Abstract
Signaling between cell membrane-bound L-type Ca2+ channels (LTCC) and ryanodine receptor Ca2+ release channels (RyR) on sarcoplasmic reticulum (SR) stores grades excitation–contraction coupling (ECC) in striated muscle. A physical connection regulates LTCC and RyR in skeletal muscle, but the molecular mechanism for coordinating LTCC and RyR in cardiomyocytes, where this physical link is absent, is unknown. Calmodulin kinase (CaMK) has characteristics suitable for an ECC coordinating molecule: it is activated by Ca2+/calmodulin, it regulates LTCC and RyR, and it is enriched in the vicinity of LTCC and RyR. Intact cardiomyocytes were studied under conditions where CaMK activity could be controlled independently of intracellular Ca2+ by using an engineered Ca2+-independent form of CaMK and a highly specific CaMK inhibitory peptide. CaMK reciprocally enhanced L-type Ca2+ current and reduced release of Ca2+ from the SR while increasing SR Ca2+ content. These findings support the hypothesis that CaMK is required to functionally couple LTCC and RyR during cardiac ECC.
Excitation–contraction coupling (ECC) in skeletal and cardiac muscle requires coordinated bidirectional interactions between cell membrane-bound L-type Ca2+ channels (LTCC) and ryanodine receptor Ca2+ release channel proteins (RyR) on intracellular sarcoplasmic reticulum (SR) Ca2+ stores (1). Substantial evidence indicates that a physical link between LTCC and RyR is essential for antegrade (2, 3) and retrograde (4) signaling in skeletal muscle type ECC. In contrast, cardiac ECC occurs in the apparent absence of a physical link (2) by a process termed Ca2+-induced Ca2+ release (5), whereby L-type Ca2+ current (ICa) traverses a subcellular microdomain (6) to trigger release of intracellular Ca2+ ([Ca2+]i) by opening RyR on SR Ca2+ stores. In addition to antegrade signaling from LTCC to RyR, Ca2+ released from the SR can dynamically regulate ICa through dual mechanisms of inactivation (7) and facilitation (8). Both [Ca2+]i-dependent inactivation and facilitation are likely important for grading ICa to determine the strength of contraction, maintain [Ca2+]i homeostasis, and to refill SR Ca2+ stores. Importantly, both RyR (9, 10) and LTCC (11, 12) are regulated by phosphorylation, raising the possibility that an appropriately positioned Ca2+-activated kinase or phosphatase could translate Ca2+ activity changes in the LTCC-RyR microdomain into coordinating signals for these molecules during cardiac ECC.
Calmodulin (CaM) kinase (CaMK) is a multifunctional serine/threonine kinase that phosphorylates cardiac RyR (9, 13–16) and colocalizes with cardiac LTCC and RyR (8). CaMK activation reduces ryanodine binding to RyR (9, 13), can decrease RyR opening probability (9, 10, 16), and facilitates ICa (17–19) by inducing a modal gating shift in LTCC favoring long openings (12). Thus, CaMK has an appropriate subcellular localization pattern and operating characteristics to be a functional link between LTCC and RyR during cardiac ECC. Based on studies from isolated LTCC (12) and RyR (9), using rabbit cardiomyocytes, we hypothesized that CaMK activation functionally couples LTCC and RyR to reciprocally reduce SR Ca2+ release and facilitate ICa during cardiac ECC. An important experimental obstacle for unraveling Ca2+-dependent signaling mechanisms during ECC has been the inability to independently control CaMK activity and [Ca2+]i. We circumvented these obstacles by using an engineered Ca2+-independent, constitutively active form of CaMK and a specific CaMK inhibitory peptide to independently control CaMK activity, ICa, and SR Ca2+ content. Here, we show that CaMK activity is required to dynamically and reciprocally “link” ICa with SR Ca2+ release during cardiac ECC.
Methods
Electrophysiology.
Electrophysiology with whole cell mode voltage clamp configuration using isolated rabbit ventricular myocytes was performed according to previously published methods (17). Briefly, cells were held at −80 mV for >5 min for adequate dialysis with pipette solution before initiating experiments. ICa was activated by stepping the cell membrane from −80 mV to +20 mV at 0.5 Hz for 300 ms. Experiments were performed at 24°C. Na+ and K+ currents were eliminated by adding Cs+ and tetraethylammonium chloride (TEA) and reducing Na+ and K+ in the pipette and bath solutions. Elimination of the residual current by nifedipine (10 μM) or Cd2+ (100 μM) confirmed that the identity of active current was ICa (data not shown). The pipette (intracellular) solution was (in mM): CsCl 120.0, Hepes 10.0, TEA 10.0, phosphocreatine 5.0, MgATP 1.0, NaGTP 1.0, and pH was adjusted to 7.2 with 1.0 N CsOH. The bath (extracellular) solution was NMDG 137.0, CsCl 25.0, Hepes 10.0, glucose 10.0, CaCl2 1.8, MgCl2 0.5, and pH was adjusted to 7.4 with 12 N HCl.
Fluo 3 Fluorescence Measurements.
Fluo 3 fluorescence measurements were used to reflect SR Ca2+ release by including the pentapotassium salt of the fluo 3 (Molecular Probes) in the pipette solution (100 μM) as previously described, with minor modifications (17). Under these conditions, elimination of SR Ca2+ release by incubation with ryanodine (10 μM) or thapsigargin (1 μM) eliminates >90% of the fluo 3 transient, indicating that the dynamic fluo 3 signal reflects Ca2+ release from the SR (data not shown). Voltage signals were low pass filtered at 50 Hz before analysis. Fluo 3 [Ca2+]i transients were normalized by the ratio of the measured voltage (F) to the baseline voltage (Fo) by using PCLAMP 6.0.3.
SR Ca2+ Content.
SR Ca2+ content was quantified as the integrated Na+/Ca2+ exchanger current (20). We perfused the myocyte during a prolonged voltage command to −80 mV with a “spritz” of modified bath solution containing caffeine (20 mM) and equimolar substitution of NaCl for N-methyl-d-glucamine, using a rapid solution exchanger (ALA, Westbury, NY). The resulting inward current was integrated by using PCLAMP 6.0.3 (Axon Instruments, Foster City, CA) and normalized for cell surface area. The rate of maximal SR Ca2+ release was estimated from the initial phase of inward Na+/Ca2+ exchanger current fit to a single exponential equation.
Inhibitory Peptides.
The CaMK inhibitory peptide AC3-I (KKALHRQEAVDCL, IC50 ≈0.5 μM) was synthesized and isolated to >95% purity by RP-HPLC (Macromolecular Resources, Fort Collins, CO). The inactive control peptide AC3-C (KKALHAQERVDCL, IC50 > 500 μM) was a generous gift from Howard Schulman (Stanford University, Stanford, CA). AC3-I is a modified CaMK substrate, and the amino acid sequence HRQEAVDCL corresponds to the autophosphorylation site (T286/287) on CaMK, except T is modified to A to prevent phosphorylation.
Constitutively Active CaMK.
Constitutively active CaMK (amino acid residues 1–380 of mouse type II, α isoform) was expressed in baculovirus, purified with a CaM affinity column as previously described (8), and used at a final concentration of 0.9 μM to approximate physiologic activity (21). The purified CaMK was made Ca2+/CaM-independent by thiophosphorylation of Thr 286 in the presence of Ca2+, CaM, and adenosine 5′-O-(3-thiotriphosphate) (8). The blank buffer does not support activation of ICa (8) (see Fig. 2) or LTCC (12) by endogenous CaMK, and was used as a control.
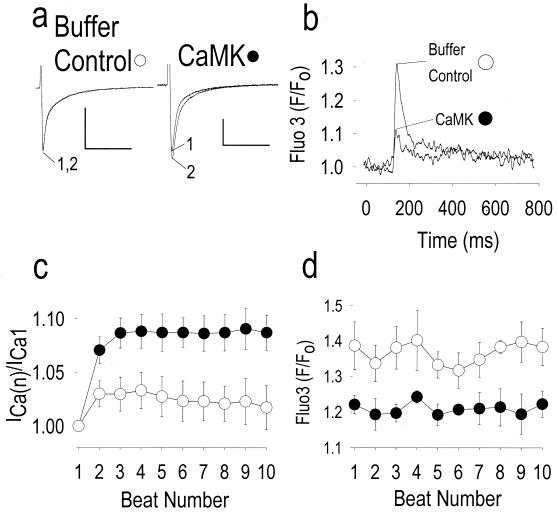
Figure 2.
Addition of constitutively active CaMK reconstitutes ICa facilitation and reciprocally reduces release of Ca2+ from intracellular stores. (a) ICa facilitation is absent in this cell dialyzed with buffer solution (Buffer Control), but is present after addition of constitutively active CaMK (CaMK). Numerals indicate the beat number as in Fig. 1, and the calibration bars represent 500 pA (vertical) and 100 ms (horizontal). (b) Averaged intracellular Ca2+ transients using the fluorescent indicator fluo 3 (see Methods), for beats 1–10 to show the sustained reduction in Ca2+ release from intracellular stores by CaMK (n = 5) compared with buffer alone (n = 5). (c) Summary of data showing ICa facilitation expressed as the ratio of peak ICa during the first to the nth beat [ICa(n)], as shown in Fig. 1. ICa facilitation was significantly increased by CaMK (n = 5) compared with control (P < 0.05 for beats 2 and 4–10, n = 5). (d) Peak fluo 3 fluorescence signals for each stimulated beat, measured simultaneously with ICa reported in c. CaMK significantly reduced peak fluo 3 fluorescence compared with control (P < 0.05 for beats 3, 5, 8–10).
Results
CaMK Inhibition Disrupts Reciprocal Signaling Between LTCC and RyR.
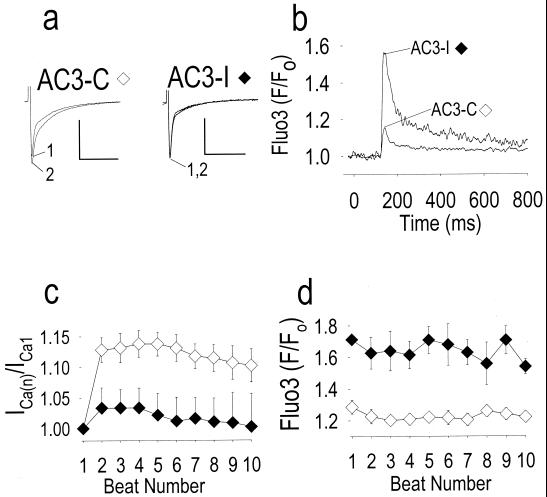
Facilitation of peak ICa occurs in ventricular myocytes, under control conditions (Fig. 1 a, Left, and c) (17–19), because of LTCC activation by CaMK (12). The [Ca2+]i transient (Fig. 1b) is primarily the result of secondary Ca2+ release from the SR because >90% of the transient was eliminated by the SR-inactivating agents ryanodine or thapsigargin (data not shown) (22). CaMK inhibition prevented ICa facilitation (Fig. 1 a, Right, and c) and enhanced the [Ca2+]i transient (Fig. 1 b and d). The enhanced SR Ca2+ release seen following CaMK inhibition (Fig. 1b) is consistent with the finding in isolated RyR that CaMK-catalyzed phosphorylation decreases RyR opening probability (9, 10). These findings strongly suggest that CaMK is required to maintain the normal relationship between ICa and SR Ca2+ release and implicate CaMK as a component of the molecular machinery for coordinating LTCC and RyR during cardiac ECC.
Figure 1.
CaMK inhibition prevents ICa facilitation and reciprocally increases release of Ca2+ from intracellular stores. (a) ICa facilitation is prominent in control cells dialyzed with an inactive peptide (AC3-C), but is markedly reduced after addition of the CaMK inhibitory peptide (AC3-I; see Methods). Numerals indicate the beat number, and the calibration bars represent 500 pA (vertical) and 100 ms (horizontal). (b) Averaged intracellular Ca2+ transients from n = 3 cells (AC3-I) and n = 10 cells (AC3-C) using the fluorescent indicator fluo 3 (see Methods) for beats 1–10 to show details of the sustained increase in release of SR Ca2+ following CaMK inhibition (AC3-I) compared with cells treated with the inactive control peptide (AC3-C). (c) Summary of data showing ICa facilitation expressed as the ratio of peak ICa during the first to the nth beat [ICa(n)]. ICa facilitation was significantly decreased by AC3-I (n = 3) compared with control (P < 0.05 for beats 2–8, n = 10). (d) Peak fluo 3 fluorescence signals for each stimulated beat, measured simultaneously with ICa reported in c. CaMK inhibition significantly increased peak fluo 3 fluorescence compared with control (P < 0.005 for all beats).
Constitutively Active CaMK Reconstitutes the Reciprocal Relationship of ICa to SR Ca2+ Release During ECC.
To further probe the role of CaMK signaling during ECC, cardiomyocytes were dialyzed with constitutively active CaMK (8). The exogenous CaMK buffer blocked the effects of activation of endogenous CaMK (8, 12) (Fig. 2a, buffer control compare with Fig. 1a, AC3-I). The constitutively active, Ca2+-independent CaMK (Fig. 2a, Right) reconstituted the control ECC phenotype (Fig. 1a, Left) by simultaneously enhancing ICa facilitation (Fig. 2 a and c) and reducing peak SR Ca2+ release (Fig. 2 b and d). Thus, CaMK supplementation reduced the apparent gain of the Ca2+-induced Ca2+ release mechanism, a result that was directly opposite to the effect of CaMK inhibition (Fig. 1), confirming that CaMK activity is required for coordinated reciprocal signaling between LTCC and RyR.
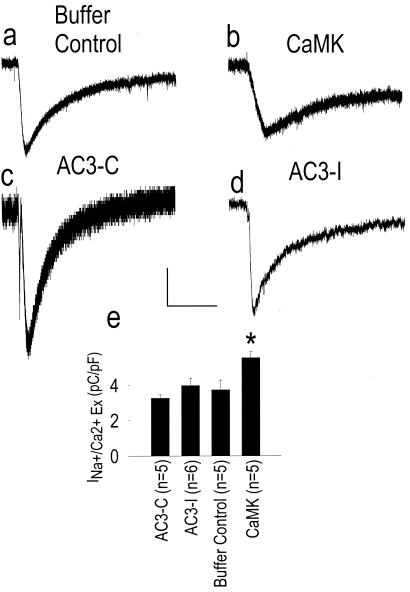
CaMK Enhances SR Ca2+ Content and Lowers the Gain of Ca2+-Induced Ca2+ Release.
Changes in SR Ca2+ stores could contribute to the reduced [Ca2+]i transient during CaMK activation, and apparent ICa facilitation could result from reduced Ca2+-dependent ICa inactivation. To assess SR Ca2+ content under these conditions, we used a rapid solution exchange system to activate SR Ca2+ release with caffeine in the presence of extracellular Na+, where the integral of the electrogenic Na+/Ca2+ exchanger current reflects the content of SR Ca2+ (20). SR Ca2+ content was significantly enhanced by supplementation with constitutively active CaMK compared with cells dialyzed with control buffer (Fig. 3), likely because of activation of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) (23, 24), and/or reduction in RyR opening (9, 10) by CaMK. In contrast, AC3-I did not significantly affect SR Ca2+ content compared with AC3-C. One possibility is that the greater [Ca2+]i transient in AC3-I treated cells (Fig. 1 b and d) favors Ca2+-dependent, phosphorylation-independent dissociation of phospholamban from SERCA (25) to increase SR Ca2+ uptake. Taken together, these data strongly suggest that CaMK directly regulates LTCC and RyR, reducing the gain of ECC.
Figure 3.
CaMK increases SR Ca2+ content. Inward Na+/Ca2+ exchanger current (INa+/Ca2+ Ex) in response to perfusion with a caffeine and Na+-containing solution in buffer control (a), CaMK (b), AC3-C (c), and AC3-I treated cardiomyocytes (d). Calibration bars indicate 50 pA (vertical) and 5 s (horizontal) for a–d. (e) Summary data for integrated INa+/Ca2+ Ex corrected for cell size from all experimental groups. *, P = 0.005, compared with cells treated with control buffer alone.
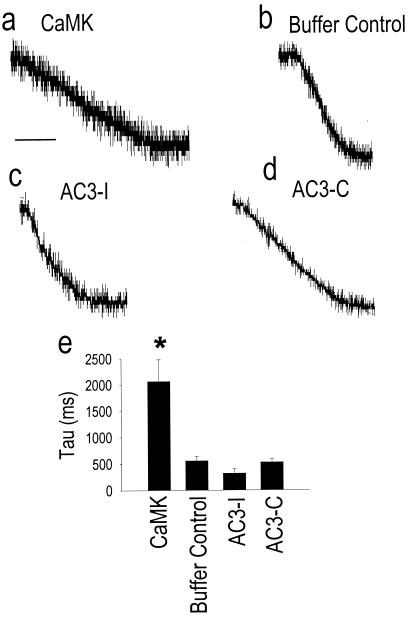
We further estimated the maximal rate of SR Ca2+ release by measuring the initial rapid phase of inward Na+/Ca2+ exchanger current. CaMK significantly slowed SR Ca2+ release compared with cells exposed to control buffer (Fig. 4 a, b, and e), further suggesting that CaMK directly reduced RyR opening during ECC, even with the increase in SR Ca2+ available for release. The finding that inward Na+/Ca2+ exchanger current was larger (Fig. 3e) and slower (Fig. 4e) in CaMK-dialyzed cells indicates that the Na+/Ca2+ exchanger was not likely saturated under these experimental conditions, and so could accurately report differences in SR Ca2+ content between experimental groups. Taken together, these findings strongly suggest that the mechanism of reciprocal regulation of ICa and SR Ca2+ release by CaMK involves reduction of RyR opening (9) and prolongation of LTCC openings (12).
Figure 4.
CaMK slows SR Ca2+ release. (a–d) The initial rapid phase of SR Ca2+ release measured from the initial inward Na+/Ca2+ exchanger current in response to rapid application of caffeine and Na+ (see Methods). Dialysis of constitutively active CaMK (CaMK) (a) significantly slowed maximal SR Ca2+ release compared with cells treated with buffer control (b). Inhibition of endogenous CaMK with the inhibitory peptide AC3-I (c) tended to enhance the maximal rate of SR Ca2+ release compared cells treated with an inactive control peptide (d), AC3-C. (e) Summary data for the time constant of initial rapid SR Ca2+ release from the same cells in Fig. 3e. The calibration bar indicates 500 ms for a and b, and 200 ms for c and d. The record heights in a–d were normalized to 1.0. *, P = 0.016, compared with cells treated with buffer alone.
Discussion
CaMK and Cardiac ECC.
The present data show that CaMK can bridge the “communication gap” between LTCC and RyR, and provide a mechanism to explain coordinated LTCC-RyR activity in cardiac ECC. Ca2+-induced Ca2+ release represents the better studied antegrade limb of cardiac ECC, but information is also communicated from the RyR to the L-type Ca2+ channel protein complex through a retrograde limb by a process termed crosstalk (7). Although inhibitory crosstalk occurs by [Ca2+]i-dependent inactivation of ICa (26), the present findings indicate that CaMK-dependent “facilitatory” crosstalk also occurs. It is likely that this facilitatory component of crosstalk is important for refilling SR Ca2+ stores by enhancing ICa while reducing SR Ca2+ release.
The present study of the role of CaMK in cardiac ECC uses a highly specific inhibitory peptide and constitutively active CaMK as experimental probes. Our finding that CaMK activity reduces the gain of cardiac ECC contrasts with a previous study where the cell membrane-permeant CaMK inhibitory compound KN-93 reduced the gain of ECC in ferret ventricular myocytes (27). In addition to inhibiting CaMK, KN-93 blocks multiple cell membrane ionic currents (28) and so could interfere with ECC independently of CaMK, for example, by inhibiting ion channel proteins on the SR that are thought to be important for balancing charges associated with Ca2+ flux during ECC. It is also possible that differences in experimental conditions may partially contribute to the discrepancy between these results. One strength of the present approach is that it employs both CaMK inhibitory and replacement strategies.
Reduction in the gain of ECC that follows addition of CaMK is similar to the ECC phenotype in experimental cardiac hypertrophy and heart failure (29). CaMK expression (30) and activity (31) are both increased in failing human hearts, raising the possibility that enhanced CaMK activity may explain the ECC phenotype in human heart failure (32), and so represent a therapeutic target. The functional properties of CaMK are partially governed by the proportion of different isoforms composing the heteromeric holoenzyme (33). Thus, changes in CaMK isoform populations that occur during cardiac development (34, 35) and disease (30) can provide a previously unrecognized dimension to regulation of cardiac ECC.
CaMK and RyR.
Although it is clear that RyR is an important substrate for CaMK actions, the effect of CaMK-catalyzed RyR phosphorylation in isolated preparations remains controversial. Some authors have reported that exogenous CaMK increases (15, 16), whereas others have found that exogenous (9, 10) and endogenous (16) CaMK reduces RyR opening or ryanodine-RyR binding (13). Our results in intact cardiomyocytes where endogenous CaMK is inhibited or exogenous Ca2+-independent CaMK is added are both consistent with the hypothesis that the net effect of CaMK activation is to reduce RyR opening.
Acknowledgments
We thank Martha Bass for expert technical assistance. We thank Dan Roden and Jeff Balser for helpful criticisms and suggestions. This work was supported by grants from the National Institutes of Health (National Heart, Lung, and Blood Institute, HL03727 and HL62494; to M.E.A.) and the American Heart Association SE affiliate (to M.E.A. and R.J.C.).
Abbreviations
- ECC
excitation–contraction coupling
- CaM
calmodulin
- CaMK
CaM kinase
- LTCC
L-type Ca2+ channels
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- ICa
L-type Ca2+ current
- [Ca2+]i
intracellular Ca2+ concentration
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tanabe T, Beam K G, Powell J A, Numa S. Nature (London) 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 2.Nakai J, Ogura T, Protasi F, Franzini-Armstrong C, Allen P D, Beam K G. Proc Natl Acad Sci USA. 1997;94:1019–1022. doi: 10.1073/pnas.94.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakai J, Sekiguchi N, Rando T A, Allen P D, Beam K G. J Biol Chem. 1998;273:13403–13406. doi: 10.1074/jbc.273.22.13403. [DOI] [PubMed] [Google Scholar]
- 4.Nakai J, Dirksen R T, Nguyen H T, Pessah I N, Beam K G, Allen P D. Nature (London) 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 5.Fabiato A, Fabiato F. J Physiol. 1975;249:469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Lopez J R, Shacklock P S, Balke C W, Wier W G. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- 7.Adachi-Akahane S, Cleemann L, Morad M. J Gen Physiol. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, MacMillan L B, McNeill R B, Colbran R J, Anderson M E. Am J Physiol. 1999;276:H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- 9.Lokuta A J, Rogers T B, Lederer W J, Valdivia H H. J Physiol. 1995;487:609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Best P M. Nature (London) 1992;359:739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- 11.Yue DT, Herzig S, Marban E. Proc Natl Acad Sci USA. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzhura I, Wu Y, Colbran R J, Balser J R, Anderson M E. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 13.Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. J Biochem. 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- 14.Hohenegger M, Suko J. Biochem J. 1993;296:303–308. doi: 10.1042/bj2960303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witcher D R, Kovacs R J, Schulman H, Cefali D C, Jones L R. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 16.Hain J, Nath S, Mayrleitner M, Fleischer S, Schindler H. Biophys J. 1994;67:1823–1833. doi: 10.1016/S0006-3495(94)80664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson M E, Braun A P, Schulman H, Premack B A. Circ Res. 1994;75:854–861. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- 18.Yuan W, Bers D M. Am J Physiol. 1994;267:H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- 19.Xiao R P, Cheng H, Lederer W J, Suzuki T, Lakatta E G. Proc Natl Acad Sci USA. 1994;91:9659–9663. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz M E, Cook S J, Chamunorwa J P, Trafford A W, Lancaster M K, O'Neill S C, Eisner D A. Circ Res. 1996;78:857–862. doi: 10.1161/01.res.78.5.857. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R C, Kranias E G. Biochemistry. 1989;28:5909–5916. doi: 10.1021/bi00440a030. [DOI] [PubMed] [Google Scholar]
- 22.Sham J S, Cleemann L, Morad M. Proc Natl Acad Sci USA. 1995;92:121–125. doi: 10.1073/pnas.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odermatt A, Kurzydlowski K, MacLennan D H. J Biol Chem. 1996;271:14206–14213. doi: 10.1074/jbc.271.24.14206. [DOI] [PubMed] [Google Scholar]
- 24.Kuschel M, Karczewski P, Hempel P, Schlegel W P, Krause E G, Bartel S. Am J Physiol. 1999;276:H1625–H1633. doi: 10.1152/ajpheart.1999.276.5.H1625. [DOI] [PubMed] [Google Scholar]
- 25.Asahi M, McKenna E, Kurzydlowski K, Tada M, MacLennan D H. J Biol Chem. 2000;275:15034–15038. doi: 10.1074/jbc.275.20.15034. [DOI] [PubMed] [Google Scholar]
- 26.Hadley RW, Lederer W J. J Physiol. 1991;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Satoh H, Ginsburg K S, Bers D M. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson M E, Braun A P, Wu Y, Lu T, Schulman H, Sung R J. J Pharmacol Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- 29.Gomez A M, Valdivia H H, Cheng H, Lederer M R, Santana L F, Cannell M B, McCune S A, Altschuld R A, Lederer W J. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 30.Hoch B, Meyer R, Hetzer R, Krause E G, Karczewski P. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Cardiovasc Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 32.Beuckelmann D J, Nabauer M, Erdmann E. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- 33.De Koninck P, Schulman H. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 34.Hagemann D, Hoch B, Krause EG, Karczewski P. J Cell Biochem. 1999;74:202–210. [PubMed] [Google Scholar]
- 35.Hoch B, Wobus A M, Krause E G, Karczewski P. J Cell Biochem. 2000;79:293–300. [PubMed] [Google Scholar]