ABSTRACT
We evaluated the utility of a three-dimensional (3-D) endoscopic system for skull base surgery. We performed a retrospective case series in a tertiary care medical center. Thirty-six patients underwent skull base (nonpituitary) resections via 3-D endoscopic system. Fifteen patients (42%) were operated for excision of malignant tumors, 19 (53%) for excision of benign lesions, and 3 (8.3%) for skull base reconstruction. The tumors involved the cribriform plate (n = 13), sphenoid sinus and planum (n = 17), clivus (n = 7), and sella (n = 7). Complete tumor resection was achieved in 31 patients and subtotal resection in two. Five patients (14%) had postoperative complications. There was one case of meningitis, and there were no cases of cerebrospinal fluid leak. The surgeon's ability to recognize anatomic structures at the skull base was evaluated using the 3-D and two-dimensional systems. The 3-D technique was superior to the conventional technique for identification of the sella, carotid prominence, optic prominence, cribriform plate, sphenoid, and fovea ethmoidalis. The two systems were equal for detection of the turbinates, clivus, maxillary, ethmoids, and frontal sinuses. Endoscopic skull base surgery with stereoscopic viewing is feasible and safe. Further studies are required to evaluate the advantage of binocular vision in skull base surgery.
Keywords: Endoscopic approach, stereoscopic view, minimal invasive, skull base, three-dimensional, surgery
Endoscopic endonasal surgery became the workhorse for treating inflammatory diseases and neoplasms involving the paranasal sinuses and skull base over the past decade.1 Endoscopic surgery provides excellent access to the anterior skull base and to the sphenoethmoidal and paranasal cavities while minimizing morbidity. Intradural and extradural tumor resection can be performed endoscopically in a single procedure that also allows adequate reconstruction of the dura.2 The technical reproducibility of the endoscopic approach has several advantages, but one of its major limitations is the lack of stereoscopic vision. A three-dimensional (3-D) image of the surgical field is essential for the perception of depth during microsurgery, and so the binocular view of the surgical field may be advantageous for the implementation of new endoscopic procedures and for popularization of this technique.3 Several new 3-D endoscopic systems have been added to our armamentarium, but questions about their actual benefit led to their slow integration into practice.4
The aims of the study were: (1) to assess the safety of the procedure, (2) to evaluate the ability of the surgeons to correctly identify specific anatomic landmarks, and (3) to estimate the adverse effects of the 3-D system on the surgical team. This is the first study that evaluates the utility of a new 3-D system dedicated to nonpituitary, endoscopic skull base surgery.
MATERIALS AND METHODS
All patients who underwent endoscopic skull base surgeries at the Tel Aviv Sourasky Medical Center Skull Base Service between June 2008 and September 2009 were eligible to enroll in this study. Of 53 endoscopic skull base operations performed in our institute using a 3-D endoscopic system, 36 patients underwent nonpituitary skull base resections. Of these, 29 underwent surgery via the expanded endoscopic approach (EEA) alone, and the other seven patients had combined EEA and frontal craniotomy. All the procedures were performed with the patients in the supine position and under general anesthesia. Verification of anatomic landmarks was by an intraoperative navigation system (Vector Vision, Brain Laboratory, Feldkirchen, Germany). A lumbar spine catheter was inserted for drainage in cases where a high-flow cerebrospinal fluid (CSF) leak was encountered during surgery. The patients were treated with a perioperative prophylactic antibiotic regimen consisting of parenteral metronidazole and cefuroxime with or without vancomycin.5 Treatment was initiated 1 hour before surgery and continued until the nasal packing was removed. Reconstruction was performed with fascia lata and fat flap in cases of a low-flow CSF leak. Inlay fascia lata and underlay nasal septal flap were used for high-flow CSF leaks, as previously described.2 A vomer bone graft was used to support the inlay fascia lata flap in two cases of large anterior skull base defects.6 The reconstruction was covered with strips of Surgicel (Ethicon Inc., Johnson and Johnson, Somerville, NJ) and fibrin glue as well as with Gelfoam® sponge (Pfizer Inc., New York, NY) to provide additional support and separation from the nasal packing. The reconstruction was supported with a No. 14 Foley catheter inflated inside the nasopharynx. The lumbar drain was removed 3 or 5 days after the operation and the nasal packing was removed on postoperative day 5 or 7, for low- and high-flow leaks, respectively. All the study patients were followed on a regular basis for 1 to 13 months (average 4.2 months) after discharge.
Endoscopic System
The 3-D endoscopic system uses 0-degree and 30-degree endoscopes with distal 3-D sensors (Visionsense Ltd., Petach-Tikva, Israel). The 3-D video endoscope is a prototype that is 150 mm in length and has an outer diameter of 5 mm at the distal end. The effective working distance is between 15 and 50 mm. The endoscopic images captured at the distal end of the scope by a charge-coupled device camera are transmitted to the computer, and the graphic data are then processed for 3-D imaging. The images are immediately sent to the display unit.
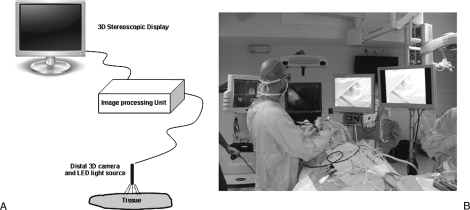
The stereoscopic technology is based on the ability to acquire and present different images to the left and right eyes simultaneously. The sensor at the tip of the 3-D endoscope is divided into hundreds of thousands of micron-sized optical elements, which create a digital map of the surgical field. A computer program is used to reconstruct natural stereovision from the raw data. The system requires the use of passive (polarizing) glasses, which, in combination with a 3-D screen, emulates the normal visual process of stereopsis, or spatial perception. Figure 1 shows a depiction of the imaging system and a picture of the operating theater.
Figure 1.
The 3-D imaging system used for endoscopic skull base operations. (A) Depiction of the 3-D system. The camera includes a miniature 3-D sensor that digitally maps the surgical field. An LED light source is mounted on the camera system to illuminate the surgical field. The signals are digitally sent to the central image processing unit. The camera control unit consists of a single PC with dedicated hardware and software. The computer is connected to a stereoscopic display. The image is acquired with polarizing glasses. (B) A picture of the operating theater. Two high-definition 2-D monitors are used (on the right), next to a 3-D monitor (middle) and navigation system (far left side). The surgeon uses specialized polarizing microscopes to gain stereoscopic view of the surgical field.
Data Collection and Analysis
The data were collected prospectively by a researcher who did not carry out any of the operations included in the study (O.W.). After each surgery, the operating surgeons from the head and neck team were asked to score their ability to identify and recognize each one of the following anatomic structures: middle turbinate, maxillary sinus, lamina papyracea, cribriform plate, ethmoidal sinuses, sphenoid sinus, clivus, fovea ethmoidalis, planum sphenoidale, sella turcica, carotid prominence, and optic nerve prominence. The score was composed of the surgeon difficulty level to recognize each landmark. Anatomic verifications were performed with the assistance of the navigation system. Scores were: (1) 3-D is inferior to the two-dimensional (2-D) system; (2) 3-D equals the 2-D system; and (3), 3-D is superior to the 2-D system. All scores were undertaken after a consensus between the surgeons had been achieved. For statistical comparison, Student t test was used. Significance was set at p < 0.01.
RESULTS
Thirty-six patients underwent endoscopic skull base surgery via a 3-D endoscopic system that was used in our department during the study period. Table 1 provides a summary of patients' demographic and clinical data, and Table 2 lists the underlying histology of their lesions. Fifteen of these patients had malignant tumors (42%) and 19 had benign lesions (53%). The remaining three patients underwent skull base reconstruction for CSF leak repair (8.3%). The initial Karnofsky score was 100 for each patient. Thirteen patients (36%) underwent at least one previous operation, and five patients (14%) underwent perioperative radiation therapy (three received preoperative and two received postoperative external beam radiation). Complete gross tumor resection was achieved in 31/33 patients (94%). One additional patient had an olfactory groove meningioma that involved the cavernous sinus and optic nerves, and 90% of the tumor was removed. The remaining patient had a clival chordoma involving both hypoglossal canals, and 95% of the tumor was removed. Tumor resection was associated with a high-flow intraoperative CSF leak in 16 cases (44%). Figures 2 and 3 show representative cases before and after tumor resection.
Table 1.
Patients' Demographics and Clinical Characterization
| Variable | n (%) |
|---|---|
| EEA, expanded endoscopic approach; FC, frontal craniotomy; SD, standard deviation. | |
| Number of patients | 36 |
| Age (y) | |
| Mean ± SD | 47.8 ± 19 |
| Median | 51 |
| Days in hospital | |
| Mean ± SD | 7.1 ± 1.8 |
| Sex | |
| Female | 12 (33) |
| Male | 24 (67) |
| Comorbidity | |
| No | 23 (64) |
| Yes | 13 (36) |
| Previous surgery | |
| No | 23 (64) |
| Yes | 13 (36) |
| Previous radiotherapy | |
| No | 33 (92) |
| Yes | 3 (8) |
| Dural involvement | |
| No | 25 (69) |
| Yes | 11 (31) |
| Brain involvement | |
| None | 31 (86) |
| Yes | 5 (14) |
| Surgical approach | |
| EEA alone | 29 (83) |
| EEA and FC | 7 (17) |
Table 2.
Histology
| Pathology | n (%) |
|---|---|
| ACC, adenoid cystic carcinoma; JNA, juvenile nasopharyngeal angiofibroma; MPNST, malignant peripheral nerve sheath tumor; SNUC, sinonasal undifferentiated carcinoma. | |
| Inverted papilloma | 13 (36) |
| Chordoma | 6 (17) |
| CSF leak | 3 (8) |
| Melanoma | 2 (5.5) |
| Encephalocele | 2 (5.5) |
| Ewing's sarcoma | 1 (2.8) |
| Craniopharyngioma | 1 (2.8) |
| Adenocarcinoma | 1 (2.8) |
| Esthesioneuroblastoma | 1 (2.8) |
| MPNST | 1 (2.8) |
| SNUC | 1 (2.8) |
| ACC | 1 (2.8) |
| Osteoma | 1 (2.8) |
| Meningioma | 1 (2.8) |
| JNA | 1 (2.8) |
| Total | 36 (100) |
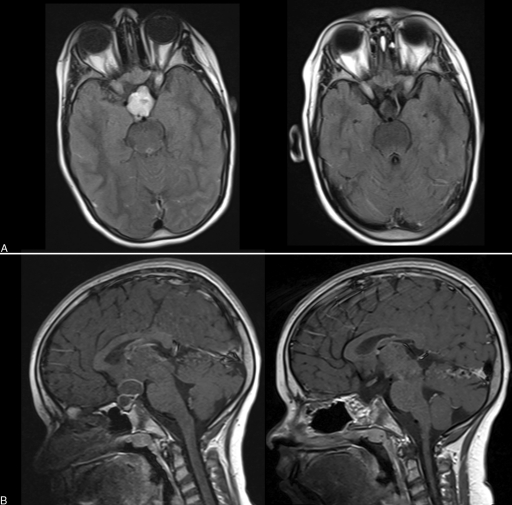
Figure 2.
Magnetic resonance imaging (MRI) of a 12-year old girl with craniopharyngioma. (A) Preoperative (left) and postoperative (right) axial plane, T1 MRI with gadolinium. (B) Sagittal plane MRI showing the tumor before (left) and after (right) the operation.
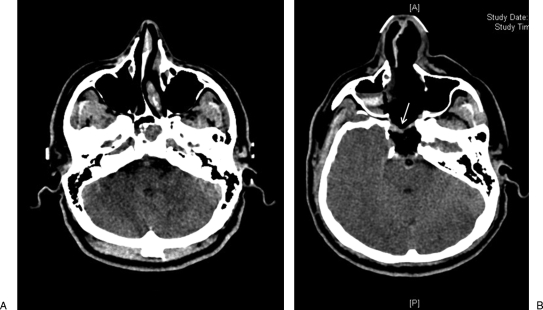
Figure 3.
Computed tomography images of a 35-year-old man with chordoma invading the dura. (A) Preoperative scan showing the tumor infiltrating the clivus. (B) Postoperative image. Reconstruction was performed with fascia lata inlay flap, fat, and nasal septal rotational flap. The arrow indicates the nasal flap rotated over the fat.
Five patients (14%) had postoperative complications (Table 3). There was one case of gram-negative meningitis (2.8%), one case of epiphora (2.8%), and no cases of pneumocephalus or postoperative CSF leak.
Table 3.
Incidence of Postoperative Complications
| Complication | n (%) |
|---|---|
| Meningitis | 1 (2.8) |
| Wound infection | 2 (5.6) |
| Epistaxis | 1 (2.8) |
| Epiphora | 1 (2.8) |
| Total | 5 (13.9) |
Next, we evaluated the 3-D endoscopic system in comparison with the conventional 2-D technique. The surgeons were asked (1) to rate their ability to identify specific anatomic structures (see Materials and Methods) and (2) to assess adverse effects of the 3-D system on the operating team. The 3-D technique was superior to the conventional technique for identification of the sellar region, carotid prominence, optic prominence, cribriform plate, fovea ethmoidalis, planum, sphenoid, and sella (n = 14 cases, p < 0.001). Both methods were equal for detection of the middle turbinate, clivus, maxillary sinus, ethmoidal sinuses, and frontal sinuses. There were no complaints of visual strains or headaches, but there was some facial discomfort that was mostly related to wearing of the glasses required for the 3-D system.
DISCUSSION
Advancements in imaging modalities and refinements in surgical and reconstructive techniques have allowed an increasing number of children and adults with skull base neoplasms to undergo curative surgical resections.7,8,9 The endoscopic approach provides an excellent access to the paranasal sinuses and skull base and, therefore, has become a popular alternative to open procedures for the treatment of benign tumors and selective malignant neoplasms. Compensation for the loss of stereoscopic vision in 2-D endoscopy is achieved by sensorial and tactile information that the surgeon receives during the manipulation of instruments and changes in 2-D visual input.
There has been significant progress in the development of new endoscopic imaging modalities, including that of distal chip cameras, 3-D endoscopes, and robotic surgery.10,11 We considered that perception of depth and space using 3-D visualization may potentially improve the surgeon's ability to perform complex tasks with minimally invasive skull base surgery. The aim of the current study was to assess the utility of a 3-D endoscopic system in a single medical center over a 1-year period. The 3-D technique allowed accurate identification of the sellar region, carotid prominence, optic prominence, cribriform plate, and fovea ethmoidalis. However, due to its narrow viewing angle, surgery in the nasal cavity, maxillary sinus, and frontal sinus was difficult compared with conventional endoscopy. Other limitations of the 3-D endoscopic system may be inferior sharpness and contrast compared with new high-definition 2-D systems. This may stem from the distal chip camera, the light-emitting device system, or the optical glasses, which cause a loss of photons transmitted to the retina. Finally, the short range of focus and the restricted viewing angle require improvement. Our surgeons did not report experiencing any adverse effects during or after surgery.
The value of 2-D and 3-D visual systems has been tested mainly for minimally invasive general surgery.12,13,14,15,16,17 The system used in the current study was described previously18 and compared with the conventional system in artificial models.19 In addition, Tabaee et al recently demonstrated the utility of 3-D endoscopy in pituitary surgery.20 The current study is the first to evaluate the utility of the 3-D technology for skull base surgery.
Few studies have specifically examined the advantage of 3-D systems over conventional endoscopy, and most of the studies involved laparoscopic procedures. Performance studies and skill tests in laparoscopic simulators revealed significantly faster performances, shorter execution time, and increased accuracy, all leading to more rapidly executed and safer endoscopic surgery under 3-D conditions.14,15,17 Fraser et al evaluated the utility of 3-D and 2-D endoscopes by using a task-based simulator paradigm.19 They reported a significantly higher level of efficiency using the 3-D system than conventional endoscopes. Others, however, reported no significant advantage of 3-D laparoscopic systems.16,21,22
We are mindful of several limitations related to the design of our study. The main one is the lack of objective measures to compare the 2-D and 3-D systems. Second, the data were collected and reported during a learning period of the prototype 3-D system.
In conclusion, the 3-D imaging system offers stereoscopic viewing while using endoscopes for surgical resections of skull base tumors. Surgery using the 3-D technique is safe and allows accurate perception of depth. It has been established that a well-trained and experienced surgeon is able to cope with the limitations of 2-D visualization. However, it is not known whether 3-D endoscopic imaging systems will have an impact on surgical efficacy, complication rates, or the learning curve of untrained surgeons. Future studies should integrate performance parameters for conclusively establishing an advantage of the 3-D system on conventional methods.
ACKNOWLEDGMENTS
We thank Esther Eshkol for editorial assistance. This work was partially supported by grants from the Israeli Cancer Association, The US-Israel Binational Science Foundation, and the Israeli Science Foundation (Z.G.).
REFERENCES
- Snyderman C H, Carrau R L, Kassam A B, et al. Endoscopic skull base surgery: principles of endonasal oncological surgery. J Surg Oncol. 2008;97:658–664. doi: 10.1002/jso.21020. [DOI] [PubMed] [Google Scholar]
- Hadad G, Bassagasteguy L, Carrau R L, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- Birkett D H, Josephs L G, Este-McDonald J. A new 3-D laparoscope in gastrointestinal surgery. Surg Endosc. 1994;8:1448–1451. doi: 10.1007/BF00187357. [DOI] [PubMed] [Google Scholar]
- Becker H, Melzer A, Schurr M O, Buess G. 3-D video techniques in endoscopic surgery. Endosc Surg Allied Technol. 1993;1:40–46. [PubMed] [Google Scholar]
- Gil Z, Patel S G, Bilsky M, Shah J P, Kraus D H. Complications after craniofacial resection for malignant tumors: are complication trends changing? Otolaryngol Head Neck Surg. 2009;140:218–223. doi: 10.1016/j.otohns.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Tabaee A, Anand V K, Brown S M, Lin J W, Schwartz T H. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope. 2007;117:1133–1137. doi: 10.1097/MLG.0b013e31805c08c5. [DOI] [PubMed] [Google Scholar]
- Gil Z, Patel S G, Singh B, et al. International Collaborative Study Group Analysis of prognostic factors in 146 patients with anterior skull base sarcoma: an international collaborative study. Cancer. 2007;110:1033–1041. doi: 10.1002/cncr.22882. [DOI] [PubMed] [Google Scholar]
- Gil Z, Patel S G, Cantu G, et al. International Collaborative Study Group Outcome of craniofacial surgery in children and adolescents with malignant tumors involving the skull base: an international collaborative study. Head Neck. 2009;31:308–317. doi: 10.1002/hed.20958. [DOI] [PubMed] [Google Scholar]
- Fliss D M, Abergel A, Cavel O, Margalit N, Gil Z. Combined subcranial approaches for excision of complex anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2007;133:888–896. doi: 10.1001/archotol.133.9.888. [DOI] [PubMed] [Google Scholar]
- Mehta R P, Cueva R A, Brown J D, et al. What's new in skull base medicine and surgery? Skull Base Committee Report. Otolaryngol Head Neck Surg. 2006;135:620–630. doi: 10.1016/j.otohns.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Hanna E Y, Holsinger C, DeMonte F, Kupferman M. Robotic endoscopic surgery of the skull base: a novel surgical approach. Arch Otolaryngol Head Neck Surg. 2007;133:1209–1214. doi: 10.1001/archotol.133.12.1209. [DOI] [PubMed] [Google Scholar]
- Peitgen K, Walz M V, Walz M V, Holtmann G, Eigler F W. A prospective randomized experimental evaluation of three-dimensional imaging in laparoscopy. Gastrointest Endosc. 1996;44:262–267. doi: 10.1016/s0016-5107(96)70162-1. [DOI] [PubMed] [Google Scholar]
- Chan A C, Chung S C, Yim A P, Lau J Y, Ng E K, Li A K. Comparison of two-dimensional vs three-dimensional camera systems in laparoscopic surgery. Surg Endosc. 1997;11:438–440. doi: 10.1007/s004649900385. [DOI] [PubMed] [Google Scholar]
- Crosthwaite G, Chung T, Dunkley P, Shimi S, Cuschieri A. Comparison of direct vision and electronic two- and three-dimensional display systems on surgical task efficiency in endoscopic surgery. Br J Surg. 1995;82:849–851. doi: 10.1002/bjs.1800820640. [DOI] [PubMed] [Google Scholar]
- Durrani A F, Preminger G M. Three-dimensional video imaging for endoscopic surgery. Comput Biol Med. 1995;25:237–247. doi: 10.1016/0010-4825(95)00001-k. [DOI] [PubMed] [Google Scholar]
- Jones D B, Brewer J D, Soper N J. The influence of three-dimensional video systems on laparoscopic task performance. Surg Laparosc Endosc. 1996;6:191–197. [PubMed] [Google Scholar]
- Pietrabissa A, Scarcello E, Carobbi A, Mosca F. Three-dimensional versus two-dimensional video system for the trained endoscopic surgeon and the beginner. Endosc Surg Allied Technol. 1994;2:315–317. [PubMed] [Google Scholar]
- Brown S M, Tabaee A, Singh A, Schwartz T H, Anand V K. Three-dimensional endoscopic sinus surgery: feasibility and technical aspects. Otolaryngol Head Neck Surg. 2008;138:400–402. doi: 10.1016/j.otohns.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Fraser J F, Allen B, Anand V K, Schwartz T H. Three-dimensional neurostereoendoscopy: subjective and objective comparison to 2D. Minim Invasive Neurosurg. 2009;52:25–31. doi: 10.1055/s-0028-1104567. [DOI] [PubMed] [Google Scholar]
- Tabaee A, Anand V K, Fraser J F, Brown S M, Singh A, Schwartz T H. Three-dimensional endoscopic pituitary surgery. Neurosurgery. 2009;64(5 Suppl 2):288–293. discussion 294–295. doi: 10.1227/01.NEU.0000338069.51023.3C. [DOI] [PubMed] [Google Scholar]
- Bergen P van, Kunert W, Buess G F. The effect of high-definition imaging on surgical task efficiency in minimally invasive surgery: an experimental comparison between three-dimensional imaging and direct vision through a stereoscopic TEM rectoscope. Surg Endosc. 2000;14:71–74. doi: 10.1007/s004649900015. [DOI] [PubMed] [Google Scholar]
- Hanna G B, Shimi S M, Cuschieri A. Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet. 1998;351:248–251. doi: 10.1016/S0140-6736(97)08005-7. [DOI] [PubMed] [Google Scholar]