ABSTRACT
Sinonasal teratocarcinosarcoma (SNTCS) is a rare, malignant neoplasm that contains both mesenchymal and epithelial components. The mortality rate for this tumor is ∼60% within 3 years, with the average survival rate being 1.7 years. Usually, this neoplasm presents with symptoms of nasal obstruction and epistaxis. Neurological symptoms from intracranial extension and dural invasion are rare presentations for this neoplasm. We present the first known case of an intracerebral metastasis of a previously resected SNTCS.
Keywords: Teratocarcinosarcoma, intracerebral, metastasis, neoplasm
Sinonasal teratocarcinosarcoma (SNTCS) is a rare malignant neoplasm consisting of primitive neuroepithelial elements with various malignant epithelial and mesenchymal components.1 Henfer and Hyams were the first to coin the term “teratocarcinosarcoma” after the clinicopathologic study of 20 patients with sinonasal tract neoplasms.2 The most common presentation for teratocarcinosarcoma is nasal obstruction and epistaxis with the average duration of symptoms being reported at 3.5 months.3,4 Overall, there is a strong male predominance with a 7:1 male to female ratio.5 Mean survival for this neoplasm has been reported at 1.7 years with a 60% mortality rate within 3 years.6 Metastasis of SNTCS is rare and has previously only been reported on the spinal axis, cervical lymph nodes, and respiratory tract.4,7 Because of the usual location in the sinonasal tract and symptomatology, this neoplasm is rarely encountered by neurosurgeons. We present the first known case of SNTCS with intracerebral metastasis managed via a multidisciplinary approach.
CASE REPORT
The patient is a 51-year-old female who was initially referred to otolaryngology clinic with chronic sinusitis. The patient described a 1-year history of anosmia and nasal airway obstruction. She also expressed a remote history of right facial pain, headaches, and epistaxis. The only positive finding on physical exam was right-sided proptosis. Magnetic resonance imaging (MRI) revealed a large, contrast enhancing heterogeneous mass in the right nasopharynx with extension through the cribriform plate and into the sphenoid sinus (Fig. 1).
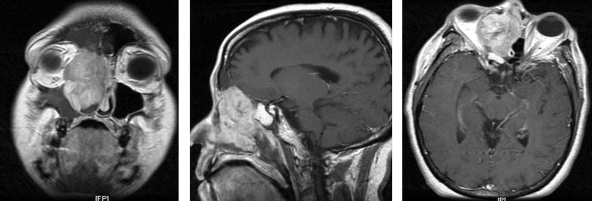
Figure 1.
(A) Coronal T1-weighted MRI showing an inhomogeneously enhancing lesion of the right sinonasal cavity. (B) Sagittal MRI showing intracranial extension with erosion through the cribriform plate. (C) Axial MRI showing invasion into the right orbit with lateral displacement of the medial rectus muscle.
Shortly afterward, the patient was brought to the operating room and had a combined craniofacial resection of the tumor. A bicoronal skin incision was made and a subgaleal flap was mobilized anteriorly toward the orbital ridge. Pericranium with a vascularized pedicle was harvested for later reconstruction of the floor of the anterior fossa. A bifrontal craniotomy was performed and extradural subfrontal access obtained. Transbasal osteotomies were performed to access the sphenoid and ethmoid sinuses. The tumor could easily be visualized extending up through the right orbital roof and caudally from the planum sphenoidale to the tuberculum sella. The tumor was peeled away from the dura, and there was no dural breach noted. Debulking of the tumor was performed from a superior to inferior manner and a thin layer of tumor was left on the periorbita for otolaryngology debulking. Once total resection of the intracranial portion of the tumor was performed, hemostasis was achieved and the pericranium was used to reconstruct the anterior floor and create a pericranial flap.
The otolaryngology service then came in to perform the transfacial portion of the case. A lateral rhinotomy with a modified Weber-Ferguson incision was performed. Once the soft tissues were elevated, a Caldwell Luc procedure was performed to confirm no tumor involvement in the maxillary sinus. After this was accomplished, the tumor was resected using a microdebrider beginning inferiorly and extending superiorly to the pericranial flap and laterally to the periorbita and the lateral nasal wall. The right nasal bone and nasal portion of the maxilla were disarticulated for access; once the tumor was debrided, a sphenoidotomy was performed and no tumor involvement was confirmed. At this point, the nasal bones were reapproximated and secured using mini plates. Postoperatively, the patient did well and was discharged to home. She received adjuvant radiation therapy and continued to do well.
Five months later, the patient returned for routine follow-up appointment and was noted to be doing well without complaints or symptoms. Surveillance computed tomography scan at the follow-up showed no recurrence of the lesion. However, she presented to the hospital 1 month later with headaches, lethargy, and confusion. An MRI revealed a heterogeneously enhancing right temporal mass (Fig. 2). The patient was admitted and underwent an image-guided temporal craniotomy for resection of the mass. During her hospital stay, she returned to her functional baseline and was discharged to home.
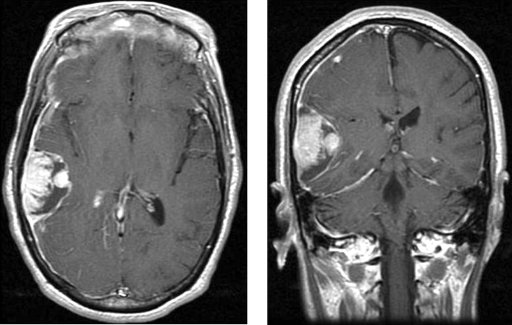
Figure 2.
Axial and coronal T1-weighted MRI depicting a heterogeneously enhancing mass with areas of hypointensity of the right temporal lobe with compressing of right lateral ventricle.
Microscopic examination showed various epithelial, neuroepithelial, and mesenchymal components as well as undifferentiated cellular clusters. Nests of benign immature squamous clusters with cytoplasmic vacuolation are identified (Fig. 3A) with surrounding primitive myxoid, fibrocollagenous, and sarcomatous stroma. Epithelial glandular structures are present with focal mucin secretions (Fig. 3B). These are strongly positive with cytokeratin. A range of benign to malignant mesenchymal areas are seen with focal fibrous area, hyalinized osteoid-like tissue, chondromyxoid foci, and undifferentiated sarcomatous areas with skeletal muscle differentiation, positive with desmin (Fig. 3C and 3D). The undifferentiated and primitive neural areas with background neurofibrillary matrix and glial cells are shown (Figs. 3E and 3F).
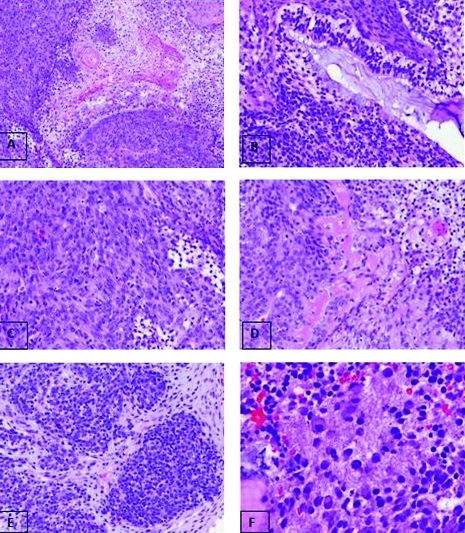
Figure 3.
Different components and structures identified in the tumor. (A) Fetal squamous epithelium, 200 × . (B) Glandular structure with columnar epithelium and mucin-secretion, 200 × . (C) Sarcomatous spindle cell area, 200 × . (D) Mesenchymal area within hyalin osteoid-like material, 200 × . (E) Clusters of undifferentiated blastomatous cells, 200 × . (F) Neural tissue with fibrillary matrix and ganglion cells, 400 × .
DISCUSSION
Teratocarcinosarcoma is a rare neoplasm with less than 65 reported cases in the literature. The term teratocarcinosarcoma was first coined by Heffner and Hymans in 1984 after they reviewed 20 cases of sinonasal tract neoplasms with mixed histological features of carcinosarcoma and teratoma.2 Along with the pathological features of SNTCS, they also observed the treatment and survival rates in their patients. It was determined that the mean survival rate was 1.7 years with a 60% mortality within 3 years.2 SNTCS has a male predominance which has been reported at 7:1 or 8:1.5,8 This neoplasm is exclusively seen in adults with a reported age range from 18 to 79 years with a mean age of ∼55 years old.5 Diagnosis of SNTCS can prove to be difficult if only a biopsy is taken because of the heterogeneity and variegated histological architecture.4 Small sample sizes can underestimate the true histology of this neoplasm and lead to a misdiagnosis such as malignant craniopharyngioma, adenocarcinoma, squamous cell carcinoma, or esthesioneuroblastoma.1
Treatment has been relegated to surgery and/or radiation. No effective chemotherapeutic regimens have yet been reported for SNTCS in part because of its highly variable histology.1,2,4 However, single modality treatment has proven to be ineffective for locoregional control. Wei et al, in a meta-analysis of 54 cases, determined that 67% of patients that had a single surgical resection and 80% of patients treated primarily with radiation had reoccurrence, metastasis, or unresponsiveness to treatment.5
Surgery in combination with radiation has only provided mixed results for control of the primary disease. Smith et al reported a review of 10 patients treated at a single institution and compared their results with the established literature.3 In their series, they found that all but one patient underwent surgery plus radiation therapy (Sx + XRT). The other patient had surgery in combination with chemotherapy (Sx + Cx). One of the nine Sx + XRT patients was lost to follow-up; however, three of the remaining eight (37.5%) available patients died of the disease process while five of eight (62.5) patients showed no evidence of disease with the longest followed up at 372 months.
Neurosurgeons rarely encounter this neoplasm because of the usual location in the nasopharyngeal tract. The most common presenting symptoms are nasal airway obstruction and epistaxis2,3,5,6,9 and to a lesser extent dysphagia and odynophagia.7 Neurological symptoms are rare with SNTCS as they do not usually invade the intracranial space. However, there are reports of neurological symptoms from SNTCS. Terasaka et al reported a case of a 66-year-old man with progressive somnolence, headaches, and apathy with an intracranial extension of an SNTCS.1 He was treated initially with a surgical resection followed by radiation therapy. Interestingly, this patient was reported to be disease free at 31 months. The only other case of SNTCS with neurological symptoms was report by Lim et al in 2008.4 They report a case of a 32-year-old man with confusion and abnormal behavior that progressed to somnolence. After surgical resection, the patient presented 7 months later with back pain, leg weakness, and urinary incontinence. The patient underwent an MRI that revealed nodular enhancement encasing the spinal cord from C6 through the level of the conus medullaris and infiltration of the lumbosacral thecal sac. To date, this is the only reported case of metastasis of SNTCS in the craniospinal axis.
We present a unique case of a woman with SNTCS with intracerebral metastasis. Our patient initially presented with the common symptoms of nasal airway obstruction and epistaxis and had a gross total craniofacial surgical resection followed by adjuvant radiotherapy. However, she later presented with atypical symptoms of headaches, lethargy, and confusion and was found to have an intracerebral metastasis. Lim et al postulated the possibility of craniospinal axis dissemination via the cerebrospinal fluid.4 Though SNTCS is rare and not often encountered by neurosurgeons, the possibility of intracranial pathology and craniospinal dissemination must be considered in regards to this neoplasm.
REFERENCES
- Terasaka S, Medary M B, Whiting D M, Fukushima T, Espejo E J, Nathan G. Prolonged survival in a patient with sinonasal teratocarcinosarcoma with cranial extension. Case report. J Neurosurg. 1998;88(4):753–756. doi: 10.3171/jns.1998.88.4.0753. [DOI] [PubMed] [Google Scholar]
- Heffner D K, Hyams V J. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses A clinicopathologic study of 20 cases. Cancer. 1984;53(10):2140–2154. doi: 10.1002/1097-0142(19840515)53:10<2140::aid-cncr2820531025>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Smith S L, Hessel A C, Luna M A, Malpica A, Rosenthal D I, El-Naggar A K. Sinonasal teratocarcinosarcoma of the head and neck: a report of 10 patients treated at a single institution and comparison with reported series. Arch Otolaryngol Head Neck Surg. 2008;134(6):592–595. doi: 10.1001/archotol.134.6.592. [DOI] [PubMed] [Google Scholar]
- Tchoyoson Lim C C, Thiagarajan A, Sim C S, Khoo M L, Shakespeare T P, Ng I. Craniospinal dissemination in teratocarcinosarcoma. J Neurosurg. 2008;109(2):321–324. doi: 10.3171/JNS/2008/109/8/0321. [DOI] [PubMed] [Google Scholar]
- Wei S, Carroll W, Lazenby A, Bell W, Lopez R, Said-Al-Naief N. Sinonasal teratocarcinosarcoma: report of a case with review of literature and treatment outcome. Ann Diagn Pathol. 2008;12(6):415–425. doi: 10.1016/j.anndiagpath.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Szudek J, Bullock M, Taylor S M. Sinonasal teratocarcinosarcoma involving the cavernous sinus. J Otolaryngol. 2005;34(4):286–288. doi: 10.2310/7070.2005.34421. [DOI] [PubMed] [Google Scholar]
- Carrizo F, Pineda-Daboin K, Neto A G, Luna M A. Pharyngeal teratocarcinosarcoma: review of the literature and report of two cases. Ann Diagn Pathol. 2006;10(6):339–342. doi: 10.1016/j.anndiagpath.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Salem F, Rosenblum M K, Jhanwar S C, Kancherla P, Ghossein R A, Carlson D L. Teratocarcinosarcoma of the nasal cavity and paranasal sinuses: report of 3 cases with assessment for chromosome 12p status. Hum Pathol. 2008;39(4):605–609. doi: 10.1016/j.humpath.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Wellman M, Kerr P D, Battistuzzi S, Cristante L. Paranasal sinus teratocarcinosarcoma with intradural extension. J Otolaryngol. 2002;31(3):173–176. doi: 10.2310/7070.2002.10895. [DOI] [PubMed] [Google Scholar]