ABSTRACT
Cerebellopontine angle lipomas are rare and attempts at surgical excision are associated with significant morbidity. Lipomatosis of nerve, the fatty infiltration of nerves, is a distinct entity. We present a case of intractible trigeminal neuralgia caused by lipomatosis of the trigeminal nerve. Clinical case: A 25-year-old male presented with severe right-sided trigeminal neuralgia. Imaging showed a lesion involving the trigeminal nerve with signal characteristics of fat. At surgery the lesion was found to be a fatty infiltration of the nerve itself. Surgery was therefore limited to arachnoid adhesiolysis. The patient remains symptom-free and neurologically intact to date. Correctly identifying these lesions as lipomatosis of nerve rather than lipoma of the cerebellopontine angle make it clear that even partial surgical excision will inevitably result in neurological deficit and should not be attempted. However, in the case of intractable trigeminal neuralgia we demonstrate that surgery can still play a role.
Keywords: Lipomatosis, trigeminal nerve, neuralgia, cerebellopontine angle, lipoma
Lipomatosis of nerve is a rare condition in which the nerve is infiltrated by mature adipocytes and fibrous tissue. It is classified under mesenchymal tumors (ICD-0 code 8850/0; WHO Classification of Tumors of the Central Nervous System) but comprehensive literature searching is confounded by multiple synonyms including hamartomatous lipomatosis, fibrolipomatous hamartoma of nerve, and neural fibrolipoma. One of the principal changes in the 2002 WHO Classification of Soft Tissue Tumors was the renaming of fibrolipomatous hamartoma of nerve (1994 WHO Classification) as lipomatosis of nerve.1,2 Essential to all descriptions is an infiltration of the epineurium by adipose and fibrous tissue in contrast to lipomas which are less likely to infiltrate nerves, although they can cause extrinsic compression.1
Lipomatosis of nerve most commonly affects peripheral nerves of the distal forearm and can present with compression symptoms. The lesions are benign but surgery for example, carpal tunnel decompression may be necessary for symptom relief. Lipomatosis of these peripheral nerves may be associated with metaplastic bone growth and is associated with macrodactyly in approximately one third of patients.1 There is only one report of lipomatosis affecting a cranial nerve (CN)3 and no previous reports have been found describing lipomatosis of the trigeminal nerve. There are several reports of cerebellopontine angle (CPA) lipomas.4
We present a case of intractable trigeminal neuralgia (TN) secondary to lipomatosis at this previously unreported site. Surgical lysis of arachnoid adhesions provided full symptomatic relief with no neurological deficit postoperatively. This case illustrates that surgery is a viable option in the treatment of symptomatic trigeminal nerve lipomatosis. Implications of the careful classification of this lesion are discussed.
CLINICAL CASE
Clinical Features
A 25-year-old male presented with a 1-year history of paroxysmal, lancinating right-sided facial pain, which had worsened in frequency and intensity over the previous month. The pain was exacerbated by light touch and talking and was confined to the territory of the second division of the trigeminal nerve (V2). The patient had no past medical history of note and no significant family history. There was no neurological deficit on examination.
Radiological Features
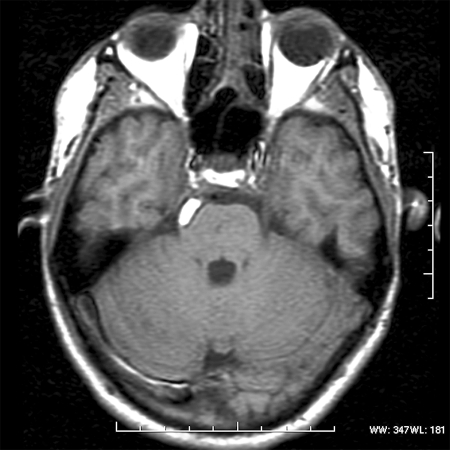
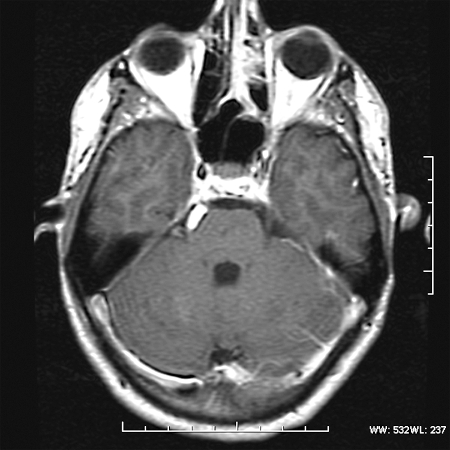
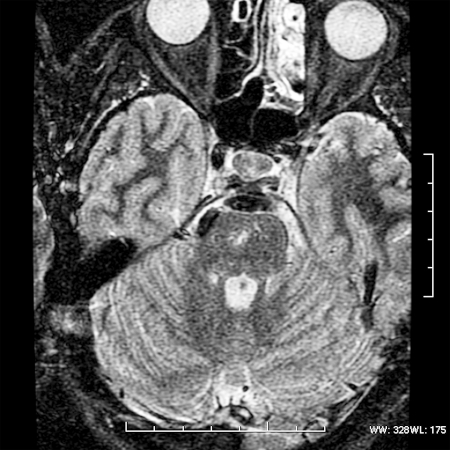
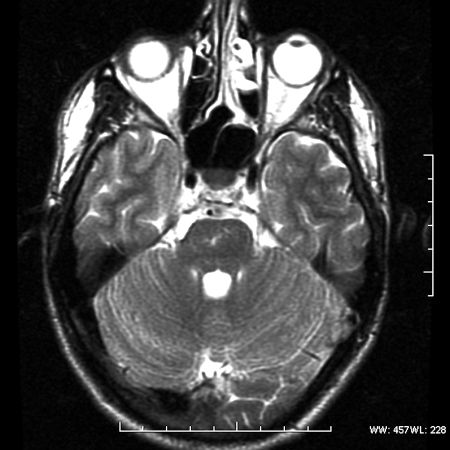
Magnetic resonance imaging (MRI) revealed a lesion expanding the right trigeminal nerve with the signal characteristics of fat. The lesion was hyperintense on T1- (Fig. 1), hypointense on T2-weighted images, and did not enhance after administration of gadolinium (Fig. 2). The lesion demonstrated characteristic signal “drop-out” on fat suppression sequences (Fig. 3) and chemical shift artifact on T2 images, confirming the fatty nature of the lesion (Fig. 4).
Figure 1.
Axial T1 MRI without gadolinium showing a hyperintense focal lesion in the right lateral pontine cistern.
Figure 2.
Axial T1 postgadolinium. No change or enhancement after contrast.
Figure 3.
Axial short tau inversion recovery MRI showing the fatty component of the lesion.
Figure 4.
Axial Fast spin echo T2 MRI. Note the hypointense band surrounding the medial portion of the lesion to the right of the pons attributed to the chemical shift artifact characteristic of fatty lesions.
Clinical Course
The initial management plan was pharmacological symptom-control and imaging follow-up. The patient was started on carbamazepine (CBZ) orally 200 mg twice daily, which was increased to 1400 mg/d to achieve symptom control. Attempts to wean the medication after episodes of symptom control were initially successful but the respite was only ever temporary and a gradual stepwise increase of CBZ was required reaching a maximum of 2000 mg/d 18 months after presentation. Due to side effects at this dose the CBZ was reduced to 1800 mg/d and gabapentin was added-in and titrated up to 900 mg/d, initially with good effect. However, the pain returned and the CBZ was increased again to 2000 mg/d and gabapentin to 2600 mg/d. The gabapentin was changed to pregabalin 300 mg/d due to side effects and amitriptyline was added at 12.5 mg/d. Almost 2 years after presentation (February 2008) the patient was admitted with a severe trigeminal crisis. He was loaded with phenytoin (18 mg/kg) but even this gave only partial relief of symptoms and he was referred for consideration of surgery in March 2008.
Surgery
A right-sided suboccipital craniectomy was performed with the patient in a park-bench position. The dura mater was opened, cerebrospinal fluid was released from the cerebellopontine cistern and the flocculus was retracted medially. Cranial nerves V to XI were identified. The inferomedial portion of the trigeminal nerve was noted to be enlarged and to contain a yellowish, soft, fatty material. Arachnoid adhesions were freed along the length of the enlarged trigeminal nerve from Meckel's cave to the trigeminal root entry zone, sufficient only to leave the nerve free from compression. Resection was not attempted as damage to the trigeminal nerve would have been inevitable. The surgical site was washed with warm saline and the craniectomy closed with autologous bone graft and fibrin glue.
Outcome
The patient awoke from surgery pain free and with no neurological deficit. Postoperative recovery was uneventful. The neuropathic pain medication was gradually weaned from the first postoperative day.
At the latest follow-up (6 months postsurgery) the patient remained completely pain free with no neurological deficit. He was not taking any analgesia.
DISCUSSION
We present a case of trigeminal nerve lipomatosis presenting with intractable TN treated effectively and safely with arachnoid adhesiolysis. We propose that distinguishing between a lipoma of the CPA and lipomatosis of the CN of the CP angle has clinical significance.
Intracranial lipomas are rare with a prevalence at autopsy ranging from 0.08 to 0.3%5 in general autopsies and rising to 0.2–0.5%5 in neuropathological studies. These prevalence data are supported by computed tomography6 and MRI7 studies. The majority (81%) of intracranial lipomas are supratentorial, 82% are in the midline, 47% near the corpus callosum and only 12% in the CPA.8 Supratentorial lipomas are frequently associated with other congenital malformations but in themselves are usually asymptomatic. It is recognized, however, that CP angle lipomas are usually isolated abnormalities9 and are often symptomatic, presenting with slowly progressive CN deficits.10 There are ∼100 case reports in the literature9 of lipomas of the CPA, comprising an estimated 0.14% of all CPA tumors9 and 0.05% of operated ones.11
The majority of cases reported as CPA lipomas in the literature present with progressive sensorineural hearing loss.4,9 Only 12 cases have been reported to present with TN5,12,13,14,15,16,17,18,19,20,21,22 (see Table 1).
Table 1.
Case Reports of Trigeminal Neuralgia Caused by Cerebellopontine Angle Lipomas
| Reference | Age (y) | M/F | Symptoms | Duration | L/R | Management | Findings | Complete/Incomplete Resection | Postoperatively |
|---|---|---|---|---|---|---|---|---|---|
| Note the intimate relationship between cranial nerves (CN) and the lipoma. Postoperative CN deficits are common and surgical approaches favor incomplete resection. AICA, anterior inferior cerebellar artery; CBZ, carbamazepine; NR, not reported; TN, trigeminal neuralgia. | |||||||||
| Budka et al5 | 26 | F | L TN, Vertigo R TN, R hearing loss | 2 | R | Surgery | Pea sized lipoma Lipomatous infiltration of acoustic nerve | Incomplete | TN relief No additional deficit |
| Graves and Schemm12 | 26 | M | TN, dizziness, tinnitus Mild hearing loss | 5 | L | Surgery | CNVII, VIII, IX, X involved. CN V compressed. Adherent to brainstem. Rhizotomy | Incomplete. Nerves embedded & vascular | TN relief Complete hearing loss |
| Rosenbloom et al13 | 28 | M | TN V2 Hypoaesthesia V1V2 Nausea dizziness Headaches L ptosis | 1 y | L | Surgery | Yellow VII VIII IX incorporated V1,V2 adjacent | Incomplete Adherent to CN, vessels, brainstem | Headache & facial pain alleviated but new hearing loss & persistent dizziness |
| Delgado Mije et al14 | 35 | F | TN V3 | NR | R | Surgery | Yellow. Adherent to brainstem, enveloping V | Incomplete CN involved | TN relief |
| Aihara et al15 | 47 | F | TN VII dysfunction | 1 y | R | Surgery | CNVII, VIII encompassed. Attached to CNV | Incomplete | NR |
| Kato et al16 | 13 | F | TN V3 | 6 m | R | Surgery | Yellow Encasing VII, VIII, AICA. V infiltrated Rhizotomy | Incomplete | TN relief. V2 V3 deficits. Transient tinnitus, hearing loss, nystagmus (3 mo) |
| Behar et al17 | 23 | M | TN V3 L ptosis | 6 m | R | CBZ Surgery | CN VII, VIII, IX, X course through mass | Incomplete (CN) | Hearing loss |
| Celik et al18 | 32 | M | TN | 8 | L | Surgery | Yellow Encasing VII, VIII Displacing V | Incomplete Trauma to VII &VIII | TN relief Facial n. palsy, hearing loss |
| Raieli et al20 | 8 | M | TN V3 Facial spasm | 2 y | R | CBZ Imaging | NR | NR | NR |
| Alafaci et al19 | 16 | F | TN V2 V3 Vertigo Hearing loss | 2 y | R | Surgery | Yellow Incorporating VII VIII AICA displaced causing compression Histo: nerve fibers traversing adipose tissue | Incomplete CN involved | TN relief Hearing loss same Temporary facial n. palsy |
| Schlierter et al21 | 24 | M | TN | NR | L | Medical | NR | NR | NR |
| Marton et al22 | 46 | M | Hearing loss (12 y) TN (10 y) severe for year | 1 y | L | Surgery | Between V and VII/VIII complex | Incomplete Arachnoid debridement only | TN relief Hearing deficit same |
CPA lipomas are often found to be intimately related to blood vessels and CN and adipocytes have been shown to infiltrate and separate nerve fibers in several cases.4,5,12,13,14,15,16,17,18,19 This intimate relationship prevents excision of the lesion without incurring significant CN deficits.
In the current case there was no extrinsic lipomatous mass but rather a fatty expansion of the trigeminal nerve itself. This is consistent with “lipomatosis of nerve” which is an infiltration of the epineurium by adipose and fibrous tissue resulting in a fusiform enlargement of the nerve with concentric perineural fibrosis.1
Lipomatosis of nerve is rare and has not been previously reported as affecting the trigeminal nerve. However, Kato and colleagues16 report a patient with TN who underwent surgery to excise a CP angle lipoma enveloping CN VII and VIII and was found, at operation, to have a swollen trigeminal nerve with subpial fatty infiltration of the trigeminal rootlets for which they performed a rhizotomy. Similarly, in one of the earliest reviews of CPA lipomas Budka5 clearly described fatty infiltration of CN VIII and IV, confirmed histologically.
Etiology and Pathogenesis
Lipomata and lipomatosis are both benign tumors of mesenchymal origin (WHO Classification ICD 8850/0). One proposed mechanism of pathogensis for CPA lipomas relates to the embryological origin of adipocytes.23 Adipocytes are mesenchymal cells derived from mesoderm or ectomesenchyme, which is in turn derived from the neural crest. The meninx primitiva, which subsequently forms the pia and arachnoid mater, is derived from the same cell population. It has been proposed that CPA lipomas are formed from remnants of meninx primitiva trapped at the pontomedullary sulcus at the time of neural tube folding.23 The adipocytes are then thought to extend in the subarachnoid space along the Virchow-Robin spaces adjacent to the pia. Adipocytes are indeed found in direct connection with the leptomeninges.5 This theory has been used to explain the intimate relationship of CPA lipomas to CN and blood vessels, which also track through these spaces. However, it does not explain the lipomatous infiltration of these nerves. The etiology of lipomatosis of nerve is unknown.1 It is possible that these trapped adipocytes surround and infiltrate the CN, though an alternative mechanism may involve the proliferation and expansion of the adipocytes found normally within nerves.24
Histopathological Features
Histopathological features of lipomatosis of nerve include infiltration of epineural and perineural compartments by adipocytes and fibrous tissue resulting in fusiform enlargement of the nerve with concentric perineural fibrosis. Immunostaining is positive for S100 but is otherwise not informative. The nerves may appear macroscopically enlarged. The adipose tissue may be found surrounding as well as infiltrating the nerve.1
Imaging
MRI of peripheral nerve lipomatosis shows thickened nerve fascicles (low intensity on T1-weighted sequences) surrounded by tissue with characteristics of fat (high signal on T1-weighted and low signal on T2-weighted sequences) with a coaxial-cable-like appearance on cross section and spaghetti-like appearance on longitudinal planes of the nerves.25 The authors suggest these findings are pathognomomic.
Although these imaging characteristics are difficult to identify at the skull base several studies of CPA lipoma imaging describe nerve complexes within fatty tissue.11,23,26,27 As with lipomas at other sites, lipomatous lesions at the CPA have been shown to be high signal on T1 with variable/low signal on T2 and signal drop-out with fat-suppression sequences.18,23,28,29 In an MR study of 44 intracranial lipomas chemical shift artifact was appreciated in all those greater than 1 cm diameter (69%).23 Chemical shift artifact can be used to confirm the presence of fat in a lesion and disappears with fat suppression. The differential diagnosis of CPA lesions with some of these features includes epidermoids, lipomatous meningiomas, and lipomatous degeneration of schwannomas but the diagnosis of lipoma or lipomatosis of nerve can usually be made confidently with imaging alone.
Management
Surgery for CPA lipomas has poor results with studies showing improvement without additional neurological deficits in only 18 to 19%4 of patients and new neurological deficits in 684 to 72%.9 In one review4 all but two patients who underwent “complete resection” had postoperative neurological deficits. This can be explained by the intimate relationship of the lesion to blood vessels and CN within the cerebellopontine cistern.
As current imaging modalities allow for confident diagnosis of CPA lipomas29 surgery is no longer indicated for diagnosis. There are no reports of malignant transformation of intracranial lipomas (although there are two reports of clinically insignificant growth4,30) so neither is surgery indicated to prevent neoplastic progression. This, combined with the high risk of postoperative neurological deficits has led most authors to advise conservative management (imaging surveillance and neurological examination) for asymptomatic cases.
However, management of symptomatic cases remains controversial. Of the 12 cases in the literature of TN associated with CPA lipomas (see Table 1) surgery was performed in 10 cases (2 medical management20,21) all using a suboccipital retromastoid approach. Surgical resection was limited in most cases due to the incorporation of CN within the mass.12,13,14,15,17,19 Of those who underwent surgery all patients had complete relief of TN but at the expense of other CN deficits, notably hearing, in six cases (see Table 1).
Some authors advocate rhizotomy,12,16 nerve decompression22 or, as in the current case, arachnoid debridement22 without lipoma resection to achieve symptom relief while avoiding additional neurological deficits. Alafaci et al19 described the lipoma causing neurovascular compression via displacement of the anterior inferior cerebellar artery19 and advise neurovascular decompression alone.
The presence of CN within the lipomatous mass is the reason that surgical resection of CPA lipomas is often incomplete11 and is associated with a high incidence of additional CN deficits postoperatively.9,10 In the case of an extrinsic mass with CN coursing through it (a classic CPA lipoma) partial excision can decompress the nerve and provide symptom relief. The surgical approach is different in true lipomatosis of nerve where the nerve is expanded by adipocytes—in such cases any attempt at surgical excision would inevitably lead to nerve damage and likely neurological deficit. However, as demonstrated in the current case, adhesiolysis can provide symptomatic relief.
CONCLUSION
We report a case of TN caused by trigeminal nerve lipomatosis. Our case provides evidence that arachnoid adhesiolysis can achieve effective, safe symptom relief.
We encourage the distinction to be made between lipomatosis of nerve (nerves filled with adipocytes) and CPA lipomas (nerves embedded in adipocytes). Once defined in this way it is clear that to attempt surgical excision of a lipomatous nerve would inevitably result in neurological deficits, whereas some true CPA lipomas may be amenable to cautious partial resection. We aim to illustrate that safe surgical treatment is possible and remains appropriate in patients with intractable symptoms. Patients should be warned of the high risk of postoperative CN deficits.
ACKNOWLEDGMENTS
ECM has received funding from The Association of Surgeons in Training (UK), Covidien and the Ethel Househam Fellowship in Neurosurgery. No author has any financial, personal or professional conflicts of interest in producing this work.
REFERENCES
- Nielsen G P. In: Fletcher CDM, Unni KK, Mertens F, editor. Pathology and Genetics of Tumors of Soft Tissue and Bone (The International Agency for Research on Cancer/WHO Classification of Tumours) Geneva: The World Health Organisation; 2002. Adipocytic tumours. pp. 24–25.
- Beck D W, Menezes A H. Lesions in Meckel's cave: variable presentation and pathology. J Neurosurg. 1987;67:684–689. doi: 10.3171/jns.1987.67.5.0684. [DOI] [PubMed] [Google Scholar]
- Berti E, Roncaroli F. Fibrolipomatous hamartoma of a cranial nerve. Histopathology. 1994;24:391–392. doi: 10.1111/j.1365-2559.1994.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Bigelow D C, Eisen M D, Smith P G, et al. Lipomas of the internal auditory canal and cerebellopontine angle. Laryngoscope. 1998;108:1459–1469. doi: 10.1097/00005537-199810000-00008. [DOI] [PubMed] [Google Scholar]
- Budka H. Intracranial lipomatous hamartomas (intracranial “lipomas”). A study of 13 cases including combinations with medulloblastoma, colloid and epidermoid cysts, angiomatosis and other malformations. Acta Neuropathol. 1974;28:205–222. doi: 10.1007/BF00719025. [DOI] [PubMed] [Google Scholar]
- Faerber E N, Wolpert S M. The value of computed tomography in the diagnosis of intracranial lipomata. J Comput Assist Tomogr. 1978;2:297–299. doi: 10.1097/00004728-197807000-00010. [DOI] [PubMed] [Google Scholar]
- Kemmling A, Noelte I, Gerigk L, Singer S, Groden C, Scharf J. A diagnostic pitfall for intracranial aneurysms in time-of-flight MR angiography: small intracranial lipomas. AJR Am J Roentgenol. 2008;190:W62–67. doi: 10.2214/AJR.07.2517. [DOI] [PubMed] [Google Scholar]
- Donati F, Vassella F, Kaiser G, Blumberg A. Intracranial lipomas. Neuropediatrics. 1992;23:32–38. doi: 10.1055/s-2008-1071309. [DOI] [PubMed] [Google Scholar]
- Tankéré F, Vitte E, Martin-Duverneuil N, Soudant J. Cerebellopontine angle lipomas: report of four cases and review of the literature. Neurosurgery. 2002;50:626–631, discussion 631–632. [PubMed] [Google Scholar]
- Zimmermann M, Kellermann S, Gerlach R, Seifert V. Cerebellopontine angle lipoma: case report and review of the literature. Acta Neurochir (Wien) 1999;141:1347–1351. doi: 10.1007/s007010050440. [DOI] [PubMed] [Google Scholar]
- Schuhmann M U, Lüdemann W O, Schreiber H, Samii M. Cerebellopontine angle lipoma: a rare differential diagnosis. Skull Base Surg. 1997;7:199–205. doi: 10.1055/s-2008-1058596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves V B, Schemm G W. Clinical characteristics and CT findings in lipoma of the cerebellopontine angle. Case report. J Neurosurg. 1982;57:839–841. doi: 10.3171/jns.1982.57.6.0839. [DOI] [PubMed] [Google Scholar]
- Rosenbloom S B, Carson B S, Wang H, Rosenbaum A E, Udvarhelyi G B. Cerebellopontine angle lipoma. Surg Neurol. 1985;23:134–138. doi: 10.1016/0090-3019(85)90330-1. [DOI] [PubMed] [Google Scholar]
- Delgado Mije D, Moro Sánchez R M, Escribano Fernández M, Azuara Muslera M V. Neuralgia of the trigeminal secondary to lipoma of the pontocerebellar angle. Med Clin (Barc) 1992;99:556. [PubMed] [Google Scholar]
- Aihara N, Nagai H, Kamiya K, Matsumoto T, Yamashita N. Cerebellopontine angle lipoma—case report. No To Shinkei. 1993;45:559–562. [PubMed] [Google Scholar]
- Kato T, Sawamura Y, Abe H. Trigeminal neuralgia caused by a cerebellopontine-angle lipoma: case report. Surg Neurol. 1995;44:33–35. doi: 10.1016/0090-3019(95)00056-9. [DOI] [PubMed] [Google Scholar]
- Behar P M, Dolan R, Dastur K, Marrangoni A G, Nayak N. Fibrovascular lipoma of the cerebellopontine angle mimicking trigeminal neuralgia. Ear Nose Throat J. 1998;77:58–60. [PubMed] [Google Scholar]
- Celik S E, Kocaeli H, Cordan T, Bekar A. Trigeminal neuralgia due to cerebellopontine angle lipoma. Case illustration. J Neurosurg. 2000;92:889. doi: 10.3171/jns.2000.92.5.0889. [DOI] [PubMed] [Google Scholar]
- Alafaci C, Salpietro F M, Puglisi E, et al. Trigeminal pain caused by a cerebellopontine-angle lipoma. Case report and review of the literature. J Neurosurg Sci. 2001;45:110–113. [PubMed] [Google Scholar]
- Raieli V, Eliseo G, Manfrè L, Pandolfi E, Romano M, Eliseo M. Trigeminal neuralgia and cerebellopontine-angle lipoma in a child. Headache. 2001;41:720–722. doi: 10.1046/j.1526-4610.2001.041007720.x. [DOI] [PubMed] [Google Scholar]
- Schlierter M, Schrey M, Schramm P. Lipoma in cerebellopontine angle. Rofo. 2007;179:1–3. doi: 10.1055/s-2007-965834. [DOI] [PubMed] [Google Scholar]
- Marton E, Basaldella L, Longatti P L. Minimal surgery for a cerebellopontine angle lipoma. J Clin Neurosci. 2009;16:129–132. doi: 10.1016/j.jocn.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Truwit C L, Barkovich A J. Pathogenesis of intracranial lipoma: an MR study in 42 patients. AJR Am J Roentgenol. 1990;155:855–864, discussion 865. doi: 10.2214/ajr.155.4.2119122. [DOI] [PubMed] [Google Scholar]
- Sunderland S. The adipose tissue of peripheral nerves. Brain. 1945;68:118–122. doi: 10.1093/brain/68.2.118. [DOI] [PubMed] [Google Scholar]
- Marom E M, Helms C A. Fibrolipomatous hamartoma: pathognomonic on MR imaging. Skeletal Radiol. 1999;28:260–264. doi: 10.1007/s002560050512. [DOI] [PubMed] [Google Scholar]
- Lenthall R, McConachie N S, Jefferson D. Cerebellopontine angle lipoma with an incidental scalp lipoma in a patient with hemifacial spasm. Eur Radiol. 2000;10:195. doi: 10.1007/s003300050032. [DOI] [PubMed] [Google Scholar]
- Dalley R W, Robertson W D, Lapointe J S, Durity F A. Computed tomography of a cerebellopontine angle lipoma. J Comput Assist Tomogr. 1986;10:704–706. doi: 10.1097/00004728-198607000-00037. [DOI] [PubMed] [Google Scholar]
- Ruggieri R M, Manfrè L, Calbucci F, Piccoli F. Therapeutic considerations in cerebellopontine angle lipomas inducing hemifacial spasm. Neurol Sci. 2000;21:329–331. doi: 10.1007/s100720070072. [DOI] [PubMed] [Google Scholar]
- Yildiz H, Hakyemez B, Koroglu M, Yesildag A, Baykal B. Intracranial lipomas: importance of localization. Neuroradiology. 2006;48:1–7. doi: 10.1007/s00234-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Rodríguez Prado N, Llorente Pendás J L, Gómez Martínez J R, et al. Cerebellopontine angle and internal auditory canal lipomas: report of four cases and review of the literature. Acta Otorrinolaringol Esp. 2004;55:126–130. doi: 10.1016/s0001-6519(04)78495-2. [DOI] [PubMed] [Google Scholar]