Abstract
Cystic fibrosis (CF) is characterized by a massive proinflammatory phenotype in the lung, caused by mutations in the CFTR gene. IL-8 and other proinflammatory mediators are elevated in the CF airway, and the immediate mechanism may depend on disease-specific stabilization of IL-8 mRNA in CF lung epithelial cells. MAPK signaling pathways impact directly on IL-8 protein expression in CF cells, and we have hypothesized that the mechanism may also involve stabilization of the IL-8 mRNA. To test this hypothesis, we have examined the effects of pharmacological and molecular inhibitors of p38, and downstream MK2, ERK1/2, and JNK, on stability of IL-8 mRNA in CF lung epithelial cells. We previously showed that tristetraprolin (TTP) was constitutively low in CF and that raising TTP destabilized the IL-8 mRNA. We therefore also tested these effects on CF lung epithelial cells stably expressing TTP. TTP binds to AU-rich elements in the 3′-UTR of the IL-8 mRNA. We find that inhibition of p38 and ERK1/2 reduces the stability of IL-8 mRNA in parental CF cells. However, neither intervention further lowers TTP-dependent destabilization of IL-8 mRNA. By contrast, inhibition of the JNK-2 pathway has no effect on IL-8 mRNA stability in parental CF cell, but rather increases the stability of the message in cells expressing high levels of TTP. However, we find that inhibition of ERK1/2 or p38 leads to suppression of the effect of JNK-2 inhibition on IL-8 mRNA stability. These data thus lend support to our hypothesis that constitutive MAPK signaling and proteasomal activity might also contribute, along with aberrantly lower TTP, to the proinflammatory phenotype in CF lung epithelial cells by increasing IL-8 mRNA stability and IL-8 protein expression.
Keywords: cytokines, chemokines, inflammation, gene regulation
cystic fibrosis (CF) is the most common fatal autosomal recessive disease in the U.S. and Europe, and is due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (5, 9, 11, 15). CF is characterized by a massive proinflammatory phenotype in the lung, due to the secretion of high levels of IL-8 and other proinflammatory cytokines and chemokines (1, 4, 6, 17, 18). IL-8 is the most potent known chemotactic agent for neutrophils (18) and is hypersecreted from CF lung epithelial cells (4). However, the CF-dependent proinflammatory molecular mechanisms in the CF airway remain poorly understood.
The expression of inflammatory genes is known to be regulated by diverse processes, including mRNA decay. In the case of CF lung epithelial cells in culture, which constitutively secrete high levels of IL-8, we have recently reported that high levels of IL-8 mRNA are sustained by mutation-dependent reduction in the AU-rich element (ARE) binding protein tristetraprolin (TTP) (2). We found that elevation in TTP directly reduced the stability of IL-8 mRNA, as well as the level of secreted IL-8 protein. However, it remains clear that low levels of TTP might not be the only mechanism underpinning CF lung inflammation. For example, several previous studies have identified a function for MAPK signaling in regulating IL-8 mRNA stability in different cell types (13). Moreover, TTP itself has been shown to coordinate with MAPK signaling pathways, as well as the proteasome, to regulate expression of TNFα (7). Therefore, we hypothesized that MAPK signaling and proteasomal activity might also contribute, along with TTP, to dysregulation of IL-8 mRNA stability in CF lung epithelial cells.
We report here that IL-8 mRNA stability in CF lung epithelial cells is differentially regulated by MAPK pathways and proteasomal activity. Our approach has been to interrogate the state of IL-8 mRNA stability, and downstream IL-8 protein expression, in terms of the effects of pharmacological and molecular inhibitors of p38, ERK1/2, JNK, and the proteasome. Briefly, we find that the effects of these interventions on IL-8 expression depend on whether the ARE-interacting protein TTP has been permanently transfected into the CF cell. Furthermore, we find that only some interventions have equivalent effects in both IB3-1 and IB3-1-TTP cells. We find virtually identical results in CFBE cells, a different CF lung epithelial cell line. Based on these findings, we suggest that the mechanism by which CFTR mutations cause hyperinflammation in the CF airway may be due to aberrant regulation of IL-8 mRNA stability by a concerted mechanism involving active MAPK signaling.
MATERIALS AND METHODS
Reagents.
LHC-8 media, MEM Alpha, FBS, Trypsin-EDTA (0.05% and 0.25%), and Lipofectamine transfection reagent were purchased from Invitrogen. Actinomycin D (ActD), puromycin, and protease inhibitor mixture were purchased from Sigma Chemical (St. Louis, MO). The pharmacological inhibitors of MAPK pathways, SB-203580, SP-600125, and U0126, as well as the proteasome inhibitor MG132, were obtained from EMD Chemicals (Gibbstown, NJ). RNA aqueous kit for isolation of RNA from CF cells was obtained from Ambion (Austin, TX). IL-8 ELISA kit and antibody against GAPDH were purchased from R&D Systems (Minneapolis, MN). Protein assay reagents were purchased from Bio-Rad Laboratories (Richmond, CA).
Cell culture and transfection.
IB3-1 CF lung epithelial cells were maintained in LHC-8 serum-free medium in humidified 5% CO2 as previously described (8). The IB3-1-TTP cells were similarly maintained in LHC-8 medium containing puromycin (0.5 μg/ml). CFBE cells (obtained from J.P. Clancy, University of Alabama, Birmingham) were maintained in MEM Alpha medium (supplemented with 10% FBS) containing 2 μg/ml puromycin in humidified 5% CO2. Transfections were performed using Lipofectamine Transfection reagent (Invitrogen).
Measurement of mRNA stability by qRT-PCR method.
RNA was isolated from the cells, and mRNA expression levels were analyzed with qRT-PCR as described earlier (2).
Statistical analysis.
Statistical analysis was performed using Excel. Significance values (P ≤ 0.05) were determined from Student's t-test, performed on the percent remaining IL-8 mRNA at 3 h. Error bars on graphs represent SE.
RESULTS
IL-8 gene expression in CF lung epithelial cells is modulated by regulation of p38-MAPK and downstream MK2 signaling pathways.
We have previously shown that both IL-8 mRNA and protein levels are high in IB3-1 cells compared with the [wild-type CFTR]-repaired control IB3-1/S9 cells, and that reduced mRNA decay directly contributes to high IL-8 expression in CF lung epithelial cells (2). Since such posttranscriptional mechanisms are known to be regulated by MAPK signaling pathways, we hypothesized that the p38 and MK2 pathways might contribute to CF-dependent IL-8 mRNA stabilization. Figure 1A shows that enhanced degradation of IL-8 mRNA occurs when the IB3-1 CF lung epithelial cells are incubated either with the p38 inhibitor SB-203580 or if transiently transfected with dominant negatives of p38 (viz, p38-AGF) or MK2 (MK2-KR). It is known that p38 activates MK2 (20). Therefore, whether the action of p38 inhibition is direct or through MK2 is not immediately evident from this experiment. However, MK2-EE, a constitutively active mutant of MK2, has only a partial effect on IL-8 mRNA stability. These data therefore strongly support the concept that IL-8 mRNA stability in CF cells is regulated by p38, as well as partially by the downstream MK2 signaling. By contrast, similar treatment of the IB3-1-TTP cells, stably expressing increased TTP protein, does not induce any significant additional instability of IL-8 mRNA (Fig. 1B) or the corresponding reduction in levels of IL-8 protein (Fig. 1C). Thus it appears that TTP-dependent and p38/MK2-dependent mechanisms can equally destabilize IL-8 mRNA in CF lung epithelial cells. However, the p38/MK2 pathway cannot further destabilize the IL-8 mRNA once high levels of TTP are also constitutively present. SB-203580 effectively inhibits p38 phosphorylation in both IB3-1 and IB3-1-TTP cells as indicated in Fig. 1D.
Fig. 1.
Effect of inhibiting p38 pathway on IL-8 mRNA stability in cystic fibrosis (CF) lung epithelial cells. IB3-1 cells (A) or IB3-1-TTP cells (B) were treated with the chemical inhibitor SB-203580 (10 μM) or transiently transfected with dominant negatives (p38-AGF, MK-EE, and MK2-KR; ∼1 μg/1.5 × 106 cells) for p38-MAPK signaling pathway. RNA was isolated from IB3-1 and IB3-1-TTP cells after actinomycin D (5 μg/ml) treatment for the indicated time intervals, and the remaining mRNA was analyzed by quantitative real-time PCR. The data reflect averages of at least 3 independent experiments (*P < 0.05 and **P > 0.05). C: IL-8 protein secreted by both cell types was analyzed by ELISA 16 h after transfection. D: the phospho-p38 protein levels were analyzed by Western blot.
Inhibition of ERK1/2 pathway reduces IL-8 mRNA stability in CF cells.
Previous studies have shown that inhibition of the ERK1/2 pathway decreases IL-8 secretion from IB3-1 CF lung epithelial cells (3). The mechanism of this effect has been shown to be independent of the NF-κB signaling pathway. However, to delineate whether posttranscriptional mechanisms might be operative, we examined the effect of ERK1/2 pathway inhibition on IL-8 mRNA stability in CF cells. Figure 2A depicts that both the ERK1/2 inhibitor U0126 and the dominant negative ERK1/2 inhibitor Mnk-1 DN significantly diminish IL-8 mRNA stability in IB3-1 cells.
Fig. 2.
Inhibition of ERK1/2 pathway in CF cells regulates stability of IL-8 mRNA. IB3-1 cells (A) or IB3-1-TTP cells (B) were treated with the chemical inhibitor U0126 (10 μM) or transiently transfected with dominant negative (Mnk-1; ∼1 μg/1.5 × 106 cells) for ERK1/2-MAPK signaling pathway. RNA was isolated from IB3-1 and IB3-1-TTP cells after actinomycin D treatment for the indicated time intervals, and the remaining mRNA was analyzed by quantitative real-time PCR. The data reflect averages of at least 3 independent experiments (*P < 0.05 and **P > 0.05). C: IL-8 protein secreted by both cell types was analyzed by ELISA 16 h after transfection. D: the phospho-ERK1/2 protein levels were analyzed by Western blot.
However, when the same interventions were tested in IB3-1 cells constitutively expressing TTP, Fig. 2B shows that no additional significant decreases in IL-8 mRNA stability could be observed. The corresponding IL-8 protein levels were also not reduced any further. We conclude that inhibition of ERK1/2 in IB3-1 cells causes IL-8 to become unstable. However, in IB3-1-TTP cells, where increased expression of TTP protein already promotes enhanced IL-8 mRNA decay (Fig. 2, B and C), inhibition of ERK1/2 does not cause further destabilization. Analyses of total protein obtained from a whole cell lysate indicate that incubation with U0126 effectively blocks ERK1/2 phosphorylation (Fig. 2D).
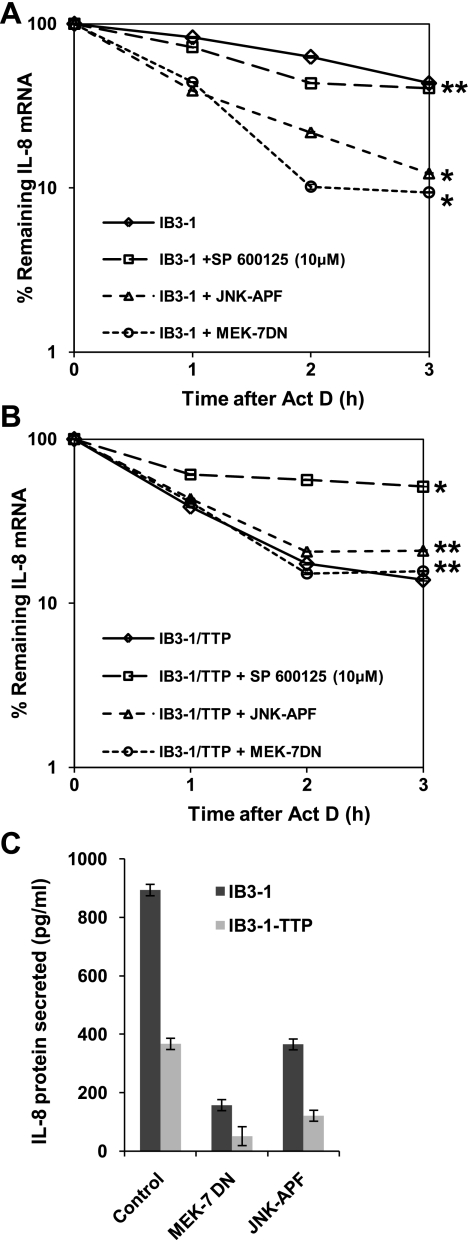
JNK-1 and JNK-2 pathways have differential effects on IL-8 mRNA stability in CF lung epithelial cells.
Inhibition of the JNK signaling pathway has been shown to reduce sodium 4-phenylbutyrate-induced IL-8 production (19). Therefore, to examine the function of JNK pathway in regulating IL-8 expression in CF lung epithelial cells, we treated the cells with the JNK-2 inhibitor SP-600125. In addition, we transfected cells with the dominant negative mutants of JNK-1, MEK-7 and JNK-APF. As shown in Fig. 3A, inhibition of JNK-2 by SP-600125 had no effect on IL-8 mRNA stability. However, expression of both dominant negatives to JNK-1 caused significantly enhanced degradation of IL-8 mRNA. By contrast, when IB3-1-TTP cells were treated with SP-600125, IL-8 mRNA was stabilized to levels comparable to that observed in the parental IB3-1 CF cells with low endogenous levels of TTP (Fig. 3B). Thus the inhibition of the JNK-2 pathway dominates over TTP function in the IB3-1-TTP cells. The corresponding IL-8 protein levels are depicted in Fig. 3C.
Fig. 3.
Inhibition of JNK-2 in CF cells has a different effect on IL-8 mRNA stability from that of p38 and ERK1/2. IB3-1 cells (A) or IB3-1-TTP cells (B) were treated with the chemical inhibitor SP-600125 (10 μM) or transiently transfected with dominant negatives (JNK-APF and MEK-7; ∼1 μg/1.5 × 106 cells) for JNK signaling pathway. RNA was isolated from IB3-1 and IB3-1-TTP cells after actinomycin D treatment for the indicated time intervals, and the remaining mRNA was analyzed by quantitative real-time PCR. The data reflect averages of at least 3 independent experiments (*P < 0.05 and **P > 0.05). C: IL-8 protein secreted by both cell types was analyzed by ELISA 16 h after transfection.
IL-8 mRNA stability exhibits a similar response to inhibition of MAPK signaling pathways.
The role of MAPK pathways in regulating IL-8 expression in IB3-1 CF lung epithelial cells was further examined in CFBE bronchial epithelial cells, another CF cell line (10). As depicted in Fig. 4A, the data indicate that IL-8 mRNA is degraded at a slower rate with ∼2.3-fold difference in half-life in CFBE cells (t1/2 = 339 min) compared with CFTR-repaired control HBE cells (t1/2 = 145 min). The corresponding IL-8 protein level also exhibits an approximately fourfold increase in CFBE cells compared with HBE control cells (Fig. 4B). Thus both IL-8 mRNA and protein expression in CFBE cells follow a phenomenon similar to our previously published observation in IB3-1 CF cells compared with IB3-1/S9 control cells. Although the absolute half-lives are likely to vary between cells, the difference between cognate pairs is important for understanding associated mechanisms. Consistently, increased stability of IL-8 is observed in the various CF cell types as indicated in relative increase in the half-life for IL-8 mRNA for a specific CF cell type compared with a cognate control (2).
Fig. 4.
Inhibition of MAPK pathways in CFBE cells has a similar effect on IL-8 mRNA stability as in IB3-1 cells. A: RNA was isolated from CFBE and HBE cells after treatment with actinomycin D (10 μg/ml) for the indicated time intervals. The remaining RNA was analyzed by quantitative real-time PCR and were normalized against that of control β-actin mRNA. The data (*P < 0.01) reflects averages of at least 3 independent experiments. B: IL-8 protein secreted by both cell types was analyzed by ELISA 16 h after transfection (P < 0.01). C: CFBE cells were treated with the chemical inhibitor SB-203580 (10 μM), U0126 (10 μM), or SP-600125 (10 μM). RNA was isolated from CFBE cells after actinomycin D (10 μg/ml) treatment for the indicated time intervals, and the remaining mRNA was analyzed by quantitative real-time PCR. The data reflect averages of at least 3 independent experiments (*P < 0.05 and **P > 0.05).
To further demonstrate that the CFBE is functionally similar to IB3-1 cells with respect to regulation of IL-8 mRNA stability, we examined the effect of inhibition of MAPK signaling pathways on IL-8 mRNA decay in CFBE cells. As shown in Fig. 4C, inhibition of both p38 and ERK1/2 pathways promotes enhanced degradation of IL-8 mRNA in CFBE cells, similar to our findings in IB3-1 CF cells (Figs. 1A and 2A). Additionally, inhibition of JNK-2 has no significant effect on IL-8 mRNA stability in CFBE cells as was observed in IB3-1 CF cells (Fig. 3A). Thus the function of MAPK pathways in the stabilization of IL-8 mRNA is a phenomenon observed in different CF lung epithelial cell lines.
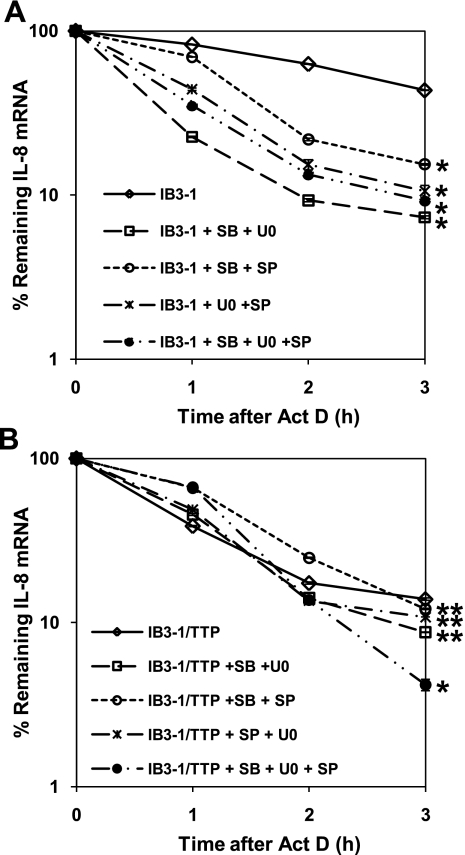
MAPK pathways concertedly and differentially affect IL-8 mRNA stability.
The MAPK pathways, p38, ERK1/2, and JNK, are known to converge on some common functions. Therefore, it is possible that in CF cells, these pathways might be dysfunctionally coordinated. As shown in Fig. 5A, the mixture of SB-203580 and U0126 maximally destabilizes IL-8 mRNA in IB3-1 cells. These levels are slightly lower than either inhibitor alone and are essentially identical to levels observed in untreated IB3-1-TTP cells. Thus these two pathways appear to act by different, non-overlapping processes on the IL-8 mRNA stability. However, as shown in Fig. 5B, adding these two inhibitors to IB3-1-TTP cells actually tends to enhance the TTP effect by slightly decreasing IL-8 mRNA stability. By contrast, the stabilization of IL-8 mRNA by SP-600125 alone is reversed by concurrent treatment with SB-203580 or U0126, or a combination of both. As depicted in Fig. 5B, similar cotreatment of IB3-1-TTP cells with the indicated combinations of MAPK inhibitors shows that the SP-600125-mediated suppression of TTP function could be overcome by concerted inhibition of the p38 and/or ERK1/2 pathways. The presence of elevated levels of TTP protein thus affects the function of the MAPK pathways on IL-8 mRNA stability.
Fig. 5.
Combined effect of inhibition of all the MAPK pathway stability of IL-8 mRNA in CF cells. IB3-1 cells (A) or IB3-1-TTP cells (B) were treated with the indicated combination of the chemical inhibitor SB-203580 (10 μM), U0126 (10 μM), and SP-600125 (10 μM) for the MAPK signaling pathways. RNA was isolated from IB3-1 and IB3-1-TTP cells after actinomycin D treatment for the indicated time intervals, and the remaining mRNA was analyzed by quantitative real-time PCR. The data reflect averages of at least 3 independent experiments (*P < 0.05 and **P > 0.05).
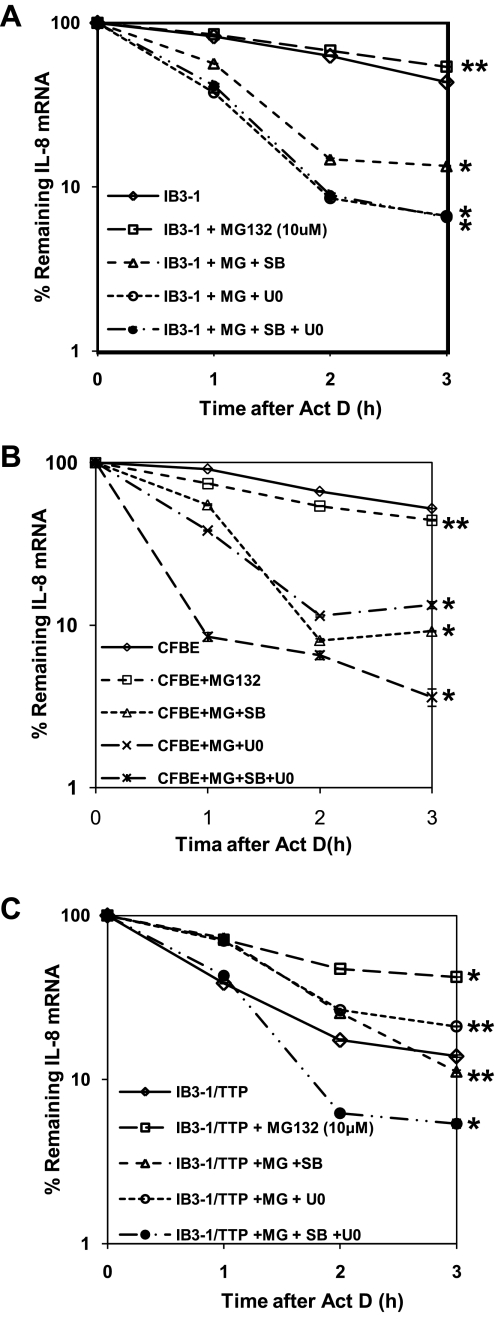
Proteasome inhibition stabilizes IL-8 mRNA in CF lung epithelial cells.
The cellular proteasome pathway is known to coordinate closely with the MAPK signaling pathways in regulating the expression of inflammatory genes (7). We therefore examined the function of the proteasome on IL-8 mRNA stability in CF lung epithelial cells. As shown in Fig. 6A, IL-8 mRNA does not undergo any significant change in stability upon inhibition of the proteasome pathway by MG-132. Moreover, to examine the coordination between the proteasome and the p38 as well as ERK1/2 MAPK signaling pathways, we treated MG-132-pretreated IB3-1 CF cells with the MAPK inhibitors alone or in combination. This was followed by actinomycin D treatment over time. As depicted in Fig. 6A, inhibiting MAPK pathways can overcome the effect of MG-132 and can reinduce instability of IL-8 mRNA. Similar observations were also established in CFBE cells as shown in Fig. 6B.
Fig. 6.
Proteasome function coordinates with MAPK pathways to modulate the stability of IL-8 mRNA in CF cells. IB3-1 cells (A) or CFBE (B) or IB3-1-TTP cells (C) were treated for 2 h with MG-132 (10 μM) followed by incubation with the indicated combination of the chemical inhibitor SB-203580 (10 μM), U0126 (10 μM), and SP-600125 (10 μM) for the MAPK signaling pathways. RNA was subsequently isolated from IB3-1 and IB3-1-TTP cells after actinomycin D treatment for the indicated time intervals, and the remaining mRNA was analyzed by quantitative real-time PCR. The data reflects averages of at least 3 independent experiments (*P < 0.05 and **P > 0.05).
Additionally, treatment of IB3-1-TTP cells by MG-132 abrogates TTP-mediated destabilization of IL-8 mRNA (Fig. 6C). However, cotreatment of these cells with SB-203580 or U0126 can relieve the inhibitory effect of MG-132 on TTP function, promoting destabilization of IL-8 mRNA to levels comparable to that with TTP alone (P < 0.001). Moreover, combined action of SB-203580 and U0126 on MG-132-treated IB3-1-TTP cells was effective in inducing IL-8 mRNA degradation at a rate faster (∼1.5-fold) than that induced by TTP alone.
DISCUSSION
These data lend further support to the concept that increased stability of IL-8 mRNA may contribute to the proinflammatory phenotype in the CF airway. We have previously shown that by increasing the aberrantly low levels of TTP in CF lung epithelial cells, we are able to lower the stability of IL-8 mRNA and IL-8 protein expression to near control levels (2). TTP is a destabilizing protein that binds to AREs in the 3′-UTR of the IL-8 mRNA. Here we show that MAPK signaling pathways may also contribute to regulation of IL-8 mRNA stability in CF cells.
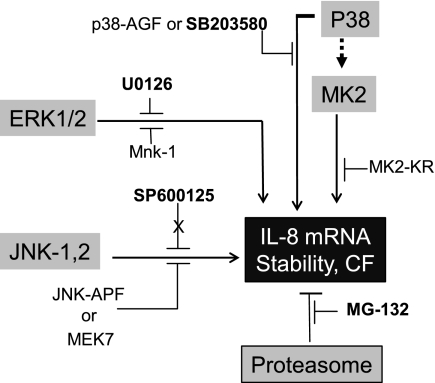
The data in this paper indicate that constitutive activation of p38 and ERK1/2 signaling pathways in CF lung epithelial cells may concertedly contribute to the mechanism(s) by which IL-8 mRNA is significantly stabilized, and consequent IL-8 protein expression is increased. We summarize the entire system in Fig. 7. This interpretation is supported by experiments in which both pharmacological inhibitors and recombinant, dominant negative mutants of different MAPKs can reduce CF-dependent IL-8 mRNA stability. In addition, our data further show that the TTP-enhanced CF cell responds to MAPK inhibition in different ways from the parental CF epithelial cell. In particular, inhibiting the JNK-2 system inhibits TTP-dependent decrease in IL-8 mRNA stability. Recent reports indicate that JNK function is required for TTP protein expression without affecting TTP mRNA levels (12). Importantly, our studies have not shown a detectable change in TTP protein level upon SP-600125 treatment of the IB3-1-TTP cells (data not shown). However, inhibition of ERK1/2 or p38 actually suppresses this JNK-2 effect on TTP action in the IB3-1-TTP cell. By contrast, inhibition of either ERK1/2 or p38 has no significant effect themselves on TTP-dependent destabilization of IL-8 in the IB3-1-TTP cell. Finally, we have shown that the cellular proteasome coordinates with MAPK signaling pathways to regulate IL-8 expression in CF cells. These data thus lend support to our hypothesis that MAPK signaling and proteasomal activity might also contribute, along with TTP, to the proinflammatory phenotype in CF lung epithelial cells by modulating IL-8 mRNA stability and IL-8 protein expression.
Fig. 7.
Concerted action of MAPK pathways and proteasome function on IL-8 mRNA stability in CF cells. IL-8 mRNA stabilization in CF lung epithelial cells as well as CF bronchial epithelial cells is promoted by constitutive activation of p38 and ERK1/2 pathways. However, inhibition of JNK-2 as well as the proteasome has no effect on IL-8 mRNA stability in these CF cells.
The destabilizing effect of TTP on IL-8 mRNA in the CF cell is dependent on the interaction of TTP protein with AREs in the 3′-UTR (2). An important mechanistic question is whether the MAPKs act in the same manner. Earlier reports indicate that airway inflammation in CF is controlled by the p38-MAPK pathway (16, 21). The stress-activated protein kinase p38 is known to drive MK2 (MAPKAPK-2) (20). MK2 activity is known to reduce the half-lives of mRNAs for TNFα and IL-6 only if the AREs in the respective 3′-UTRs are also present (14). Therefore, when p38 is inhibited by SB-203580, the action may be by inhibition of downstream MK2. To explore this question in more detail, we also inhibited MK2 with dominant negative MK2-KR. Consistently, this intervention resulted in the exact same effect as inhibiting p38: IL-8 stability was reduced in IB3-1 cells, but had no further effect on reduced IL-8 mRNA instability found in the IB3-1-TTP cells. It is therefore possible that p38 acts via MK2 and an as yet unknown further downstream signaling process to stabilize IL-8 mRNA in CF cells. The fact that inhibiting either p38 or MK2 fails to further destabilize IL-8 mRNA in IB3-1-TTP cells suggests that the downstream effects of the p38/MK2 system may act by a similar CF-specific ARE-interaction mechanism.
Due to the presence of similar AU-rich motifs in the 3′-UTR, both IL-8 and TNFα mRNA are regulated through similar posttranscriptional mechanisms by TTP (7). The regulation of TNFα mRNA stability by TTP in mouse macrophage cells has also been shown to occur via a proteasome-dependent mechanism involving MAPK signaling pathways (7). Consistently, we find that both the proteasome and the MAPK signaling pathways interplay to regulate IL-8 mRNA stability in the CF cells, albeit by different mechanisms. As expected and has been previously shown for other ARE-containing mRNAs (7), inhibition of proteasome function in CF cells stabilized IL-8 mRNA. This effect was insensitive to, and dominant over, TTP function, as those CF cells stably expressing increased levels of TTP also exhibited stable IL-8 mRNA levels upon inhibition of proteasome activity. Moreover, synergistic inhibition of the proteasome and the MAPK signaling pathways successfully reduced IL-8 expression by destabilizing IL-8 mRNA. We therefore conclude that targeting IL-8 mRNA stability at multiple mechanistic steps, including MAPK signaling, proteasome function, and TTP activity, may become novel areas for developing candidate CF therapies.
GRANTS
This study was supported by USU-Intramural Funds (R. Biswas), the National Institutes of Health [NO1-HV28187 (H. B. Pollard) and RO1-DK-053051 (H. B. Pollard)], and the Cystic Fibrosis Foundation (R. Biswas and H. B. Pollard).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The views expressed are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences, the Department of the Defense, or the United States government.
ACKNOWLEDGMENTS
We thank Cara Olsen (Biostatistics Consulting Center, USU) for helping with data analyses. We also thank Paul Anderson (Brigham & Women's Arthritis Center, Boston, MA) and Thomas A. Hamilton (Cleveland Clinic Foundation, Cleveland, OH) for dominant negative clones for p38, ERK1/2, and JNK pathways and Perry Blackshear (National Institute of Environmental Health Sciences, Durham, NC) for providing the pCMV.hTTP.tagHA plasmid used for generating IB3-1-TTP stable cell lines.
REFERENCES
- 1. Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutierrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 156: 1197–1204, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Balakathiresan NS, Bhattacharyya S, Gutti U, Long RP, Jozwik C, Huang W, Srivastava M, Pollard HB, Biswas R. Tristetraprolin regulates IL-8 mRNA stability in cystic fibrosis lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L1012–L1018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boncoeur E, Criq VS, Bonvin E, Roque T, Henrion-Caude A, Gruenert DC, Clement A, Jacquot J, Tabary O. Oxidative stress induces extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase in cystic fibrosis lung epithelial cells: potential mechanism for excessive IL-8 expression. Int J Biochem Cell Biol 40: 432–446, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 152: 2111–2118, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63: 827–834, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Dean TP, Dai Y, Shute JK, Church MK, Warner JO. Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr Res 34: 159–161, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Deleault KM, Skinner SJ, Brooks SA. Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol 45: 13–24, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Eidelman O, Srivastava M, Zhang J, Leighton X, Murtie J, Jozwik C, Jacobson K, Weinstein DL, Metcalf EL, Pollard HB. Control of the proinflammatory state in cystic fibrosis lung epithelial cells by genes from the TNF-alphaR/NFkappaB pathway. Mol Med 7: 523–534, 2001 [PMC free article] [PubMed] [Google Scholar]
- 9. Frizzell RA. Ten years with CFTR. Physiol Rev 79: S1–S2, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Hentchel-Franks K, Lozano D, Eubanks-Tarn V, Cobb B, Fan L, Oster R, Sorscher E, Clancy JP. Activation of airway cl− secretion in human subjects by adenosine. Am J Respir Cell Mol Biol 31: 140–146, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Kopito RR. Biosynthesis and degradation of CFTR. Physiol Rev 79: S167–S173, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Korhonen R, Linker K, Pautz A, Forstermann U, Moilanen E, Kleinert H. Post-transcriptional regulation of human inducible nitric-oxide synthase expression by the Jun N-terminal kinase. Mol Pharmacol 71: 1427–1434, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Muselet-Charlier C, Roque T, Boncoeur E, Chadelat K, Clement A, Jacquot J, Tabary O. Enhanced IL-1beta-induced IL-8 production in cystic fibrosis lung epithelial cells is dependent of both mitogen-activated protein kinases and NF-kappaB signaling. Biochem Biophys Res Commun 357: 402–407, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk HD, Holtmann H, Kollias G, Gaestel M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem 277: 3065–3068, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Pollard HB. Anatomic genomics: systems of genes supporting the biology of systems. Anat Rec 259: FMIII–IX, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Raia V, Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Auricchio S, Cimmino M, Cavaliere M, Nardone M, Cesaro A, Malcolm J, Quaratino S, Londei M. Inhibition of p38 mitogen activated protein kinase controls airway inflammation in cystic fibrosis. Thorax 60: 773–780, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richman-Eisenstat JB, Jorens PG, Hebert CA, Ueki I, Nadel JA. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol Lung Cell Mol Physiol 264: L413–L418, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 19: 429–438, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Roque T, Boncoeur E, Saint-Criq V, Bonvin E, Clement A, Tabary O, Jacquot J. Proinflammatory effect of sodium 4-phenylbutyrate in deltaF508-cystic fibrosis transmembrane conductance regulator lung epithelial cells: involvement of extracellular signal-regulated protein kinase 1/2 and c-Jun-NH2-terminal kinase signaling. J Pharmacol Exp Ther 326: 949–956, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Stokoe D, Campbell DG, Nakielny S, Hidaka H, Leevers SJ, Marshall C, Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J 11: 3985–3994, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaman MM, Gelrud A, Junaidi O, Regan MM, Warny M, Shea JC, Kelly C, O'Sullivan BP, Freedman SD. Interleukin 8 secretion from monocytes of subjects heterozygous for the deltaF508 cystic fibrosis transmembrane conductance regulator gene mutation is altered. Clin Diagn Lab Immunol 11: 819–824, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]