Abstract
Purpose
The frequently elevated activities of the c-src and c-yes products in human epithelial tumors suggest that these activated tyrosine kinases have tumorigenic functions analogous to the v-src and v-yes oncogene products. Studies of v-src transformed fibroblasts have identified many of the effectors of this potent oncogene, however since c-src and c-yes lack the mutational and promiscuous activities of their retroviral oncogene homologues, their presumptive tumorigenic functions in human epithelial tumors are more subtle, less well defined, and await identification of possible effectors more directly relevant to epithelial cells.
Experimental Design
We recently identified a transmembrane glycoprotein named Trask that is expressed in epithelial tissues but not fibroblasts and is phosphorylated by src kinases in mitotic epithelial cells. In this study we have surveyed the expression and phosphorylation of Trask in many human epithelial cancer cell lines and surgical tissues and tumors.
Results
Trask is widely expressed in human epithelial tissues but its phosphorylation is tightly regulated and restricted to detached mitotic cells or cells undergoing physiologic shedding. However abberant Trask phosphorylation is seen in many epithelial tumors from all stages including pre-invasive, invasive, and metastatic tumors. Trask phosphorylation requires src kinases, and is also aberrantly hyperphosphorylated in the src-activated PyMT mouse epithelial tumors and dephosphorylated by the src inhibitor treatment of these tumors.
Conclusions
The widespread phosphorylation of Trask in many human epithlelial cancers identifies a new potential effector of src kinases in human epithelial tumorigenesis.
Introduction
The oncogenic potential of Src kinases has been recognized for more than three decades stemming from the identification of the v-src tyrosine kinase oncogene as the tumorigenic driver of the Rous sarcoma virus (reviewed in (1)). v-src or engineered activating mutants of c-src are highly transforming in experimental models (2, 3). While the mutational activation of Src kinases is extremely rare in human tumors, Src and Yes show increased activity in many human epithelial cancers including cancers of the colon, breast, pancreas, and lung (reviewed in (4)). This is recapitulated in mouse models of epithelial cancer where Src and Yes kinases are activated in PyMT induced and in Neu-induced mammary epithelial tumors (5, 6). The essential role of Src in the PyMT model is confirmed as these tumors are suppressed in a src-null background (7). However the mechanisms that lead to the activation of Src kinases in human tumors remain undefined, and an essential role for Src kinases in human cancers remains presumptive at this point and awaits further definition.
Numerous lines of evidence suggest that the function of Src kinases may be particularly important for invasion and metastasis in human cancers. The increased invasive and metastatic properties of ErbB2-induced epithelial tumors are associated with increased expression and activity of Src and this can be reverted by pharmacologic inhibitors or dominant negative mutants of Src kinase (8). Increased invasive properties are similarly conferred to intestinal epithelial cells by overexpression of c-src (9). Treatment of cancer cells with Src-selective inhibitors reduces their invasive and migratory properties with much less effect on their proliferative attributes (10, 11). In mouse orthotopic models of human epithelial cancer, the inactivation of Src kinases identifies a function more important to invasion and metastasis than proliferative growth (12, 13). Cellular substrates of Src that are thought to mediate the invasive and migratory properties conferred by overactive Src include adhesion signaling proteins including certain integrins, focal adhesion complex proteins, and certain extracellular and membrane proteases (reviewed in (14)). Much of this evidence comes from the analysis of cellular changes induced by the highly transforming v-src oncogene product.
Although the evidence that Src and Yes are functionally important in human tumors is compelling, mechanistic exploration of this function has proven to be complex and challenging. A large body of evidence has been derived from mechanistic studies of cell transformation by v-src in fibroblast models (reviewed in (14, 15)). However the v-src oncogene product has considerable structural and functional differences from the c-src and c-yes gene products of human tumors and much more potent transforming activity compared with the human proto-oncogenes. In fact c-src is not transforming, even when overexpressed, and it’s mutational activation is very rarely seen in human cancers (reviewed in (16)). Therefore the tumor promoting functions of c-src and c-yes may be much more subtle than their viral oncogene homologs. In addition, the fibroblast models do not faithfully represent the full spectrum of human cancers. In fact the increased activity of Src kinases is mostly seen in the very common human epithelial cancers as well as the much rarer mesenchymal tumors that are modeled by experimental fibroblast systems. Epithelial cells differ significantly from mesenchymal cells in their interactions with each other and with the extracellular matrix. They form semi-permanent attachment structures such as hemidesmosomes and tight junctions as well as unidirectional attachment to basement membrane proteins. These attributes define the epithelial tissue architecture and are not present in mesenchymal cells. Cell migration and mitosis require dissociation of these and other structures and specialized mechanisms are most likely involved to allow epithelial cell detachment and migration. As such, experimental epithelial cell models may provide mechanistic insight into the role of Src kinases in epithelial tumors not appreciated from fibroblast models and there may be important Src effectors in human epithelial tumors not evident or even expressed in fibroblasts.
Our studies have focused on epithelial cell models and epithelial cancers with wildtype Src family kinases. These models may reveal mechanisms and markers not readily apparent in fibroblast models of v-src transformation, yet possibly highly relevant to the most common human malignancies. In this effort, we previously identified Trask, a substrate of Src kinases that is phosphorylated during epithelial cell mitosis (17). Trask is a type 1 transmembrane glycoprotein seen in multicellular organisms from vertebrates to mammalians, and unique in the human genome with no homologous family members and its cellular or biochemical functions are not readily predictable by comparative homology. Its structure contains a large extracellular domain containing two CUB domains and a smaller intracellular region containing a proline rich region. Trask is expressed as a 140kd membrane glycoprotein but can be cleaved by the membrane serine protease MT-SP1 within the extracellular region to yield a smaller 85kd Trask protein (17). The functional relevance of this cleavage is not yet understood, but transcriptional profiling analysis of numerous normal and tumor tissues reveals that the expression of Trask and MT-SP1 is tightly linked and particularly elevated in tumors (18, 19). Trask was also identified through independent efforts as CDCP1 in microarray analyses of RNA transcripts overexpressed in colon cancers compared with adjacent normal tissues (20) (21). In another line of study, a subtractive immunization screen designed to identify antibodies against more metastatic variants of HEp3 carcinoma cells identified SIMA135, identical to Trask/CDCP1, as a putative marker of metastasis, although further analysis in panels of tumor cell lines found no correlation with metastatic activity (22). We identified Trask/CDCP1 by virtue of it being the predominant tyrosine phosphorylated and Src inhibitor suppressible protein in detached mitotic epithelial cells (17). Trask is a substrate of Src and Yes kinases in vitro and in cells and its phosphorylation can be inhibited by several classes of Src-selective tyrosine kinase inhibitors (17). In inducible transfection models we found that Trask overexpression and constitutive phosphorylation stabilize a detached cellular phenotype and poor ability to spread on plastic.
In the current study, we report a much broader context for the physiologic phosphorylation of Trask. Although Trask is expressed in many epithelial tissues, its phosphorylation is tightly regulated and only seen in mitotic cells or in cells underoing physiologic shedding. However Trask phosphorylation is seen in most cancer cell lines in the anchorage-independent state, and widely seen in human pre-invasive, invasive, and metastatic epithelial cancers. The widespread phosphorylation of Trask in cancers identifies a new src-driven pathway, linked with the anchorage-independent attribute of epithelial tumorigenesis.
Materials and Methods
Cell Culture and reagents
All cell lines were obtained from the American Type Culture Collection. Cells were grown in a 1:1 mixture of DMEM:F12 media supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100µg/ml streptomycin, and 4mM glutamine, and incubated at 37 C in 5%CO2. MCF10A cells were grow in DMEM:F12 media supplemented with 5% donor horse serum, 0.5 ug/ml hydrocortisone, 10 ug/ml insulin, 20 ng/ml epidermal growth factor, 100 U/ml penicillin, 100 ug/ml streptomycin, and 4nM glutamine. To force cells into suspension, cells were washed in PBS and exposed to a 0.05% solution of trypsin or a 2mM solution of EDTA in Hank’s buffer. When required to maintain cells in suspension and prevent spreading and attachment, cells were spun down, resuspended in growth media, and cultured in ULC plates (Corning) for growth. To harvest lysates, cells were rapidly scraped on ice at fixed timepoints and lysed in RIPA buffer. Anti-phosphotyrosine antibodies (PY99) were purchased from SantaCruz Biotechnology, Inc (SantaCruz, CA). Polyclonal anti-Trask antibodies were generated by immunizing rabbits with a recombinant full length Trask intracellular domain. Monoclonal anti-Trask antibodies were generated by immunizing mice with a recombinant full length Trask extracellular domain and recognize both cleaved and uncleaved forms of Trask. Anti-phospho-Trask antibodies were generated against a phospho-peptide immunogen containing sequences centered around phosphorylated tyrosine 743 of Trask in rabbits and affinity purified on a phospho-peptide column. PP1 was purchased from EMD-Calbiochem (San Diego, CA). Dasatinib was purchased from Bristol-Myers Squibb (New York, NY) and purified for in vitro and in vivo use (see supplementary methods). pcDNA4-MycTrask was constructed as previously described (17). Total cellular lysates were harvested in modified RIPA buffer (10 mM Na phosphate (pH 7.2), 150 mM NaCl, 0.1% SDS, 1% NP40, 1% Na deoxycholate, protease inhibitors and 1mM sodium orthovanadate). For western blotting, 50 ug of each lysate was separated by SDS-PAGE, transferred to membrane, and immunoblotted using appropriate primary and secondary antibodies and enhanced chemoluminescence visualization. Immuno precipitations were performed by incubating 300ug of cellular lysate with the indicated antibodies overnight at 4C. Immune complexes were precipitated by incubation with protein-G sepharose beads, washed several times in RIPA buffer, and boiled in sample buffer.
Immunohistochemical studies
Deparaffinized sections were rehydrated and antigen retrieval was performed by 15 minutes incubation in warm trypsin followed by microwave in 10mM citrate buffer for total of 10 minutes in 1 minute intervals. Slides were then washed and blocked with 3% H2O2 followed by blocking in goat serum and primary incubation at 4C overnight. Secondary staining was performed using biotinylated goat anti-rabbit antibodies (Vector Labs, Burlingame, CA) and colorized using Vectastain ABC Kit (Vector Labs) and 3,3'-diaminobenzidine (DAB)-H2O2 substrate (Sigma, St, Louis, MO). Slides were then counterstained with hematoxylin, dehydrated through graded alcohols and xylene and mounted. Slides were studied and imaged under brightfield microscopy. All staining procedures included positive and negative controls. Controls were prepared from formalin-fixed paraffin embedded cell buttons of cell lines with well defined expression and phosphorylation of Trask by immunoblotting techniques. For Trask immunostains the positive control was MDA-MB-468 cells and the negative control was MCF-7 cells. For phospho-Trask immunostains the positive control was MDA-MB-468 cells and the negative controls were MCF-7 cells and dasatinib-treated MDA-MB-468 cells. MDA-468 cells have abundant expression and phosphorylation of Trask by western blotting. MCF-7 cells have no expression of Trask by western blotting or by RT-PCR. Staining intensity was scored and agreed upon by two investigators according to the following definitions: 0 indicates no visible expression (similar to negative controls). 1+ indicates expression that is faint but is identifiable and above background and negative controls. 2+ indicates moderate expression that is clearly evident but also clearly less intense than positive controls. 3+ indicates intense expression similar to positive controls. Only intensity was used for scoring. Percentages of cells were not a parameter used for scoring since Trask phosphorylation has focal characteristics in tissues that is relevant to cell behavior, and some degree of focal variability may also be due to uneven fixation artifacts. Immunohistochemically stained tissue sections were viewed and imaged using an Olympus BX41 brightfield microscope fitted with a DP70 digital camera. Images were acquired using the Olympus DP Controller software and gamma adjusted for optimal representation.
Animal studies
Animals were handled according to protocols approved by the University of California, San Francisco, Institutional Animal Care and Use Committee. Ex-vivo infection of mouse mammary epithelial cells (MECs) was performed as previously described (23). Briefly, primary MECs were harvested from 10–12 week donor mice and infected in vitro with pMIG-PyMT on two successive days and transplanted into cleared mammary fat pads of 3 week old recipient mice. Tumors developed in about 3 months. Tumor bearing mice were treated with dasatinib or vehicle control administered by Alzet Osmotic Pumps (Cupertino, CA) at a rate of 0.4 mg/day of dasatinib. Mice were sacrificed and tumors rapidly dissected and immediately fixed in formalin for immunohistochemical studies or snap frozen for western blotting studies. Pancreatic orthotopic models were generated as follows. The mouse pancreas was surgically exposed through an abdominal excision under anesthesia, and 250,000 luciferase-expressing L3.6pl tumor cells implanted directly into the pancreas, and the abdomen closed. Tumors were visible by in vivo imaging within 2–3 weeks and grossly palpable by 4–5 weeks with a very high take-rate at which time the mice were euthanized and tumors rapidly excised and snap frozen for western blotting.
Results
Trask expression and frequent phosphorylation in human cancer cell lines
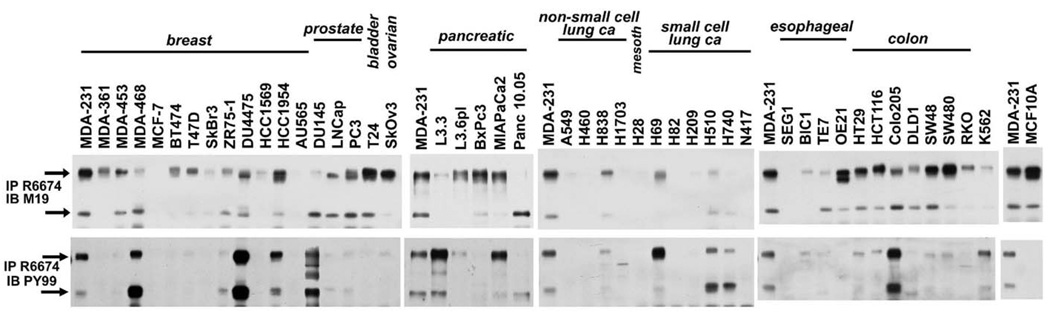
The expression and phosphorylation of Trask was surveyed in a panel of human epithelial cancer cell lines (figure 1). Trask is widely expressed in most of these cancer cell lines, although some lung cancer cell lines lack expression of Trask. Interestingly many cancer cell lines show aberrant phosphorylation of Trask, some at lower levels (such as MDA-231 or A549), and some at high levels (such as MDA-468 or Colo205). Cancer cell lines vary in their morphologic characteristics in tissue culture. Interestingly we found a correlation between Trask phosphorylation and the spread or suspended morphology of cells. Certain epithelial cancer cells which, when cultured in vitro, grow naturally in suspension or in semi-suspension show high phosphorylation of Trask (examples: MDA-468, Du4475, Colo205, H69, H510,H740). MCF10A untransformed breast epithelial cells have abundant expression of Trask but undetectable phosphorylation.
Figure 1.
The expression and phosphorylation of Trask were determined and compared in a diverse panel of epithelial cancer cell lines. Total cell lysates were immunoprecipitated with rabbit anti-Trask antibodies and immunoblotted with mouse anti-Trask antibodies or anti-phosphotyrosine antibodies. The MDA-231 cell lysate was included in all blots as a basis to allow comparison of Trask expression and phosphorylation across different blots. The MCF10A cell lysate is also included at the far right as an example of untransformed epithelial cells. Arrows indicate the 140kd and 85kd forms of Trask.
Tumor growth in vivo induces Trask phosphorylation
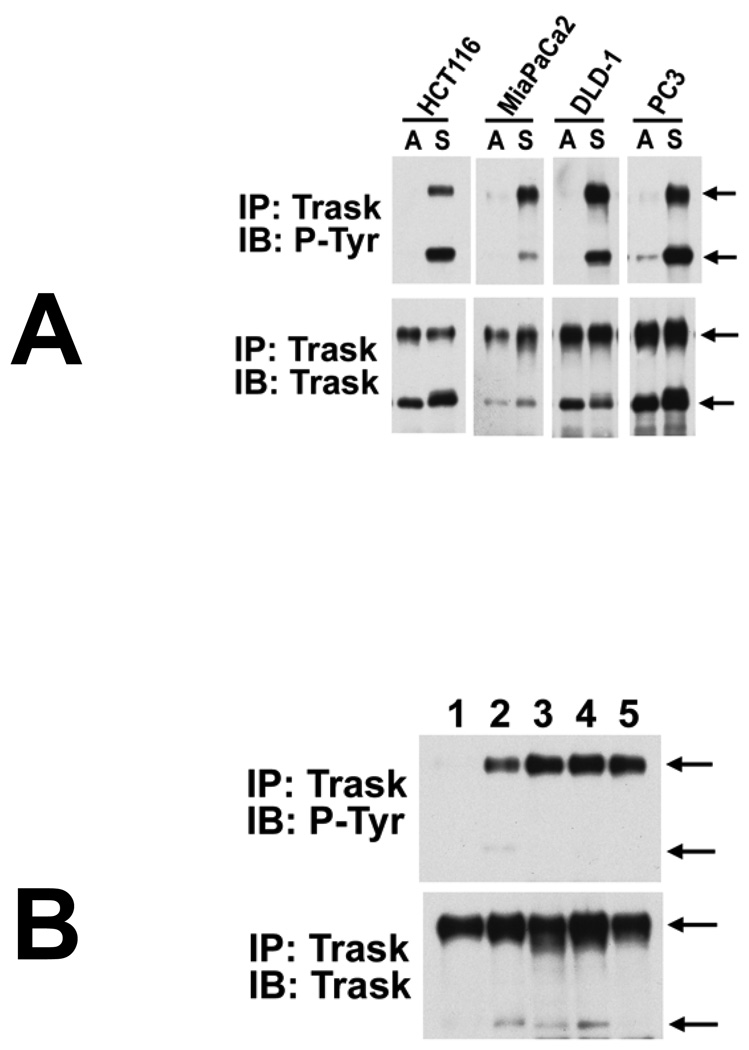
Although constitutive phosphorylation of Trask is not seen in many cancer cell lines when grown in monolayer, Trask phosphorylation can be induced in these cancer cells when deprived of anchorage. The phosphorylation of Trask is shown for four different types of cancer cells cultured in suspension (figure 2A). Since growth in vitro in monolayer is very different than growth in vivo, we compared and contrasted the in vitro and in vivo phosphorylation of Trask in L3.6pl pancreatic cancer cells. In these cells, Trask is minimally phosphorylated when grown in monolayer spread onto plastic, but phosphorylated when grown in suspension (figure 2B, lanes 1,2). When these cells are grown as an orthotopic tumor model in the mouse pancreas in vivo, Trask is phosphorylated, reflecting the adhesion-independent nature of tumorigenic growth in vivo (figure 2B, lanes 3,4). Trask is phosphorylated in the primary tumor as well as in a metastatic tumor (figure 2B, lanes 3,4,5).
Figure 2.
A) The HCT116 and DLD-1 (colon cancer), MiaPaCa2 (pancreatic cancer), and PC3 (prostate cancer) epithelial cancer cells were harvested either while adherent (labelled A) or 2 hours after being forced into suspension by EDTA (labelled S). Cell lysates were analyzed for the expression and phosphorylation of Trask as indicated. Arrows indicate the 140kd and 85kd forms of Trask. B) The expression and phosphorylation of Trask was determined and compared in L3.6pl-luc pancreatic cancer cells under different in vitro and in vivo circumstances. These L3.6pl-luc cells express luciferase for use in in vivo imaging. All immunoprecipitates are from equal amounts of cell lysate. Lane 1 and 2 correspond to lysates from adherent and suspended cells respectively, growing in vitro. These cells were also grown orthotopically within the pancreas of nude mice and their growth monitored by in vivo imaging as described in methods. Well established pancreatic tumors from two different sacrificed mice were harvested, snap frozen, and their lysates used in lanes 3,4. A peritoneal metastasis from one of the mice was removed and lysate used in lane 5. These data comparing the same cancer cells in vitro and in vivo show that despite the more restricted phosphorylation of Trask in the in vitro growth model, their tumorigenic growth in vivo is characterized by maximal and constitutive phosphorylation of Trask.
Trask expression and phosphorylation in human tissues and tumors
To study the expression and phosphorylation of Trask in vivo, we raised anti-Trask and anti-pY743-Trask polyclonal antibodies. Conditions for immunohistochemical staining of paraffin embedded tissues were established and the specificity of these immunostains for Trask and phospho-Trask were confirmed using formalin-fixed paraffin embedded positive and negative controls from cell lines that express or don’t express Trask, and from cell lines with constitutively phosphorylated Trask or dephosphorylated Trask due to Src inhibitor pre-treatment (supplementary materials). In tyrosine mutation studies we identified that Trask is phosphorylated on multiple tyrosine residues simultaneously, including Tyrosines 707, 734, and 743 (data not shown). Our attempts to raise anti-p-Trask antibodies were most successful against Tyr743. The expression and phosphorylation of Trask was surveyed in a panel of archival human epithelial cancer surgical or biopsy specimens and a number of control normal biopsies or excisions by immunohistochemical studies. Trask is widely expressed in many normal epithelial tissues but not detectable by immunohistochemical analysis in mesenchymal tissues or central nervous sytem tissues (data not shown). The expression and phosphorylation of Trask in tissues and cancers of the breast, colon, and lung are shown in Tables 1,2,3.
Table 1.
Sections from the indicated archival tumor tissue sources were prepared and stained with anti-Trask and anti-phospho-Trask antibodies. The staining intensity was scored by two observers according to procedures and definitions described in methods. All immunostains and their analyses were conducted using well defined positive and negative controls as described in methods.
| Breast tissue and cancer | ||||
|---|---|---|---|---|
| Case | tissue | disease subtype | Trask score | P-Trask score |
| 1 | breast | normal | 2+* | 0 |
| 2 | breast | normal | 2+* | 0 |
| 3 | breast | normal | 2+* | 0 |
| 4 | breast | normal | 2+* | 0 |
| 5 | breast | normal | 2+* | 0 |
| 6 | breast | normal | 2+* | 0 |
| 7 | breast | normal | 2+* | 0 |
| 8 | breast | normal | 2+* | 0 |
| 9 | breast | normal | 2+* | 2+ some areas, hyperplasia |
| 10 | breast | LCIS | 0/1+ | no tumor, but 2+ in nl&ADH |
| 11 | breast | DCIS+LCIS | 2+ LCIS, no DCIS | 3+ LCIS, also 3+ col hyp, ?also in nl |
| 12 | breast | DCIS+LCIS | 1+ DCIS, 1+ LCIS | 0 |
| 13 | breast | DCIS+IDC+ILC | 2+ DCIS, 2+IDC, 2+ LN met | 0 DICS, 0 IDC, no tumor in LN |
| 14 | breast | IDC+DCIS | 3+ DCIS, 3+ IDC, 2+ LN met | 0 DCIS, 0 IDC, 0 LN met |
| 15 | breast | IDC+DCIS | 1+ DCIS, 1+ IDC, 2+ LN met | 0 DCIS, 2+ IDC, 0 LN met |
| 16 | breast | IDC+DCIS | 2+ DICS, 2/3+ IDC, 1+ LN met | 0 DICS, 0 IDC, 0 LN met |
| 17 | breast | IDC+DCIS | 2+ IDC | 2+ IDC (in emboli) |
| 18 | breast | IDC+DCIS | 1+ DCIS, 1+ IDC | 2+ DCIS (basal layer), 0 IDC |
| 19 | breast | IDC+DCIS+IDC+DCIS | 3+ DCIS, 3+ IDC, 3+ LN met | 3+ DCIS (focal), 3+ IDC, 0 LN met |
| 20 | breast | IDC+DCIS+LCIS | 2+ DICS, 1/2+ IDC | 3+ DCIS, 2+ LCIS, 2+ IDC |
| 21 | breast | IDC+LCIS | 1+ LCIS, 1+ IDC | 2+ IDC (invading), 0 LCIS |
| 22 | breast | ILC | 0 | 0 |
| 23 | breast | ILC+LCIS | 2+ LCIS, 2+ IDC, 2+ LN met | 3+ DCIS, 2+ LCIS, 3+ IDC, 0 LN met |
| 24 | breast | IDC + ILC | 0 ILC, 1+ IDC | 2+ in migrating ILC, 2+ IDC |
| 25 | breast | IDC | 2+ | 0 |
| 26 | breast | IDC | 3+ | 2+ |
| 27 | breast | LN mets | 0/1+ | 2+ LN met |
| 28 | breast | LN mets | 1+ | very rare 2+ |
| 29 | breast | liver mets | 2/3+ | 1+ |
| 30 | breast | liver mets | 0 | 3+ |
| 31 | breast | lung met | 2+ | 3+ |
| 32 | breast | liver mets | 3+ | insuff. spec |
| 33 | breast | liver mets | 3+ | 0 |
| 34 | breast | liver mets | 3+ | 0 |
| 35 | breast | liver mets | 3+ | 0 |
| 36 | breast | liver mets | 3+ | 0 |
luminal > basal
LN = lymph node
IDC = infiltrating ductal cancer
ILC = infiltrating lobular cancer
DCIS = ductal carcinoma in situ
LCIS = lobular carcinoma in situ
Table 2.
Sections from the indicated archival tumor tissue sources were prepared and stained with anti-Trask and anti-phospho-Trask antibodies. The staining intensity was scored by two observers according to procedures and definitions described in methods. All immunostains and their analyses were conducted using well defined positive and negative controls as described in methods.
| Colon tissue and cancer | ||||
|---|---|---|---|---|
| Case | tissue | disease subtype | Trask score | P-Trask score |
| 1 | colon | normal | 2+* | 0 crypts (3+ mitoses) / 2+ apex |
| 2 | colon | normal | 2+* | 0 crypts (3+ mitoses) |
| 3 | colon | normal | 2+* | 0/1+ in crypts / 2+ apex |
| 4 | colon | normal | 2+* | 0 crypts (3+ mitoses) / 2+ apex |
| 5 | colon | normal | 2+* | 0 crypts / some 2+ apex |
| 6 | colon | normal | 2+* | 0 crypts / 1+ apex |
| 7 | colon | normal | 2+* | 0 crypts / 2+ apex |
| 8 | colon | normal | 2+* | 0 crypts / some 2+ apex |
| 9 | colon | normal | 2+* | 0 crypts (3+ mitoses) / 2+ apex |
| 10 | colon | normal | 2+* | 0 crypts / 2+ apex |
| 11 | colon | normal | 2+* | 0 crypts / 2+ apex |
| 12 | colon | normal | 2+* | 0 crypts / 2+ apex |
| 13 | colon | normal | 2+* | 0 crypts / 2+ apex |
| 14 | colon | FAP | 3+ patchy | 3+ patchy |
| 15 | colon | FAP | 3+apical/1+ crypts / nl colon=3+ | 3+ patchy |
| 16 | colon | tubular adenoma | 1+ | 3+ |
| 17 | colon | tubulovillous adenoma | 1+ focal | 3+ patchy |
| 18 | colon | tubular adenoma | 2+ | 3+ |
| 19 | colon | tubular adenoma | 2+ | 3+ |
| 20 | colon | tubular adenoma | 2+ | 3+ scattered |
| 21 | colon | tubular adenoma | 2+ | 3+ |
| 22 | colon | tubular adenoma | 3+ | 1+ |
| 23 | colon | tubular adenoma | 3+ | 1+ |
| 24 | colon | villous adenoma | patches of 0, areas of 2+ | 3+ patchy |
| 25 | colon | tubular adenoma | patches of 0, areas of 2+ | 3+ patchy |
| 26 | colon | tubular adenoma + cancer | 2+ | 3+ in cancer, 0 in adenoma |
| 27 | colon | tubular adenoma + cancer | 1/2+ | 0 cancer, 3+ adenoma |
| 28 | colon | cancer | 3+ | 1+ focal |
| 29 | colon | cancer | 2+ | 2+ focal |
| 30 | colon | cancer | 2+ | 3+ focal |
| 31 | colon | cancer | 1+ | 3+ |
| 32 | colon | cancer | 1/2+ | 3+ |
| 33 | colon | cancer | 2+ | 3+ focal |
| 34 | colon | cancer + LNs | 1/2+ | 2+ |
| 35 | colon | cancer + LNs | 3+ tumor / 2+ LN | 2+ tumor / 2+ LN |
| 36 | colon | cancer + LNs | 1+ tumor / 1+ LN | mostly 0, scattered 3+ |
| 37 | colon | liver mets | 2+ | 3+ |
| 38 | colon | liver mets | 1+ | 3+ |
| 39 | colon | liver mets | 1+ | 0 |
| 40 | colon | liver mets | 2+ | 3+ only very focal |
| 41 | colon | liver mets | 1+ | 0 |
| 42 | colon | liver mets | 1+ | 3+ |
| 43 | colon | liver mets | 1+ | 3+ |
| 44 | colon | liver mets | 3+ | 1 = focal |
| 45 | colon | liver mets | 2+ | 2+ |
| 46 | colon | liver mets | 2+ | 0/ rare 3+ cells |
| 47 | colon | liver mets | 2+ | 0 |
| 48 | colon | lung mets | 2+ | 3+ focal |
| 49 | colon | lung mets | 3+ | 2+ |
| 50 | colon | lung mets | 1+ | 0 |
| 51 | colon | lung mets | 2+ | 2+ focal |
| 52 | colon | lung | 3+ | 0 |
| 53 | colon | liver met | 1+ | 3+ |
| 54 | colon | liver met | 3+ | 3+ |
| 55 | colon | liver met | 2+ | 3+ |
uniform throughout
Table 3.
Sections from the indicated archival tumor tissue sources were prepared and stained with anti-Trask and anti-phospho-Trask antibodies. The staining intensity was scored by two observers according to procedures and definitions described in methods. All immunostains and their analyses were conducted using well defined positive and negative controls as described in methods.
| Lung tissue and cancer | ||||
|---|---|---|---|---|
| Case | tissue | disease subtype | Trask score | P-Trask score |
| 1 | lung | normal lung | 3+* | 0 |
| 2 | lung | normal lung | 2+* | 0, 3+ detached / mitotic cells |
| 3 | lung | normal lung | 2+* | 0 |
| 4 | lung | normal lung | 3+* | 0, 3+ rare detaching cells |
| 5 | lung | normal lung | 2+* | 0, 3+ in mitoses |
| 6 | lung | normal lung | 3+* | 0 |
| 7 | lung | normal lung | 3+* | 0 |
| 8 | lung | normal lung | 2+* | 0 |
| 9 | lung | normal lung | 3+* | 0 |
| 10 | lung | normal lung | 2+* | 0 |
| 11 | lung | adenoca | 3+ | patchy 1+ |
| 12 | lung | adenoca | 2+ | 0 |
| 13 | lung | adenoca | 2+ | 3+ |
| 14 | lung | adenoca | 3+ | 0 |
| 15 | lung | adenoca | 2+ | 0/1+ |
| 16 | lung | adenoca | 3+ | patchy 2+ |
| 17 | lung | adenoca | 3+ | 0 |
| 18 | lung | adenoca / BAC | 2+ | 0 |
| 19 | lung | BAC | 2+ | 0 |
| 20 | lung | BAC | 0/1+ | 0 |
| 21 | lung | BAC | 0/1+ | 0 |
| 22 | lung | large cell | 3+ | 2+ |
| 23 | lung | squamous cell | 3+ | 0 |
| 24 | lung | squamous cell | 1+ | 1+ |
| 25 | lung | squamous cell | 3+ | patchy 3+ |
| 26 | lung | squamous cell | 3+ | 3+ |
| 27 | lung | squamous cell | 3+ | patchy 3+ |
| 28 | lung | squamous cell | 2+ | 0 |
| 29 | lung | squamous cell | 3+ | 3+ |
| 30 | lung | small cell ca | 1+ | 0 |
| 31 | lung | small cell ca | 1+ | 0 |
| 32 | lung | small cell ca | 1+ | 0 |
| 33 | lung | small cell ca | 2+ | 2/3+ |
| 34 | lung | small cell ca | 1+ | 0 |
| 35 | lung | small cell ca | 1+ | 3+ |
| 36 | lung | small cell ca | 1+ | 3+ |
bronchioles
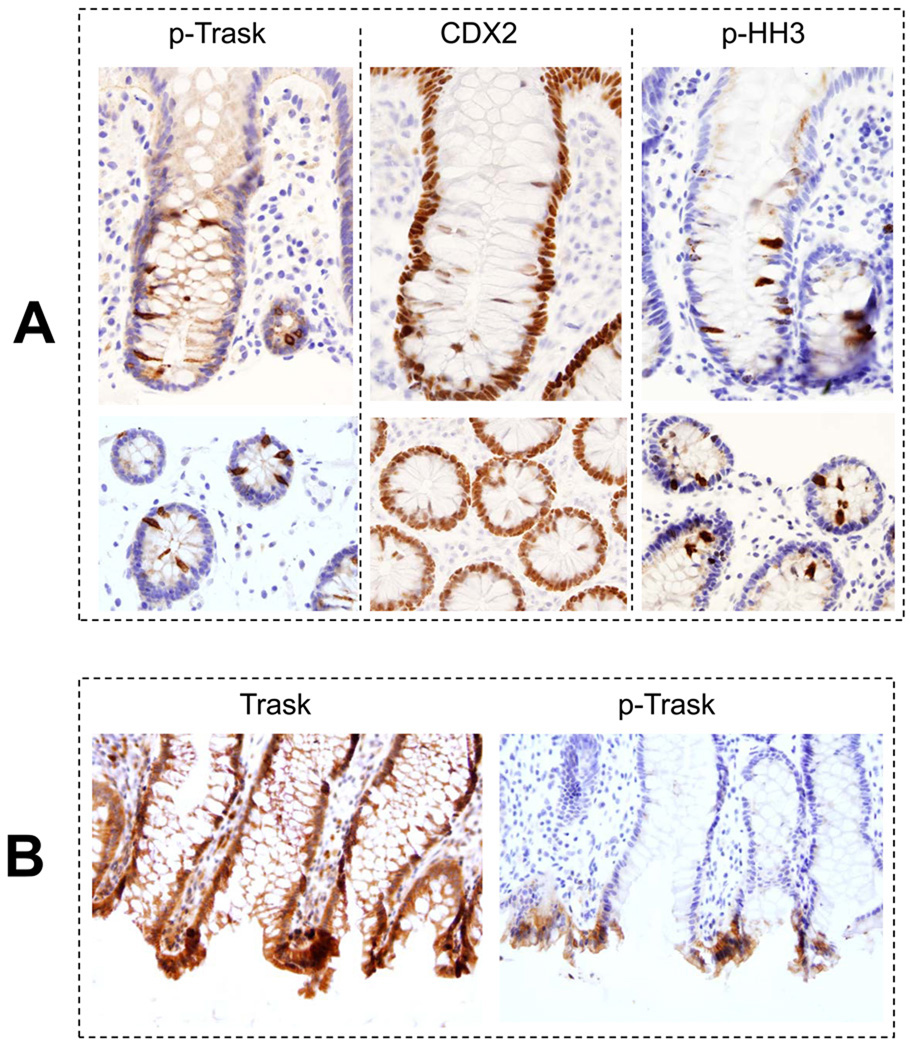
Anti-phospho-Trask immunostains showed no evidence of Trask phosphorylation in normal epithlelial tissues (Table 1,2,3). However detached mitotic cells have intense Trask phosphorylation. This is best seen in colonic crypts where there is high mitotic activity (figure 3A). The mitotic nature of these detached and hyperphosphorylated cells was confirmed by immunostaining with anti-phospho-histone H3 antibodies, and their colon epithelial identity verified by CDX2 staining (figure 3A). In addition, there is frequent non-mitotic phosphorylation of Trask at the apices of intestinal villi (figure 3B). Apical cells are frequently shed into the lumen and the apical cells with Trask phosphorylation are likely cells that are about to be shed. Intestinal epithelial cells are continuously replaced by mitotic activity within the crypts and exfoliation at the apex.
Figure 3.
A) Sections from normal colon biopsies were prepared and stained with anti-phospho-Trask antibodies. The staining intensity was scored by two observers according to procedures and definitions described in methods. All immunostains and their analyses were conducted using well defined positive and negative controls as described in methods. Trask phosphorylation is seen in occasional mitotic cells. This is shown here in the mitotically active crypt regions of normal colonic mucosa. The detaching cells in the crypts were confirmed to be epithelial cells and not extrinsic cells as shown by staining with the intestinal epithelial differentiation marker CDX2 and confirmed to be mitotic cells by the mitotic marker phospho-histone H3. B) Trask phosphorylation is also seen in some clusters of cells at the apices of colonic villi where shedding commonly occurs.
Trask expression and frequent phosphorylation in human cancers
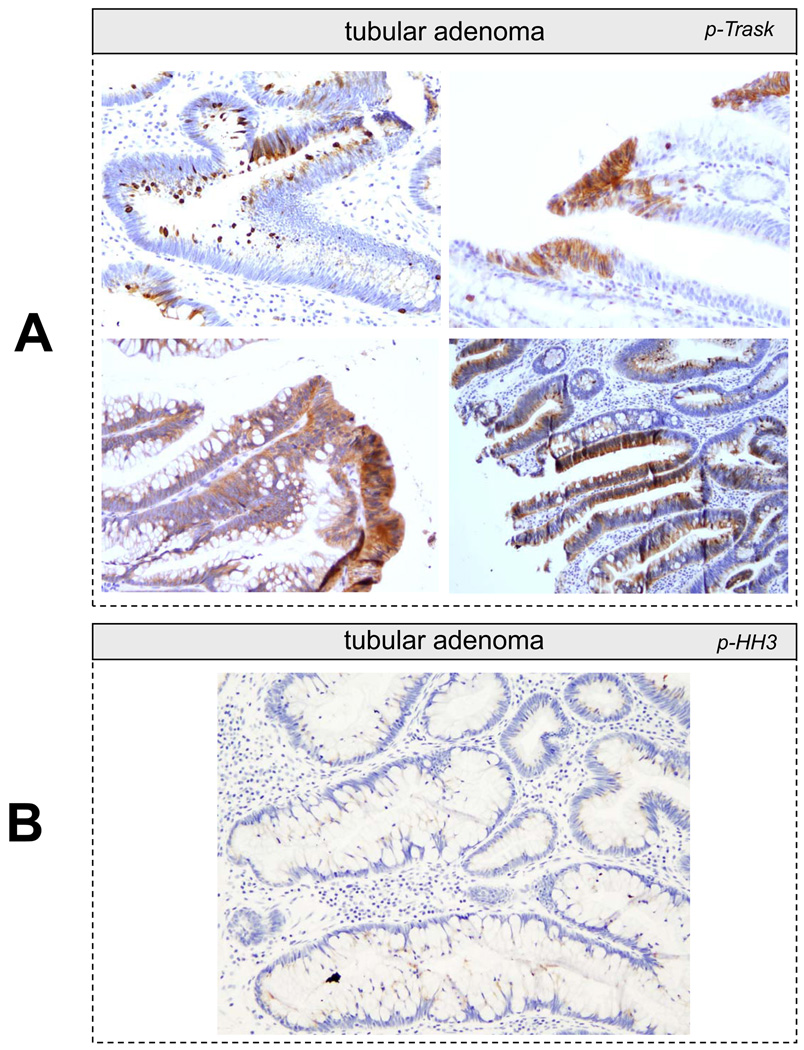
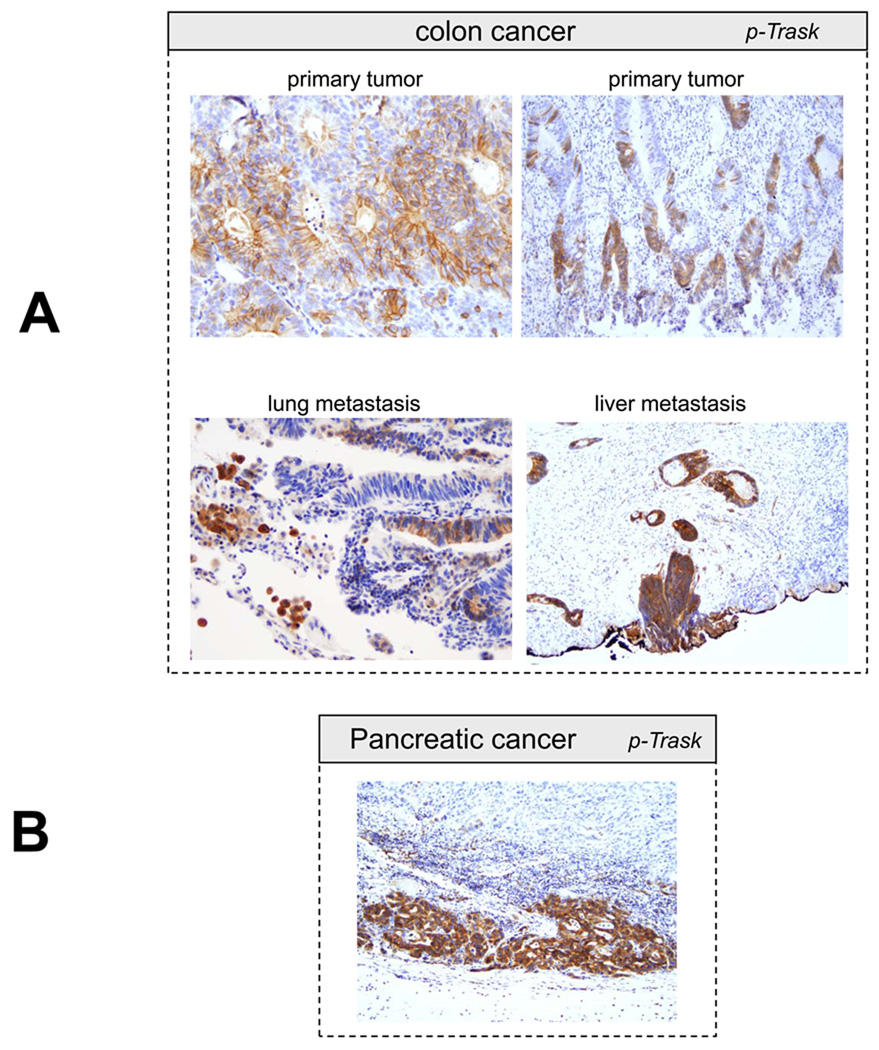
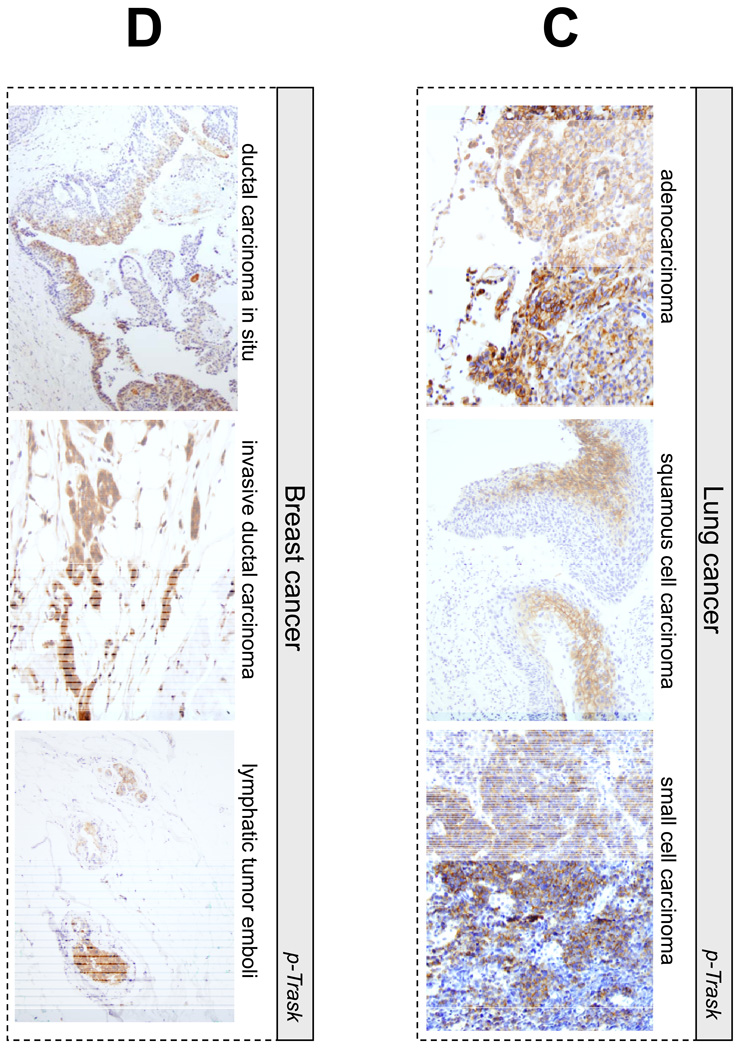
Trask is expressed in most epithelial cancers that we surveyed. In addition, Trask phosphorylation is seen in many cancers. Trask phosphorylation is seen in early pre-invasive cancers, such as tubular adenomas of the colon and rare cases of ductal carcinoma in situ of the breast (figure 4A,5D), as well as invasive primary tumors, and tumors at sites of distant metastases (figure 5A–D). In some tubular adenomas there is abundant tumor cell shedding with high Trask phosphorylation (figure 4A). These Trask phosphorylated detached cells are not mitotic cells as confirmed by p-HH3 staining (figure 4B) and they are not at the apices of the villi where physiological shedding is seen in normal colons. Rather they reveal an abnormal interphase detachment and shedding in these pre-invasive cancers. In some tumors, Trask phosphorylation is seen in focal areas and in a patchy distribution, while other tumors show a more widespread phosphorylation of Trask. Trask phosphorylation is also seen in lymphatic tumor emboli (figure 5D). The widespread phosphorylation of Trask seen in tumors is not due to the mitotic phase, as evidenced by the counterstain which shows that most of the tumor cells are in interphase. Intense phosphorylation of Trask is seen in some cancers (figure 5).
Figure 4.
A) Representative images from immunostains of colon tubular adenomas is shown. In contrast to normal colonic crypts, where Trask phosphorylation is restricted to mitotic cells, in adenomas there is abundant Trask phosphorylation in many clusters and foci of tumor cells. There is also abundant tumor cell luminal shedding. B) The frequent luminal shedding in adnomas is non-mitotic as confirmed by the absence of phospho-Histone H3 staining.
Figure 5.
Representative images from phospho-Trask immunostains are shown for a number of subtypes of epithelial cancers as indicated.
Trask is a unique Src substrate
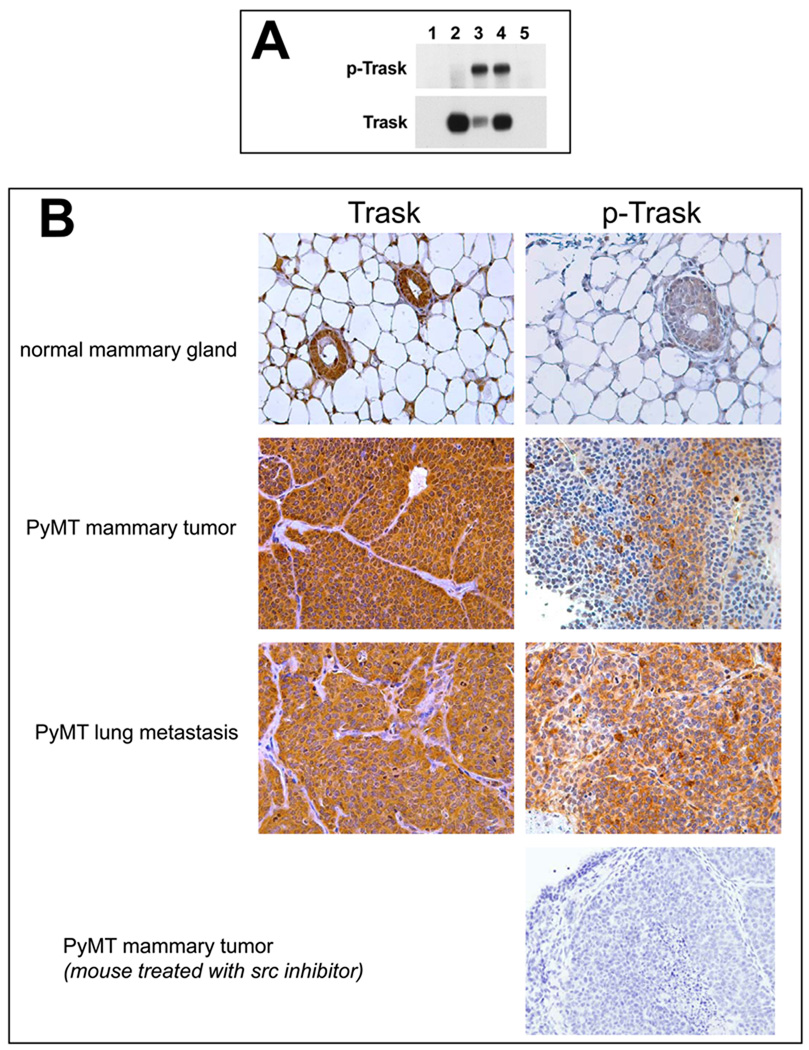
The phosphorylation of Trask is tightly linked with Src kinases and in contrast to many of the well known substrates of Src kinases, Trask may be a unique subtrate of Src kinases or at minimum, uniquely dependent on Src kinases for tyrosine phosphorylation. Although the transfection and overexpression of Trask in all cells that we have tested induces its phosphorylation, its overexpression in SYF (Src/Yes/Fyn null) cells shows no evidence of phosphorylation (figure 6A), suggesting that Src kinases are essential for Trask phosphorylation. The co-transfection of c-src or c-yes into SYF cells restores Trask phosphorylation, further confirming the essential role of src kinases in Trask phosphorylation (figure 6A).
Figure 6.
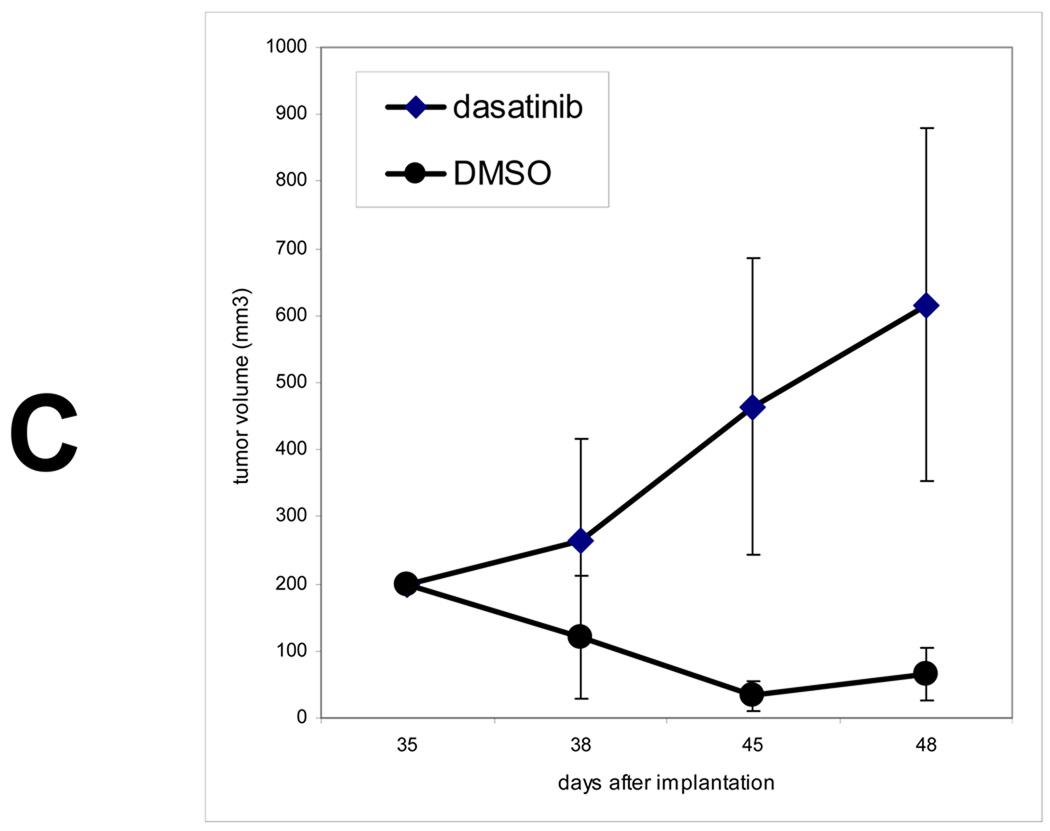
A) SYF cells were transfected with a pcDNA4-MycTrask expression vector and either Src or Yes expressing vectors and the expressed Trask protein was immunoprecipitated with anti-myc antibodies and immunoblotted with anti-phosphotyrosine or anti-Trask antibodies. Lanes correspond to 1) untransfected cells, 2) cells transfected with pcDNA4-MycTrask and pCMV6 vector, 3) cells transfected with pcDNA4-MycTrask and pCMV6-src, 4) cells transfected with pcDNA4-MycTrask and pCMV6-yes. Lane 5 contains the same lysate as lane 4 but was immunoprecipitated with IgG control. B) PyMT induced mammary tumors were generated in mice as described in Methods and allowed to grow to about 2.5 cm in size. Mice were sacrificied and tumor tissues were fixed in formalin and lung metastases were dissected, identified and fixed. Sections of normal mammary tissue from control mice as well as sections from PyMT induced tumors and their lung metastases were studied by immunohistochemical analyses to determine the expression and phosphorylation of Trask. Tumors from mice treated with the Src inhibitor dasatinib for 1 week were also harvested for analysis. Trask phosphorylation is induced in this tumor model and dephosphorylated by Src inhibitor therapy. C) Mice were orthotopically implanted with PyMT induced syngeneic tumors and after tumors were established at approximately one month, 30 tumor-bearing mice were randomized to two arms and treated with dasatinib or DMSO control and tumor sizes were measured twice weekly.
To further confirm the link between Src and Trask phosphorylation in vivo, we studied the phosphorylation of Trask in a model of Src-induced epithelial tumorigenesis. Mouse mammary tumors induced by the expression of the Polyoma middle T (PyMT) antigen are driven by the PyMT induced activation of Src (7). We established an orthotopic model of PyMT-driven mouse by ex-vivo PyMT retroviral infection of mouse mammary epithelium and re-implantation into the mammary fat pad. Trask expression and phosphorylation were studied in these tumors and their lung metastases by immunohistochemical methods. Trask is expressed in the normal mammary epithelium, but not phosphorylated (figure 6B). However PyMT-induced mammary tumors show significant phosphorylation of Trask, particularly in lung metasases (figure 6B). The phosphorylation of Trask in these tumors is due to the activation of Src kinases by PyMT and can be suppressed by treating the mice with Src inhibitors (figure 6B). The phosphorylation of Trask is not seen in all parts of the tumor, but rather in a patchy and focal distribution. The growth of these tumors is highly suppressible by Src inhibitors as expected from their Src-driven biology (figure 6C).
Discussion
A principal function of epithelial cells is adherence to tissue level architecture. At its most basic level, this architecture consists of monolayers of attached cells against a basement membrane. This highly structured organization in epithelial tissues allows them to serve as protective barriers and function to interface with the luminal or outside environment. Certain cellular functions require disengagement from substratum, including functions such as cell migration during development or wound repair, mitotic cell division, and physiologic shedding. Disengagement from substratum requires the activation of autonomous signaling mechanisms not regulated by tissue architecture. The activation of such anchorage-independent programs and the ability to defy tissue-level imposed programs are also important parts of epithelial tumorigenesis. Much of the signaling that underlies the anchorage-dependency and independency of epithelial cells occurs through tyrosine phosphorylation. Here we report that a common signaling pathway that characterizes the anchorage independent growth of epithelial tumor cells is the Src family kinase phosphorylation of their transmembrane substrate Trask.
In untransformed epithelial cells, the phosphorylation of Trask is tightly regulated and Trask phosphorylation is only seen during circumstances of mitotic detachment or physiological shedding such as seen at the apex of the intestinal villi. Trask phosphorylation can be induced in vitro by forced detachment and growth in suspension. In contrast to the tightly regulated phosphorylation of Trask in untransformed epithelial cells and tissues, we find that the Src phosphorylation of Trask is commonly seen in many epithelial cancers and cancer cell lines. We see Trask phosphorylation in all stages of cancer including pre-invasive cancers such as tubular adenomas of the colon, as well as invasive, and metastatic cancers. As such, the abberant phosphorylation of Trask is not an activity acquired in later stages of invasive or metastatic cancers. It is possible that Trask phosphorylation in pre-invasive cancers identifies those pre-invasive tumors that are at high risk for invasion and dissemination. Future studies may attempt to test that hypothesis, however this may be too simplistic a hypothesis, considering what we know so far about the functions of Trask. It is likely that both the phosphorylation and the dephosphorylation of Trask are important functions in migrating cells and likely also in invading and metastasizing cells and some level of regulation would be optimal for these tumorigenic functions. As such, Trask phosphorylation may be a marker of cellular activities at certain points in time or certain tumor areas. Consistent with this, we see only focal phosphorylation of Trask in many tumors. The spacial focality we seen in the immunostains may merely reflect a snapshot in time captured at the moment of tissue acquisition and fixation, and may be evidence of a wider temporal plasticity in Trask phosphorylation reflecting the dynamic nature of tumor cell adhesion and migration characteristics in vivo that we are unable to appreciate in these snapshot analyses. The cellular attributes that Trask phosphorylation may be reporting could be disrupted cell-stromal or cell-cell interactions, changes in cytoskeletal signaling, or activation of anoikis resistance. We have evidence that Trask phosphorylation is regulated by cell-cell and cell-matrix engagement (manuscript in submission). Several studies have reported the activation of Src kinases when epithelial cells detach from matrix and its function in averting anoikis when detached (24–26). The specific role of Trask/CDCP1 in promoting anoikis resistance was recently demonstrated in a lung cancer cell line (27).
The apparent deregulation of Trask in pre-invasive epithelial tumors is interesting but not altogether surprising. Indeed, increased activity of both Src or Yes is an early event in colon neoplasia and seen very frequently in pre-invasive lesions of the colon (28–30). The finding of Trask phosphorylation in pre-invasive tumors suggests that some of the molecular and possibly phenotypic attributes of invasive or metastatic epithelial cancers are already present in pre-invasive tumors. Consistent with this, the activation of Src and Yes in pre-invasive tumors of the colon is seen predominantly in tumors at highest risk for progression to invasive cancer (28, 29). The deregulation of Trask in pre-invasive tumors could have phenotypic consequences, but these would likely be clinically undetectable with little symptomatic consequence. These could include phenotypes such as shedding of tumor cells. However due to the intact nature of the basement membrane in pre-invasive tumors, the shedding would be entirely into the epithelial lumen which would be of little clinical or symptomatic consequence to affected patients. Indeed we do find significant interphase tumor cell shedding in tubular adenomas which we do not see in normal colon tissues (figure 4A). Trask phosphorylation could also increase the survival of luminally shed cells. Consistent with this, exfoliated tumor cells are detected much more succesfully in the stools of patients with tubular adenomas or colon cancers compared with the stools of patients with inflammatory diseases of the colon or normal colons (31, 32) and exfoliated breast epithelial cells are detected in nipple aspirates of women with pre-neoplastic lesions of the breast (33). Although not clinically significant in pre-invasive disease, the phenotypic consequence of deregulated Trask phosphorylation may be much more significant after progression to invasive disease, since the ability to survive and migrate within connective tissues, lymphatics, and the bloodstream can be mediated through Trask phosphorylation. Consistent with this, we do see Trask phosphorylation in tumor emboli (figure 5D).
When we use the same tumor cells to compare in vitro and in vivo growth, we find that Trask phosphorylation in the in vivo model resembles the anchorage-deprived state of the in vitro model, not the adherent monolayer growth (figure 2B). This is not surprising, since it is widely recognized that the growth of tumor cell lines in flattened monolayers on tissue culture treated plasticware represents an artificial state with many differences from their growth in vivo.
The mechanisms by which Trask may promote survival in suspended epithelial cells are currently unknown. Trask/CDCP1 was found to bind PKC delta in a phosphorylation-dependent manner through the C2 domain of PKC delta (34). PKC delta is thought to regulate apoptosis although its role may be complex and both pro-apoptotic and anti-apoptotic functions have been attributed to PKC delta (35). The functions of PKC delta may be context-dependent. In murine mammary epithelial cells, PKC delta activation promotes the ability to survive and grow in an anchorage-independent manner (36). Almost surely Trask regulates functions other than survival, since we have previously shown that the forced overexpression and over phosphorylation of Trask retains detached epithelial cells in suspension and prevents them from re-spreading on substrate which suggests that Trask phosphorylation impacts cytoskeletal signaling as well (17). We have been studying the role of Trask in cytoskeletal signaling and have identified mechanistic links between Trask and components of the actin cytoskeleton, although this evidence is only preliminary at this point.
The fact that the phosphorylation of Trask is entirely dependent on src kinases may suggest its use as an in vivo marker of the activity of Src kinases. Its phosphorylation in PyMT induced tumors and in many human cancers is consistent with this. However it is unlikely that the level of Trask phosphorylation faithfully reflects the activity of Src kinases. In the tumor cell panel reported here, the level of Trask phosphorylation varies widely and does not parallel the much more uniform activity of Src kinases previously published by ourselves and others (37, 38). In addition, although epithelial cells undergoing mitosis or detachment show modest increases in Src kinase activity (39), the abundant phosphorylation of Trask seen in these circumstances greatly exceeds increases in Src kinase activities (26). In addition, Trask phosphorylation appears to be partly dependent on Trask expression, and its phosphorylation can be induced by its overexpression ((17) and manuscript in preparation). Clearly, there are many variables other than the steady state activity of Src kinases that regulate the Src-mediated phosphorylation of Trask.
In summary, Trask is a recently identified Src substrate that may be highly relevant to epithelial tumorigenesis. The evidence indicates that Trask is a principal marker of anchorage-independent signaling in epithelial cells and its Src-driven phosphorylation is tightly regulated in epithelial cells. The deregulation of Trask phosphorylation is a common and early event in epithelial tumorigenesis and further mechanistic exploration of its signaling functions should be of high priority.
Statement of translational relevance
Src inhibitors are being studied in patients with epithelial malignancies. This class of anti-tumor agents presents unique challenges in clinical testing and development, principally because the tumorigenic functions and downstream molecular effectors of src, and the expected biological outcomes of src inhibitor therapy are not yet well described. In this paper we present a novel Src substrate that is abberantly phosphorylated in epithelial cancers. Trask phosphorylation is uniquely linked with Src kinases, relatively unique to epithelial tissues, and physiologically relevant to the anchorage independent state in epithelial cells. These attributes make Trask an important new biomarker that may not only be an effector of activated src kinases in epithelial tumors, but may have predictive or pharmacodynamic utility in the clinical development of src inhibitors in epithelial cancers.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health grant CA113952 and the American Cancer Society RSG-0213901CDD. DS is supported by a Susan G. Komen for the Cure postdoctoral fellowship. CHW is supported by a California Breast Cancer Research Program Post-doctoral fellowship. We wish to acknowledge the services of the Immunohistochemical Core, the Tissue Core, and the Mouse Pathology Core facilities of the UCSF Comprehensive Cancer Center in this work, and in particular the technical assistance of Lorretta Chan of the immunohistochemical core.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Martin GS. The road to Src. Oncogene. 2004;23:7910–7917. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright CA, Eckhart W, Simon S, Kaplan PL. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987;49:83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- 3.Jove R, Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- 4.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 5.Muthuswamy SK, Muller WJ. Activation of Src family kinases in Neu-induced mammary tumors correlates with their association with distinct sets of tyrosine phosphorylated proteins in vivo. Oncogene. 1995;11:1801–1810. [PubMed] [Google Scholar]
- 6.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Molecular and Cellular Biology. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy CT, Muthuswamy SK, Cardiff RD, Soriano P, Muller WJ. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes & Development. 1994;8:23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- 8.Tan M, Li P, Klos KS, et al. ErbB2 promotes Src synthesis and stability: novel mechanisms of Src activation that confer breast cancer metastasis. Cancer Res. 2005;65:1858–1867. doi: 10.1158/0008-5472.CAN-04-2353. [DOI] [PubMed] [Google Scholar]
- 9.Pories SE, Hess DT, Swenson K, et al. Overexpression of pp60c-src elicits invasive behavior in rat colon epithelial cells. Gastroenterology. 1998;114:1287–1295. doi: 10.1016/s0016-5085(98)70435-4. [DOI] [PubMed] [Google Scholar]
- 10.Vultur A, Buettner R, Kowolik C, et al. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther. 2008;7:1185–1194. doi: 10.1158/1535-7163.MCT-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito H, Gardner-Thorpe J, Zinner MJ, Ashley SW, Whang EE. Inhibition of tyrosine kinase Src suppresses pancreatic cancer invasiveness. Surgery. 2003;134:221–226. doi: 10.1067/msy.2003.224. [DOI] [PubMed] [Google Scholar]
- 12.Yezhelyev MV, Koehl G, Guba M, et al. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004;10:8028–8036. doi: 10.1158/1078-0432.CCR-04-0621. [DOI] [PubMed] [Google Scholar]
- 13.Boyer B, Bourgeois Y, Poupon MF. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 2002;21:2347–2356. doi: 10.1038/sj.onc.1205298. [DOI] [PubMed] [Google Scholar]
- 14.Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- 15.Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src's hold over actin and cell adhesions. Nature Reviews Molecular Cell Biology. 2002;3:233–245. doi: 10.1038/nrm779. [Review] [119 refs] [DOI] [PubMed] [Google Scholar]
- 16.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM. Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene. 2005;24:5333–5343. doi: 10.1038/sj.onc.1208582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatt AS, Welm A, Farady CJ, Vasquez M, Wilson K, Craik CS. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci U S A. 2007;104:5771–5776. doi: 10.1073/pnas.0606514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt AS, Takeuchi T, Ylstra B, et al. Quantitation of membrane type serine protease 1 (MT-SP1) in transformed and normal cells. Biol Chem. 2003;384:257–266. doi: 10.1515/BC.2003.029. [DOI] [PubMed] [Google Scholar]
- 20.Scherl-Mostageer M, Sommergruber W, Abseher R, Hauptmann R, Ambros P, Schweifer N. Identification of a novel gene, CDCP1, overexpressed in human colorectal cancer. Oncogene. 2001;20:4402–4408. doi: 10.1038/sj.onc.1204566. [DOI] [PubMed] [Google Scholar]
- 21.Perry SE, Robinson P, Melcher A, et al. Expression of the CUB domain containing protein 1 (CDCP1) gene in colorectal tumour cells. FEBS Lett. 2007;581:1137–1142. doi: 10.1016/j.febslet.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Hooper JD, Zijlstra A, Aimes RT, et al. Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene. 2003;22:1783–1794. doi: 10.1038/sj.onc.1206220. [DOI] [PubMed] [Google Scholar]
- 23.Welm AL, Sneddon JB, Taylor C, et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A. 2007;104:7570–7575. doi: 10.1073/pnas.0702095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Windham TC, Parikh NU, Siwak DR, et al. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- 25.Wei L, Yang Y, Zhang X, Yu Q. Altered regulation of Src upon cell detachment protects human lung adenocarcinoma cells from anoikis. Oncogene. 2004;23:9052–9061. doi: 10.1038/sj.onc.1208091. [DOI] [PubMed] [Google Scholar]
- 26.Loza-Coll MA, Perera S, Shi W, Filmus J. A transient increase in the activity of Src-family kinases induced by cell detachment delays anoikis of intestinal epithelial cells. Oncogene. 2005;24:1727–1737. doi: 10.1038/sj.onc.1208379. [DOI] [PubMed] [Google Scholar]
- 27.Uekita T, Jia L, Narisawa-Saito M, Yokota J, Kiyono T, Sakai R. CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma. Mol Cell Biol. 2007;27:7649–7660. doi: 10.1128/MCB.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cartwright CA, Meisler AI, Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proceedings of the National Academy of Sciences USA. 1990;87:558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pena SV, Melhem MF, Meisler AI, Cartwright CA. Elevated c-yes tyrosine kinase activity in premalignant lesions of the colon. Gastroenterology. 1995;108:117–124. doi: 10.1016/0016-5085(95)90015-2. [see comments] [DOI] [PubMed] [Google Scholar]
- 30.Iravani S, Mao W, Fu L, et al. Elevated c-Src protein expression is an early event in colonic neoplasia. Lab Invest. 1998;78:365–371. [PubMed] [Google Scholar]
- 31.Villa E, Dugani A, Rebecchi AM, et al. Identification of subjects at risk for colorectal carcinoma through a test based on K-ras determination in the stool. Gastroenterology. 1996;110:1346–1353. doi: 10.1053/gast.1996.v110.pm8613038. [DOI] [PubMed] [Google Scholar]
- 32.Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219–1227. doi: 10.1053/gast.2000.19580. [DOI] [PubMed] [Google Scholar]
- 33.Wrensch MR, Petrakis NL, Miike R, et al. Breast Cancer Risk in Women With Abnormal Cytology in Nipple Aspirates of Breast Fluid. 2001:1791–1798. doi: 10.1093/jnci/93.23.1791. [DOI] [PubMed] [Google Scholar]
- 34.Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 36.Grossoni VC, Falbo KB, Kazanietz MG, de Kier Joffe ED, Urtreger AJ. Protein kinase C delta enhances proliferation and survival of murine mammary cells. Mol Carcinog. 2007;46:381–390. doi: 10.1002/mc.20287. [DOI] [PubMed] [Google Scholar]
- 37.Moasser MM, Srethapakdi M, Sachar KS, Kraker AJ, Rosen N. Inhibition of Src Kinases by a Selective Tyrosine Kinase Inhibitor Causes Mitotic Arrest. Cancer Res. 1999;59:6145–6152. [PubMed] [Google Scholar]
- 38.Biscardi JS, Belsches AP, Parsons SJ. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog. 1998;21:261–272. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 39.Park J, Meisler AI, Cartwright CA. c-Yes tyrosine kinase activity in human colon carcinoma. Oncogene. 1993;8:2627–2635. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.