Abstract
Metabolomics is an emerging component of systems biology that may be a viable strategy for the identification and validation of physiologically relevant biomarkers. Nuclear magnetic resonance (NMR) spectroscopy allows for establishing quantitative data sets for multiple endogenous metabolites without preconception. Sepsis-induced acute lung injury (ALI) is a complex and serious illness associated with high morbidity and mortality for which there is presently no effective pharmacotherapy. The goal of this study was to apply 1H-NMR based quantitative metabolomics with subsequent computational analysis to begin working towards elucidating the plasma metabolic changes associated with sepsis-induced ALI. To this end, this pilot study generated quantitative data sets that revealed differences between patients with ALI and healthy subjects in the level of the following metabolites: total glutathione, adenosine, phosphatidylserine, and sphingomyelin. Moreover, myoinositol levels were associated with acute physiology scores (APS) (ρ = −0.53, P = 0.05, q = 0.25) and ventilator-free days (ρ = −0.73, P = 0.005, q = 0.01). There was also an association between total glutathione and APS (ρ = 0.56, P = 0.04, q = 0.25). Computational network analysis revealed a distinct metabolic pathway for each metabolite. In summary, this pilot study demonstrated the feasibility of plasma 1H-NMR quantitative metabolomics because it yielded a physiologically relevant metabolite data set that distinguished sepsis-induced ALI from health. In addition, it justifies the continued study of this approach to determine whether sepsis-induced ALI has a distinct metabolic phenotype and whether there are predictive biomarkers of severity and outcome in these patients.
Keywords: biomarkers, bioinformatics, apoptosis, oxidant stress, glutathione, adenosine, phosphatidylserine, sphingomyelin, myoinositol
sepsis is one of the leading causes of death in the Western world, and the incidence of sepsis is expected to rise since sepsis is a disease of the elderly and the U.S. population is aging (43, 44). The systemic inflammatory response of sepsis leads to acute lung injury (ALI) in ∼30% of patients (43, 44). The pathogenesis of ALI is extraordinarily complex and involves the loss of homeostasis of a number of processes. Despite our improving understanding of its pathophysiology, sepsis-induced ALI remains a challenging clinical problem for which there are no predictive biomarkers of susceptibility or severity and no effective pharmacotherapy. For these reasons, sepsis-induced ALI lends itself to biomarker discovery, but our knowledge and application of systems biology to this critical illness is still evolving (12, 21).
Unlike genomics and proteomics, quantitative metabolomics is in its infancy. It focuses on the identification of metabolites and measurement of metabolite concentration and is an emerging component of the systems biology approach for the discovery of clinically relevant biomarkers and potential therapeutic targets (45). A principle of metabolomics is that many human diseases are caused or result from an abnormal metabolic state (25). Metabolites represent a reading of the functional state of the physiological environment, and metabolomics is a unique tool of discovery because it identifies multiple endogenous markers from a single sample without preconception or selection bias (31). Initial differences in metabolites may be predictive of disease severity or clinical outcome, and changes over time may be useful in characterizing therapeutic response. Quantitative metabolite data sets can also be linked to biological events using bioinformatics platforms that map metabolic reactions and identify associated genes (25, 45, 47). This information can be employed to generate hypotheses related to mechanisms of disease pathogenesis or potential targets of drug action as well as gene function (30). In this context, measurement of endogenous plasma metabolites reflect those across different tissues, so changes in metabolites in the blood, a readily accessible biofluid, are indicative of the metabolome of the host (2).
Metabolite data can be acquired by a number of different analytical platforms, but high-resolution nuclear magnetic resonance (NMR) spectroscopy, specifically 1H-NMR, and mass spectrometry are the most common (2, 13, 34). Although mass spectrometry is more sensitive (in terms of the detection of low-abundant metabolites), 1H-NMR spectroscopy can identify and quantify multiple metabolites (often called “global metabolic profile”) from a single sample, can be used for liquid or solid samples with minimal sample preparation, and is cost effective and robust. In addition, nearly all major classes of metabolites have characteristic NMR spectra, which makes this technique very useful for metabolite fingerprinting. Even though no such fingerprint exists for sepsis-induced ALI, we have demonstrated this principle in our published work of metabolomics in an experimental model of inflammatory cytokine-induced lung injury (35).
In this pilot study, we extended our 1H-NMR-based metabolomics approach to test its feasibility to identify and quantify physiologically relevant metabolites of sepsis-induced ALI. To accomplish this, we established 1H-NMR-based metabolic profiles from existing baseline plasma samples from patients enrolled in the University of Michigan ALI Specialized Center of Clinically Oriented Research (SCCOR) randomized trial of granulocyte/macrophage colony-stimulating factor (GM-CSF) in the treatment of ALI. We followed quantitative metabolomics with a computational analysis to identify the reactions that involve each of the differentiating metabolites and the associated enzymes and genes.
MATERIALS AND METHODS
Patient enrollment.
The study received approval from the Institutional Review Board (IRB) of the University of Michigan Health System and by the IRB at each study site. All mechanically ventilated patients in the participating intensive care units at the University of Michigan Medical Center (Critical Care Medicine Unit, Surgical Intensive Care Unit, Cardiothoracic Surgery Intensive Care Unit) that met criteria for ALI or acute respiratory distress syndrome (ARDS) by the ERS/ATS consensus definition were considered for enrollment (7). In addition to the exclusion criteria incorporated in this definition, patients were excluded if: 1) <18 years of age; 2) >7 days had elapsed since onset of ALI/ARDS; 3) there was evidence of preexisting chronic respiratory failure; 4) the patient was neutropenic (absolute neutrophil count <1,000 cells/mm3); 5) there was a history of hematological malignancy or bone marrow transplantation; 6) the patient had entered other therapeutic trials; or 7) there was a decision by the patient (or his/her legally authorized decision maker) or attending physician to forego aggressive care.
Enrollment and randomization.
After informed consent was obtained from the patient or the patient's legal surrogate, demographic data and physiological measurements were collected from the time of entry into the study. Blood samples were collected from all patients in the morning between days 0 and 3 from the time of ALI diagnosis and in all cases before randomization. The window for randomization and initiation of study drug infusion was 3–7 days after meeting criteria for ALI/ARDS. Patients were randomized (1:1) to receive either GM-CSF or placebo. Following randomization, subjects received recombinant human GM-CSF (250 μg/M2) or placebo, administered by slow intravenous infusion over 4 h, once daily for 14 days. Study drug infusions were held on days on which the white blood cell count was >40,000 cells/mm3 or the PaO2/FiO2 (P/F) ratio was <80. Standardized ventilator management was based on the ARDS Network low stretch protocol, targeting a tidal volume of 6 ml/kg based on ideal body weight and a plateau pressure less than 35 mmHg. Weaning from mechanical ventilation was standardized, based on the ARDS Network protocol. This protocol was implemented by respiratory therapists and used pressure-support ventilation.
Each subject was assessed at study initiation and then daily by the study coordinators, and study parameters were recorded, including survival, requirement for mechanical ventilation, P/F ratio, acute physiology score (APS), hemodynamics, and laboratory studies. Ventilator-free days (VFD) was defined as the number of days within the first 28 days after enrollment on which the patient was alive and breathing without ventilatory assistance, so long as the period of unassisted breathing lasted at least 48 h. The study was approved by a data safety and monitoring board assembled by the National Heart, Lung, and Blood Institute. The data safety and monitoring board reviewed the study for safety on a regular basis.
Healthy volunteers.
We obtained blood from healthy volunteers to generate a set of reference metabolite values using the same NMR platform as that used for our patient samples. Following written informed consent, morning, nonfasting blood samples were acquired by direct venipuncture from healthy, medication-free, nonsmoking men and women of at least 21 years of age.
Plasma from patients and healthy volunteers was acquired from heparin-preserved whole blood samples following centrifugation and was stored (−80°C) until the time of extraction and NMR assay at the University of Colorado at Denver.
Sample preparation for 1H-NMR spectroscopy.
Plasma samples were thawed on ice and extracted using a dual methanol-chloroform extraction (for protein precipitation and separation of hydrophilic and lipophilic fractions) as previously described (33). This eliminates macromolecules (e.g., proteins) and establishes a fused metabolic profile for water-soluble and lipid metabolites. Briefly, 0.5 ml of ice-cold plasma was mixed with 1 ml of chloroform:methanol (1:1 vol/vol) and centrifuged. The supernatant (organic phase) was collected, and the pellet was resuspended with 0.5 ml of chloroform-methanol and centrifuged. The supernatants were combined, and 0.5 ml of ice-cold water was added to “wash out” remaining water-soluble metabolites from the organic phase. After 15 min at −20°C, the upper (aqueous) phase was removed and added to the remaining pellet (to wash out remaining water-soluble metabolites from the pellet), 1 ml of water was added, and the sample was centrifuged and freeze-dried overnight. The water-soluble extracts were then dissolved in 0.5 ml of D2O, centrifuged, and transferred into 5-mm NMR tubes. The lipid-rich methanol-chloroform fraction (bottom phase after low temperature exposure) was evaporated using a high-speed vacuum centrifuge; dried lipid extracts were dissolved in 0.6 ml of deuterated methanol:chloroform (1:2 vol/vol) and centrifuged and transferred into 5-mm NMR tubes. All reagents were purchased Sigma-Aldrich (St. Louis, MO).
Quantitative 1H-NMR spectroscopy on plasma extracts.
All one- and two-dimensional NMR spectra were obtained on a Bruker 500 MHz DRX spectrometer equipped with Bruker TopSpin software (Bruker Biospin, Fremont, CA) using an inverse 5-mm TXI probe (tuned for proton detection). For metabolite identification in plasma water-soluble and lipid extracts, a two-dimensional (2D)-H, C-HSQC (heteronuclear single quantum correlation) NMR technique was used. The experiments were acquired with 512 increments and 400 scans per increment, using 90 degree pulse and a recovery delay of 1 s. The spectral width was 10 ppm in the proton dimension and 140 reference for both carbon (21 ppm) and proton (1.32 ppm) axes. For metabolite quantification (proton NMR), a standard water presaturation pulse program “zgpr” was used to suppress water residue signal with a power level of 61 dB. The total number of acquisitions was 64, and the pulse delay of 12.8 s (for fully relaxed proton NMR spectra) was applied between acquisitions for fully relaxed 1H-NMR spectra resulting in a total acquisition time of 12 min per each water-soluble and lipid NMR spectrum. An external standard, trimethylsilylpropionic acid (TMSP-d4; 0.6 mmol/l for water-soluble and 1.2 mmol/l for lipid extracts), sealed in a thin, glass capillary, was used for metabolite quantification and as a chemical shift reference (0 ppm).
For postprocessing spectral analysis and metabolite signal integration, the Bruker 1DWINNMR program (Bruker Biospin) was used (33, 35, 41). After Fourier transformation (with line broadening of 0.2 Hz), exponential multiplication, and phase correction, the baseline correction was applied for each metabolite NMR peak of interest. The 1H-NMR peaks for single metabolites were identified and referred to a metabolite chemical shift library. After performing line broadening (0.2 Hz), exponential multiplication, and Fourier transformation, each spectrum was phase and baseline corrected. The chemical shift was set to 0 ppm for TMSP (the external standard). The absolute concentrations of metabolites were calculated according to the formula:
where Cx = metabolite concentration (μmol/ml); Ix = integral of metabolite 1H peak; Nx = number of protons in metabolite 1H peak (from CH, CH2, CH3, etc.); C = TMSP concentration (0.6 or 1.2 mmol/l; see text); I = integral of TMSP 1H peak at 0 ppm (:9 since TMSP has 9 protons); V = volume of the extract (ml); and M = volume of plasma sample (ml).
Statistical analyses.
Candidate differentiating metabolites of sepsis-induced ALI detected by 1H-NMR spectroscopy were defined as those that were different from those of healthy volunteers using an unpaired Student's t-test and a false discovery rate of ≤5%. The plasma metabolite levels of placebo and GM-CSF-treated patients were also compared using an unpaired Student's t-test. For the analysis, APS and P/F ratios acquired at study initiation, but before the administration of study drug, were used (Table 1) (1, 5). The association between metabolites and APS, P/F ratios, VFD, and mortality in sepsis-induced ALI patients was assessed by Spearman's rank correlation analysis. For this exploratory study, we defined the critical significance level (α) as 0.05 (15). Based on this and a beta of 0.2, the sample size of 13 patients was randomly selected from a larger cohort of 94 SCCOR study patients enrolled at the time this study was carried out. Statistical analyses were performed using SPSS (SAS, Cary, NC), and multiple comparison testing was accounted for by estimating the false discovery rate (FDR; q value) using R software (version 2.11.1 with R library extension qvalue_1.1 tar.gz http://genomics.princeton.edu/storeylab/qvalue/) (6, 36, 37). The conservative single lambda value and smoother approach was used in which lambda was set at zero, which defaults the pi_0 value to 1. This software generates adjusted P values (q values) that account for the P value distribution generated by multiple comparisons and represents the percent of significant tests that will result in a false positive.
Table 1.
Demographics of enrolled ALI SCCOR patients in metabolomics pilot study
| Patients | |
|---|---|
| Sample size | 13 |
| Sex (%) | |
| Male | 69 |
| Female | 31 |
| Age | 55.4 + 16.1 |
| Group (%) | |
| Placebo | 38.5 |
| GM-CSF | 61.5 |
| Etiology (%) | |
| Sepsis | 46.2 |
| Pneumonia | 30.8 |
| Aspiration | 7.7 |
| Pancreatitis | 15.4 |
| Any sepsis (%) | 92.3 |
| APS* | 59.3 + 13.7 |
| Qualifying PaO2/FIO2 ratio | 136 + 19.1 |
Data are means +SD.
Acute Physiology Score; component of the APACHE III severity of illness scoring system.
Computational data analysis.
We used Metscape, the metabolic network analysis and visualization tool (http://www.metscape.ncibi.org/tryplugin.html) (19), to generate the gene and enzyme networks associated with each of the differentiating metabolites. Metscape is a plugin for Cytoscape (http://www.cytoscape.org/). This tool uses Kyoto Encyclopedia of Genes and Genomes (KEGG) ID as the primary compound identifier and contains nearly 2,700 compounds, 870 enzymes, 1,400 genes, and 3,000 metabolic reactions involved in over 70 human-specific metabolic pathways defined in the Edinburgh human metabolic network (25). The user can enter or upload a list of compound names or KEGG IDs to generate networks consisting of the reactions and compounds associated with the query compounds. The tool also provides information about enzymes, genes, and pathways related to the query. We chose to use Metscape because it allowed the uploading of our experimental data and the visualization of changes over a set of experimental conditions.
RESULTS
Patients, healthy volunteers, and sample size.
Enrollment into the SCCOR study began at the University of Michigan in July 2004. The demographics and clinical and initial physiological characteristics of these ALI patients are shown in Table 1. All 13 patients were white. Sepsis and pneumonia were most commonly identified as the precipitating event leading to ALI/ARDS. Five of the 13 patients were from the placebo control group, and 8 of the 13 patients were randomized to the GM-CSF treatment arm. All patients were critically ill, with a mean (+SD) APS at enrollment of 59.3 + 13.7. Respiratory failure was severe, with mean P/F ratio less than 200, indicative of ARDS, rather than ALI. There were no gender differences in age, APS, VFD, or P/F ratio.
The mean (+SD) age of the healthy volunteers was 31.3 + 9.3 years; one-half of the subjects were male and all were white, except one female who was Asian.
Metabolites.
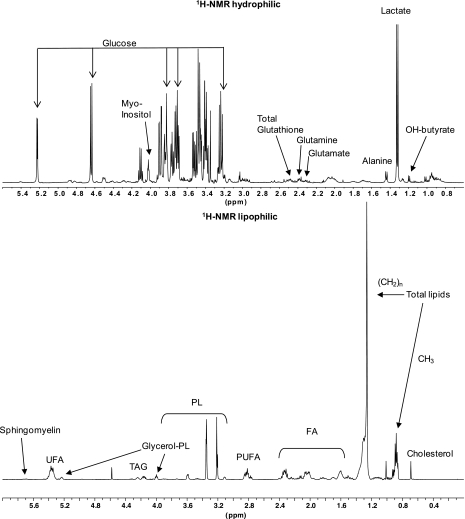
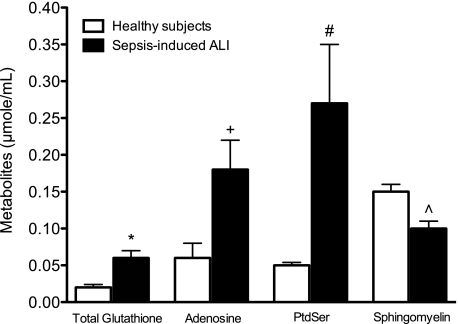
Over 40 metabolites were identified and quantified by 1H-NMR from each data set (lipophilic and hydrophilic fractions) of each plasma sample (Table 2). 1H-NMR spectroscopy (Fig. 1) identified biologically relevant metabolites from mechanically ventilated patients with confirmed sepsis-induced ALI. The resulting quantitative metabolomic data showed differences in the levels of total glutathione, adenosine, phosphatidylserine (PtdSer), and sphingomyelin (Fig. 2) compared with healthy volunteers. The t-test comparisons revealed four metabolites with effect sizes (Cohen's D-statistic) ranging from 1.0–2.8. These large effect sizes provide evidence of a sufficient sample size to detect meaningful differences between metabolite levels of patients with sepsis-induced ALI and healthy controls that are not due to chance. In all cases, the FDRs were ≤5%. Collectively, these changes reflect the complex pathology and provide evidence for the involvement of a number of processes including oxidant stress (glutathione), energy balance (adenosine), apoptosis (PtdSer), and endothelial barrier function (sphingomyelin), in the pathogenesis of sepsis-induced ALI (8, 11, 24, 27, 28, 38, 40, 42).
Table 2.
Absolute concentrations of plasma endogenous metabolites [μmol/ml] calculated from 1H- (water-soluble and lipid fraction) spectra of healthy control subjects and patients with sepsis-induced ALI
| Water-Soluble Metabolites | Healthy Controls (n = 6) | Sepsis-Induced ALI (n = 13) |
|---|---|---|
| Aromatic AA | 1.97 + 1.04 | 4.51 + 1.79 |
| Adenine | 0.28 + 0.14 | 0.36 + 0.28 |
| Adenosine* | 0.06 + 0.04 | 0.19 + 0.17 |
| Glucose A | 1.33 + 0.46 | 1.65 + 0.82 |
| Glucose B | 1.60 + 0.57 | 2.48 + 0.89 |
| Myoinositol | 0.26 + 0.14 | 0.18 + 0.13 |
| Polyols_Sugars | 10.36 + 3.22 | 21.09 + 5.98 |
| TMAO+Betaine | 0.04 + 0.02 | 0.04 + 0.02 |
| Cholines | 0.03 + 0.01 | 0.06 + 0.03 |
| Tyrosine | 0.07 + 0.04 | 0.05 + 0.04 |
| Creatine+Crn | 0.10 + 0.04 | 0.21 + 0.12 |
| GSH+Lysine | 0.64 + 0.31 | 0.38 + 0.19 |
| Citrate | 0.10 + 0.04 | 0.22 + 0.06 |
| Total Glutathione* | 0.02 + 0.01 | 0.06 + 0.05 |
| Glutamine | 0.06 + 0.02 | 0.13 + 0.06 |
| Succinate | 0.02 + 0.01 | 0.03 + 0.01 |
| Glutamate | 0.44 + 0.09 | 0.54 + 0.18 |
| Total CH2-CH3 | 1.20 + 0.32 | 2.61 + 0.57 |
| Acetate | 0.05 + 0.03 | 0.07 + 0.03 |
| Lysine+Arginine | 0.28 + 0.10 | 0.36 + 0.17 |
| Alanine | 0.29 + 0.06 | 0.17 + 0.08 |
| Lactate | 1.26 + 0.63 | 1.28 + 0.42 |
| OH-Butyrate | 0.06 + 0.02 | 0.11 + 0.06 |
| Val, Leu, Ile | 1.89 + 0.72 | 2.29 + 0.42 |
| Total Glucose | 1.86 + 0.70 | 2.66 + 0.90 |
| Lipid Metabolites | ||
| Sphingomyelin* | 0.15 + 0.03 | 0.10 + 0.04 |
| PUFA+MUFA | 5.40 + 0.79 | 4.62 + 2.39 |
| TAG | 0.72 + 0.22 | 1.77 + 0.89 |
| Glycerol-P-Lipids | 0.88 + 0.13 | 1.09 + 0.31 |
| PtdSerine* | 0.05 + 0.01 | 0.27 + 0.28 |
| PtdCholine | 1.09 + 0.16 | 0.91 + 0.27 |
| Total cholines | 1.73 + 0.26 | 1.36 + 0.45 |
| PtdEthanolamine | 0.03 + 0.01 | 0.04 + 0.02 |
| PUFA | 5.39 + 1.02 | 3.95 + 2.13 |
| FA | 10.29 + 1.48 | 8.46 + 3.54 |
| CH2n-Lipids | 81.52 + 11.76 | 75.79 + 33.82 |
| Total Lipids | 29.29 + 4.84 | 18.38 + 6.98 |
| Cholesterol | 3.02 + 0.39 | 1.60 + 0.56 |
| [PUFA/MUFA] | 1.00 + 0.10 | 0.86 + 0.12 |
Data are means +SD.
P and q values ≤0.05.
PUFA, polyunsaturated fatty acids; MUFA, monounsaturated fatty acids; TMAO, trimethylamine N-oxide.
Fig. 1.
Representative 1H-NMR spectra of plasma water- and lipid-soluble metabolites. FA, fatty acids; OH-butyrate, hydroxybutyrate; PL, phospholipids; PUFA, polyunsaturated fatty acids; TAG, triacylglycerol; UFA, unsaturated fatty acids.
Fig. 2.
Differentiating metabolites of mechanically ventilated sepsis-induced acute lung injury (ALI) patients (n = 13) compared with healthy subjects (n = 6). These metabolites are associated with oxidant stress (total glutathione), loss of ATP homeostasis (adenosine), apoptosis (PtdSer), and disruption of endothelial barrier function (sphingomyelin). Data are the means +SE. *P = 0.03 (q = 0.05); +P = 0.02 (q = 0.04); #P = 0.02 (q = 0.04); and ∧P = 0.006 (q = 0.02).
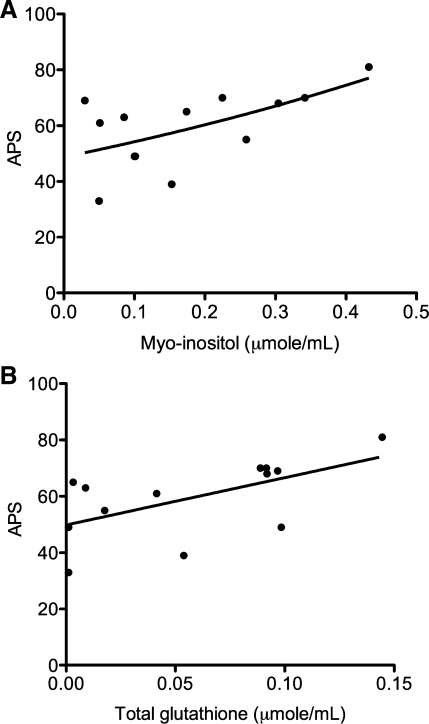
We combined the metabolite data from placebo and GM-CSF treatment groups. We were justified in doing so because metabolite data were obtained from pretreatment plasma samples, the analysis of the data from the randomized trial indicated that administration of GM-CSF did not significantly alter either VFD or 28-day survival compared with placebo control, and there were no differences between the metabolite levels of placebo and GM-CSF-treated patients. The results of this analysis suggested associations between metabolites and indicators of disease severity. Specifically, myoinositol was inversely associated with VFD (ρ = −0.73, P = 0.005, q = 0.01). Myoinositol and total glutathione were positively associated with APS (ρ = 0.53, P = 0.05; Fig. 3A and ρ = 0.56, P = 0.04; Fig. 3B). The FDRs of these latter associations were 25% compared with 70% for other metabolites, which, in our opinion, warrants further investigation to determine whether myoinositol and total glutathione levels could be used to discriminate disease severity.
Fig. 3.
Possible associations between acute physiology score (APS) and myoinositol (A) and total glutathione (B). The associations, assessed by Spearman's rank correlation, suggest that myoinositol (ρ = 0.53; P = 0.05; q = 0.25) and total glutathione (ρ = 0.56; P = 0.04; q = 0.25) may be markers of sepsis-induced ALI severity.
Computational data analysis.
To gain additional insight about the metabolic context of the differentiating metabolites of sepsis-induced ALI, we built metabolic networks associated with each of them using Metscape (Fig. 4, A–D). Each network consists of the compound nodes (substrates, products, and cofactors) and edges that represent biochemical reactions. Metscape also provided information about the enzymes that catalyze the reactions and the genes that encode them (Supplementary Table S3; Supplemental Material for this article is available online at the Journal website).
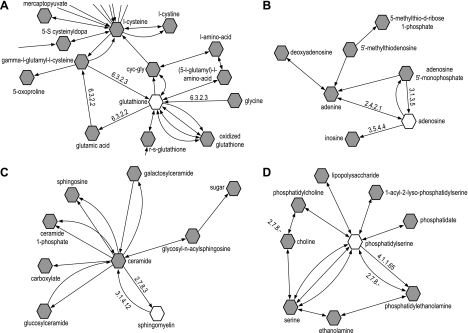
Fig. 4.
Bioinformatics analysis of differentiating metabolites identified 4 distinct metabolic networks associated with total glutathione (A), adenosine (B), sphingomyelin (C), and phosphatidylserine (D). Networks were generated using the bioinformatics platform Cytoscape (http://www.cytoscape.org) with the Metscape plugin (http://metscape.ncibi.org/tryplugin.html). Hexagons represent compounds, and numbers are the enzyme commission (EC) number for enzymes involved in respective reactions.
The glutathione network (Fig. 4A) revealed the synthesis of glutathione from glutamate, cysteine, and glycine in a reaction catalyzed by two enzymes, γ-glutamylcysteine synthetase (EC6.3.2.2) and GSH synthetase (EC6.3.2.3). The adenosine network (Fig. 4B) showed the key enzymes that regulate adenosine production and metabolism, adenosine deaminase (ADA; EC.3.5.4.4), which converts adenosine to inosine, and ecto-5′-nucleotidase (EC3.1.3.5), which regulates adenosine production. Sphingomyelin is a precursor of ceramide. Sphingomyelinase (EC3.1.4.12) catalyzes the cleavage of the phosphodiester bond in sphingomyelin to form ceramide and phosphocholine (Fig. 4C). Phosphatidylserine can be synthesized from phosphatidylcholine or phosphatidylethanolamine by two phosphatidylserine synthases (PSS1 and PSS2). The conversion of phosphatidylserine to phosphatidylethanolamine is catalyzed by phosphatidylserine decarboxylase (EC4.1.1.65) (Fig. 4D).
DISCUSSION
The results of this pilot study show that sepsis-induced ALI caused measurable changes in biologically relevant metabolites of the human metabolome. These data support the idea that quantitative 1H-NMR-spectroscopy metabolomics is a viable tool for the identification of potential novel biomarkers of ALI. This is evidenced by the ability of this approach to distinguish a metabolic phenotype of an illness from that of health. This is a first and necessary step of quantitative metabolomics because unlike chemometric metabolomics, which focuses on pattern recognition, quantitative metabolomics measures metabolite concentration (3, 10, 20, 45). This approach requires reference values from healthy subjects to make confident conclusions about changes in specific metabolite concentrations as well as their physiological relevance. It is not yet clear whether the changes in metabolites identified here are specific for ALI or are a manifestation of broader systemic inflammation. These data serve as the basis for planned future studies that will be designed to determine if quantitative metabolomics differ between patients with sepsis-induced ALI, patients with sepsis but without ALI, and those with other critical illnesses. A recent chemometric metabolomic study of traumatic critically ill patients identified changes in sugars, carbohydrates, and amino acids as discriminators of illness (26). These were not evident in our patient population.
The need for metabolite concentration data from healthy controls is further exemplified by the fact that quantitative metabolomics is still an emerging strategy. Despite rapid progress in analytical instrumentation, the “normal” ranges of metabolites from NMR spectra have not been established. The lack of analytical standards and plasma metabolite reference ranges derived from a large database of controls make the classification of metabolite concentrations as normal or abnormal particularly difficult. This is one of the challenges being tackled by the Human Metabolome Project (www.hmdb.ca), which is compiling reference range data and is developing bioinformatics tools to assist in mapping the human metabolome (18, 46–48). Progress is being made, but the available data have not obviated the need for within-study healthy controls because, presently, normal values are reported from various studies that have used a range of different analytical platforms.
The feasibility of plasma metabolomics is demonstrated by the yielding of meaningful metabolite data from the analysis of a single plasma sample. This supports that acquisition of these data do not require access to the tissue or airway compartments. In this regard, it has been our experience that the use of bronchoalveolar lavage fluid is not feasible for metabolomic analysis because it is primarily composed of protein, and metabolite levels are low. Alternatively, because plasma interacts with all tissues, it represents a physiological “average” of the host's biochemical information (2).
While we acknowledge that our findings will need to be confirmed using a larger sample size, they are the first of their kind, and their novelty and importance are illustrated by the results of our computational analysis which identified four distinct metabolic networks. This analysis identified key enzymes, responsible for the metabolism of the four differentiating metabolites of sepsis-induced ALI, as well as genes that encode these enzymes. The relevance of this analysis is exemplified by the pathway linked to adenosine in which ADA encodes adenosine deaminase (EC.3.5.4.4), the enzyme that catalyzes the irreversible deamination of adenosine to inosine and regulates intra- and extracellular concentrations of adenosine (Fig. 4B). A known polymorphism of ADA results in diminished enzyme activity, which may lead to high levels of adenosine (4). There are adverse consequences of prolonged elevated levels of adenosine including the propagation of lung inflammation and the activation of apoptosis signaling pathways (17, 32, 49). Conversely, adenosine may enhance barrier function and be an important and necessary adaptive response to lung injury (14, 16, 23). One possible direction for future work is to see if the expression of this or any other genes encoding key enzymes regulating the metabolism of the four metabolites that we identified are altered in sepsis-induced ALI patients. This approach could be coupled with targeted metabolomics studies to quantify the levels of the metabolites that are known to be the precursors or products of these four differentiating metabolites.
This study also puts forth the hypothesis that the differentiating and physiologically relevant metabolites, myoinositol and total glutathione, may be associated with indicators of disease severity or outcome. In this regard, we found no treatment effect on any of the associations with the exception of myoinositol and VFD. By treatment analysis, the association between myoinositol and VFD in GM-CSF-treated patients was significant (P = 0.02), but it was not in placebo-treated patients. While a treatment effect is possible, this is most likely driven by the fact that 40% of the patients who had no VFD (VFD = 0) were in the GM-CSF group and only 15% were in the placebo group. Despite this limitation, these data provide the basis for testing these associations in a larger cohort. We also expect that an increased sample size and advancing technology (e.g., complementary use of NMR with liquid chromatography-mass spectrometry) and approaches to metabolite detection will result in the identification of additional metabolites of sepsis-induced ALI (22). While no single analytical technique presently exists that can identify all the metabolites in the metabolome, improved sensitivity of 1H-NMR spectroscopy permits the detection of many metabolites that are representative of important pathways that are altered by disease processes (13). This concept is substantiated by the results of our computational analysis.
We acknowledge that there are possible confounding variables that we could not control in our study. These include pharmacotherapy, diet, age, nutritional status, and concomitant illnesses, all of which may have impacted the metabolome of our patients. These parameters are limitations to all reported metabolomics studies. In particular, even though GM-CSF did not influence VFD and survival and metabolite levels were not different between GM-CSF- and placebo-treated patients, we cannot completely eliminate the possibility of a measurable effect of this cytokine on the metabolites measured, which may have confounded correlations with functional outcomes. Controlling for and identifying confounding variables is an important consideration for all metabolomics investigations, not just for ALI/ARDS studies. However, it is important to recognize that all biological and environmental parameters cannot be controlled in metabolic studies of human populations (29). This would require enormous sample sizes and could stifle the practical application of metabolomics to the human situation and critical illnesses in particular.
In summary, this exploratory study demonstrated that 1H-NMR-based quantitative metabolomics is a feasible approach for the identification of physiologically relevant metabolites associated with processes involved in the pathogenesis of sepsis-induced ALI (oxidant stress, energy homeostasis, apoptosis, and endothelial barrier function). They corroborate our present understanding of the complex pathophysiology of sepsis-induced ALI and expand our knowledge by linking individual metabolites to reactions and genes using a computational network analysis. These data can be used to develop novel hypotheses for testing in experimental and clinical models. Future studies will be directed at confirming these results in a larger cohort of patients and determining whether ALI has a metabolic signature that is unique of sepsis and other critical illnesses.
GRANTS
This work was supported, in part, by the University of Michigan College of Pharmacy (K. A. Stringer) and a Specialized Center for Clinical Research award (SCCOR) from the National Heart, Lung, and Blood Institute (P50-HL-074924; R. Paine and T. J. Standiford). The University of Colorado NMR Metabolomics Core was partly supported by the Department of Anesthesiology, the NCI P30-CA-046934 Cancer Center Core and UL1-RR-025780 CTSA grants (N. J. Serkova).
DISCLOSURES
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Cancer Institute, or the National Institutes of Health. No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Andrea L. Merz for technical assistance with sample preparation for NMR analysis.
REFERENCES
- 1. Afessa B, Gajic O, Keegan MT. Severity of illness and organ failure assessment in adult intensive care units. Crit Care Clin 23: 639–658, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ala-Korpela M. 1H NMR spectroscopy of human blood plasma. Prog Nuclear Magnetic Res Spect 27: 475–554, 1995 [Google Scholar]
- 3. Ala-Korpela M. Critical evaluation of 1H NMR metabonomics of serum as a methodology for disease risk assessment and diagnostics. Clin Chem Lab Med 46: 27–42, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Battistuzzi G, Iudicone P, Santolamazza P, Petrucci R. Activity of adenosine deaminase allelic forms in intact erythrocytes and in lymphocytes. Ann Hum Genet 45: 15–19, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Beck DH, Taylor BL, Millar B, Smith GB. Prediction of outcome from intensive care: a prospective cohort study comparing Acute Physiology and Chronic Health Evaluation II and III prognostic systems in a United Kingdom intensive care unit. Crit Care Med 25: 9–15, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc 57: 289–300, 1995 [Google Scholar]
- 7. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med 30: 60–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4: 499–502, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed 18: 143–162, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Chang DW, Hayashi S, Gharib SA, Vaisar T, King ST, Tsuchiya M, Ruzinski JT, Park DR, Matute-Bello G, Wurfel MM, Bumgarner R, Heinecke JW, Martin TR. Proteomic and computational analysis of bronchoalveolar proteins during the course of the acute respiratory distress syndrome. Am J Respir Crit Care Med 178: 701–709, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coen M, Holmes E, Lindon JC, Nicholson JK. NMR-based metabolic profiling and metabonomic approaches to problems in molecular toxicology. Chem Res Toxicol 21: 9–27, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Cohen ES, Law WR, Easington CR, Cruz KQ, Nardulli BA, Balk RA, Parrillo JE, Hollenberg SM. Adenosine deaminase inhibition attenuates microvascular dysfunction and improves survival in sepsis. Am J Respir Crit Care Med 166: 16–20, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regul Integr Comp Physiol 287: R247–R249, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Factor P, Mutlu GM, Chen L, Mohameed J, Akhmedov AT, Meng FJ, Jilling T, Lewis ER, Johnson MD, Xu A, Kass D, Martino JM, Bellmeyer A, Albazi JS, Emala C, Lee HT, Dobbs LG, Matalon S. Adenosine regulation of alveolar fluid clearance. Proc Natl Acad Sci USA 104: 4083–4088, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14: 1315–1323, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Frolkis A, Knox C, Lim E, Jewison T, Law V, Hau DD, Liu P, Gautam B, Ly S, Guo AC, Xia J, Liang Y, Shrivastava S, Wishart DS. SMPDB: the small molecule pathway database. Nucleic Acids Res 38: D480–D487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao J, Tarcea VG, Karnovsky A, Mirel BR, Weymouth TE, Beecher CW, Cavalcoli JD, Athey BD, Omenn GS, Burant CF, Jagadish HV. Metscape: a cytoscape plug-in for visualizing and interpreting metabolomic data in the context of human metabolic networks. Bioinformatics 26: 971–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. German JB, Hammock BD, Watkins SM. Metabolomics: building on a century of biochemistry to guide human health. Metabolomics 1: 3–9, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamp R, Sun X, Garcia JG. Making genomics functional: deciphering the genetics of acute lung injury. Proc Am Thorac Soc 5: 348–353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One 5: e10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu Q, Harrington EO, Newton J, Casserly B, Radin G, Warburton R, Zhou Y, Blackburn MR, Rounds S. Adenosine protected against pulmonary edema through transporter- and receptor A2-mediated endothelial barrier enhancement. Am J Physiol Lung Cell Mol Physiol 298: L755–L767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol 77: 1763–1772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma H, Sorokin A, Mazein A, Selkov A, Selkov E, Demin O, Goryanin I. The Edinburgh human metabolic network reconstruction and its functional analysis. Mol Syst Biol 3: 135, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao H, Wang H, Wang B, Liu X, Gao H, Xu M, Zhao H, Deng X, Lin D. Systemic metabolic changes of traumatic critically ill patients revealed by an NMR-based metabonomic approach. J Proteome Res 8: 5423–5430, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Martin TR, Hagimoto N, Nakamura M, Matute-Bello G. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc 2: 214–220, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuda N, Yamamoto S, Takano KI, Kageyama SI, Kurobe Y, Yoshiwara Y, Takano Y, Hattori Y. Silencing of Fas-associated death domain protects mice from septic lung inflammation and apoptosis. Am J Respir Crit Care Med 179: 806–815, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Nicholson JK. Global systems biology, personalized medicine and molecular epidemiology. Mol Syst Biol 2: 52, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov 1: 153–161, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Roessner U, Bowne J. What is metabolomics all about? Biotechniques 46: 363–365, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Rounds S, Yee WL, Dawicki DD, Harrington E, Parks N, Cutaia MV. Mechanism of extracellular ATP- and adenosine-induced apoptosis of cultured pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 275: L379–L388, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Serkova N, Fuller TF, Klawitter J, Freise CE, Niemann CU. H-NMR-based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney Int 67: 1142–1151, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Serkova NJ, Niemann CU. Pattern recognition and biomarker validation using quantitative 1H-NMR-based metabolomics. Expert Rev Mol Diagn 6: 717–731, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Serkova NJ, Van Rheen Z, Tobias M, Pitzer JE, Wilkinson JE, Stringer KA. Utility of magnetic resonance imaging and nuclear magnetic resonance-based metabolomics for quantification of inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 295: L152–L161, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Storey JD. A direct approach to false discovery rates. J Royal Stat Soc 64: 479–498, 2002 [Google Scholar]
- 37. Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: a unified approach. J Royal Stat Soc 66: 187–205, 2004 [Google Scholar]
- 38. Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 294: L632–L641, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Tarnopolsky MA, Ruby BC. Sex differences in carbohydrate metabolism. Curr Opin Clin Nutr Metab Care 4: 521–526, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal 10: 739–753, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Tissot van Patot MC, Murray AJ, Beckey V, Cindrova-Davies T, Johns J, Zwerdlinger L, Jauniaux E, Burton GJ, Serkova NJ. Human placental metabolic adaptation to chronic hypoxia, high altitude: hypoxic preconditioning. Am J Physiol Regul Integr Comp Physiol 298: R166–R172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uhlig S, Gulbins E. Sphingolipids in the lungs. Am J Respir Crit Care Med 178: 1100–1114, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 27: 337–349, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Wenzel RP. Treating sepsis. N Engl J Med 347: 966–967, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Wishart DS. Quantitative metabolomics using NMR. Trends Anal Chem 27: 228–237, 2008 [Google Scholar]
- 46. Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD, Psychogios N, Dong E, Bouatra S, Mandal R, Sinelnikov I, Xia J, Jia L, Cruz JA, Lim E, Sobsey CA, Shrivastava S, Huang P, Liu P, Fang L, Peng J, Fradette R, Cheng D, Tzur D, Clements M, Lewis A, De Souza A, Zuniga A, Dawe M, Xiong Y, Clive D, Greiner R, Nazyrova A, Shaykhutdinov R, Li L, Vogel HJ, Forsythe I. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res 37: D603–D610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, Fung C, Nikolai L, Lewis M, Coutouly MA, Forsythe I, Tang P, Shrivastava S, Jeroncic K, Stothard P, Amegbey G, Block D, Hau DD, Wagner J, Miniaci J, Clements M, Gebremedhin M, Guo N, Zhang Y, Duggan GE, Macinnis GD, Weljie AM, Dowlatabadi R, Bamforth F, Clive D, Greiner R, Li L, Marrie T, Sykes BD, Vogel HJ, Querengesser L. HMDB: the Human Metabolome Database. Nucleic Acids Res 35: D521–D526, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xia J, Wishart DS. MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res 38, Suppl: W71–W77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou Y, Mohsenin A, Morschl E, Young HW, Molina JG, Ma W, Sun CX, Martinez-Valdez H, Blackburn MR. Enhanced airway inflammation and remodeling in adenosine deaminase-deficient mice lacking the A2B adenosine receptor. J Immunol 182: 8037–8046, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.