cortex and some subcortical centers in hyperbaric oxygen. Electroencephalogr Clin Neurophysiol 5: 23–27, 1967 [DOI] [PubMed] [Google Scholar]
- 33. Sapov IA. The toxic effect of oxygen on the lungs. Biull Eksp Biol Med 35: 40–45, 1953 [Google Scholar]
- 34. Schilling CW, Adams BH. A study of the convulsive seizures caused by breathing oxygen at high pressure. US Naval Mod Bull 31: 112–121, 1933 [Google Scholar]
- 35. Sedý J, Zicha J, Kunes J, Jendelová P, Syková E. Mechanisms of neurogenic pulmonary edema development. Physiol Res 57: 499–506, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Shanklin DR, Berman HF. The influence of hyperbaric oxygen on hyaline membrane disease in newborn rabbit. South Med J 3: 1443–1448, 1963 [Google Scholar]
- 37. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85, 1985 [DOI] [PubMed] [Google Scholar]
- 38. Torbati D, Simon A, Ranade A. Frequency analysis of EEG in rats during the pre-convulsive period of O2 poisoning. Aviat Space Environ Med 52: 598–603, 1981 [PubMed] [Google Scholar]
- 39. Wang Y, Fice DS, Yeung PKF. A simple high-performance liquid chromatography assay for simultaneous determination of plasma norepinephrine, epinephrine, dopamine and 3,4-dihydroxyphenyl acetic acid. J Pharm Biomed Anal 21: 519–525, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Weaver LK, Howe S, Snow GL, Deru K. Arterial and pulmonary arterial hemodynamics and oxygen delivery/extraction in normal humans exposed to hyperbaric air and oxygen. J Appl Physiol 107: 336–345, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Wood JD, Stacey NE, Watson WJ. Pulmonary and central nervous system damage in rats exposed to hyperbaric oxygen and protection there from by gamma-aminobutyric acid. Can J Physiol Pharmacol 43: 405–410, 1965 [DOI] [PubMed] [Google Scholar]
- 42. Zierler KL. Circulation times and the theory of indicator dilution methods for determining blood flow and volume. In: Handbook of Physiology. Circulation. Washington, DC: Am. Physiol. Soc., 1962, sect. 2, vol. I, chapt. 18, p. 585–615 [Google Scholar]