Abstract
Although a highly effective vaccine against smallpox, vaccinia virus (VV) is not without adverse events, some of which can be life-threatening, particularly in immunocompromised individuals. We have recently demonstrated that the immunogenicity and protective efficacy of Dryvax® in immunocompetent mice is preserved even when co-administered with ST-246, an orally bioavailable small-molecule inhibitor of orthopoxvirus egress and dissemination. In addition, ST-246 markedly reduced the reactogenicity of the smallpox vaccine ACAM2000 and the highly neurovirulent VV strain Western Reserve (VV-WR). Here, we evaluated the impact of ST-246 co-administration on ACAM2000 reactogenicity, immunogenicity, and protective efficacy in seven murine models of varying degrees of humoral and cellular immunodeficiency: BALB/c and B-cell deficient (JH-KO) mice depleted of CD4+ or CD8+ or both subsets of T cells. We observed that ST-246 reduced vaccine lesion severity and time to complete resolution in all of the immunodeficient models examined, except in those lacking both CD4+ and CD8+ T cells. Although VV-specific humoral responses were moderately reduced by ST-246 treatment, cellular responses were generally comparable or slightly enhanced at both 1 and 6 months post-vaccination. Most importantly, in those models in which vaccination given alone conferred protection against lethal VV challenge, similar levels of protection were observed at both time points when vaccination was given with ST-246. These data suggest that, with the exception of individuals with irreversible, combined CD4+ and CD8+ T-cell deficiency, ST-246 co-administered at the time of vaccination may help reduce vaccine reactogenicity –even in those lacking humoral immunity– without impeding the induction of protective immunity.
Keywords: ST-246, ACAM2000, immunodeficient mice
1. INTRODUCTION
After an aggressive global vaccination campaign conducted by the World Health Organization (WHO) using vaccinia virus (VV), smallpox was declared eradicated in 1980 [1]. Although the eradication was achieved in the absence of knowledge about the protective immunity elicited by VV, vaccination is believed to confer protection against smallpox disease since it induces robust and long-lasting humoral and cellular immune responses that cross-recognize highly homologous antigens expressed by variola virus (VARV), its causative agent [1, 2]. Despite its extinction in nature, however, smallpox as a catastrophic epidemic disease remains a concern owing to the potential for accidental or deliberate release of VARV as an act of war or bioterrorism [3]. Other pathogenic poxviruses occurring in nature, such as monkeypox and cowpox virus, also occasionally infect human populations and pose serious health threats. Furthermore, VV continues to be intensively studied for the generation of live recombinant vaccines against cancer and infectious diseases, such as human immunodeficiency virus (HIV).
Routine immunization of the civilian population in the United States with the smallpox vaccine Dryvax was discontinued in 1972, when it became apparent that the risk from vaccine-associated adverse events outweighed the possibility of contracting naturally occurring smallpox. For instance, a 10-state study in 1970 [4] found that there were ~1254 complications per 1 million primary vaccinations, although the last reported case of smallpox in the US was 1949 [1]. The most common adverse reactions to smallpox vaccination that do not require specific treatment include fever, headache, fatigue, myalgia, chills, nausea, malaise, viral lymphangitis, regional lymphadenopathy, and pain, pruritis, edema and intense erythema at the vaccination site, which are consistent with acute viral illness resulting from active VV replication [5, 6]. Although these symptoms are mostly mild to moderate and resolve by day 15 post-vaccination (p.v.), more than a third of Dryvax vaccinees have been reported to be sick enough to miss work, school, sleep, or recreational activities [5]. More serious adverse events, including accidental auto- or contact-inoculation, erythema multiforme, generalized vaccinia, postvaccinial encephalitis, myopericarditis, eczema vaccinatum, progressive vaccinia, fetal vaccinia, and death, have also been reported in the past (reviewed in [6]).
Due to the rare but real risk for serious or life threatening complications, the smallpox vaccine is contraindicated for millions of people and their contacts with congenital or acquired immunodeficiencies or a history of atopic dermatitis, chronic skin disease, or heart disease [7]. For example, progressive vaccinia (PV) is a rare, severe, and often lethal adverse event that occurs primarily in individuals with immunological defects in cell-mediated immunity (CMI) and rarely in those with agammaglobulinemia (reviewed in [6, 8]). In PV, the initial vaccination lesion or “take” does not heal and continues to expand progressively with or without dissemination to distant skin areas and internal organs. Disease severity is dependent on the degree of T-cell deficiency and is most pronounced, and nearly always fatal, in those with extreme T-cell defects and severe combined (B- and T-cell) immunodeficiency (SCID). Most vaccinations occur without complications in persons with antibody deficiency but intact CMI, such as Bruton-type agammaglobulinemia patients who lack mature B cells, supporting the critical role of CMI in preventing PV [9]. In patients with CMI deficiency but intact humoral responses, such as Nezelof syndrome (congenital thymic dysplasia with normal antibody levels), PV is usually fatal, but is typically confined to skin progression without viremic spread, possibly due to antibody neutralization of the virus in the blood [1, 6]. PV is particularly severe and lethal in patients with combined CMI and antibody deficiency, such as congenital Swiss-type agammaglobulinemia that is characterized by low or absent antibody levels and thymic dysplasia [1]. Individuals who develop profound T-cell suppression due to an unrelated disease, such as cancer or AIDS, or are being treated with immunosuppressive drugs, such as for organ transplantation or autoimmune disease, are also at increased risk for PV. Unfortunately, the subset(s) and frequency of T cells that are needed to protect against PV are not known due to the absence of suitable techniques for differentiating and measuring CMI during the smallpox eradication era.
In the event of an outbreak, the Advisory Committee on Immunization Practices has recommended that individuals with a high-risk exposure to VARV be vaccinated regardless of contraindications, since those at greatest risk for experiencing serious complications from vaccination are also at greatest risk for death from smallpox [10]. Compared to that during the eradication campaign [4], much higher numbers of adverse events would be expected to occur today because of the significantly increased prevalence of persons living with known contraindications, which is currently estimated to include up to 25% of the U.S. population [11]. Given that the majority of adverse reactions are associated with uncontrolled VV replication and/or dissemination, co-administration of antiviral agents may play an essential role in preventing or reducing the heightened risk of adverse events in contraindicated populations.
ST-246 is an orally bioavailable, low-molecular-weight compound that is potently and selectively active against a broad spectrum of Orthopoxviruses (OPV), including VV, ectromelia, cowpox, camelpox, monkeypox (MPXV), and VARV [12–14]. In virus-infected cells in vitro, ST-246 inhibits plaque formation and significantly reduces the formation of extracellular enveloped virus (EEV or EV), without affecting the production of intracellular mature virus (IMV or MV) [12]. By targeting the VV F13L gene product and its highly conserved homologs in other OPV, ST-246 blocks the double membrane wrapping of MV to form intracellular enveloped virus (IEV), thus interfering with the production of cell-associated enveloped virus (CEV) and EV [12, 15]. In vivo, ST-246 has shown efficacy in all immunocompetent animal models of OPV-induced disease tested to date, including MPXV and VARV in non-human primates (NHPs), even when treatment is delayed up to 72 hours post-infection [12, 16–19]. It significantly inhibits systemic virus dissemination from the site of inoculation and leads to an overall reduction in virus replication and shedding in target organs [12, 16, 17]. ST-246 has additionally shown protective efficacy in mice deficient in either CD4+ or CD8+ T cells, but not both, regardless of the presence or absence of B-cell deficiency [20]. Most importantly, multiple phase I clinical trials in healthy volunteers have demonstrated that ST-246 has favorable pharmacokinetic and safety profiles with no evident negative adverse reactions to prevent its use in humans [21, 22].
We [17, 23] and others [24] have previously demonstrated that vaccine lesion severity and time to complete healing is markedly reduced in mice and NHPs vaccinated with ACAM2000 or VV-WR when concurrently treated with ST-246. Importantly, the co-administration of ST-246 with Dryvax [23] or ACAM2000 [24] did not compromise the induction of protective immunity from subsequent challenge with lethal doses of VV in mice or MPXV in NHPs, respectively. Recently, ST-246 has been used in humans along with VIGIV (vaccinia immune globulin intravenous) or with VIGIV and cidofovir (a cytosine analog with anti-OPV activity in vitro and in vivo) for the successful treatment of: 1) Dryvax-associated contact eczema vaccinatum developed by a 2-year old child [25]; 2) ACAM2000-associated PV developed by a 20-year old military vaccinee with acute myelogenous leukemia (AML) [26]; and 3) recombinant VV (Copenhagen strain)-associated contact adverse events (26 VV lesions, pronounced redness and edema, and adenopathy) experienced by a 35-year old woman who was immunosuppressed due to multiple medications, including a suppressor of T- and B-cell proliferation and a tumor necrosis factor-α (TNF-α) blocker, for the treatment of inflammatory bowel disease [27]. Taken together, the above observations suggest that without negative impact on vaccine efficacy, co-administration ST-246 at the time of vaccination might help reduce the risk of vaccine-associated adverse reactions, particularly those arising from uncontrolled EV dissemination and infection. Here, we expand on previous observations in immunocompetent animals by evaluating the impact of ST-246 on ACAM2000 reactogenicity, immunogenicity, and protective efficacy in mice with T-cell, B-cell, or combined immunodeficiencies.
2. MATERIALS AND METHODS
2.1. Viruses and cell lines
VV-WR and BSC-40 (African green monkey kidney cell line) and A20 (B-cell lymphoma cell line from BALB/c mice) cells were purchased from American Type Culture Collection (ATCC; Manassas, VA). VV strain International Health Department-J (VV-IHD-J) was from a stock originally provided by S. Dales (The Rockefeller University, New York). All cell culture media and reagents were from Invitrogen (Carlsbad, CA), unless otherwise specified. BSC-40 and A20 cells were cultured as previously described [23]. VV-WR and VV-IHD-J were propagated in BSC-40 cells, purified by two cycles of sucrose gradient centrifugation, and titered by plaque assay, as described [28]. The ACAM2000 vaccine was kindly provided by Acambis, Inc. (Cambridge, MA) as a lyophilized preparation of purified, live VV containing 6–8 mM HEPES (pH 6.5–7.5), 2% human serum albumin, 0.5–0.7% sodium chloride, and 5% mannitol. Ten vaccine vials were reconstituted in 0.3 ml of diluent (50% (v/v) glycerin and 0.25% (v/v) phenol in water) per vial, pooled to create a single stock, aliquoted into small volumes, and kept frozen at −80 °C until use. The titer of the stock vaccine determined by plaque assay was 1×108 PFU/ml.
2.2. Mice
Animal studies were conducted in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee of Oregon State University (Corvallis, OR). Six-week-old female BALB/c and 4- to 6-week-old female, homozygous JH-knockout (JH-KO) mice were purchased from Taconic (Oxnard, CA). JH-KO mice have targeted deletion of all J segments of the immunoglobulin (Ig) heavy-chain locus (JH). As a result, they have neither mature (surface Ig-bearing) B cells in the bone marrow and periphery nor detectable IgM or IgG in the sera [29]. However, T-cell development appears to proceed normally and a proportional, rather than absolute, increase in splenic T-cell number is observed due to the B-cell deficit.
2.3. In vivo depletion of CD4+ and/or CD8+ T cells
GK1.5 and 2.43 hybridomas secreting rat anti-mouse CD4 and CD8 monoclonal antibodies (mAbs), respectively, were obtained from ATCC and grown as described previously [20]. Secreted mAbs were purified by HiTrap Protein G (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) affinity chromatography and diluted in endotoxin-free PBS before administration to mice. Endotoxin contamination was confirmed to be very low (<0.2 EU/ml) using Chromo-limulus amebocyte lysates assay (Associates of Cape Cod, Inc., East Falmouth, MA). CD4+ and/or CD8+ T-cell depletion was achieved by intraperitoneal (i.p.) injection of 0.2 mg of GK1.5 and/or 0.5 mg of 2.43 antibodies in a total volume of 0.5 ml PBS, as we [20] and others [30, 31] have reported previously. Initial injections were given consecutively on days −5, −4, and −3 before vaccination (on day 0) to ensure prior cellular depletion. Thereafter, the injections were repeated every 7 days (days +4, +11, etc.) to continually maintain depletion. For short-term immunity analysis at 1 month post-vaccination (p.v.), cellular depletion was maintained until the termination of the lethal VV-WR challenge phase of the experiment. For long-term immunity analysis at 6 months p.v., the last antibody treatment during the vaccination phase was given to the majority of the mice on day 52 p.v. However, in those mice depleted of both CD4+ and CD8+ T cells that continued to show visible viral lesion(s), antibody treatment was terminated on day 66 p.v. This was because lesions continued to appear and it was assumed that these mice were unable to completely clear the virus due to their severely immunocompromised state. During the analysis phase of the long-term study, antibody injections were resumed on days 177, 178, and 179 and immune assays were performed on day 182 followed by VV-WR challenge on day 184 p.v. The antibody injection was repeated thereafter every 7 days (days +186, +193, etc.) until the termination of the challenge study on day 212 p.v. In all the studies, mice that did not receive antibody injections were used as non-depleted (N.D.) controls. Cellular depletion, as confirmed by flow cytometric analysis of peripheral blood cells and/or splenocytes, was consistently >99% (Figure S1 and data not shown).
2.4. ACAM2000 vaccination and ST-246 treatment
A “standard” dose of ~2.5 ×105 PFU was used for vaccination in order to closely mimic the dose administered to humans. The reconstituted vaccine was further diluted in PBS such that the intended dose was delivered in a 10 μl volume. The vaccination was performed via epidermal scarification by applying the virus at the base of the tail and scratching the droplet into the skin ~30 times with a 25-gauge needle, as described previously [23]. For administration by oral gavage, ST-246 was prepared as a 20 mg/ml suspension in an aqueous solution containing 0.75% methylcellulose (Sigma, St. Louis, MO) and 1% Tween 80 (Sigma). Beginning immediately before vaccination, mice were treated once daily with either 100 mg/kg of ST-246 or an equal volume of vehicle for 14 consecutive days. This dose and duration of ST-246 treatment has been demonstrated in several studies to be effective in protecting mice from lethal respiratory infection with VV [12, 16, 17]. In addition, there is no overt toxicity, such as body weight changes or clinical signs, associated with repeated oral dosing of mice with 100 mg/kg/day of ST-246, which has a no-observable-adverse-effect level (NOAEL) established at 2,000 mg/kg of body weight in mice [12, 19]. For each time point of the study (i.e., 1 or 6 months p.v.), two groups of 4 to 5 mice were set up in parallel for each treatment (vehicle or ST-246) arm of vaccine recipients: one group to be used for ex vivo analysis of immune responses and another to be used for evaluation of protective immunity from lethal VV-WR challenge. Naïve (unvaccinated) mice were used as negative controls in both immune assay and challenge experiments. The formation, progression, severity, and healing of vaccine-induced lesions was documented by photographing tails on days 3, 7, 10, 14, 17, 21, and 28 p.v. Mice that were moribund due to vaccine-induced systemic disease were humanely euthanized.
2.5. Virus challenge and clinical disease and survival monitoring
On day 30 or 184 p.v., mice were lightly anesthetized by 3% isoflurane in oxygen and intranasally (i.n.) inoculated with 10 times the 50% lethal dose (LD50) of VV-WR (7.91×105 PFU for 11 week old mice and 1.26×108 PFU for 6 month old mice) in 10 μl of PBS. The LD50 for each time point was determined prior to challenge according to the method of Reed and Muench [32] in age matched mice in order to account for age-based susceptibility to poxvirus disease [33]. Afterwards, the mice were monitored daily for signs of illness and survival and scored as 0 (normal), 1 (slightly ruffled), 2 (significantly ruffled), 3 (significantly ruffled, hunched posture and/or conjunctivitis), 4 (score of 3 combined with difficulty in breathing/moving/socializing), and 5 (death). Additionally, the weight and body temperature of each mouse was taken immediately before challenge and on alternate days thereafter. Those with serious breathing difficulty, inability to stand or move (disease index score of 4), or greater than 30% loss of starting body weight were humanely euthanized.
2.6. Sample collection and processing
Mice were euthanized by CO2 asphyxiation and blood samples were collected by cardiac puncture in sodium citrate-dextrose solution (Sigma). The blood was centrifuged at 14,000 RPM, 4 °C for 5 minutes and the plasma was collected and stored at −80°C until use. Spleens were collected in ice-cold Dulbecco’s phosphate-buffered saline (DPBS; Mediatech, Inc., Manassas, VA) and red blood cell-free single-cell suspensions were prepared, as previously described [23]. All samples from vaccinated mice were collected, processed, and analyzed individually.
2.7. Enzyme-linked immunosorbent assay (ELISA)
VV-WR-specific ELISA was performed as described previously [23] with slight modifications. Briefly, 96-well MaxiSorp surface plates (Nalge Nunc International, Rochester, NY) were coated with 5×105 PFU/well of purified VV-WR overnight at 4 °C, blocked with 5% casein (Fisher Scientific) buffer, and incubated with 8 five-fold serial dilutions of plasma at room temperature (RT) for 2 hours. The plates were then incubated at RT with a 1:10,000 dilution of HRP-conjugated goat anti-mouse IgG antibody (Bio-Rad, Hercules, CA) for 1 hour and developed by adding tetramethylbenzidine-hydrogen peroxide substrate (Pierce Biotechnology, Rockford, IL). After 15 minutes of incubation, color development was stopped by adding 2N sulfuric acid and absorbance was measured at 450 nm. Endpoint titers (reciprocals of plasma dilutions two standard deviations above the average absorbance in naïve mice plasma) were then determined from the average values of duplicate wells by using XLFit curve-fitting software for Excel (ID Business Solutions, Alameda, CA).
2.8. Plaque reduction neutralization titer 50 (PRNT50) and comet tail inhibition assays
Both assays were performed using heat-inactivated plasma (56 °C for 30 min) as described previously [23]. Briefly, for PRNT50 assay, a fixed amount of VV-WR (~38 PFU) was incubated overnight at 37 °C/5% CO2 with 8 two-fold dilutions of plasma in DMEM-2.5 (DMEM supplemented with 2.5% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 1 mM sodium pyruvate). The antibody-virus mix was then used to infect monolayers of BSC-40 cells in 24-well plates for 1.5 hrs at 37 °C/5% CO2 and overlayed with DMEM-2.5 media containing 1% methylcellulose. After 48 hrs, the monolayers were stained with 0.1% crystal violet in 5% methanol to enumerate plaques. PRNT50 was then calculated as the reciprocal of the highest dilution of test plasma that resulted in a 50% reduction in plaque count compared with naïve mice plasma wells, by using XLFit software. For comet tail inhibition assays, monolayers of cells in 6-well plates were first infected for 2 hours with ~65 PFU of VV-IHD-J, which releases significantly higher levels of EV than VV-WR, in 0.5 ml of DMEM-2.5. The cells were then overlaid with 1.0 ml of liquid culture media (DMEM-2.5) in order to allow the release and dissemination of virus from infected cells and formation of satellite plaques (comet tails). Subsequently, 60 μl of test plasma, which results in a 1:25 final dilution in the culture media, was added to the wells. After 40 hrs of incubation, the monolayers were stained with crystal violet to visualize plaques and comet tails and make qualitative assessment of inhibition.
2.9. Pentamer Staining
The published 9-mer peptides KYGRLFNEI (A52R75-83: H-2Kd) and SPYAAGYDL (F2L26-34: H-2Ld) [34] were used for quantification of VV-specific CD8+ T cells. APC-conjugated major histocompatibility complex (MHC) class I pentamers containing these peptides were custom synthesized by ProImmune (Bradenton, FL) and used for direct ex vivo staining according to the supplied protocol. Briefly, 2×106 splenocytes resuspended in FACS buffer (DPBS containing 0.1% sodium azide and 0.1% BSA) were incubated with 5 μl of aggregate-free pentamers in the dark, at RT, for 10 minutes. The cells were then surface stained with PE-conjugated anti-CD8 antibody, fixed in paraformaldehyde (PFA), resuspended in FACS buffer, and analyzed on a FACSCanto flow cytometer using FACSDiVa software (BD, San Jose, CA). A live lymphocyte gate was set based on forward scatter (FSC) and side scatter (SSC) parameters and ~500,000 events were acquired for each sample.
2.10. Intracellular cytokine staining (ICCS)
As described previously [23], 1×106 splenocytes were co-cultured with 3×105 mock- or VV-WR-infected (6-hour infection at an MOI of 5 PFU/cell), syngeneic A20 cells in 96-well flat bottom plates in the presence of Brefeldin A (3 μg/ml; eBioscience, San Diego, CA) and recombinant human interleukin-2 (IL-2; 29 units/ml; BD). After 16-18 hours of culture at 37 °C/5% CO2, cells from duplicate wells were pooled and incubated with anti-CD16/32 (BD) antibodies for 20 minutes to block Fc receptors, surface stained with anti-CD4-Alexa Fluor 700 (eBioscience) and CD8-PerCP (BD) antibodies for 30 minutes, and then fixed with 4% PFA (eBioscience) for 20 minutes at 4 °C. After membrane permeabilization with 0.1% saponin (eBioscience), the fixed cells were intracellularly stained with anti-interferon-γ (IFN-γ)-PE (BD) and IL-2-Alexa Fluor 488 (eBioscience) antibodies, fixed, resuspended in FACS buffer, and analyzed by flow cytometry. Approximately 100,000 events were acquired for each sample using a live lymphocyte gate.
2.11. Cytokine secretion analyses by cytometric bead array (CBA)
Splenocytes were co-cultured with mock- or VV-WR-infected A20 cells the same way as described above for ICCS, but without the addition of Brefeldin A and IL-2. As described previously [23], culture supernatants were collected after 48 hrs of stimulation and tested for the presence of the cytokines interferon (IFN)-γ, TNF-α, IL-2, IL-4, and IL-5 using TH1/TH2 CBA kits per the manufacturer’s (BD) instructions. In short, beads coated with capture antibodies specific for each cytokine were mixed with recombinant cytokine standards or supernatant samples, and PE-conjugated detection antibodies. After 2 hr incubation at RT, the beads were washed, resuspended in wash buffer (PBS containing serum proteins and detergent; BD), and analyzed by flow cytometry. Approximately 1,500 events were collected and the concentration of each cytokine was calculated with CBA Analysis Software (BD).
2.12. Statistical analysis
All statistical analyses were performed using SigmaStat 3.1 software (San Jose, CA). Data from vehicle and ST-246-treated mice within each experimental group of ACAM2000-vaccinated mice were compared using unpaired Student’s t-test. The Mann-Whitney Rank Sum Test was employed for the comparison of disease scores after VV-WR challenge. A p-value of <0.05 was considered statistically significant.
3. RESULTS
3.1. Effect of ST-246 on vaccine lesion formation, progression, and healing in immunodeficient mice
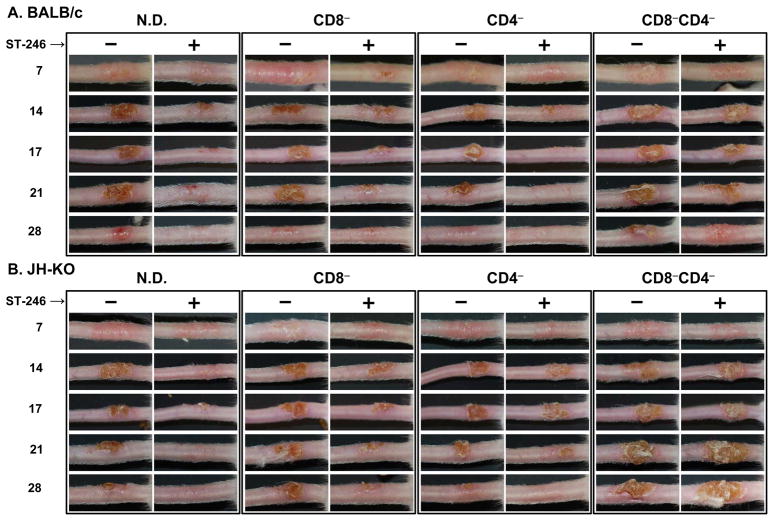
Formation of “Jennerian vesicle/pustule” or vaccine “take” by day 7 p.v. is the gold standard marker of successful human vaccination, which is considered as an evidence of acquired immunity against smallpox [2]. Although the maculopapular stage is not evident, mice vaccinated by epidermal scarification of the tail exhibit a similar progression of lesion development as in humans [23, 35]. Given that immunodeficient individuals are contraindicated for smallpox vaccination, we evaluated whether ST-246 given as an adjunct to smallpox vaccination in B- and/or T-cell deficient mice could prevent or reduce vaccine-associated adverse reactions. BALB/c (Figure 1A) and JH-KO (Figure 1B) mice that were depleted of CD4+, CD8+, or both CD4+ and CD8+ T cells were vaccinated with ~2.5×105 PFU of ACAM2000 and concurrently treated orally with vehicle or 100 mg/kg of ST-246 for 14-consecutive days. The mice were then monitored for lesion formation and progression at the vaccination site and for the potential development of satellite lesions and systemic disease due to virus dissemination from the inoculation site. As evident in Figure 1, vaccine take was not inhibited by ST-246 treatment since all of the mice, regardless of their humoral and/or cellular deficiency, developed Jennerian vesicles by day 7 p.v. as did vehicle-treated mice. However, vaccine lesion severity was noticeably reduced in all ST-246-treated groups with the exception of CD4−CD8−/BALB/c and CD4−CD8−/JH-KO mice, although there was individual variability among the 4-5 mice within all groups. Even in the CD4−CD8− mice, the severity of the vaccine lesions were less pronounced in ST-246-treated mice at early time points p.v., but not at or beyond day 14. Overall, in both vehicle- and ST-246-treated mice, lesions were slightly more severe in mice of the JH-KO than BALB/c background. Compared to non-depleted (N.D.) mice, lesion severity was slightly better (in CD4−), slightly worse (in CD8−), or significantly more pronounced (in CD4−CD8−) depending on the type(s) of T cells depleted.
Figure 1. Effect of ST-246 on vaccine lesion formation and progression in immunodeficient mice.
BALB/c (A) or B-cell deficient (JH-KO; B) mice were initially depleted of CD4+ and/or CD8+ T cells by i.p. injection of anti-CD4 and/or anti-CD8 mAbs for 3 consecutive days (days −5, −4, and −3) prior to vaccination (on day 0). Thereafter, cellular depletions were maintained for the duration of the experiment by once-weekly mAb injections (days +4, +11, etc). The mice were vaccinated by epidermal scarification of the tail with ~2.5×105 PFU of ACAM2000 and treated by oral gavage with vehicle or 100 mg/kg of ST-246 for 14 consecutive days. Naïve (unvaccinated) and vaccinated non-depleted (N.D.) mice, which were not treated with antibody, served as controls. Photographs of the tails were taken on days 7, 14, 17, 21, and 28 post-vaccination to document vaccine-induced lesion severity. Although there were differences in the degree of lesion severity among mice within a group (n = 4–5), especially in CD8−CD4− cohorts, representative tail lesions are shown.
With the exception of CD4−CD8− mice, it was also clearly evident that the majority of ST-246-treated mice in all of the other groups showed markedly accelerated (~7-14 days earlier) resolution of their lesions than their counterparts in vehicle-treated mice. However, regardless of treatment with vehicle or ST-246 treatment, all of the N.D., CD4−, and CD8− mice in both BALB/c and JH-KO background eventually cleared their primary lesions without the appearance of secondary (satellite) lesions or the manifestation of systemic disease, such as body hair ruffling, significant weight loss, or death (data not shown). The time to lesion clearance in the majority (>60%) of vehicle-treated N.D., CD4−, and CD8− mice was 35, 28, and 35 days, respectively, in BALB/c mice and 35 days for all three groups in JH-KO mice. The corresponding days in ST-246-treated mice were 21, 17, and 28 days, respectively, in BALB/c mice and 21, 28, and 28 days in JH-KO mice. Interestingly, despite the severity of their cellular defect, the majority of CD4−CD8−/BALB/c mice in both vehicle- and ST-246-treated groups also cleared their lesions by day 45 with only 2/10 and 1/10 mice, respectively, exhibiting vaccination site or satellite tail lesions on the day that antibody treatment was terminated (day 66 p.v). On the contrary, in CD4−CD8−/JH-KO mice, only 2/10 and 4/10 of the vehicle and ST-246-treated mice, respectively, were free of visible lesions by day 66 p.v. The rest of these CD4−CD8−/JH-KO mice had vaccine site lesions and/or satellite lesions on their tails and/or toes, of which some experienced slight ruffling and weight loss, suggesting their failure to clear the inoculum virus. Additionally, 3/10 and 1/10 of the vehicle- and ST-246-treated groups, respectively, eventually succumbed to systemic disease from their vaccination on days 41, 45, 64, and 41 p.v. with multiple lesions (tail, toe, footpad, mouth, and/or nose) and significant ruffling, weight loss, and respiratory distress. After termination of antibody treatment on day 66 p.v., no additional deaths were observed in the CD4−CD8−/JH-KO groups and the mice eventually cleared their lesions and were available for long-term studies at 6 months p.v. Taken together, the above data suggest that cellular rather than humoral immunity plays a principal role in controlling virus replication at the site of vaccination. Furthermore, even in a condition of B-cell deficiency, ST-246 is effective in reducing the severity and magnitude of vaccine-induced lesions in the presence of either CD4+ or CD8+ T cells, but not in the absence of both.
Although viremia after smallpox vaccination is regarded as rare and transient in immunocompetent individuals [36], it has been reported to occur in vaccinated children with immunological deficiencies and disseminated infection [1, 37]. In order to evaluate the impact of ST-246 co-administration on the level and persistence of detectable virus in the blood, we used quantitative real-time PCR (qPCR; [17]) to quantify VV DNA on days 3, 7, 14, 21, and 28 p.v. (Supplemental Table 1). With the exception of CD4−CD8−/JH-KO mice, VV DNA was undetectable or below the lower limit of quantification (LLOQ; 3,500 genomes/ml) in all vaccine/vehicle groups on days 3, 14, 21, and 28 p.v. VV DNA levels above the LLOQ occurred on days 7, 14, and 21 p.v. in CD4−CD8−/JH-KO mice while it occurred only on day 7 p.v. in all other groups. Overall, the numbers of mice with detectable VV DNA were lower in vaccine/ST-246 than in vaccine/vehicle recipients in all groups. The most pronounced effect of ST-246 was observed in CD4−CD8−/JH-KO mice on days 7 and 14 p.v. At those two time points, vaccine/ST-246 mice exhibited 12- and 20-fold reductions in VV DNA levels relative to vaccine/vehicle mice.
Auto or contact transmission of shed virus is a risk that has necessitated the covering of the vaccination site with semipermeable dressings. We have previously demonstrated that in ACAM2000-vaccinated immunocompetent mice, ST-246 treatment resulted in a considerable reduction in virus shedding from and virus levels within the vaccination site [17]. In this study, lesion swabs were taken similarly on days 3, 7, 14, 21, and 28 p.v. and qPCR was used to determine viral load. On day 3 p.v., VV DNA levels in lesion swabs were below the LLOQ (2.942 [Log10] genomes/swab) (data not shown). However, viral titers were above the LLOQ in all mice on day 7 p.v., which were considerably lower in ST-246-treated mice (Supplemental Figure 2). On this day, there were approximately 5-, 525-, 30-, 34-, 4-, 4-, 14-, and 567-fold differences in geometric mean titers in N.D., CD8−, CD4−, and CD4−CD8− groups in BALB/c and JH-KO mice, respectively. Except in CD4−CD8− mice, virus shedding was also relatively lower in ST-246-treated vaccinees beyond day 7 p.v. In CD4−CD8− BALB/c and JH-KO mice, however, there were no detectable differences between vaccine/vehicle and vaccine/ST-246 recipients beyond day 7 p.v. Collectively, the above observations suggest that virus dissemination and shedding after ACAM2000 infection and replication at the vaccination site appears to be better controlled in the presence of ST-246, as long as either the CD4+ or CD8+ T-cell population is intact. However, in the absence of both subsets of T cells, the impact of ST-246 on virus shedding appears to be limited to early time points p.v.
3.2. Short- and long-term protective efficacy of ACAM2000 given in combination with ST-246 in immunodeficient mice
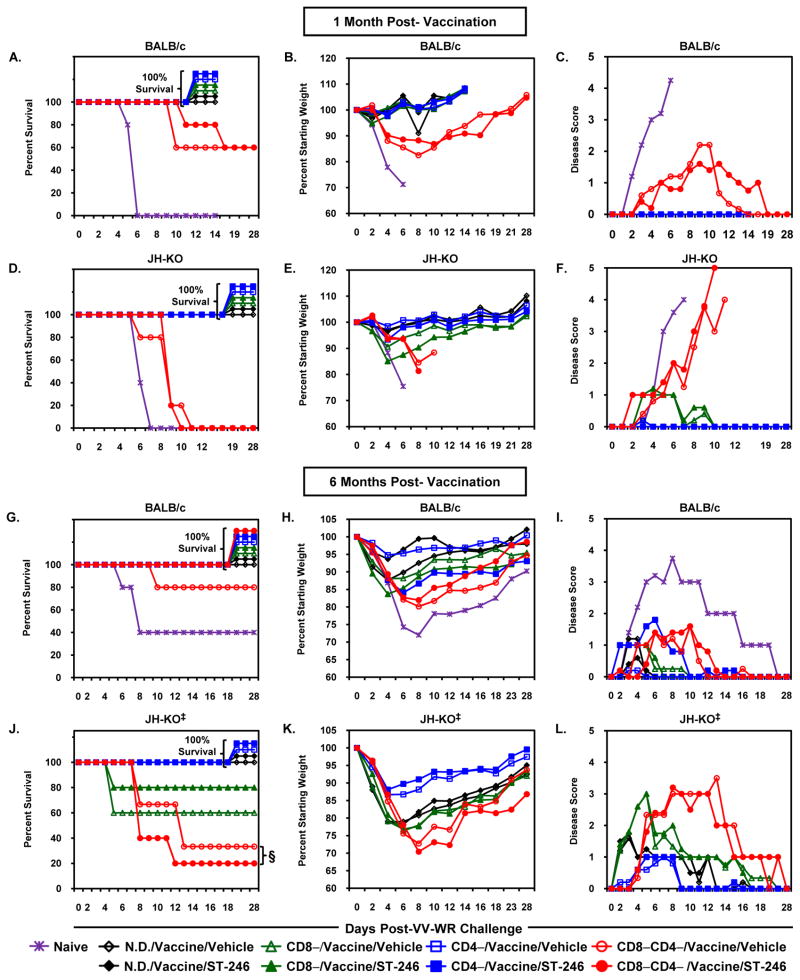
Although cidofovir co-administered with Dryvax effectively reduces the size and severity of lesions, it significantly interferes with vaccine-elicited immune responses and immunity to monkeypox [38]. To evaluate the impact of ST-246 on the induction of short- or long-term protective immunity in immunodeficient mice, ACAM2000-vaccinated mice were challenged i.n. with 10 LD50 of VV-WR at 1 or 6 months p.v. and then monitored for survival, maintenance of pre-challenge weight and temperature, and other clinical signs of illness. All of the naïve N.D. BALB/c and JH-KO mice used as controls at 1 month p.v. succumbed to viral disease by day 6 to 7 post-infection (p.i.; Figures 2A and 2D). These unvaccinated mice lost up to 30% of their weights (Figures 2B and 2E), exhibited extensive ruffling, hunched posture, conjunctivitis, and respiratory distress (Disease score [DS] = 4; Figures 2C and 2F), and were humanely euthanized. Whereas all of the vaccinated N.D, CD4−, and CD8− groups in both BALB/c and JH-KO mice survived without any major sequela, only 60% of the CD4−CD8−/BALB/c and none of the CD4−CD8−/JH-KO survived the lethal challenge. Clinical signs of disease in those mice that succumbed were similar to that seen in challenged, naïve mice. Consistent with previous observations, all the mice that did not survive lethal challenge also exhibited a continuous decline in body temperature with some approaching 25 °C at the time of euthanasia (data not shown). Contrarily, all mice that survived maintained body temperatures above 34.5 °C throughout the monitoring period. Most importantly, there were no differences in p.i. survival between vaccine/vehicle and vaccine/ST-246 mice in all of the immunocompetent and immunodeficient groups. The degree of all clinical signs of disease, including temperature, were comparable –except the slightly enhanced weight loss evident in vaccine/ST-246 recipient CD8−/JH-KO mice– with no statistically significant differences being observed at any day p.i. The above data demonstrate that ST-246 co-administration did not interfere with ACAM2000-elicited short-term protective immunity.
Figure 2. Short- and long-term protective efficacy of ACAM2000 given in combination with ST-246 in immunodeficient mice.
BALB/c (A-C;G-I) and JH-KO (D-F;J-L) mice were depleted of CD4+, CD8+, or both subsets of T cells, vaccinated, and concurrently treated with vehicle or ST-246 as described in Figure 1. One (A-F) or six months (G-L) post-vaccination, the mice were i.n. challenged with age-specific 10 LD50 of VV-WR and monitored for survival (A,D,G,J), weight loss (B,E,H,K), and degree of clinical disease symptoms (C,F,I,L). For challenge at 1 month p.v., cellular depletion was maintained throughout the vaccination and challenge periods. For challenge at 6 months p.v., antibody treatment was discontinued on day 66 p.v. (although some CD8−CD4− mice still had unhealed lesions), reinitiated five days prior to challenge, and continued throughout the monitoring period (i.e., 28 days). The graphs represent average values for 4-5 mice/group. Vaccinated mice treated with vehicle are indicated by open symbols while those treated with ST-246 are shown as closed symbols. ‡ Naïve JH-KO mice were not included in the 6 months challenge study. § In the CD8−CD4−/JH-KO group, there were only three vaccine/vehicle mice available for virus challenge since two mice in the group died from vaccine-induced systemic disease on days 41 and 45 p.v., while all five vaccine/ST-246 survived until and beyond cessation of depleting antibody treatment on day 66 p.v.
Since older mice are more resistant to VV-WR-induced disease [33], the LD50 for the virus was determined in age-matched mice two weeks prior to the 6 months time point (data not shown). Regardless, 40% of the naïve BALB/c mice still survived the 10 LD50 challenge (1.26×108 PFU; Figure 2G), although they exhibited significant weight loss (up to 26.6%; Figure 2H) and clinical signs of disease (DS = 3; Figure 2I) while maintaining normal body temperatures (data not shown). Those that succumbed to disease on day 6 to 7 p.i., however, lost up to 31.1% of their weight, had DS ≥ 4, and failed to maintain normal body temperatures. In vaccine/vehicle recipients, all of the N.D., CD4−, and CD8−/BALB/c mice, N.D and CD4−/JH-KO mice, as well as 80% of CD4−CD8−/BALB/c, 60% CD8−/JH-KO, and 33% of CD4−CD8−/JH-KO mice survived the challenge (Figures 2G and 2J). Other than it being 20% higher in CD4−CD8−/BALB/c and CD8−/JH-KO mice and 13% lower in CD4−CD8−/JH-KO mice that received vaccine/ST-246, percent survival was the same as in vaccine/vehicle recipients in all other groups. In N.D./BALB/c mice, weight losses (but not disease scores) were higher and statistically significant (days 2 to 10 p.i.) in vaccine/vehicle than vaccine/ST-246 mice. In contrast, vaccine/ST-246 mice exhibited increased and statistically significant weight losses (days 2 to 18 p.i.) and disease scores (days 2 to 7 p.i.) in CD4−/BALB/c mice. Except in the above two groups (N.D./BALB/c and CD4−/BALB/c), there were no statistically significant differences between vaccine/vehicle and vaccine/ST-246 recipients in both weight losses and disease symptoms during the 28-day follow-up period (Figure 2H, 2I, 2K, and 2L). Overall, the above data demonstrate that long-term protective immunity induced by ACAM2000 given in combination with ST-246 closely mirrors that of the vaccine given alone.
3.3. Humoral responses following ACAM2000 vaccination and treatment with ST-246
In vaccinated mice, anti-VV IgG responses appear by day 10, increase markedly by day 14, gradually increase to maximal titers by day 270 p.v., and remain high afterwards [35]. To assess the impact of ST-246 on the magnitude and anti-viral activity of ACAM2000-elicited humoral responses in immunodeficient mice, plasma samples were collected at 1 and 6 months p.v. and evaluated using ELISA and neutralization assays against the two infectious forms of VV: MV and EV. Purified MV VV-WR was used for both ELISA and PRNT50 assays, which measure the ability of plasma antibodies to neutralize MV and block infection of cells. Consistent with their genetic deficiency in mature B-cells and lack of antibody production, there was no detectable VV-specific reactivity in the plasma of any naïve or vaccinated JH-KO mice by either ELISA or PRNT50 assays (Table 1). Unvaccinated BALB/c mice also did not develop any detectable antibody responses (data not shown). At both time points p.v., immunocompetent N.D./BALB/c mice in both vaccine/vehicle and vaccine/ST-246 groups showed high ELISA titers (GMTs of 111,008 vs. 34,853 and 212,962 vs. 146,130 at 1 and 6 months p.v., respectively) and strong (GMTs of 2,124 vs. 982 and 6,289 vs. 4,030 at 1 and 6 months p.v., respectively) MV-neutralizing activity. When compared to vaccine/vehicle recipients, both ELISA and PRNT50 GMT were lower in vaccine/ST-246 recipients at both time points p.v., which was statistically significant at 1 month, but not 6 months, p.v. In CD8−/BALB/c mice, antibody responses were also lower in vaccine/ST-246 mice at both time points p.v., but the differences were not statistically significant. Given the critical role of CD4+ T-cell help in generating and maintaining humoral immunity, VV-specific antibody responses in both CD4− and CD4−CD8−/BALB/c mice at 1 month p.v. were either absent (MV neutralization GMT <25) or extremely low (ELISA GMT <322) in both vaccine/vehicle and vaccine/ST-246 mice. Although antibody responses were detectable and higher in these mice at 6 months p.v., the levels were still significantly lower than those in N.D. and CD8−/BALB/c mice. This suggests that in these CD4− and CD4−CD8−/BALB/c mice, virus/virus antigen clearance was not complete by the time depleting antibody treatment was terminated (day 52 or 66 p.v.). As a result, the recovery of the T-cell population while virus/virus antigen is persistent may have contributed to the induction of VV-specific antibody responses and their marked detection at 6 months p.v. Regardless, even in these two groups, both ELISA and PRNT50 GMT were lower, but not statistically different, in vaccine/ST-246 as compared to vaccine/vehicle recipients.
Table 1.
Vaccinia virus-specific antibody responses following ACAM2000 vaccination given with or without ST-246.
| Vaccinated Groups | IgG ELISA Endpoint Titer (×103)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Month Post-Vaccination |
6 Months Post-Vaccination§ |
|||||||||
| Vehicle | 95% CIb | ST-246 | 95% CI | P- valuec | Vehicle | 95% CI | ST-246 | 95% CI | P- value | |
| N.D./BALB/c | 111.01 | [63.83, 192.75] | 34.85 | [24.27, 50.00] | 0.001 | 212.96 | [68.55, 660.69] | 146.13 | [34.20, 625.17] | 0.586 |
| CD8−/BALB/c | 62.66 | [9.29, 422.67] | 28.92 | [8.11, 103.04] | 0.331 | 136.69 | [74.13, 252.35] | 72.73 | [31.41, 168.66] | 0.131 |
| CD4−/BALB/c | 0.11 | [0.02, 0.68] | 0.06 | [0.05, 0.08] | 0.316 | 1.55 | [0.60, 3.99] | 0.59 | [0.10, 3.36] | 0.214 |
| CD8−CD4−/BALB/c | 0.32 | [0.08, 1.28] | 0.25 | [0.07, 0.90] | 0.699 | 11.51 | [1.87, 70.79] | 5.84 | [0.61, 55.85] | 0.534 |
| All JH-KO Groups‡ | <20 | – | <20 | – | – | <20 | – | <20 | – | – |
| Vaccinated Groups |
Plaque Reduction Neutralization Titer50(×103)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Month Post-Vaccination |
6 Months Post-Vaccination§ |
|||||||||
| Vehicle | 95% CI | ST-246 | 95% CI | P- value | Vehicle | 95% CI | ST-246 | 95% CI | P- value | |
| N.D./BALB/c | 2.12 | [0.97, 4.65 ] | 0.98 | [0.60, 1.60] | 0.049 | 6.29 | [2.57, 15.42] | 4.03 | [0.78, 20.75] | 0.527 |
| CD8−/BALB/c | 0.75 | [0.12, 4.51] | 0.38 | [0.11, 1.26] | 0.360 | 4.20 | [2.28, 7.74] | 1.73 | [0.49, 6.12] | 0.118 |
| CD4−/BALB/c | <25 | – | <25 | – | – | 0.25 | [0.08, 0.74] | 0.16 | [0.02, 1.33] | 0.628 |
| CD8−CD4−/BALB/c | <25 | – | <25 | – | – | 1.27 | [0.17, 9.29] | 0.39 | [0.07, 2.33] | 0.254 |
| All JH-KO Groups‡ | <25 | – | <25 | – | – | <25 | – | <25 | – | – |
Geometric mean titer (GMT) of 4-5 mice per group. Purified VV-WR was used to perform both assays using heat-inactivated plasma.
CI = Confidence Interval
Vaccine/vehicle vs. vaccine/ST-246 comparison using Student’s t -test on the log-transformed data. P -value <0.050 is considered statistically significant.
For mice with healed vaccine lesions and no satellite lesions, antibody treatment was discontinued on day 52 p.v. Otherwise, treatment was terminated on day 66 even though some CD8−CD4− mice still had unhealed vaccine and/or satellite lesions, which eventually cleared after the T-cell depletion was stopped.
Includes N.D./JH-KO, CD8−/JH-KO, CD4−/JH-KO, and CD8−CD4−/JH-KO mice.
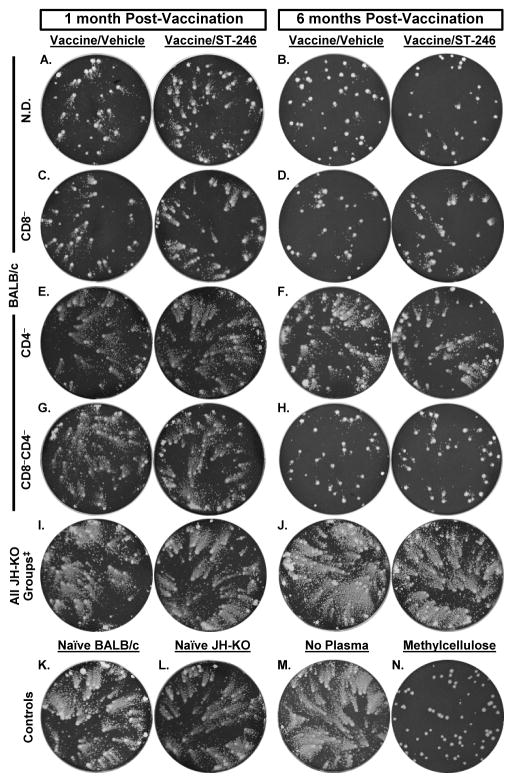
Given that EV plays an important role in VV dissemination within an infected host and its transmission to contacts [39], optimal protection against OPV-induced disease requires Abs to EV in addition to MV [40, 41]. Hence, we evaluated the impact of ST-246 on the induction of anti-EV neutralizing antibodies using comet tail inhibition assay (Figure 3 and Supplemental Table 2). The production and release of large amounts of EV from BSC-40 cells primarily infected with VV-IHD-J results in the formation of numerous small, secondary satellite plaques that resemble comet tails. Comet tail formation is inhibited by the presence of EV-, but not MV-, specific antibodies that block EV release from infected cells [42]. Plasma from naïve BALB/c (Figure 3K) and naïve or vaccinated JH-KO mice (Figures 3L, 3I, and 3J) did not inhibit the formation of comet tails at any time p.v. On the contrary, comet tails were significantly inhibited by vaccinated N.D. (Figures 3A and 3B) and CD8− (Figures 3C and 3D) BALB/c mice plasma at both time points p.v., although it was qualitatively lower in CD8− mice. At 1 month p.v., comet tails were not blocked by plasma from CD4− (Figures 3E) or CD4−CD8− (Figures 3G) BALB/c mice. However, at 6 months p.v., there was slight comet inhibition in CD4−/BALB/c mice (Figure 3F), while inhibition was marked in CD4−CD8-/BALB/c mice (Figure 3H), perhaps due to a humoral immune response elicited after cessation of depleting antibody treatment (day 52 or 66 p.v.) and recovery of T cells. Most importantly, in all groups in which comet tail inhibition was observed, the degree of inhibition was similar in both vaccine/vehicle and vaccine/ST-246 recipients at both time points p.v. Collectively, the above discussed data suggest that although the total VV-reactive and MV-neutralizing antibody levels were moderately reduced as a result of ST-246 co-administration, ACAM2000-elicited EV-neutralizing capability appears to be maintained to qualitatively comparable levels.
Figure 3. Inhibition of comet tail formation by EV-specific antibodies.
Plasma samples were obtained from parallel groups of BALB/c (A-H) and JH-KO (I-J) immunodeficient mice described in Figure 2 that were sacrificed at one or six months post-vaccination. For the 1 month time point, cellular depletion was maintained until the day of sacrifice. For the 6 months time point, depleting antibody treatment was reinitiated five days prior to sacrifice. Pooled plasma from 4-5 mice/group were diluted 1:25 in BSC-40 cell cultures that were first infected with ~65 PFU of VV-IHD-J for 2 hours and overlayed with liquid medium. After 40 hours of incubation, the monolayers were stained with 0.1% crystal violet to visualize plaques and comet tails. Wells to which no plasma (M) or naïve BALB/c (K) or JH-KO (L) plasma were added, served as negative controls. Wells containing infected monolayers that were overlaid with 2% methylcellulose (N) were used as positive controls for complete inhibition of comet formation. ‡ Indicate representative wells for all JH-KO groups including N.D./JH-KO, CD8−/JH-KO, CD4−/JH-KO, and CD8−CD4−/JH-KO since none of these groups inhibited comet formation and, thus, showed similar patterns. See Supplementary Table 2 for a qualitative scoring of the extent of comet tail inhibition.
3.4. Comparison of VV-specific CD8+ T-cell levels by pentamer staining
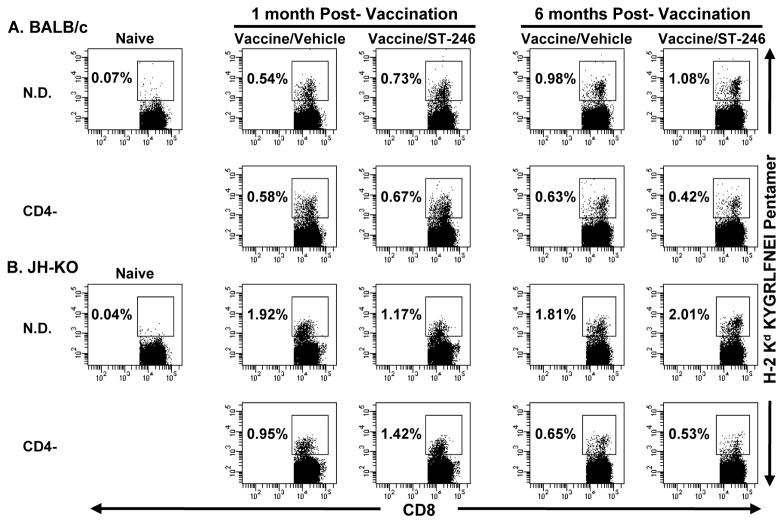
As in primary human vaccinees, VV-specific CD4+ and CD8+ T-cell responses in mice are detectable by day 7, peak by day 14, decline sharply by day 30 p.v., and remain at this level during the memory phase [43, 44]. To examine the impact of ST-246 on the induction of cellular immune responses in immunodeficient mice, we compared the levels of VV-specific CD8+ T cells in the spleen at 1 and 6 months p.v. This was done by ex vivo flow cytometric analysis using MHC class I pentamers loaded with two known CD8+ T-cell epitopes: KYGRLFNEI(H-2Kd: A52R75–83) or SPYAAGYDL (H-2Ld: F2L26–34) [34]. At both time points p.v. and in all vaccinated groups with intact CD8+ T-cell compartments, there was no detectable F2L pentamer staining above background levels seen in naïve mice (0.04−0.07%; data not shown). On the contrary, substantial numbers of A52R75–83-specific CD8+ T cells were detected in the spleens of vaccinated, but not naïve, BALB/c and JH-KO mice at both time points p.v. (Figure 4A and 4B). In immunocompetent BALB/c mice, these pentamer+CD8+ T-cells represented ~10.2% and ~24.3% of the total IFN-γ+/CD8+ T-cell response (Figure 5A and E) at 1 and 6 months p.v., respectively. Except in CD4− mice at 6 months p.v., the percentages of pentamer+CD8+ T cells were higher in JH-KO mice than BALB/c mice. Except in CD4-/BALB/c mice at 1 month p.v., the percentages of pentamer+CD8+ T cells were also higher in N.D. than CD4−/BALB/c or CD4−/JH-KO mice. Importantly, pentamer+CD8+ T-cell reactivity was comparable in vaccine/vehicle and vaccine/ST-246 recipients with two notable exceptions. At 1 month p.v., vaccine/vehicle recipients showed a ~1.6-fold increase in pentamer+CD8+ T-cells in N.D./JH-KO mice, while vaccine/ST-246 recipients showed a ~1.5-fold increase in CD4−/JH-KO mice. The above data demonstrate that the induction and maintenance of ACAM2000-elicited memory CD8+ T-cells is not hampered by ST-246 co-administration, even in the absence of CD4+ T-cell help.
Figure 4. Ex vivo analysis of the magnitude of VV peptide-specific CD8+ T-cell responses.
Single-cell suspensions were prepared from spleens obtained from the same BALB/c (A) and JH-KO (B) immunodeficient mice described in Figure 3, which were sacrificed at one or six-months post-vaccination and concurrent treatment with vehicle or ST-246. Pooled splenocytes from 4-5 mice/group were consecutively stained with KYGRLFNEI (H-2Kd: A52R75-83) peptide-loaded pentamer and anti-CD8 antibody and analyzed by flow cytometry. Dot plots represent CD8+ T cells subgated on live lymphocytes identified by FSC and SSC parameters. Naïve splenocytes were used as negative controls to set the pentamer+ gate. Percentages represent the frequency of pentamer+ cells in the CD8+ T-cell population. Although splenocytes from CD8− and CD8−CD4−/BALB/c and JH-KO mice were also analyzed similarly, their plots are not shown, since the depletion of the CD8+ T-cell population was >99%.
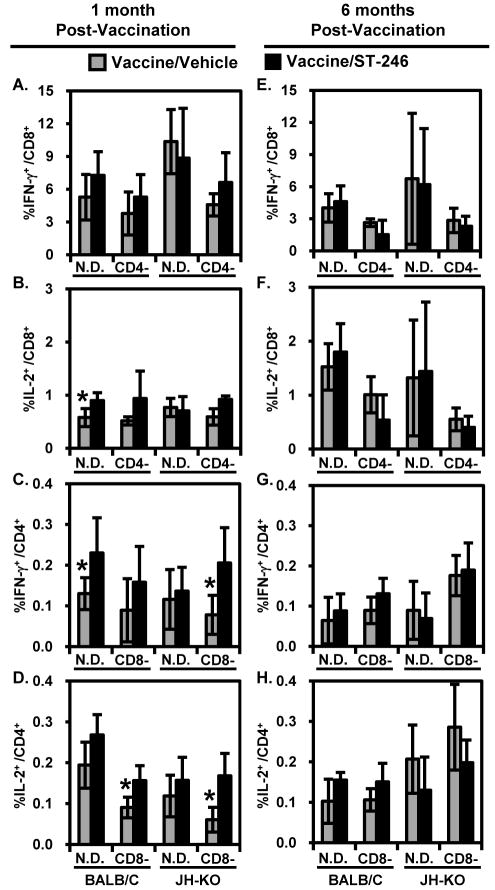
Figure 5. Intracellular cytokine staining detection of VV-specific CD8+ and CD4+ T-cell responses.
Splenocyte single-cell suspensions described in Figure 4 were stimulated in vitro with mock- or VV-WR-infected syngeneic A20 cells in the presence of IL-2 and Brefeldin A. After 16-18 hours of co-culture, the cells were surface stained with anti-CD4 and anti-CD8 antibodies, fixed, permeabilized, intracellularly stained with anti-IL-2 and -IFN-γ antibodies, and then analyzed by flow cytometry. Live lymphocytes were first identified and gated by FSC and SSC parameters and then subgated based on CD8 or CD4 expression. Panels depict the background (mock stimulated)-subtracted frequency of VV-specific CD8+ (A,B,E,F) or CD4+ (C,D,G,H) T cells expressing the indicated cytokines, as the arithmetic mean ± standard deviation of individually analyzed spleens (n = 4-5). These frequencies were <0.02% for both T-cell subsets in the spleens of naïve BALB/c and JH-KO mice (not shown). Although splenocytes from all of the immunodeficient groups were analyzed similarly, data corresponding to the depleted T-cell subset(s) is not shown due to >99% depletion efficiency. *P-value < 0.05.
3.5. Functional characterization of vaccine-induced CD4+ and CD8+ T-cell responses
Smallpox vaccination induces a robust, VV-specific CD4+ and CD8+ T-cell response with a TH1-dominant cytokine profile, i.e., IL-2, IFN-γ, and TNF-α [45, 46]. To characterize the cellular immunogenicity of ACAM2000 co-administered with ST-246 in immunodeficient mice, we assayed both short- and long-term VV-specific cellular responses by intracellular cytokine staining (ICCS; Figure 5A-5H) and cytokine production assays (Figure 6A-6H) after in vitro stimulation of splenocytes with mock- or VV-WR-infected A20 cells. A20 cells serve as antigen-presenting cells (APCs) since they process and present VV-derived antigens in the context of both MHC class I and II molecules [47]. Mock-infected A20 cells were used as negative controls to subtract background responses. At both 1 and 6 months p.v., considerable levels of VV-specific IFN-γ- and IL-2-producing CD4+ and CD8+ T cells were detected in the spleens of vaccinated (Figure 5A-5H), but not naïve (<0.02% for both cytokines and subsets of T cells; data not shown) BALB/c or JH-KO mice. The majority of IFN-γ and IL-2 producing cells at both time points p.v. and in both BALB/c and JH-KO mice were CD8+ rather than CD4+ T cells. Although a similar percentage of CD4+ T cells produced IL-2 and IFN-γ, proportionally more CD8+ T cells produced IFN-γ than IL-2 and the average levels of IFN-γ+CD8+ T cells were comparatively higher at 1 than 6 months p.v. It has been demonstrated that due to the decreased survival of VV-specific CD8+ T cells, a much smaller CD8+ T-cell memory pool is generated in the absence of CD4+ T-cell help [48]. Consistent with this observation, the frequencies of IFN-γ+CD8+ T cells was ~1.5 to 2.4-fold lower in CD4−/BALB/c and CD4−/JH-KO mice than their non-depleted counterparts (Figure 5A and 5E). Most importantly, the average proportions of cytokine-producing CD4+ and/or CD8+ T cells in vaccine/ST-246 recipients were generally comparable or slightly better those that in vaccine/vehicle recipients, especially at 1 month p.v., with no statistically significant differences in most groups. In the few cases where statistically significant differences were observed between the two vaccine groups, such as percentages of IL-2+/CD8+ or IFN-γ+/CD4+ T cells in N.D./BALB/c mice at 1 month p.v., the responses were higher in vaccine/ST-246 recipients.
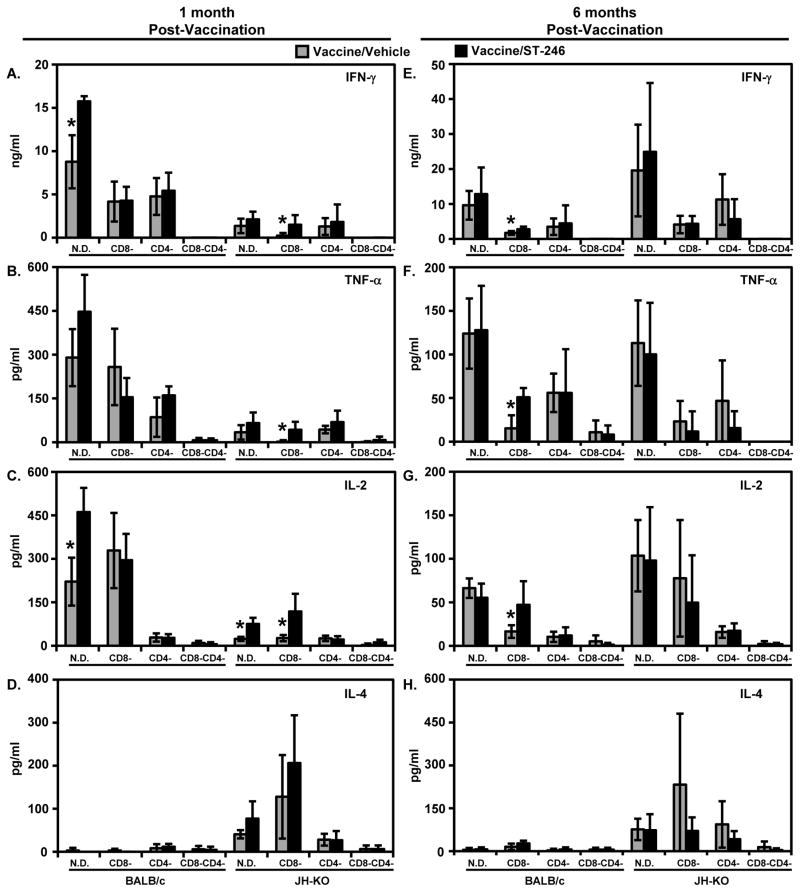
Figure 6. Quantification of VV-specific secretion of IFN-γ (A,E), TNF-α (B,F), IL-2 (C,G), and IL-4 (D,H) by in vitro-stimulated splenocytes.
Splenocyte single-cell suspensions described in Figure 4 were co-cultured with mock- or VV-WR-infected syngeneic A20 cells without the addition of BFA or IL-2. After 48 hours of stimulation, cell-free supernatants were collected and analyzed by TH1/TH2 cytokine CBA. The arithmetic mean ± standard deviation of background (mock stimulated)-subtracted values from individually analyzed spleens (n = 4–5) is shown. These values were less than the minimum quantifiable levels of the CBA assay (i.e., 20 pg/ml) in similarly stimulated splenocytes from naïve BALB/c and JH-KO mice (not shown). Although the VV-specific IL-5 secretion was also assayed, it was <20 pg/ml in all the indicated groups (not shown). *P-value < 0.05.
TH1 cytokines produced by immune cells have been shown to play a critical role in resistance to poxvirus infections, while TH2 cytokines, such as IL-4, seem to exacerbate infections [49]. To further evaluate the impact of ST-246 co-administration on the magnitude and profile of the recall cytokine response in immunodeficient mice, cell-free supernatants from in vitro re-stimulated splenocytes were assayed for IL-2, IFN-γ, TNF- α, IL-4, and IL-5 secretion. As in the ICCS assay, mock-infected A20 cells were used as negative controls to subtract background responses. At both time points p.v., cytokine production in response to stimulation with VV-WR was at or near background levels in splenocytes from naïve BALB/c and JH-KO mice (i.e., <20 pg/ml; data not shown). In sharp contrast, splenocytes from vaccinated BALB/c and JH-KO mice at both time points p.v. responded to VV-WR stimulation with a cytokine profile dominated by the TH1 cytokines IFN-γ (Figures 6A and 6E), TNF-α (Figures 6B and 6F), and IL-2 (Figures 6C and 6G). Given its role as a key mediator in VV clearance [49], IFN-γ secretion levels were significantly higher than any of the other four cytokines. Low levels of VV-specific IL-4 secretion (Figures 6D and 6H) were also detected in vaccinated JH-KO, but not BALB/c mice, whereas IL-5 levels were at or near background levels in all groups of mice and at both time points p.v. (data not shown). At both time points p.v., TH1 cytokine levels were the highest in non-depleted BALB/c and JH-KO mice, while all cytokine levels were very low and near background levels in CD4−CD8−/BALB/c and CD4−CD8−/JH-KO mice, implicating CD4+ and/or CD8+ T cells as the sources/inducers of the detected cytokines. Similar to observations in ICCS, the patterns of splenic cytokine responsiveness in vaccine/ST-246 recipients were generally comparable or slightly better than those in vaccine/vehicle recipients, especially at 1 month p.v., with no statistically significant differences in most groups. In the few cases where statistically significant differences were observed between the two vaccine groups, such as IFN-γ and IL-2 production in N.D./BALB/c mice at 1 month p.v., the responses were higher in vaccine/ST-246 recipients. The ICCS and cytokine release data above complimentarily demonstrate that concurrent treatment with ST-246 does not interfere with ACAM2000-mediated induction of short- and long-term cellular immunity that is characterized by a TH1 cytokine profile and is known to provide resistance against VV infection.
4. DISCUSSION
The heightened concern about bioterrorism has led to the stockpiling of ~200 million doses of ACAM2000 in the Strategic National Stockpile to vaccinate the US population in the event of a smallpox emergency. According to the CDC’s response plan and guidelines for smallpox emergency, surveillance and containment strategies (i.e., quarantine and ring vaccination) may be supplemented with large-scale vaccination in both affected and unaffected communities [50]. In these circumstances, even individuals with contraindications, such as those with acquired or inherited immunodeficiency, may receive the smallpox vaccine although they are highly susceptible to severe and life-threatening adverse events. It had been conservatively estimated that mass vaccination with the first-generation vaccine Dryvax may result in 125 to 500 deaths and be accompanied by thousands of serious adverse events [51]. The potential extent of these outcomes with the use of the second-generation vaccine ACAM2000 is not currently known since the small numbers (2,983 [52]) of individuals vaccinated during clinical trials make it difficult to determine the relative risk upon mass vaccinations (3). Regardless, there is a pressing need for alternative safe vaccines and/or for combinational antiviral therapies that can help prevent, reduce, and/or treat potential adverse events without compromising the induction of protective immunity.
To avoid the risk associated with replication-competent smallpox vaccines, two attenuated vaccine strains (MVA and LC16m8) were developed during the 1970s [1]. Although they were safe and well-tolerated in immunocompromised persons, their protective efficacy is not known since they were not tested against endemic smallpox. Protein and DNA subunit vaccines may offer potential safe alternatives [51, 53], but their reactogenicity, immunogenicity, and protective efficacy in humans is not yet known. In the event of adverse events, empiric (but uncontrolled) evidence suggests that, if given promptly after symptoms appear, VIG treatment may result in improved prognosis and reduced mortality in some vaccinated patients [6]. For those who do not respond to VIG treatment or are near death, cidofovir has been recommended [7]; however, its clinical effectiveness is unknown and its use is significantly limited due to the potential for renal toxicity with as few as one or two doses [54]. In addition, there is a drawback to co-administrating cidofovir with Dryvax in that it interferes with vaccine immunogenicity and protective immunity to MPXV [38].
Alternatively, as an expansion of its current development as a post-exposure prophylactic or therapeutic for smallpox disease, we have recently proposed the use of ST-246 as an adjunct to smallpox vaccination in order to reduce the risk of adverse events in contraindicated populations as well as mitigate the less serious local and systemic symptoms in healthy populations [23]. Our previous data in immunocompetent mice [17, 23], combined with data in immunodeficient mice presented here, provide strong support for this indication, since lesion severity and time to resolution (Figure 1), as well as VV DNA levels in the blood (Table S1) and VV DNA shedding from the vaccination site (Figure S2), are better controlled if vaccinated mice were concurrently treated with ST-246. The early impact of ST-246 on these parameters – which are related to acute viral replication and dissemination prior to the activation of the immune response to control them – has important implications for vaccine safety in that Dryvax-induced serious dermal complications generally occur at, or just after, “robust” reactions characterized by unusually large and painful primary lesions [55]. That is, since ST-246 reduces vaccine lesion severity and hastens healing, it might help stave off the risk of developing a robust reaction and its associated consequences. Our data in immunodeficient mice further suggest that even though ST-246 may keep virus dissemination and replication in check early and lead to an accelerated resolution of lesions (Figure 1), eventually, the vaccine-induced cellular immune response appears to be responsible for effective virus clearance and complete lesion healing. This is based on our observation that despite their inability to mount humoral immune responses, JH-KO mice with intact CD4+ or CD8+ T cells in both vaccine/vehicle and vaccine/ST-246 recipients managed to clear the infection established by the vaccine virus. This is consistent with data in rhesus macaques showing that, despite the absence of VV-neutralizing antibody due to depletion of CD4+ T cells induced by simian immunodeficiency virus (SIV) or simian/human immunodeficiency virus (SHIV) infection, Dryvax-induced lesions are eventually cleared and progressive vaccinia is rare [56]. Meanwhile, JH-KO mice lacking both subsets of T cells either succumbed to severe systemic disease (3/10 in vaccine/vehicle and 1/10 in vaccine/ST-246) or failed to clear the vaccine virus for more than two months p.v., i.e., until cessation of depleting antibody treatment that allowed recovery of T cells. Given that children with agammaglobulinemia could be vaccinated safely with Dryvax [57], our data suggest that ST-246 will be efficacious in preventing/reducing vaccine-associated adverse reactions in humans with partial (but not combined) deficiency in cellular immunity without regard to the status of their humoral immunity. However, even those with severe, acquired cellular deficiency may benefit from virus dissemination and replication kept in check by uninterrupted treatment with ST-246, if cell-mediated immune function can be restored by recovery from the underlying disease or temporary withdrawal of immunosuppressive therapies. The recent successful treatment of PV that developed in a 20-year old military service member, who developed severe cellular deficiency after receiving chemotherapy for AML diagnosed 2-weeks after ACAM2000 vaccination [26], lends strong support to this view. In the patient, the primary lesion did not heal and expanded from a size of 1×1 cm to 4×4 cm with satellite lesions forming at later stages. After combination treatment with VIG, topical imiquimod, a lipid-conjugate of cidofovir (CMX001), and topical and oral ST-246, the patient’s condition improved substantially, the lesion showed significant healing, and viral DNA was below levels of detection in swabs taken from the primary/satellite lesions by day 104 p.v. Most notably, along with periodic infusions of VIGIV, ST-246 was administered daily for 75 days, which appear to have allowed time for the recovery of some of the patient’s cellular immune response that led to the healing of his primary and satellite lesions.
The other crucial advantage of the use of ST-246 as an adjunct to smallpox vaccination is that it does not hamper the induction of VV-specific immune responses, especially cellular responses and protective immunity against lethal respiratory challenge with VV-WR, which mimics the natural route of smallpox infection. Since eradication of smallpox was successfully achieved without the determination of the protective immunity elicited by vaccination, the correlates of protection against smallpox are unknown and continue to be debated [2]. Once induced after vaccination or primary infection, humoral responses alone appear to be sufficient for protection against reinfection [30, 31, 58, 59]. However, in the absence of effective humoral responses, T cells are necessary and sufficient and both CD4+ and CD8+ T-cell-mediated immunity can contribute to protection against OPV disease [20, 30, 31, 58]. The relative importance of cellular versus humoral immunity in recovery from primary poxvirus infection is further highlighted by our recent observation that ST-246-treated (but not vehicle-treated) unvaccinated JH-KO mice depleted of CD4+ or CD8+ (but not both) T cells survived lethal challenge, if treatment is initiated immediately [20]. That is, antibody responses can be dispensable for natural resistance against respiratory poxvirus infection and that either CD4+ or CD8+ T cells alone can sufficiently mediate protection. Most relevantly to smallpox vaccination, it was recently demonstrated that the superior T-cell-mediated immune response induced by skin scarification, but not conventional injection routes (such as the intramuscular route proposed for the MVA), is responsible for providing complete protection against subsequent VV challenge independent of neutralizing antibodies [60]. Given that the magnitude and functional profile of the VV-specific T-cell response induced by ACAM2000 administered alone is essentially preserved in the presence of ST-246 (Figures 4, 5, and 6), it is not surprising that protective immunity is also maintained to comparable levels in mice with or without a specific immunodeficiency (Figure 2). Although MV neutralizing antibody titers were somewhat lower in vaccine/ST-246 recipients, the titers are substantially higher than the minimum pre-existing human PRNT50 of 1:20 [61] or 1:32 [62] that have been deduced to protect against contracting smallpox disease. In addition, studies in vaccinated mice showing that mice with MV neutralizing antibody titers of 14 (Dryvax) or 53 (ACAM1000) are fully protected from lethal respiratory challenge with cowpox virus [63] suggest that the antibody titers in vaccine/ST-246 recipients are sufficiently high enough to provide similar levels of protection from lethal challenge. In contrast to MV-specific neutralization, both vaccine/vehicle and vaccine/ST-246 recipients were similarly effective in their ability to inhibit comet tail formation (Figure 3). The critical role of EV for VV spread within an infected host suggests that the maintenance of qualitatively comparable levels of EV neutralizing capability in the presence of ST-246 may be more important than MV-neutralizing antibodies for in vivo protection against poxvirus challenge. This view is supported by data showing that, although vaccination with inactivated VV induces high levels of MV-neutralizing antibody, it is not used as a safer alternative because it fails to prevent the progression of a lethal infection, as EV-neutralizing antibodies are not induced [1]. Above all, given that a clinical take remains as the only universally accepted correlate of successful human vaccination [2] and ST-246 did not prevent take formation (Figure 1: day 7 p.v.), the preservation of vaccine-induced protective immunity is rather expected. The importance of this correlation is further supported by a study in mice showing that even those vaccinated with a 1000-fold dilution of the standard human dose of Lister strain vaccine were protected against lethal VV-WR respiratory challenge, so long as a take was evident [35].
It has been clearly demonstrated that ST-246 prevents EV formation and dissemination without inhibiting primary infection or the production of MV virions and MV- and EV-associated antigens in infected cells [12, 15]. Therefore, the amount of viral antigens that are available within infected APCs immediately following scarification and thus can be processed and presented directly (or transferred to other APCs for indirect presentation) to CD4+ and CD8+ T-cells may not be significantly affected by the presence of ST-246. This is not unprecedented in that even in the replication-deficient vaccine MVA, production of both early and late viral proteins is unimpaired, even though no progeny virions are produced later [64]. These early events appear to be critical for immune cell priming and activation since VV-infected dendritic cell (DC) antigen presentation to draining lymph node naïve T cells occurs as rapidly as 6 hrs following localized infection, but declines sharply during 6 to 24 hrs and becomes undetectable after 48 hrs [65]. Hence, even with the reduced level of virus spread and replication expected later at the vaccination site in vaccine/ST-246 recipients, the induction of protective immunity is maintained probably because ST-246 does not interfere with the migration of infected skin DCs or the infection of lymph node resident DCs during the initial hours following virus inoculation.
In summary, in the event of a bioterroristic smallpox attack, there will likely be no medical exemption given to exposed, contraindicated individuals, since risks associated with smallpox disease would far outweigh potential risks associated with vaccination. In this scenario, if used as an adjunct to vaccination, ST-246 would be expected to allow the elicitation of vaccine-induced protective immunity and also exert its antiviral effect on VARV C17L (VV F13L ortholog) protein to prevent the development of smallpox disease [14]. In a national program of preparedness against such an attack, vaccination of military personnel – which is mandatory for designated service members and employees except those with contraindications – was re-initiated in 2002 and continues to this date [66]. To date, more than 1.4 million service personnel have been vaccinated ([67]). Although most occurred at rates below the eradication campaign rates, several adverse events, including progressive vaccinia (ACAM2000; 1 case), contact eczema vaccinatum (Dryvax; 1 case), postvaccinial encephalitis (Dryvax; 1 case), generalized vaccinia (Dryvax [43 cases] and ACAM2000 [1 case]), erythema multiforme (Dryvax; 1 case), inadvertent self- or contact-inoculation (Dryvax [73 and 61 cases, respectively] and ACAM2000 [1 contact case]), myopericarditis (Dryvax [140 cases]), and a “linked” death (Dryvax; 1 case), have already been reported despite the military’s rigorous screening, exclusion of contraindicated personnel (~10%), and infection-control safeguards [25-27, 51, 52, 66, 68, 69]. Hence, in the unfortunate event that apparently healthy military personnel or their civilian contacts with undiagnosed immunosuppressive conditions, such as cancer, atopic dermatitis, autoimmunity, or HIV-infection, are inadvertently vaccinated, immediate and continued administration of ST-246 could play a critical role in recovery from severe complications [25-27]. The study of vaccine-related complications is limited by the lack of suitable animal models that faithfully replicate those observed in humans. Although severe vaccine complications were observed only in a small number of CD4−CD8−/JH-KO mice in this study, we used the seven cohorts of immunodeficient mice to model the spectrum of immunocompromised populations that are believed to be at increased risk for complications and thus are designated as contraindicated populations. Future studies could examine ST-246 efficacy in other mouse models, such as eczema vaccinatum in atopic dermatitis mice [70] and disseminated PV in cyclophosphamide-treated mice [71], in addition to our ongoing studies in NHP models of immunodeficiency [56, 59].
Supplementary Material
JH-KO mice were treated with the indicated antibodies at weekly intervals after an initial 3-day treatment as described in Materials and Methods. On the day of vaccination (A-D) or one (E-H) or 2 months (I-L) post-vaccination, whole blood samples collected in sodium citrate were stained with a combination of anti-CD8 and anti-CD4 antibodies and analyzed by flow cytometry. Representative dot plots from individually analyzed samples (n = 4-5) for each group is shown. The percentages indicate the proportion of cells in each quadrant based on live lymphocyte gating on FSC and SSC parameters. Cellular depletion was similarly efficient in BALB/c mice (not shown). † Data not available, since the mice succumbed to viral disease by day 7 post-VV-WR challenge.
BALB/c and JH-KO mice were depleted of CD4+, CD8+, or both CD4+ and CD8+ T cells and vaccinated and concurrently treated with vehicle or ST-246 as described in Figure 1. Lesion swabs of the vaccination sites were taken from the same groups of mice as in Table S1 on days 3 (data not shown), 7 (A), 14 (B), 21 (C), and 28 (D) by gently rolling dry sterile Q-Tips and collecting the cotton tips into 0.5 ml of DPBS. Virus genome copy titers were then determined by qPCR using VV-encoded ribonucleotide reductase (I4L)-specific primers as described previously [17]. Mean ± standard deviation of the log-transformed genome copy values from individually assayed mice (4-5 mice/group) is shown.– – – Lower limit of quantification (LLOQ) = 2.942 [log10] genome copies/swab (0.5 ml). Day 3 data not shown since ~90% of sample values were below the LLOQ. Virus titers below the LLOQ were assigned values of 2.942. For N.D/JH-KO mice, the data represents the average of two independent experiments. Values from vaccine/vehicle mice were compared with vaccine/ST-246 mice within each group using Student’s t-test on the log-transformed data. **P-value <0.01 and *P-value <0.05.
Acknowledgments
This work was supported by Small Business Innovation Research grant R43AI075747 from the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and Its Eradication. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]
- 2.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006 Jun;211:320–37. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy RB, Ovsyannikova I, Poland GA. Smallpox vaccines for biodefense. Vaccine. 2009 Nov 5;27 Suppl 4:D73–9. doi: 10.1016/j.vaccine.2009.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis. 1970 Oct;122(4):303–9. doi: 10.1093/infdis/122.4.303. [DOI] [PubMed] [Google Scholar]
- 5.Frey SE, Couch RB, Tacket CO, Treanor JJ, Wolff M, Newman FK, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002 Apr 25;346(17):1265–74. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- 6.Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003 Jul 15;37(2):251–71. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 7.Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm Rep. 2003 Feb 21;52(RR-4):1–28. [PubMed] [Google Scholar]
- 8.Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003 Mar 15;36(6):766– 74. doi: 10.1086/374244. [DOI] [PubMed] [Google Scholar]
- 9.Kempe CH. Studies smallpox and complications of smallpox vaccination. Pediatrics. 1960 Aug;26:176–89. [PubMed] [Google Scholar]
- 10.Rotz LD, Dotson DA, Damon IK, Becher JA. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. MMWR Recomm Rep. 2001 Jun 22;50(RR-10):1–25. [PubMed] [Google Scholar]
- 11.Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002 Mar-Apr;5(2):84–90. [PubMed] [Google Scholar]
- 12.Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005 Oct;79(20):13139–49. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duraffour S, Snoeck R, de Vos R, van Den Oord JJ, Crance JM, Garin D, et al. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antivir Ther. 2007;12(8):1205–16. [PubMed] [Google Scholar]
- 14.Smith SK, Olson VA, Karem KL, Jordan R, Hruby DE, Damon IK. In vitro efficacy of ST-246 against smallpox and monkeypox. Antimicrob Agents Chemother. 2009 Mar;53(3):1007–12. doi: 10.1128/AAC.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Honeychurch KM, Yang G, Byrd CM, Harver C, Hruby DE, et al. Vaccinia virus p37 interacts with host proteins associated with LE-derived transport vesicle biogenesis. Virol J. 2009 Apr 28;6(1):44. doi: 10.1186/1743-422X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quenelle DC, Buller RM, Parker S, Keith KA, Hruby DE, Jordan R, et al. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob Agents Chemother. 2007 Feb;51(2):689–95. doi: 10.1128/AAC.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berhanu A, King DS, Mosier S, Jordan R, Jones KF, Hruby DE, et al. ST-246 Inhibits In Vivo Poxvirus Dissemination, Virus Shedding, and Systemic Disease Manifestation. Antimicrob Agents Chemother. 2009 Dec;53(12):4999–5009. doi: 10.1128/AAC.00678-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huggins J, Goff A, Hensley L, Mucker E, Shamblin J, Wlazlowski C, et al. Non- human primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009 Apr 6;53(6):2620–5. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan R, Goff A, Frimm A, Corrado ML, Hensley LE, Byrd CM, et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob Agents Chemother. 2009 May;53(5):1817–22. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosenbach DW, Berhanu A, King DS, Mosier S, Jones KF, Jordan RA, et al. Efficacy of ST-246 versus lethal poxvirus challenge in immunodeficient mice. Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):838–43. doi: 10.1073/pnas.0912134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan R, Tien D, Bolken TC, Jones KF, Tyavanagimatt SR, Strasser J, et al. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob Agents Chemother. 2008 May;52(5):1721–7. doi: 10.1128/AAC.01303-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan R, Chinsangaram J, Bolken TC, Tyavanagimatt SR, Tien D, Jones KF, et al. Safety and Pharmacokinetics of the Anti-Orthopoxvirus Compound ST-246(R) following Repeat Oral Dosing in Healthy Adult Subjects. Antimicrob Agents Chemother. 2010 Apr 12;54(6):2560–6. doi: 10.1128/AAC.01689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosenbach DW, Jordan R, King DS, Berhanu A, Warren TK, Kirkwood-Watts DL, et al. Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination. Vaccine. 2008 Feb 13;26(7):933–46. doi: 10.1016/j.vaccine.2007.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvera PM, Peters E, Bassler J, Gong G, Lin S, Hebblewaite D, et al. Impact of concurrent ST-246 administration on ACAM2000 vaccine efficacy in cynomolgus macaques. 7th ASM Biodefense Emerging Dis Res Meet, abstr190 (H); 2009. [Google Scholar]
- 25.Vora S, Damon I, Fulginiti V, Weber SG, Kahana M, Stein SL, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008 May 15;46(10):1555–61. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Progressive Vaccinia in a Military Smallpox Vaccinee — United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(19):532–6. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Human Vaccinia Infection After Contact with a Raccoon Rabies Vaccine Bait — Pennsylvania, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(43):1204–7. [PubMed] [Google Scholar]
- 28.Earl PL, Cooper N, Wyatt S, Moss B, Carroll MW. Preparation of cell cultures and vaccinia virus stocks. In: Ausubel MF, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. 2. New York: John Wiley and Sons; 1998. pp. 16.16.4–16.16.6. [Google Scholar]
- 29.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993 Jun;5(6):647–56. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 30.Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9458–63. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and Humoral Immunity against Vaccinia Virus Infection of Mice. J Immunol. 2004 May 15;172(10):6265–71. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 32.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27(3):493–7. [Google Scholar]
- 33.Subrahmanyan TP. A study of the possible basis of age-dependent resistance of mice to poxvirus diseases. Aust J Exp Biol Med Sci. 1968 Jun;46(3):251–65. doi: 10.1038/icb.1968.20. [DOI] [PubMed] [Google Scholar]
- 34.Tscharke DC, Woo WP, Sakala IG, Sidney J, Sette A, Moss DJ, et al. Poxvirus CD8+ T-cell determinants and cross-reactivity in BALB/c mice. J Virol. 2006 Jul;80(13):6318–23. doi: 10.1128/JVI.00427-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melamed S, Paran N, Katz L, Ben-Nathan D, Israely T, Schneider P, et al. Tail scarification with Vaccinia virus Lister as a model for evaluation of smallpox vaccine potency in mice. Vaccine. 2007 Nov 7;25(45):7743–53. doi: 10.1016/j.vaccine.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Cummings JF, Polhemus ME, Hawkes C, Klote M, Ludwig GV, Wortmann G. Lack of vaccinia viremia after smallpox vaccination. Clin Infect Dis. 2004 Feb 1;38(3):456–8. doi: 10.1086/381101. [DOI] [PubMed] [Google Scholar]
- 37.Keidan SE, McCarthy K, Haworth JC. Fatal generalized vaccinia with failure of antibody production and absence of serum gamma globulin. Arch Dis Child. 1953 Apr;28(138):110–6. doi: 10.1136/adc.28.138.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei H, Huang D, Fortman J, Wang R, Shao L, Chen ZW. Coadministration of cidofovir and smallpox vaccine reduced vaccination side effects but interfered with vaccine-elicited immune responses and immunity to monkeypox. J Virol. 2009 Jan;83(2):1115–25. doi: 10.1128/JVI.00984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Payne LG, Kristensson K. Extracellular release of enveloped vaccinia virus from mouse nasal epithelial cells in vivo. J Gen Virol. 1985 Mar;66( Pt 3):643–6. doi: 10.1099/0022-1317-66-3-643. [DOI] [PubMed] [Google Scholar]
- 40.Boulter EA, Zwartouw HT, Titmuss DH, Maber HB. The nature of the immune state produced by inactivated vaccinia virus in rabbits. Am J Epidemiol. 1971 Dec;94(6):612–20. doi: 10.1093/oxfordjournals.aje.a121360. [DOI] [PubMed] [Google Scholar]
- 41.Law M, Putz MM, Smith GL. An investigation of the therapeutic value of vaccinia immune IgG in a mouse pneumonia model. J Gen Virol. 2005 April 1;86(4):991–1000. doi: 10.1099/vir.0.80660-0. [DOI] [PubMed] [Google Scholar]
- 42.Vanderplasschen A, Hollinshead M, Smith GL. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J Gen Virol. 1997 Aug;78( Pt 8):2041–8. doi: 10.1099/0022-1317-78-8-2041. [DOI] [PubMed] [Google Scholar]
- 43.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008 May;28(5):710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Ferrier-Rembert A, Drillien R, Meignier B, Garin D, Crance JM. Safety, immunogenicity and protective efficacy in mice of a new cell-cultured Lister smallpox vaccine candidate. Vaccine. 2007 Nov 28;25(49):8290–7. doi: 10.1016/j.vaccine.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 45.Amara RR, Nigam P, Sharma S, Liu J, Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J Virol. 2004 Apr;78(8):3811–6. doi: 10.1128/JVI.78.8.3811-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007 Jun 11;204(6):1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington LE, Most Rv R, Whitton JL, Ahmed R. Recombinant vaccinia virus- induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002 Apr;76(7):3329–37. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novy P, Quigley M, Huang X, Yang Y. CD4 T Cells Are Required for CD8 T Cell Survival during Both Primary and Memory Recall Responses. J Immunol. 2007 December 15;179(12):8243–51. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 49.Ramshaw IA, Ramsay AJ, Karupiah G, Rolph MS, Mahalingam S, Ruby JC. Cytokines and immunity to viral infections. Immunol Rev. 1997 Oct;159:119–35. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Smallpox Response Plan and Guidelines (Version 3.0) 2003 http://www.bt.cdc.gov/agent/smallpox/response-plan/index.asp.
- 51.Artenstein AW, Grabenstein JD. Smallpox vaccines for biodefense: need and feasibility. Expert Rev Vaccines. 2008 Oct;7(8):1225–37. doi: 10.1586/14760584.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beachkofsky TM, Carrizales SC, Bidinger JJ, Hrncir DE, Whittemore DE, Hivnor CM. Adverse Events Following Smallpox Vaccination With ACAM2000 in a Military Population. Arch Dermatol. 2010 Jun;146(6):656–61. doi: 10.1001/archdermatol.2010.46. [DOI] [PubMed] [Google Scholar]
- 53.Berhanu A, Wilson RL, Kirkwood-Watts DL, King DS, Warren TK, Lund SA, et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J Virol. 2008 Apr;82(7):3517–29. doi: 10.1128/JVI.01854-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilead. VISTIDE® (cidofovir injection) 1996 http://www.gilead.com/pdf/vistide.pdf.
- 55.Kempe CH, Berge TO, England B. Hyperimmune vaccinal gamma globulin; source, evaluation, and use in prophylaxis and therapy. Pediatrics. 1956 Aug;18(2):177–88. [PubMed] [Google Scholar]
- 56.Edghill-Smith Y, Venzon D, Karpova T, McNally J, Nacsa J, Tsai WP, et al. Modeling a safer smallpox vaccination regimen, for human immunodeficiency virus type 1-infected patients, in immunocompromised macaques. J Infect Dis. 2003 Oct 15;188(8):1181–91. doi: 10.1086/378518. [DOI] [PubMed] [Google Scholar]
- 57.Good RA, Zak SJ, Condie RM, Bridges RA. Clinical investigation of patients with agammaglobulinemia and hypogammaglobulinemia. Pediatr Clin North Am. 1960 May;7:397–433. doi: 10.1016/s0031-3955(16)30945-2. [DOI] [PubMed] [Google Scholar]
- 58.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4590–5. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005 Jul;11(7):740–7. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 60.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010 Jan 17;16(2):224–7. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarkar JK, Mitra AC, Mukherjee MK. The minimum protective level of antibodies in smallpox. Bull World Health Organ. 1975;52(3):307–11. [PMC free article] [PubMed] [Google Scholar]
- 62.Mack TM, Noble J, Jr, Thomas DB. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972 Mar;21(2):214–8. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 63.Monath TP, Caldwell JR, Mundt W, Fusco J, Johnson CS, Buller M, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)--a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004 Oct;8(Suppl 2):S31–44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10847–51. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002 Mar;3(3):265–71. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 66.Military Vaccine (MILVAX) Agency. Smallpox vaccination saftey summary: DoD smallpox vaccination program. 2007 http://smallpox.mil/event/spsafetysum.asp.
- 67.Military Vaccine (MILVAX) Agency. Smallpox Vaccination Program. 2008 www.smallpox.army.mil/documents/Smallpox_QA.pdf.
- 68.Centers for Disease Control and Prevention. Vaccinia virus infection after sexual contact with a military smallpox vaccinee --- washington, 2010. MMWR Morb Mortal Wkly Rep. 2010 Jul 2;59(25):773–5. [PubMed] [Google Scholar]
- 69.U.S. Department of Defense. Panels find vaccines may relate to reservist’s illness, death. 2003 http://www.defenselink.mil/releases/release.aspx?releaseid=5799.
- 70.Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, Tagaya Y, et al. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J Exp Med. 2009 Jun 8;206(6):1219–25. doi: 10.1084/jem.20082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neyts J, Leyssen P, Verbeken E, De Clercq E. Efficacy of cidofovir in a murine model of disseminated progressive vaccinia. Antimicrob Agents Chemother. 2004 Jun;48(6):2267–73. doi: 10.1128/AAC.48.6.2267-2273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
JH-KO mice were treated with the indicated antibodies at weekly intervals after an initial 3-day treatment as described in Materials and Methods. On the day of vaccination (A-D) or one (E-H) or 2 months (I-L) post-vaccination, whole blood samples collected in sodium citrate were stained with a combination of anti-CD8 and anti-CD4 antibodies and analyzed by flow cytometry. Representative dot plots from individually analyzed samples (n = 4-5) for each group is shown. The percentages indicate the proportion of cells in each quadrant based on live lymphocyte gating on FSC and SSC parameters. Cellular depletion was similarly efficient in BALB/c mice (not shown). † Data not available, since the mice succumbed to viral disease by day 7 post-VV-WR challenge.
BALB/c and JH-KO mice were depleted of CD4+, CD8+, or both CD4+ and CD8+ T cells and vaccinated and concurrently treated with vehicle or ST-246 as described in Figure 1. Lesion swabs of the vaccination sites were taken from the same groups of mice as in Table S1 on days 3 (data not shown), 7 (A), 14 (B), 21 (C), and 28 (D) by gently rolling dry sterile Q-Tips and collecting the cotton tips into 0.5 ml of DPBS. Virus genome copy titers were then determined by qPCR using VV-encoded ribonucleotide reductase (I4L)-specific primers as described previously [17]. Mean ± standard deviation of the log-transformed genome copy values from individually assayed mice (4-5 mice/group) is shown.– – – Lower limit of quantification (LLOQ) = 2.942 [log10] genome copies/swab (0.5 ml). Day 3 data not shown since ~90% of sample values were below the LLOQ. Virus titers below the LLOQ were assigned values of 2.942. For N.D/JH-KO mice, the data represents the average of two independent experiments. Values from vaccine/vehicle mice were compared with vaccine/ST-246 mice within each group using Student’s t-test on the log-transformed data. **P-value <0.01 and *P-value <0.05.