ABSTRACT
We report two cases of complex middle cerebral artery aneurysms that were surgically treated using the orbitopterional approach in a two-piece method. The objective of this work is to discuss the usefulness of the orbitopterional approach in the surgical management of large and giant middle cerebral artery aneurysms. A 32-year-old man with a giant aneurysm and a 50-year-old woman with a large and complex aneurysm presented with subarachnoid hemorrhages. Both aneurysms were successfully clipped through an orbitopterional approach. This approach permits a more basal view of the vascular structures with only a minor retraction of frontal lobe. It also increases the view angle and amount of working space available. This approach should be considered as an alternative to the classic pterional craniotomy for the surgical management of such complex lesions.
Keywords: Orbitopterional approach, giant aneurysm, middle cerebral artery, subarachnoid hemorrhage
Giant aneurysms of the middle cerebral artery (MCA) represent 13% of all giant intracranial aneurysms.1 Approximately 4% of the aneurysms located within the MCA are giant and occur most frequently at the bifurcation.2 The prognosis of these aneurysms is very poor. The rate of rupture is high, and this risk increases over time. The annual rupture rate for a giant aneurysm located in the anterior circulation is 8%, and intra-aneurysmal thrombosis does not preclude rupture or rebleeding of such lesions.3,4,5 Complications that contribute to the morbidity and mortality of these aneurysms include the progressive enlargement of the aneurysm with local mass effects and distal ischemic events.1,6 It is therefore generally indicated to treat large and giant aneurysms. The available therapeutic options include surgery, endovascular therapy, or a combination of both.6,7,8,9,10
In the present article, the authors report on two cases of complex MCA aneurysms with previous subarachnoidal hemorrhages that were treated by surgical clipping using the orbitopterional approach. We discuss the various methods of treatment of these complex aneurysms with an emphasis on the possible surgical approaches and the advantages of an orbital osteotomy.
CASE REPORTS
Case 1
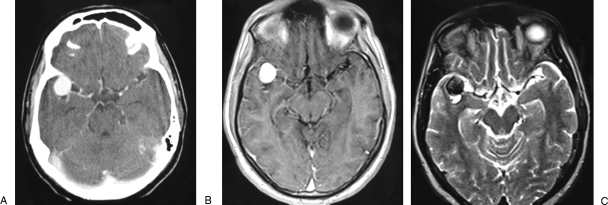
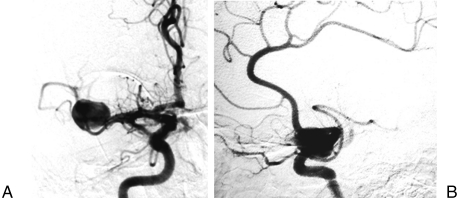
A previously healthy 32-year-old man was admitted to a regional hospital after an episode of sudden headache with loss of consciousness and seizures. He was diagnosed with a subarachnoidal hemorrhage, and radiological and angiographic investigation showed a giant aneurysm of the right MCA. The patient was referred to our hospital 3 months after the bleed for specialized treatment. At admission, the neurological examination revealed only a mild central facial palsy on the left side. Computed tomography (CT) and magnetic resonance imaging demonstrated a lesion in the right Sylvian fissure, which was indicative of a giant aneurysm of the MCA (Fig. 1). Cerebral angiography detected a giant aneurysm at the bifurcation of the right MCA (Fig. 2). The superior trunk was not seen and the collateral pial anastomoses supplying its vascular territory were mainly comprised of branches of the anterior cerebral artery (ACA).
Figure 1.
(A) Contrast-enhanced computed tomography scan. (B) Axial T1-weighted contrast-enhanced magnetic resonance (MR) image and (C) axial T2-weighted MR image showing an aneurysm inside the right Sylvian fissure.
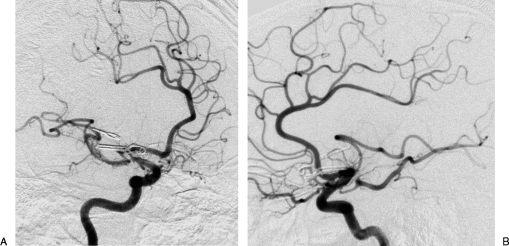
Figure 2.
(A) Front and (B) lateral views of the right carotid angiography revealing the giant aneurysm of the middle cerebral artery.
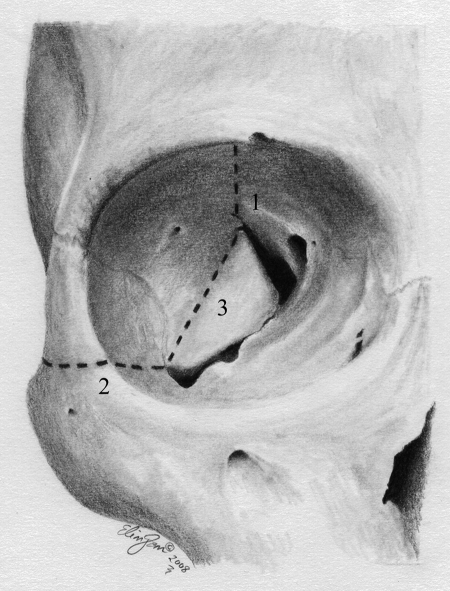
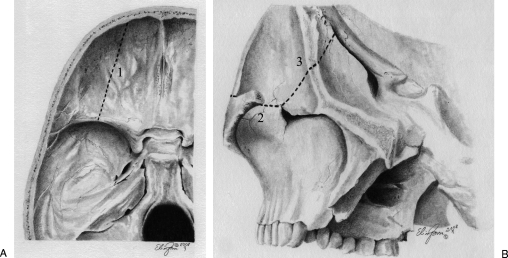
We opted for direct surgical treatment of the aneurysm by a right orbitopterional approach in a two-piece method. First, a pterional craniotomy was made as described by Yasargil11 with small modifications,12,13 and in so doing we preserved the superficial temporal artery for a possible bypass procedure. The dura was dissected free from the sphenoid wing and anterior and temporal fossa, and the periorbita was dissected from the roof and lateral wall of the orbit. Second, a superolateral orbital osteotomy was performed by using a high-speed drill and a small chisel. The first cut started laterally to the supraorbital notch and ended at the lateral aspect of the superior orbital fissure. The second cut began at the orbital rim in the inferior point of the lateral wall, parallel to the zygomatic arch, and was directed toward the inferior orbital fissure. The last cut connected the two previous ones and extended from the superior to the inferior orbital fissures (Figs. 3 and 4). After the dural opening, the superficial layer of the Sylvian fissure was opened and exposed the aneurysm. The aneurysm filled the fissure, and gaining space by splitting it was not possible. The frontal lobe was slightly retracted and the carotid artery and carotid bifurcation were seen. The MCA was dissected distally to the point of the aneurysm. The lesion measured ∼3 cm across its greatest diameter. It was further dissected with care and the inferior trunk was identified. A temporary occlusion of the M1 segment and the inferior trunk was performed under mild hypothermia and a continuous propofol infusion. The aneurysmal sac was opened, and a thrombectomy was performed. Further dissection around the aneurysm was facilitated by internal decompression, and we identified a thin thrombosed superior trunk arising from the aneurysmal sac. A small clip was applied distally to the superior trunk, and it was subsequently transected. Two straight clips were applied to the broad neck of the aneurysm, and the aneurysmal sac was resected. We decided not to perform a bypass because the superior trunk was very thin and totally thrombosed and because the preoperative angiography showed a good collateral circulation from the ACA branches.
Figure 3.
Artistic representation showing the orbital view of osteotomies (dotted lines). The numbers represent the order of cuts.
Figure 4.
Artistic representation of the osteotomies. (A) Intracranial view of the first cut: dotted line 1. (B) Second and third cuts showed by a combined extra- and intracranial view after the pterional craniotomy. The zygomatic arch was removed to show the inferior orbital fissure.
The patient's postoperative course was uneventful. A CT scan showed a small ischemic area in the temporal posterior region with no apparent clinical significance, and the postoperative cerebral angiography showed no evidence of continued aneurysm filling (Fig. 5). The patient was discharged from hospital on postoperative day 7 with no new deficits. At the follow-up evaluation after 1 year, temporal muscle atrophy was present but there were no cosmetic bone defects or other complications.
Figure 5.
(A) Oblique and (B) lateral projections of the postoperative right carotid angiography showing complete exclusion of the aneurysm from the circulation.
Case 2
A 50-year-old woman was admitted to another institution with a history of a sudden, severe headache, nausea, and a seizure. Nuchal rigidity was observed, and a CT scan revealed a subarachnoid hemorrhage. The patient was referred to our hospital 1 month after this episode for treatment. The neurological examination was normal and a cerebral angiogram showed a large aneurysm of ∼2 cm in the bifurcation of the right MCA (Fig. 6).
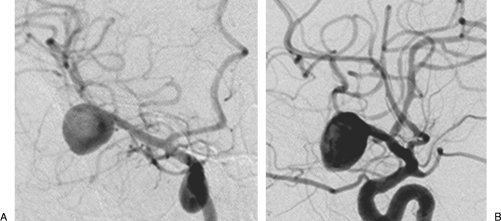
Figure 6.
(A) Oblique and (B) lateral projections of the right carotid angiography demonstrating a large middle cerebral artery aneurysm.
The aneurysm was clipped by an orbitopterional approach in a two-piece method as described in case 1. After opening the Sylvian fissure and basal cisterns, the aneurysm and superior trunk were identified. With mild hypothermia and a continuous propofol infusion, a temporary clip was placed on the M1 segment of the MCA. After this maneuver, the aneurysmal sac softened, which facilitated further dissection. The aneurysm had a large complex neck and was clipped with three clips in a tandem technique with preservation of the superior and inferior trunks.
The postoperative course was uneventful, and the control cerebral angiography showed complete exclusion of the aneurysm from circulation (Fig. 7). At the follow-up assessment after 6 months, a frontal paresis was verified but no bone defects were present.
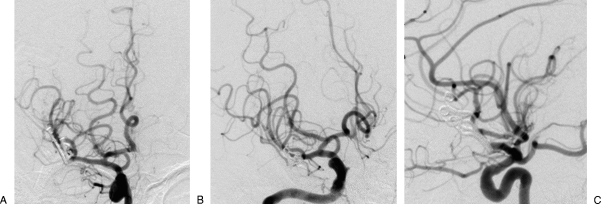
Figure 7.
(A) Front, (B) oblique, and (C) lateral views from the postoperative angiography revealing the complete obliteration of the aneurysm.
DISCUSSION
Treatment is indicated whenever possible to improve the severe natural history of large and giant aneurysms. Despite advances in microsurgery and endovascular techniques, treatment of these lesions continues to be a challenge. Endovascular treatment with coil embolization has a significant incidence of rebleeding after coiling and is often associated with both a low complete occlusion rate and a high recanalization rate with coil compaction. Embolization with a liquid polymer with or without stenting has a low complete occlusion rate, and recanalization can also occur. Endovascular deconstructive techniques can be used in selected cases, which are generally associated with a revascularization procedure.9,14,15,16,17,18,19
When compared with endovascular treatments of large and giant aneurysms, surgery has a better rate for the complete obliteration of the aneurysm. Morbidity and mortality related to the surgical treatment of giant aneurysms of the anterior circulation are comparable with those of endovascular techniques. Therefore, surgery should be considered as the treatment of choice for these aneurysms if it is feasible. The main objectives of surgery are the exclusion of the aneurysm from the circulation, preservation of blood flow in the parent vessels, and relief of the mass effect of the aneurysm.10,19,20
The best method of microsurgical occlusion is by the direct clipping of the aneurysmal neck. Because the necks of these lesions are frequently wide, broad, and thickened, the application of several clips may be necessary to complete the occlusion. When direct clipping is not possible, other techniques, such as the excision of the aneurysm with an anastomosis of the parent vessels or parent vessel occlusion and trapping, with or without a revascularization procedure, should be used.10,21,22,23,24,25
Classically, the giant and large aneurysms of the MCA were surgically treated through a pterional craniotomy with extensive drilling of the sphenoid wing.2,20,26,27 With development of skull base techniques that expand the exposure of the pterional approach and minimize brain retraction, some authors advocate the use of cranial base approaches for giant and complex aneurysms of the anterior circulation.10,22,28,29,30
The orbitopterional approach is a modification of the orbitozygomatic approach, where a pterional craniotomy is combined with a superolateral orbitotomy and preservation of the zygomatic arch.31,32,33 It can be accomplished in one- or two-piece methods. In the two-piece method, the pterional craniotomy is made first in the standard fashion, and a superolateral orbitotomy is then performed separately. The one-piece method combines the pterional craniotomy and the orbital osteotomy into one bone flap.32,34,35,36 We prefer the two-piece method where the orbital osteotomies can be performed more posteriorly, which diminishes the need for orbital reconstruction and the risk of a blind fracture of the orbital roof.
Possible complications of this approach are cosmetic deformity, enophthalmos, pulsatile exophthalmos, palpebral ptosis, and blindness. We observed a frontal paresis in one case and atrophy of the temporalis muscle in the other. No cosmetic bone deformities or other major complications were seen.
Alaywan and Sindou37 described an increase in the view angle of 75% with the orbital osteotomy when compared with the pterional craniotomy alone, using the optic-carotid complex as a target. Schwartz and colleagues,38 using a frameless stereotactic device, also showed a significant increase in surgical exposure with an orbital osteotomy. The area of exposure of the pterional trans-Sylvian approach was increased from 26 to 39%.
The cranial approaches combined with an orbital osteotomy are mainly indicated for lesions of the sellar, suprasellar, and parasellar regions, along with the anterior fossa and orbit.32,34,39,40 Sindou et al41 also reported the utilities of these approaches for lesions of the Sylvian fissure and mesial temporal area. Other studies have shown the advantage of this approach for accessing anterior communicating artery aneurysms.35,39,40,42
We conducted a literature review on orbital osteotomies in the surgical treatment of complex aneurysms of the MCA (Table 1). Ikeda et al43 reported a case of a giant fusiform aneurysm at the horizontal portion of the MCA in a child, which was approached by an orbitopterional craniotomy. Sekhar et al44 published their series of complex MCA aneurysms that needed reconstruction, and four of them (two giant, one large, and one small) were treated by an orbitopterional approach. Sindou and Alaywan33 reported a case of a giant aneurysm and observed that the orbital removal facilitated the exposure of the neck. Smith et al30 and Origitano et al28 recommended the use of orbitocranial approaches to treat complex aneurysms of the MCA.
Table 1.
Summary of Published Cases of Complex MCA Aneurysm Approached by Orbitopterional Craniotomy
| Author and Year | Age (y)/Sex | Size | Surgical Technique | Approach-Related Complications | Outcome |
|---|---|---|---|---|---|
| —, not reported; GOS, Glasgow Outcome Scale; MCA, middle cerebral artery; SVG, saphenous vein graft. | |||||
| Smith et al 198930 | 61/M | — | — | Frontalis weakness | — |
| 32/F | — | — | None | — | |
| 31/F | — | — | Temporalis atrophy | — | |
| 54/M | — | — | None | — | |
| Sindou and Alaywan 199033 | — | Giant | Clipping + aneurysm sac resection | None | — |
| Origitano et al 199328 | 54/M | — | — | Displacement of cranial bone flap | — |
| Ikeda et al 199443 | 13/M | Giant | Clipping + reinforcement of aneurysm remnant with oxycellulose and cyanoacrylate glue | — | Rebleeding after 3 mo requiring new surgery; GOS: 4 |
| Sekhar et al 200544 | 49/F | Giant | Clipping + bypass with SVG | None | Internal capsule infarct; GOS: 4 |
| 72/F | Giant | Excision of aneurysm + aneurysmorrhaphy | None | Clotting of aneurysmorrhaphy requiring new surgery; GOS: 5 | |
| 51/F | Large | Excision of aneurysm + branch reimplant | None | GOS: 5 | |
| 49/F | Small | Excision of aneurysm + arterial patch on MCA bifurcation | Paresis of III, V, and VII cranial nerves | GOS: 5 | |
| Present study | 32/M | Giant | Clipping + aneurysm sac resection | Frontal paresis | GOS: 5 |
| 50/F | Large | Clipping | Temporalis muscle atrophy | GOS: 5 | |
The main advantages of an orbitopterional approach for complex MCA aneurysms are: (1) it provides multidirectional viewing of the aneurysm, which makes surgical dissection possible via multiple routes; (2) it involves minimal brain retraction; and (3) it enables access to the aneurysm via the shortest possible distance from the surface.28,30,33,41 We also observed that this approach enabled a greater angle of view and working space around the internal carotid artery and MCA, which allowed for an earlier dissection of these structures and proximal vascular control of the aneurysm. The increased working space also made the dissection and clipping of the aneurysm less difficult when compared with the pterional approach.
In conclusion, we report two cases of complex MCA aneurysms that were successfully clipped via an orbitopterional approach. This approach permits an increase in the angle of visualization and effective working space with the need for only minimal brain retraction, which facilitates the proximal control, dissection, and clipping of aneurysms. It is a safe procedure that we now consider as the optimal option in the surgical management of large and giant complex MCA aneurysms. For complex but small MCA aneurysms, we still prefer to use the classical pterional approach without orbital osteotomy.
ACKNOWLEDGMENTS
We wish to thank Regina Levy, M.D., and Marcus Acioly, M.D., for their critical review and suggestions.
REFERENCES
- Anson J A. In: Awad IA, Barrow DL, editor. Giant Intracranial Aneurysms. Park Ridge, IL: AANS Publications Committee; 1997. Epidemiology and natural history. pp. 23–33.
- Dashti R, Hernesniemi J, Niemelä M, et al. Microneurosurgical management of middle cerebral artery bifurcation aneurysms. Surg Neurol. 2007;67:441–456. doi: 10.1016/j.surneu.2006.11.056. [DOI] [PubMed] [Google Scholar]
- Khurana V G, Piepgras D G, Whisnant J P. Ruptured giant intracranial aneurysms. Part I. A study of rebleeding. J Neurosurg. 1998;88:425–429. doi: 10.3171/jns.1998.88.3.0425. [DOI] [PubMed] [Google Scholar]
- Nakase H, Shin Y, Kanemoto Y, Ohnishi H, Morimoto T, Sakaki T. Long-term outcome of unruptured giant cerebral aneurysms. Neurol Med Chir (Tokyo) 2006;46:379–384. discussion 384–386. doi: 10.2176/nmc.46.379. [DOI] [PubMed] [Google Scholar]
- Wiebers D O, Whisnant J P, Huston J, III, et al. International Study of Unruptured Intracranial Aneurysms Investigators Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- Choi I S, David C. Giant intracranial aneurysms: development, clinical presentation and treatment. Eur J Radiol. 2003;46:178–194. doi: 10.1016/s0720-048x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Hamburger C, Schönberger J, Lange M. Management and prognosis of intracranial giant aneurysms. A report on 58 cases. Neurosurg Rev. 1992;15:97–103. doi: 10.1007/BF00313502. [DOI] [PubMed] [Google Scholar]
- Ponce F A, Albuquerque F C, McDougall C G, Han P P, Zabramski J M, Spetzler R F. Combined endovascular and microsurgical management of giant and complex unruptured aneurysms. Neurosurg Focus. 2004;17:E11. doi: 10.3171/foc.2004.17.5.11. [DOI] [PubMed] [Google Scholar]
- Sluzewski M, Menovsky T, Rooij W J van, Wijnalda D. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003;24:257–262. [PMC free article] [PubMed] [Google Scholar]
- Spetzler R F, Riina H A, Lemole G M., Jr Giant aneurysms. Neurosurgery. 2001;49:902–908. doi: 10.1097/00006123-200110000-00022. [DOI] [PubMed] [Google Scholar]
- Yasargil M G. In: Yasargil MG, editor. Microneurosurgery: Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms. New York: Thieme Medical Publishers; 1984. Interfascial pterional (frontotemporosphenoidal) craniotomy. pp. 215–233.
- Oliveira E, Tedeschi H. In: Sekhar LN, Oliveira E, editor. Cranial Microsurgery. Approaches and Techniques. New York: Thieme Medical Publishers; 1999. Pterional and pretemporal approaches. pp. 124–129.
- Spetzler R F, Lee K S. Reconstruction of the temporalis muscle for the pterional craniotomy. Technical note. J Neurosurg. 1990;73:636–637. doi: 10.3171/jns.1990.73.4.0636. [DOI] [PubMed] [Google Scholar]
- Gonzalez N R, Duckwiler G, Jahan R, Murayama Y, Viñuela F. Challenges in the endovascular treatment of giant intracranial aneurysms. Neurosurgery. 2006;59(5 Suppl 3):S113–S124. discussion S3–S13. doi: 10.1227/01.NEU.0000237559.93852.F1. [DOI] [PubMed] [Google Scholar]
- Li M-H, Li Y-D, Fang C, et al. Endovascular treatment of giant or very large intracranial aneurysms with different modalities: an analysis of 20 cases. Neuroradiology. 2007;49:819–828. doi: 10.1007/s00234-007-0257-6. [DOI] [PubMed] [Google Scholar]
- Molyneux A J, Cekirge S, Saatci I, Gál G. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol. 2004;25:39–51. [PMC free article] [PubMed] [Google Scholar]
- Murayama Y, Nien Y L, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- Nguyen T N, Hoh B L, Amin-Hanjani S, Pryor J C, Ogilvy C S. Comparison of ruptured vs unruptured aneurysms in recanalization after coil embolization. Surg Neurol. 2007;68:19–23. doi: 10.1016/j.surneu.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Parkinson R J, Eddleman C S, Batjer H H, Bendok B R. Giant intracranial aneurysms: endovascular challenges. Neurosurgery. 2006;59(5 Suppl 3):S103–S112. discussion S3–S13. doi: 10.1227/01.NEU.0000237410.32115.C9. [DOI] [PubMed] [Google Scholar]
- Gewirtz R J, Awad I A. Giant aneurysms of the anterior circle of Willis: management outcome of open microsurgical treatment. Surg Neurol. 1996;45:409–420. discussion 420–421. doi: 10.1016/0090-3019(95)00437-8. [DOI] [PubMed] [Google Scholar]
- Drake C G, Peerless S J. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141–162. doi: 10.3171/jns.1997.87.2.0141. [DOI] [PubMed] [Google Scholar]
- Lawton M T, Spetzler R F. Surgical strategies for giant intracranial aneurysms. Acta Neurochir Suppl. 1999;72:141–156. doi: 10.1007/978-3-7091-6377-1_12. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy B A, Salehi S A, Mindea S A, Batjer H H. Selective cerebral revascularization as an adjunct in the treatment of giant anterior circulation aneurysms. Neurosurg Focus. 2003;14:e4. doi: 10.3171/foc.2003.14.3.5. [DOI] [PubMed] [Google Scholar]
- Peerless S J, Drake C G. Treatment of giant cerebral aneurysms of the anterior circulation. Neurosurg Rev. 1982;5:149–154. doi: 10.1007/BF01742677. [DOI] [PubMed] [Google Scholar]
- Peerless S J, Ferguson G G, Drake C G. Extracranial-intracranial (EC/IC) bypass in the treatment of giant intracranial aneurysms. Neurosurg Rev. 1982;5:77–81. doi: 10.1007/BF01743477. [DOI] [PubMed] [Google Scholar]
- Heros R C, Fritsch M J. Surgical management of middle cerebral artery aneurysms. Neurosurgery. 2001;48:780–785. discussion 785–786. doi: 10.1097/00006123-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Yasargil M G. In: Yasargil MG, editor. Microneurosurgery: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. New York: Thieme Medical Publishers; 1984. Middle cerebral artery aneurysms. pp. 124–164.
- Origitano T C, Anderson D E, Tarassoli Y, Reichman O H, al-Mefty O. Skull base approaches to complex cerebral aneurysms. Surg Neurol. 1993;40:339–346. doi: 10.1016/0090-3019(93)90148-t. [DOI] [PubMed] [Google Scholar]
- Sekhar L N, Kalia K K, Yonas H, Wright D C, Ching H. Cranial base approaches to intracranial aneurysms in the subarachnoid space. Neurosurgery. 1994;35:472–481. discussion 481–483. doi: 10.1227/00006123-199409000-00016. [DOI] [PubMed] [Google Scholar]
- Smith R R, Al-Mefty O, Middleton T H. An orbitocranial approach to complex aneurysms of the anterior circulation. Neurosurgery. 1989;24:385–391. doi: 10.1227/00006123-198903000-00013. [DOI] [PubMed] [Google Scholar]
- Hakuba A, Liu S S, Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271–276. doi: 10.1016/0090-3019(86)90161-8. [DOI] [PubMed] [Google Scholar]
- Lemole G M, Jr, Henn J S, Zabramski J M, Spetzler R F. Modifications to the orbitozygomatic approach. Technical note. J Neurosurg. 2003;99:924–930. doi: 10.3171/jns.2003.99.5.0924. [DOI] [PubMed] [Google Scholar]
- Sindou M, Alaywan M. [Orbital and/or zygomatic removal in an approach to lesions near the cranial base. Surgical technique, anatomic study and analysis of a series of 24 cases] Neurochirurgie. 1990;36:225–233. (French) [PubMed] [Google Scholar]
- Al-Mefty O. Supraorbital-pterional approach to skull base lesions. Neurosurgery. 1987;21:474–477. doi: 10.1227/00006123-198710000-00006. [DOI] [PubMed] [Google Scholar]
- Andaluz N, Loveren H R Van, Keller J T, Zuccarello M. Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery. 2003;52:1140–1148. discussion 1148–1149. [PubMed] [Google Scholar]
- Andaluz N, Loveren H R van, Keller J T, Zuccarello M. The one-piece orbitopterional approach. Skull Base. 2003;13:241–245. doi: 10.1055/s-2004-817701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaywan M, Sindou M. Fronto-temporal approach with orbito-zygomatic removal. Surgical anatomy. Acta Neurochir (Wien) 1990;104:79–83. doi: 10.1007/BF01842824. [DOI] [PubMed] [Google Scholar]
- Schwartz M S, Anderson G J, Horgan M A, Kellogg J X, McMenomey S O, Delashaw J B., Jr Quantification of increased exposure resulting from orbital rim and orbitozygomatic osteotomy via the frontotemporal transsylvian approach. J Neurosurg. 1999;91:1020–1026. doi: 10.3171/jns.1999.91.6.1020. [DOI] [PubMed] [Google Scholar]
- Delashaw J B, Jr, Jane J A, Kassell N F, Luce C. Supraorbital craniotomy by fracture of the anterior orbital roof. Technical note. J Neurosurg. 1993;79:615–618. doi: 10.3171/jns.1993.79.4.0615. [DOI] [PubMed] [Google Scholar]
- Jane J A, Park T S, Pobereskin L H, Winn H R, Butler A B. The supraorbital approach: technical note. Neurosurgery. 1982;11:537–542. [PubMed] [Google Scholar]
- Sindou M, Emery E, Acevedo G, Ben-David U. Respective indications for orbital rim, zygomatic arch and orbito-zygomatic osteotomies in the surgical approach to central skull base lesions. Critical, retrospective review in 146 cases. Acta Neurochir (Wien) 2001;143:967–975. doi: 10.1007/s007010170001. [DOI] [PubMed] [Google Scholar]
- Figueiredo E G, Deshmukh P, Zabramski J M, et al. Quantitative anatomic study of three surgical approaches to the anterior communicating artery complex. Neurosurgery. 2005;56(2 Suppl):397–405. discussion 397–405. doi: 10.1227/01.neu.0000156549.96185.6d. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Yamashita J, Higashi S. Giant fusiform aneurysm at the horizontal portion of the middle cerebral artery in a child—case report. Neurol Med Chir (Tokyo) 1994;34:30–34. doi: 10.2176/nmc.34.30. [DOI] [PubMed] [Google Scholar]
- Sekhar L N, Stimac D, Bakir A, Rak R. Reconstruction options for complex middle cerebral artery aneurysms. Neurosurgery. 2005;56(1 Suppl):66–74. discussion 66–74. doi: 10.1227/01.neu.0000144210.44405.e0. [DOI] [PubMed] [Google Scholar]